Abstract
A multiplex real-time polymerase chain reaction procedure was developed to identify the most prevalent clinical isolates of Salmonella enterica subsp. enterica. Genes from the rfb, fliC, fljB, and viaB groups that encode the O, H, and Vi antigens were used to design 15 primer pairs and TaqMan probes specific for the genes rfbJ, wzx, fliC, fljB, wcdB, the sdf-l sequence, and invA, which was used as an internal amplification control. The primers and probes were variously combined into six sets. The first round of reactions used two of these sets to detect Salmonella O:4, O:9, O:7, O:8, and O:3,10 serogroups. Once the serogroups were identified, the results of a second round of reactions that used primers and probes for the flagellar antigen l genes, 1,2; e,h; g,m; d; e,n,x; and z10, and the Vi gene were used to identify individual serovars. The procedure was standardized using 18 Salmonella reference strains and other enterobacteria. The procedure's reliability and sensitivity was evaluated using 267 randomly chosen serotyped Salmonella clinical isolates. The procedure had a sensitivity of 95.5% and was 100% specific. Thus, our technique is a quick, sensitive, reliable, and specific means of identifying S. enterica serovars and can be used in conjunction with traditional serotyping. Other primer and probe combinations could be used to increase the number of identifiable serovars.
Information about the geographic distribution of Salmonella serovars is necessary for the epidemiological surveillance of salmonellosis1 (http://www.who.int/salmsurv/general/documents/GSS_STRATEGICPLAN2006_10.pdf; accessed April 27, 2009). Typhimurium, Enteritidis, Typhi, Braenderup, Newport, Saintpaul, and Hadar represent 82.9% of the prevalent serovars isolated from clinical samples in Colombia, and these serovars are also prevalent in other regions of the world.1,2 Presently, S. enterica serotyping uses the Kauffman-White scheme, which involves first performing agglutination reactions between specific antibodies and 46 O-surface polysaccharides to identify the serogroup, and then, within a serogroup, identifying the corresponding serovar(s) according to the results of agglutination reactions involving 119 phase 1 and 2 H flagellar antigens, and the Vi capsular antigen2,3,4 and the corresponding antibodies. This method of serotyping has limited application, however, owing to the need to produce antiserums, its variable quality, and its high cost. Additionally, about 5–8% of the isolates can only be partially typed or not typed at all owing to the presence of a capsule, a rough phenotype, or a lack of flagella.3,4,5
During the past decade, molecular methods that use polymerase chain reaction (PCR) amplification of S. enterica DNA sequences to determine serogroups6,7 and serovars have been developed.8,9,10 Multiplex PCR has also been used to identify serovars in clinical samples and food.11,12,13,14,15 The SefA gene, the IE1L and IE1R insertion elements, and the Sdf-I sequence have been used to identify S. enterica serovar Enteritidis in different matrixes.14,15,16 Other published PCR gene targets have included 1) the rfb genes that encode the glycosyl synthase and transferase enzymes of O-polysaccharide biosynthesis17,18,19; 2) the rfbJ and abe genes that encode the enzymes involved in the synthesis of abequose (a sugar present in the O:4 and O:8 serogroups20; 3) the wzx gene that encodes a flippase21; 4) the fliC and fliB genes that encode the proteins of the phase 1 and 2 H antigens that make up part of the variable Salmonella flagellar domain and are expressed one at a time owing to the variation mechanism of the flagellar phase that is regulated by the fljBA operon22,23; 5) the Vi antigen, which is encoded by a number of genes found in the ViaB locus, including wcdB, which is specific for the Typhi serovar.24
Nevertheless, the aforementioned PCR studies have not used an algorithm derived from the KW scheme, which would dictate the order of the PCR reactions so that the serogroup would be identified first and then the individual serovars. Such a system could be used to readily detect the serovars isolated most frequently from clinical samples. The aim of current work was to develop multiplex real-time PCR (MRT-PCR) assays, using specific primers and TaqMan probes that are to be used in conjunction with the aforementioned algorithm, to identify the more prevalent clinical S. enterica isolates.
Materials and Methods
Strains
Eighteen Salmonella serovar reference strains were used to standardize the MRT-PCR procedure (S. Typhimurium ATCC 14028, S. Typhi NTCC 9001, S. Enteritidis CDC 64, S. Weltevreden INS 210/2004, S. Dublin INS-Canada 4, S. Braenderup CDC H9812, S. Hadar CDC 06-07, S. Newport INS-Canada F5-5, S. Heidelberg CDC 16, S. Saintpaul CDC 108, S. Gallinarum ISP-278-74, S. Mbandaka World Health Organization 5,7, S. Muenchen CDC 1966, S. Kentucky CDC 2865-56, S. Agona CDC 006/111-19-92, S. Anatum CDC 49 287 b2k, S. Javiana CDC 160, S. Derby CDC 002); 262 serotyped Salmonella isolates and 5 non-Salmonella enterobacteria isolates were blindly and randomly chosen to evaluate the procedure. These bacterial isolates belong to the collection of the Microbiology Group at the Instituto Nacional de Salud, Colombia. All isolates were stored at −70°C in 20% skim milk (Difco, Becton Dickinson, Franklin Lakes, NJ).
DNA Extraction
The bacterial strains were subcultured in brain heart infusion broth (Difco, Becton Dickson) for 18 hours at 36°C. The bacteria of the cultures were lysed at 98°C for 10 minutes in the infusion broth and then centrifuged at 20,442 × g for 5 minutes to isolate the bacterial DNA from the supernatants. The DNA concentration used for MRT-PCR assays was 1 ng/μl.
Primer and TaqMan Probe Design
For this study, 15 sets of primer pairs and TaqMan probes were designed. The sequences of the rfb, fliC, fljB, viaB genes and the invA gene, and the Sdf-I sequence were obtained from GenBank, National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/GenBank; accessed May 2, 2007). Multiple alignments using ClustalW (EMBL-EBI; http://www.ebi.ac.uk/clustalw/index.html; accessed May 2, 2007) were performed, and the primer sequences and the TaqMan probes specific for the Typhimurium, Saintpaul, Enteritidis, Typhi, Braenderup, Newport, and Hadar serovars were designed using the Beacon Designer v7.0 (PREMIER Biosoft International) software. The specificities of the primers and probes was evaluated in silico using the Blastn (http://blast.ncbi.nlm.nih.gov/Blast.cgi; accessed May 2007) and ClustalW algorithms, and the sequences that might form dimers and secondary structures were assessed using OligoAnalyzer software (http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/; accessed May-August, 2007). The probes were labeled at their 5′ ends with one of the reporter fluorophores, FAM (6-carboxyfluorescein), Cal Fluor Red-560 (CFR560), Cal Fluor Orange-610 (CFO610), or Quasar-670, and at their 3′ ends with a Black Hole Quencher sequestering molecule. The primers and the probes were synthesized by Biosearch Technologies, Inc. Novato, CA. As a strategy to identify the serovars, an algorithm was developed based on the KW scheme, which involves first identifying the O antigens of the serogroup and then, within a serogroup, identifying the serovars (Figure 1). Primers and probes for the O:4; O:9; O:7; O:8; and O:3,10 serogroups, and the phase l; 1,2; e,h; g,m; d; e,n,x; and z10 flagellar antigens, were distributed among six mixtures named: BDE, C1C2z10, TypSp, En, Ty, and BraeNHad. Each mixture also included the invA primers and probe set as an internal amplification control.25 Table 1 shows the compositions of the mixtures.
Figure 1.
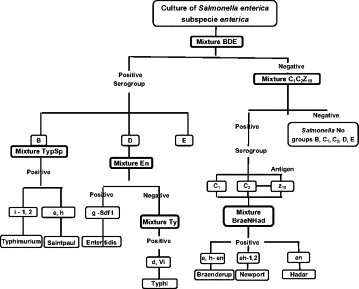
The algorithm derived from the Kaufmann-White scheme, which dictates the order of the MRT-PCR reactions so that the S. enterica subsp. enterica serogroups are first identified followed by the identification of serotypes.
Table 1.
TaqMan Probes and Oligonucleotide Primers Used
| Mixture name | Designation | Sequence | Amplicon position | Gene/NCBI access no. |
|---|---|---|---|---|
| BDE | rfbJ-Tq | 5′-FAM-TCTCTTATCTGTTCGCCTGTTGT-3′ | 305 | rfbJ |
| rfbJ-F | 5′-GCATTTACCACATCATCTAC-3′ | 275 | AE008792 | |
| rfbJ-R | 5′-GCGATTAGAGCATGTATATG-3′ | 424 | ||
| O:9.wzx-Tq | 5′-CFO560-CTTATTATTGCCGCTTCCTCTTGG-3′ | 37 | Wzx | |
| O:9.wzx-F | 5′-GTTGGTTAGAATTCCAAGAC-3′ | 15 | M65054 | |
| O:9.wzx-R | 5′-AAAGAGTAAATACAGCGTATG-3′ | 154 | ||
| O:3,10.wzx-Tq | 5′-CFR610-AATCTAGCCATTCGTTGCTGACT-3′ | 1411 | wzx | |
| O:3,10.wzx-F | 5′-CATGGTTCTTTATAAGCATATTC-3′ | 1328 | X60665 | |
| O:3,10.wzx-R | 5′-TACTCATACACACTTGTATAAAG-3′ | 1435 | ||
| C1C2Z10 | O:7.wzx-Tq | 5′-FAM-ACATAAGCACAGTCACAACCTGG-3′ | 835 | wzx |
| O:7.wzx-F | 5′-TTCTTAATTTAAGCTACGTCAC-3′ | 764 | M84642 | |
| O:7.wzx-R | 5′-CACTAGCTGTAATAGCATTAAC-3′ | 865 | ||
| O:8.abe-Tq | 5′-CFO560-CTCGGCACTCCAACCTAATCG-3′ | 834 | abe | |
| O:8.abe-F | 5′-AGAAACGCATAGTAGTAGTAAG-3′ | 720 | X61917 | |
| O:8.abe-R | 5′-TTTCACACCCTTCTCAATAG-3′ | 858 | ||
| fliC.Z10-Tq | 5′-CFR610-CACTACCGTCGCAGCTTCTG-3′ | 936 | fliCZ10 | |
| fliC.Z10-F | 5′-ATCAAGTAGTGTTCAGGATG-3′ | 864 | AY353472 | |
| fliC.Z10-R | 5′-ACCATTCTTATCAGTGTACG-3′ | 963 | ||
| TypSP | fliC.i-Tq | 5′-CFO560-ACTCTTGCTGGCGGTGCGACTTCC-3′ | 772 | fliCi |
| fliC.i-F | 5′-GTTGATAAGACGAACGGTGAGG-3′ | 748 | D13689 | |
| fliC.i-R | 5′-CTGCTGTCAATGCGGCTTTAG-3′ | 875 | ||
| fljB.1,2-Tq | 5′-FAM-CGCCAGCCGCAAGGGTTACTGTAC-3′ | 782 | fljB1,2 | |
| fljB.1,2-F | 5′-TGTTACTATTGGTGGCTTTACTGG-3′ | 708 | AF045151 | |
| fljB.1,2-R | 5′-CAGCAGGCATTGTGGTCTTAG-3′ | 809 | ||
| fliC.eh-Tq | 5′-CFR610-TACCGTCTACGCCACCAAGT-3′ | 1129 | fliCeh | |
| fliC.eh-F | 5′-TAATGTAACCACTTATACTGATTC-3′ | 1056 | AY649703 | |
| fliC.eh-R | 5′-TTACCGTCGATAGTAACAAC-3′ | 1157 | ||
| Ty | fliC.d-Tq | 5′-FAM-CACCGCCTGTTCTGAAGTTATGT-3′ | 1219 | fliCd |
| fliC.d-F | 5′-TCTGAAGTTGTTACTGCTAC-3′ | 1141 | L21912 | |
| fliC.d-R | 5′-TTATCTGTATTAACCTCTTTAAGC-3′ | 1244 | ||
| wcdB-Tq | 5′-CFO560-CTCCAACTGCCACATTATAGACCT-3′ | 799 | wcdB | |
| wcdB-F | 5′-GATTCAGGCCAATCTATTATC-3′ | 723 | D14156 | |
| wcdB-R | 5′-AAGCTCATTTAACGAAGTTC-3′ | 822 | ||
| En | fliC.g-Tq | 5′-CFR610-CCTGAACAGACAACTCACGCAC-3′ | 295 | fliCg,m |
| fliC.g-F | 5′-TAACGACGGCATTTCTATTG-3′ | 204 | AY649709 | |
| fliC.g-R | 5′-GATCGGAATCAGAGTTAGTC-3′ | 325 | ||
| Sdf.1-Tq | 5′-FAM-AGTAAATCAGCCTGTTGTTGCTC-3′ | 239 | Sdf-1 | |
| Sdf.1-F | 5′-CTCAGATTCAGGGAGTATATC-3′ | 208 | AF370707 | |
| Sdf.1-R | 5′-TTCGTTCTTCTGGTACTTAC-3′ | 317 | ||
| BraeNHad* | fljB.enx-Tq | 5′-CFO560-AGCACCGAATGATACAGCCC-3′ | 795 | fliCenx |
| fljB.enx-F | 5′-TGTAAGTGGTTATACCGATG-3′ | 702 | AY353305 | |
| fljB.enx-R | 5′-CCTGTAACAGTAGATTTAGTTG-3′ | 821 | ||
| Internal control† | invA-Tq | 5′-Quasar670-TACTGCTCGTAATTCGCCGC-3′ | 1773 | InvA |
| invA-F | 5′-TAACCTTGTGGAGCATATTC-3′ | 1692 | M90846 | |
| invA-R | 5′-GAATAACATCCTCAACTTCAG-3′ | 1804 |
Tq, TaqMan.
The BraeNHad mixture contained the primers and TaqMan probe for the fliC.eh, fljB.enx, and fljB.1,2 genes.
Each mixture included the primers and the TaqMan probe for invA amplification.
MRT-PCR Amplification Conditions
To standardize the MRT-PCR assays, the DNA of the Salmonella reference strains were used, and the primer and TaqMan probe sets were first individually tested and then tested in mixtures.25,26 All reactions were done in 20 μl of the master mix, 1× DyNAmo Probe qPCR (Finnzymes, Finland). The optimum concentrations of the primers in each reaction mixture were: BDE: rfbJ, 600 nmol/L; O:9.wzx, 100 nmol/L; O:3,10.wzx, 600 nmol/L; invA, 200 nmol/L; C1C2-Z10: O:7.wzx, 600 nmol/L; O:8 rfbJ, 100 nmol/L; fliC.z10, 500 nmol/L; invA, 200 nmol/L; TypSp: fliC.i, 500 nmol/L; fljB.1,2, 400 nmol/L; fliC.e,h, 100 nmol/L; invA, 200 nmol/L; Ty: fliC.d, 500 nmol/L; wcdB, 100 nmol/L; invA, 200 nmol/L; En: Sdf.1, 300 nmol/L; fliC.g, 300 nmol/L; invA 400 nmol/L; BraeNHad: fliC.eh, 400 nmol/L; fliC.enx, 100 nmol/L; fljB.1,2, 600 nmol/L; invA, 200 nmol/L. Each reaction mixture also contained 100 nmol/L corresponding TaqMan probe (Biosearch Technologies, Inc.) and 1 ng/μl of the bacterial DNA template.
All reactions used the same amplification conditions and were performed in a CDF-3240 Chromo 4 four-color real-time apparatus (Bio-Rad, Hercules, CA) with the following cycles: first cycle, 95°C for 15 minutes; 35 cycles, 94°C for 10 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. The fluorescence of a mixture was recorded after the extension step for each reaction cycle. The results were analyzed using Opticon Monitor software (version 3.1.32, Bio-Rad). Each MRT-PCR run included the following samples: 1) a positive control, ie, DNA from a known serovar; 2) a negative control, ie, a reaction mixture minus the bacterial DNA template and the internal amplification control (invA); and 3) DNA samples from the serovars that were to be typed.
The thermocycler wells were selected including the samples, the internal control of amplification, the positive and negative controls in the arithmetic scale, and then in the logarithmic scale. The threshold fluorescence level was taken to be just the sum of the fluorescence generated by the negative control, by samples containing intact probes, by the samples used for the control curves, and by positive samples just before the start of amplification. The threshold cycle value (Ct) was defined as the cycle for which the fluorescence was overlapped the threshold level.
The reproducibility of the technique was determined using duplicate runs of the positive control that were performed on different days. The standard deviation was used as the dispersion measurement for the Ct, with an acceptance criterion of 2 SD.
Data Analysis
The Epi-info v.6.0 software (Centers for Disease Control and Prevention, Atlanta, GA) was used to calculate the sensitivities and the specificities of the MRT-PCR reactions.
Results
The sequences of the genes that encode the O:4 (rfbJ), O:9 (wzx), O:7 (wzx), O:8 (abe), and O:3,10 (wzx) serogroups are dissimilar, which allowed us to use them to design unique primers and TaqMan probes. Conversely, the sequences of the genes that encode the G complex antigens (g,m; g,p; g,m,t) and for the flagellar phase 1 complex antigens (1,2; 1,5; 1,6) are about 95–98% identical, which precluded designing unique primers and TaqMan probes for those genes. As alternatives, we prepared primers and TaqMan probes for the Sdf-l chromosomal sequence, as described by Agron and colleagues,16 and the fliC.g gene to identify the Enteritidis and Dublin serovars.22 The sequences of the EN complex genes were 98% identical so that a single set of primers and probe was used to identify both the e,n,x and e,n,z15 antigens (Table 1). The positive amplification curves OR were similar to those of the positive controls in that Ct values of less than 25 cycles were found.
The sets of primer pairs and probe for the reference strains were tested, and each was shown to be specific for its reference strain. Once the specificities had been established, the MRT-PCR conditions for the mixtures were optimized. The serovars were identified using the six MRT-PCR mixtures according to the order diagrammed in Figure 1. First, a serogroup was assigned using the data obtained from the BDE and C1C2Z10 MRT-PCR reactions, and depending on the result, the mixtures that would identify the serovar antigens was selected and tested. Using this strategy, only two rounds of MRT-PCR reactions were needed to identify the Typhimurium, Saintpaul, Enteritidis, Typhi, Braenderup, Newport, and Hadar serovars.
The serotyping and MRT-PCR results were in agreement for 255 of the 267 isolates (95.5%). Table 2 shows the percentage agreement between the serotyping and MRT-PCR results for the individual serovars. Overall, MRT-PCR had a sensitivity of 95.5%, and a specificity of 100%. Discrepancies between the serotyping and MRT-PCR results were found for 12 isolates, the serogroup was not identified for 9 isolates, and the serovar was not identified for three isolates. The discrepancies are related to technical errors that occurred while handling the samples (n = 3) and misinterpretations of the amplification curves (n = 9).
Table 2.
Agreement Between the Results of the Serotyping and the MRT-PCR Runs for the Test (Randomly and Blindly Chosen) Cases
| Serotyping |
MRT-PCR |
|||||
|---|---|---|---|---|---|---|
| Serogroup | Serovar | Antigenic formula | No.* | Result | No.* | Agreement (%) |
| B | Typhimurium | 1,4,[5],12:i:1,2 | 36 | B; i; 1,2 | 36 | 100 |
| Saintpaul | 1,4,[5],12:e,h:1,2 | 29 | B; e,h; 1,2 | 29 | 100 | |
| Agona | 1,4,5,12:f,g,s:[1,2] | 1 | B; -;- | 1 | 100 | |
| Derby | 1,4,5,12:f,g:[1,2] | 1 | B; -; - | 1 | 100 | |
| Paratyphi B | 1,4,5,12:b:1,2 | 2 | B; -;1,2 | 2 | 100 | |
| Sandiego | 4,5,12:e,h:enz15 | 1 | B; e,h; - | 1 | 100 | |
| C1 | Braenderup | 6,7,14:e,h:e,n,z15 | 26 | C1; e,h; n,Z15 | 26 | 100 |
| Infantis | 6,7:r:1,5 | 1 | C1; -;- | 1 | 100 | |
| Virchow | 6,7:r:1,2 | 1 | C1; -; 1,2 | 1 | 100 | |
| Isangi | 6,7,14:d:1,5 | 2 | C1; -;- | 2 | 100 | |
| Livingstone | 6,7,14:d:l,w | 1 | C1; -; - | 1 | 100 | |
| Montevideo | 6,7,14:g,m[p],s:[1,2,7] | 2 | C1; -;- | 2 | 100 | |
| Mbandaka | 6,7,14:z10:e,n,z15 | 3 | C1; z10; e,n,z15 | 3 | 100 | |
| Virchow | 6,7:r:1,2 | 1 | C1; -; 1,2 | 1 | 100 | |
| C2 | Newport | 6,8,20:e,h:1,2 | 28 | C2; e,h; 1,2 | 27 | 96.4 |
| Hadar | 6,8:z10:e,n,x | 28 | C2; z10; e,n,x | 26 | 92.8 | |
| Kentucky | 6,8:i:z6 | 3 | C2; -; - | 3 | 100 | |
| Bovismorbificans | 6,8,20:r,[i]:1,5 | 1 | C2; -;- | 1 | 100 | |
| Corvalis | 8,20:z4,z23:z6 | 1 | C2; -;- | 1 | 100 | |
| Kottbus | 6,8:e,h:1,5 | 1 | C2; e,h;- | 1 | 100 | |
| Manhattan | 6,8:d:1,5 | 2 | C2; d;- | 2 | 100 | |
| Muenchen | 6,8:d:1,2 | 1 | C2; -; 1,2 | 1 | 100 | |
| D | Typhi | 9,12,[Vi]:d:- | 27 | D; Vi; d:- | 25 | 92.6 |
| Enteritidis | 1,9,12:g,m:- | 26 | D; g; m; Sdf-I | 25 | 96.1 | |
| Grupo D | 1,9,12:-:- | 3 | D; -; - | 0 | 0 | |
| Dublin | 1,9,12,[Vi]:g,p:- | 28 | D; g; - | 27 | 96.4 | |
| Javiana | 1,9,12:l,z28:1,5 | 2 | D; -; - | 0 | 0 | |
| Panama | 1,9,12:l,v:1,5 | 2 | D; -; - | 2 | 100 | |
| E | Muenster | 3,10:e,h:1,5 | 1 | E; -; - | 1 | 100 |
| L | Grupo L | 21:-:- | 1 | -; -;- | 1 | 100 |
| Other Genus | A. hydrophila | N/A | 1 | Negative | 1 | 100 |
| Citrobacter freundii | N/A | 1 | Negative | 1 | 100 | |
| Escherichia coli | N/A | 1 | Negative | 1 | 100 | |
| K. pneumoniae | N/A | 1 | Negative | 1 | 100 | |
| Shigella sonnei | N/A | 1 | Negative | 1 | 100 | |
| Total | 267 | 255 | 95.4 | |||
N/A, not applicable.
Number of isolate serovars of interest.
Discussion
For this study, an MRT-PCR system was developed to identify the Typhimurium, Enteritidis, Typhi, Saintpaul, Braenderup, Newport, and Hadar serovars, which are prevalent in clinical samples in Colombia and other regions of the world.2,27 This new approach uses an algorithm, based on the Kauffman-White scheme that allows the serogroup to be identified first and then the serovar. This algorithm allows for the optimization of the composition of the primer and probe mixtures of the MRT-PCR assays.
Other studies have shown that molecular typing using PCR has certain advantages over serotyping in that only molecular typing can identify isolates that cannot be typed owing to the loss of the O antigen in rough strains, the presence of a capsule, and/or the absence of flagella.28,29,30 Herrera-León and colleagues9,10 developed an approach consisting of three rounds of multiplex PCR reactions for the identification of the six serovars (Enteritidis, Typhimurium, Hadar, 4,5,12:i:-, Ohio, Infantis) that make up 79.8% of the Salmonella serovars isolated from Spaniards.31 Some of these isolates are prevalent in other regions of the world.1 For example, in Colombia, the most common serovars isolated from Colombians are Typhimurium, Enteritidis, Typhi, Braenderup, Dublin, Newport, Saintpaul, Uganda, Anatum, and Hadar, representing 82.9% of the total isolates (Instituto Nacional de Salud, http://www.ins.gov.co; accessed April 28, 2007). Our method has the advantage that only two rounds of MRT-PCR reactions are needed for serovar identification (Figure 1), and the results are highly sensitive (95.5%) and specific (100%).
According to McQuiston and colleagues22 the sequences of the Salmonella fliC and fljB genes (encoding the phase 1 and 2 flagellar antigens) are highly conserved at the 5′ and 3′ termini, whereas the sequences of the central regions are variable. However, this finding is true only for the genes that encode H antigens that are not part of the flagellar complex—the sequences of genes that encode proteins of the complex are very similar, which precludes using those sequences to identify serovars.
Echeita and colleagues8 reported the design of a universal (sense) primer and specific (antisense) primers for each of the phase 1 complex antigens (1,2; 1,5; 1,6; and 1,7), when we examined the specificities of the primer sequences using the Blastn algorithm, however, it was determined that some of the primers could amplify unspecific DNA associated with the same complex genes of different bacterial genera. Therefore, the current study included primers and a probe for the Sdf-l sequence, as described by Agron et al,16 as well as primers and a probe for fliC-g to identify Enteritidis. In doing so, we obtained 96% agreement between the serotyping and MRT-PCR results. Thus, it is necessary to design probes that target sequences that are distinct from those genes encoding the O, H, and Vi antigens for Derby, Agona, Essen and Hato serovars to distinguish these antigens from others that make up part of a complex.8 Amplification of invA did not occur when enterobacteria DNAs were used as templates. Therefore, as expected, invA is specific for Salmonella.29,30
The fact that there was not 100% agreement between the serotyping and MRT-PCR results may be a consequence of sequence variations within the gene wzx, which identifies the O:9 serogroup, and within the genes fliC and fljB, as reported.21,22,32 Because these genetic variations exist, it is possible that the primers that we designed to target these genes may have failed to do so. However, because our MRT-PCR procedure was shown to be highly sensitive (95.5% sensitivity), it has potential as a complementary or alternative technique to serotyping. By recombining the designed primers and probes to produce new mixtures, our MRT-PCR procedure, could be used to identify Salmonella serovars other than those used in this study.
In conclusion, the aforementioned work by ourselves and others demonstrates that MRT-PCR is a useful adjunct to traditional serotyping, because it can identify Salmonella serovars that the latter approach misses. Additionally, as MRT-PCR procedures for the detection of S. enterica in food have been reported,12,13,14,15 it is possible that our MRT-PCR procedure might be used to identify Salmonella serovars in clinical samples and food.
Acknowledgements
We are grateful to Dr. Silvia Restrepo (Universidad de los Andes, Bogotá, Colombia) for her support.
Footnotes
Supported by the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología, “Francisco José de Caldas,” Colciencias (Code project: 3256-04-18105), by the Instituto Nacional de Salud, Colombia, and by the Instituto Colombiano de Medicina Tropical–Universidad CES.
Contributor Information
Nélida Muñoz, Email: nmunoz@ins.gov.co.
Nora Cardona-Castro, Email: ncardona@ces.edu.co.
References
- 1.Herikstad H, Motarjemi Y, Tauxe R. Salmonella surveillance: a global survey of public health serotyping. Epidemiol Infect. 2002;29:1–8. doi: 10.1017/s0950268802006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop R, Fields P, Plikaytis B, Braden C, Tauxe R. Salmonella surveillance summary, 2005. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2007. [Google Scholar]
- 3.Seyfarth AM, Lo Fo Wong DMA, Aarestrup FM, Wegener HC. The external quality assurance system of the WHO Global Salmonella Surveillance and Laboratory Support Project. World Health Organization; Geneva: 2003. [Google Scholar]
- 4.Popoff M: WHO Collaborating Centre for Reference and Research on Salmonella, Institute Pasteur, Paris, France, 2001: Antigenic Formulas of the Salmonella Serovars, 8th ed. 2001
- 5.Samuel G, Reeves P. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr Res. 2003;338:2503–2519. doi: 10.1016/j.carres.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Luk J, Kongmuang U, Reeves P, Lindberg A. Selective amplification of abequose and paratose synthase genes (rfb) by polymerase chain reaction for identification of Salmonella major serogroups (A. O:4, O:8, and O:9) J Clin Microbiol. 1993;31:2118–2123. doi: 10.1128/jcm.31.8.2118-2123.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald C, Sherwood R, Gheesling L, Brenner F, Fields P. Molecular analysis of the rfb O antigen gene cluster of Salmonella enterica serogroup O: 6,14 and development of a serogroup-specific PCR assay. Appl Environ Microbiol. 2003;69:6099–6105. doi: 10.1128/AEM.69.10.6099-6105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Echeita M, Herrera S, Garaizar J, Usera M. Multiplex PCR-based detection and identification of the most common Salmonella second-phase flagellar antigens. Res Microbiol. 2002;153:107–113. doi: 10.1016/s0923-2508(01)01295-5. [DOI] [PubMed] [Google Scholar]
- 9.Herrera-Leon S, McQuiston J, Usera M, Fields P, Garaizar J, Echeita M. Multiplex PCR for distinguishing the most common phase-1 flagellar antigens of Salmonella spp. J Clin Microbiol. 2004;42:2581–2586. doi: 10.1128/JCM.42.6.2581-2586.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera-León S, Ramiro R, Arroyo M, Díez R, Usera M, Echeita M. Blind comparison of traditional serotyping with three multiplex PCRs for the identification of Salmonella serovars. Res Microbiol. 2007;158:122–127. doi: 10.1016/j.resmic.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez J, Sota M, Vivanco A, Perales I, Cisterna R, Rementeria A, Garaizar J. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J Clin Microbiol. 2004;42:1734–1738. doi: 10.1128/JCM.42.4.1734-1738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trafny E, Kozłowska K, Szpakowska M. A novel multiplex PCR assay for the detection of Salmonella enterica serovar Enteritidis in human feces. Lett Appl Microbiol. 2006;43:673–679. doi: 10.1111/j.1472-765X.2006.02007.x. [DOI] [PubMed] [Google Scholar]
- 13.Malorny B, Bunge C, Helmuth R. A real-time PCR for the detection of Salmonella Enteritidis in poultry meat and consumption eggs. J Microbiol Methods. 2007;70:245–251. doi: 10.1016/j.mimet.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Seo K, Valentin-Bon I, Brackett R, Holt P. Rapid, specific detection of Salmonella Enteritidis in pooled eggs by real-time PCR. J Food Prot. 2004;67:864–869. doi: 10.4315/0362-028x-67.5.864. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Yeh O. Designing of polymerase chain reaction primers for the detection of Salmonella Enteritidis in foods and fecal samples. Lett Appl Microbiol. 2002;34:422–427. doi: 10.1046/j.1472-765x.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- 16.Agron P, Walker R, Kinde H, Sawyer S, Hayes D, Wollard J, Andersen G. Identification by subtractive hybridization of sequences specific for Salmonella enterica serovar Enteritidis. Appl Environ Microbiol. 2001;67:4984–4991. doi: 10.1128/AEM.67.11.4984-4991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curd H, Liu D, Reeves P. Relationships among the O-antigen gene clusters of Salmonella enterica groups O:4. D1, D2, and D3. J Bacteriol. 1998;180:1002–1007. doi: 10.1128/jb.180.4.1002-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X, Brahmbhatt H, Quigley N, Reeves P. Structure and sequence of the rfb (O antigen) gene cluster of Salmonella serovar Typhimurium (strain LT2) Mol Microbiol. 1991;5:695–713. doi: 10.1111/j.1365-2958.1991.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Romana L, Reeves P. Sequences and structural analysis of the rfb (O antigen) gene cluster from a serogroup O:7 Salmonella enterica strain. J Gen Microbiol. 1992;138:1843–1855. doi: 10.1099/00221287-138-9-1843. [DOI] [PubMed] [Google Scholar]
- 20.Xiang S, Haase A, Reeves P. Variation of the rfb gene clusters in Salmonella enterica. J Bacteriol. 1993;175:4877–4884. doi: 10.1128/jb.175.15.4877-4884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Cole R, Reeves P. An O-antigen processing function for Wzx (RfbX): a promising candidate for O-unit flipase. J Bacteriol. 1996;178:2102–2107. doi: 10.1128/jb.178.7.2102-2107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQuiston J, Parrenas R, Ortiz-Rivera M, Gheesling L, Brenner F, Fields P. Sequencing and comparative analysis of flagellin genes fliC, fljB, and flpA from Salmonella. J Clin Microbiol. 2004;42:1923–1932. doi: 10.1128/JCM.42.5.1923-1932.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto S, Kutsukake K. FljA-mediated posttranscriptional control of phase 1 flagellin expression in flagellar phase variation of Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188:958–967. doi: 10.1128/JB.188.3.958-967.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto Y, Li N, Yokoyama H, Ezaki T. Complete nucleotide sequence and molecular characterization of ViaB region encoding Vi antigen in Salmonella Typhi. J Bacteriol. 1993;175:4456–4465. doi: 10.1128/jb.175.14.4456-4465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoorfar J, Malorny B, Abdulmawjood A, Cook N, Wagner M, Fach P. Practical considerations in design of internal amplification controls for diagnostic PCR assays. J Clin Microbiol. 2004;42:1863–1868. doi: 10.1128/JCM.42.5.1863-1868.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malorny B, Hoorfar J, Bunge C, Helmuth R. Multicenter validation of the analytical accuracy of Salmonella PCR: towards an international standard. Appl Environ Microbiol. 2003;69:290–296. doi: 10.1128/AEM.69.1.290-296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen S, Bishop R, Brenner F, Roels T, Bean N, Tauxe R. The changing epidemiology of Salmonella: trends in serovars isolated from humans in the United States, 1987–1997. J Infect Dis. 2001;183:753–761. doi: 10.1086/318832. [DOI] [PubMed] [Google Scholar]
- 28.Hoorfar J, Baggesen D, Porting P. A PCR-based strategy for simple and rapid identification of rough presumptive Salmonella isolates. J Microbiol Methods. 1999;35:77–84. doi: 10.1016/s0167-7012(98)00108-0. [DOI] [PubMed] [Google Scholar]
- 29.Hoorfar J, Ahrens P, Radstrom P. Automated 5 nuclease PCR assay for identification of Salmonella enterica. J Clin Microbiol. 2000;38:3429–3435. doi: 10.1128/jcm.38.9.3429-3435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malorny B, Paccassoni E, Fach P, Bunge C, Martin A, Helmuth R. Diagnostic real-time PCR for detection of salmonella in food. Appl Environ Microbiol. 2004;70:7046–7052. doi: 10.1128/AEM.70.12.7046-7052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echeita M, Aladueña A, Díez R, Arroyo M, Cerdán F, Gutiérrez R, de la Fuente M, González-Sanz R, Herrera-León S, Usera M. Distribución de los serotipos y fagotipos de Salmonella de origen humano aislados en España en 1997–2001. Enferm Infecc Microbiol Clin. 2005;23:127–134. doi: 10.1157/13072161. [DOI] [PubMed] [Google Scholar]
- 32.Dauga C, Zabrovskaia A, Grimont P. Restriction fragment length polymorphism analysis of some flagellin genes of Salmonella enterica. J Clin Microbiol. 1998;36:2835–2843. doi: 10.1128/jcm.36.10.2835-2843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


