Abstract
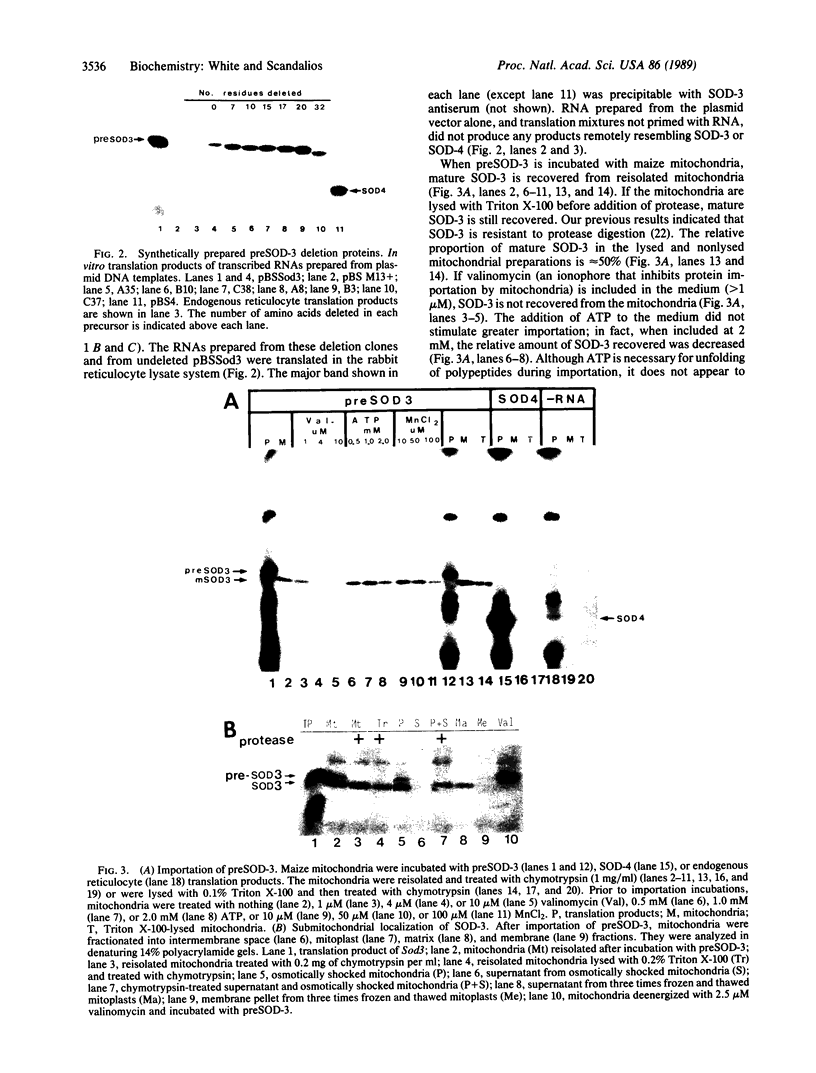
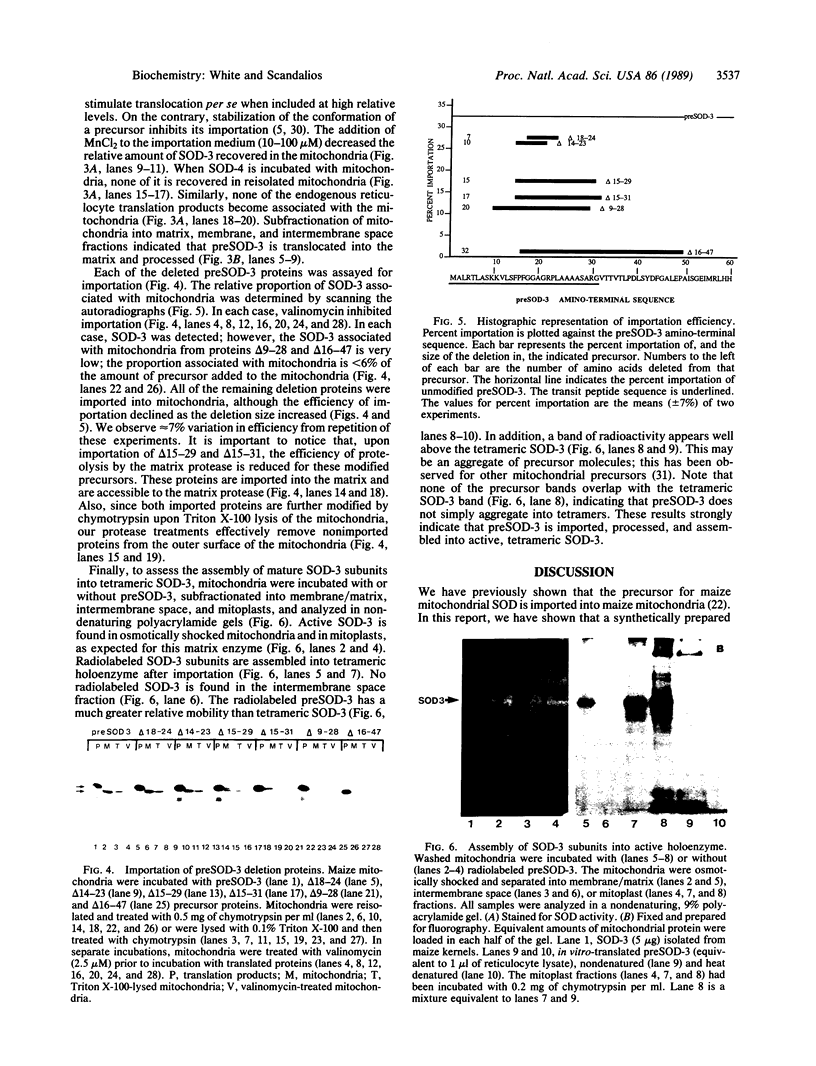
The maize mitochondrial superoxide dismutase (SOD; EC 1.15.1.1), a nuclear gene product, has been previously shown to be imported into maize mitochondria. The cDNA for maize mitochondrial SOD was subcloned into a vector containing the T7 promoter. Deletions were made in the transit peptide coding region of the cDNA. The undeleted and deleted proteins were synthetically produced by transcription and translation in vitro. Undeleted preSOD-3 is translocated into isolated maize mitochondria with an efficiency of approximately 30%. Mature SOD-3 subunits are recovered from the matrix but not the membranes of subfractionated mitochondria. These subunits are assembled into the tetrameric holoenzyme. The modified SOD-3 precursors are imported into mitochondria at lower efficiencies than undeleted preSOD-3. The relative import efficiency appears to be dependent upon the deletion size. To our knowledge such analysis of a plant mitochondrial precursor protein has not been reported previously.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., McCammon M. T., Montgomery D. L., Douglas M. G. Sequences required for delivery and localization of the ADP/ATP translocator to the mitochondrial inner membrane. Mol Cell Biol. 1986 Feb;6(2):626–634. doi: 10.1128/mcb.6.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison D. S., Schatz G. Artificial mitochondrial presequences. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9011–9015. doi: 10.1073/pnas.83.23.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J. A., Chandlee J. M., Scandalios J. G. Purification and Partial Characterization of a Genetically-Defined Superoxide Dismutase (SOD-1) Associated with Maize Chloroplasts. Plant Physiol. 1983 Sep;73(1):31–35. doi: 10.1104/pp.73.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J. A., Scandalios J. G. Isolation and characterization of the cytosolic and mitochondrial superoxide dismutases of maize. Arch Biochem Biophys. 1981 Feb;206(2):249–264. doi: 10.1016/0003-9861(81)90089-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Douglas M. G. The role of protein structure in the mitochondrial import pathway. Unfolding of mitochondrially bound precursors is required for membrane translocation. J Biol Chem. 1987 Nov 15;262(32):15605–15609. [PubMed] [Google Scholar]
- Colman A., Robinson C. Protein import into organelles: hierarchical targeting signals. Cell. 1986 Aug 1;46(3):321–322. doi: 10.1016/0092-8674(86)90650-1. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G. Transient involvement of signal recognition particle and its receptor in the microsomal membrane prior to protein translocation. Cell. 1983 Dec;35(3 Pt 2):677–685. doi: 10.1016/0092-8674(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Pollock R. A., Rosenberg L. E. Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell. 1986 Feb 14;44(3):451–459. doi: 10.1016/0092-8674(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The amino-terminal region of an imported mitochondrial precursor polypeptide can direct cytoplasmic dihydrofolate reductase into the mitochondrial matrix. EMBO J. 1984 Dec 20;3(13):3149–3156. doi: 10.1002/j.1460-2075.1984.tb02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Tobin E. M. Transit peptides of nuclear-encoded chloroplast proteins share a common amino acid framework. EMBO J. 1986 Jan;5(1):9–13. doi: 10.1002/j.1460-2075.1986.tb04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Hoeben P., Tropschug M., Neupert W. The carboxyl-terminal two-thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria. J Biol Chem. 1987 Nov 5;262(31):14851–14854. [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steeg H., Oudshoorn P., Van Hell B., Polman J. E., Grivell L. A. Targeting efficiency of a mitochondrial pre-sequence is dependent on the passenger protein. EMBO J. 1986 Dec 20;5(13):3643–3650. doi: 10.1002/j.1460-2075.1986.tb04694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- White J. A., Scandalios J. G. In vitro synthesis, importation and processing of Mn-superoxide dismutase (SOD-3) into maize mitochondria. Biochim Biophys Acta. 1987 Oct 8;926(1):16–25. doi: 10.1016/0304-4165(87)90178-4. [DOI] [PubMed] [Google Scholar]
- White J. A., Scandalios J. G. Isolation and characterization of a cDNA for mitochondrial manganese superoxide dismutase (SOD-3) of maize and its relation to other manganese superoxide dismutases. Biochim Biophys Acta. 1988 Nov 10;951(1):61–70. doi: 10.1016/0167-4781(88)90025-5. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., Brändli A. W., Pesold-Hurt B., Blank D., Schatz G. Transport of proteins to the mitochondrial intermembrane space: the 'matrix-targeting' and the 'sorting' domains in the cytochrome c1 presequence. EMBO J. 1987 Aug;6(8):2433–2439. doi: 10.1002/j.1460-2075.1987.tb02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. P., Schatz G. Transport of proteins to the mitochondrial intermembrane space: the 'sorting' domain of the cytochrome c1 presequence is a stop-transfer sequence specific for the mitochondrial inner membrane. EMBO J. 1987 Aug;6(8):2441–2448. doi: 10.1002/j.1460-2075.1987.tb02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]








