Abstract
Objectives. We aimed to compare the rate of apoptosis after cardiopulmonary bypass (CPB) and cardioplegic arrest during coronary artery bypass grafting (CABG) surgery between atrial and ventricular tissue.
Methods. During CABG surgery with CPB and cardioplegic arrest, sequential biopsies were taken from the right atrial appendage and left ventricular anterior wall before CPB and after aortic cross clamp release. Change in number of apoptotic cells and biochemical markers of myocardial ischaemia and renal dysfunction were assessed.
Results. CPB was associated with a transient small, but significant increase in CK (1091±374%), CK-MB (128±38%), troponin-T (102±13%) and NT-proBNP (1308±372%) levels (all: p<0.05). A higher number of apoptotic cells as assessed by caspase-3 staining was found in the ventricular biopsies taken after aortic cross clamp release compared with the biopsies taken before CPB (5.3±0.6 vs. 14.0±1.5 cells/microscopic field, p<0.01). The number of apoptotic cells in the atrial appendage was not altered during CPB. Correlation between the duration of aortic cross clamp time and the change in caspase-3 positive cells in the left ventricular wall was of borderline significance (r of 0.58, p=0.08). Similar results were obtained from TUNEL staining for apoptosis.
Conclusion. CABG surgery with CPB and cardioplegic arrest is associated with an elevated rate of apoptosis in ventricular but not in atrial myocardial tissue. Ventricular tissue may be more sensitive to detect changes than atrial tissue, and may be more useful to investigate the protective effects of therapeutic intervention. (Neth Heart J 2010;18:236–42.)
Keywords: Coronary Artery Bypass, Apoptosis, Heart vetricules, Heart Arrest, Heart Atria, Reperfusion Injury
Coronary artery bypass grafting (CABG) surgery with cardiopulmonary bypass (CPB) and cardioplegic arrest is the most commonly used cardiac surgery procedure in the Western world.1 This procedure is associated with periods of oxygen deprivation and possibly a damaging effect to the Heart. Restoration of coronary blood flow after release of the aortic cross clamp leads to ischaemia-reperfusion injury. Subsequent production of reactive oxygen species results in cardiomyocyte death due to apoptosis.2-4 These changes may cause (transient) cardiac dysfunction,5,6 and therefore limiting the duration of cardioplegic arrest may reduce cell injury.
We hypothesised that CPB and cardioplegic arrest (in the setting of CABG) would cause a considerable increase in the number of apoptotic cells in the Heart. We therefore studied the presence of apoptosis in hearts of patients undergoing CABG surgery with CPB and cardioplegic arrest and we aimed to compare the rate of apoptosis after CPB and cardioplegic arrest during CABG surgery between atrial and ventricular tissue.
Methods
Patients
Patients were eligible for this study when aged between 18 and 80 years, when admitted for elective CABG surgery with CPB and cardioplegic arrest for the first time for two or more vessel coronary artery disease, and who met all the other inclusion and none of the exclusion criteria as listed in table 1. This study was approved by the Institutional Review Board of the University Medical Center Groningen. Each participating patient has signed informed consent.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
| Accepted for CABG surgery with CBP and cardioplegic arrest for 2- and 3-vessel coronary artery disease |
| Primary CABG surgery |
| Haemoglobin concentration ≥7.2 mmol/l and ≤9.7 mmol/l |
| Man or woman between 18 to 80 years of age |
| Exclusion criteria |
| Off-pump CABG surgery |
| Previous MI of the anterior wall |
| LVEF ≤40% |
| Chronic kidney disease (serum creatinine >106 mmol/ml for all females, >133 mmol/ml for black males, >115 mmol/ml for non-black males |
| Atrial fibrillation |
| Grand mal seizure within 1 year of enrolment |
| Malignant hypertension |
| Previous treatment with rhEPO |
| Blood transfusion <12 weeks before randomisation |
| Polycythemia vera |
| Pregnancy/breast feeding |
| Severe valvular disease (including pulmonary and tricuspid) or left ventricular outflow obstruction, which requires surgery |
| Pulmonary hypertension |
| Concomitant inflammatory or malignant disease |
| Presence of other serious medical conditions |
| Participation in any investigational device or drug trial(s) or receiving other investigational agent(s) within 30 days prior to enrolment. |
CABG=coronary artery bypass graft surgery, CPB=cardiopulmonary bypass, MI=myocardial infarction, LVEF=left ventricular ejection fraction, rhEPO=recombinant human erythropoietin.
Anaesthesia
General anaesthesia was provided according to a fixed protocol.7 Premedication consisted of oral diazepam 10 to 15 mg two hours preoperatively. After insertion of peripheral venous and arterial cannulae under local anaesthesia, general anaesthesia was induced with sufentanil 0.5 to 1 μg/kg and midazolam 0.05 to 0.1 mg/kg. Tracheal intubation was achieved with pancuronium 0.1 mg/kg and the lungs were ventilated with air and oxygen (fraction of inspired oxygen [FiO2] = 0.4). Anaesthesia was maintained with sufentanil, pancuronium and infusion of midazolam 0.1 mg/kg/h. A flow-directed continuous cardiac output catheter was inserted in the right internal jugular vein, and an indwelling bladder catheter was used for urine collection. After induction of anaesthesia the patients received cefuroxim 1500 mg (clindamycin 600 mg when allergic to penicillin).
Fluid management
Hydroxyethyl starch 6% and saline were used to maintain mean arterial pressure (MAP) >60 mmHg and to maintain filling pressure and cardiac output. Transfusion of packed red blood cells was administered at haemoglobin levels <5.0 mmol/l. According to standard care in our clinic, intravenous insulin therapy was started at serum glucose levels >8.0 mmol/l. Before institution of CPB, an activated clotting time of >400 seconds was produced by administration of heparin (starting dose 3 mg/kg). Patient characteristics were recorded prospectively. Diuretics, mannitol and aprotinin were not used during the entire study period.
Cardiopulmonary bypass
CPB was provided using a Stockert roller pump with an open reservoir, a hollow fibre oxygenator (Cobe optima), an arterial filter (Affinity, Medtronic) and an inline blood monitoring system (CDI500, Terumo). The CPB flow was maintained at 2.4 l/min/m2 and the circuit was primed with 1000 ml Ringer’s lactate, 500 ml Haes 200/0.5 10% and 50 mg heparin. Nasopharyngeal temperature was lowered to 32°C. MAP was maintained between 60 and 90 mmHg. Deviations were corrected with phenylephrine and nitroglycerin.
Surgery and biopsies
Surgery was performed by experienced cardiothoracic surgeons. All patients underwent CABG surgery with mild hypothermia (32 to 34°C). Cardioplegia in all patients was standardised to the crystalloid St. Thomas’ solution. The atrial biopsies were taken from the right atrial appendage and closed with prolene 4.0. Care was taken not to squeeze the biopsy places with forceps. Ventricular tissue samples were collected from the left anterior ventricular wall parallel to the left anterior descending artery, each from separate sites. Under an angle of approximately 30° using a 24 Gauge tru-cut needle ventricular biopsies were taken. Part was put in 4% paraformaldehyde for 24 hours, after which it was processed for paraffin embedding for immunohistological assessment. The second part was snap frozen in liquid nitrogen for RNA isolation. The ventricular biopsy site was closed using prolene 4.0 suture with teflon pledges. Before closure of the chest, biopsy sites were checked for bleeding.
Study design and mode of sampling biopsies
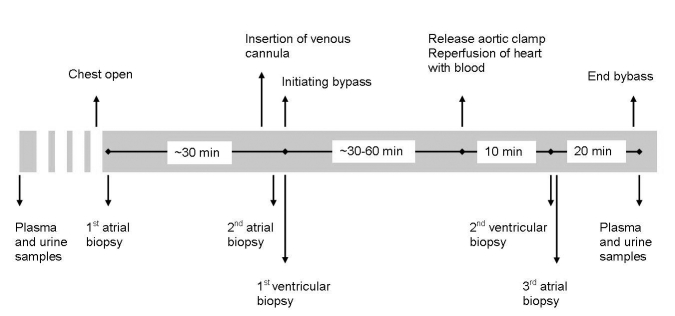
Tissue samples were collected at three different time points during surgery (figure 1). Immediately after opening the chest, a baseline atrial biopsy was collected. During placement of the venous cannula (20 to 40 minutes later) a second atrial biopsy was collected. Immediately after initiation of bypass the first ventricular biopsy was obtained. Ten minutes after aortic cross clamp release, the second ventricular biopsy was taken. Finally, a third atrial biopsy was obtained immediately after harvesting the second ventricular tissue sample. Additionally plasma and urine were collected for up to five days postprocedure to examine release of markers of both myocardial ischaemia and of renal function.
Figure 1.
Study design. The figure shows the study design as described in the text.
Monitoring and measurements during surgery
Blood pressure, heart rate, electrocardiogram, cardiac output and mixed venous oxygen saturation were monitored according to routine clinical practice. Blood and urine samples were collected at different time points. One day before surgery, blood and urine were collected for screening. Furthermore, pre-surgery, post-surgery, and at 2, 5, 12, 24, 48, 72 and 120 hours after surgery, blood and urine samples were collected. Markers of myocardial ischaemia (creatinine kinase (CK), CK-myoglobin (CK-MB), troponin-T, N-terminal-pro brain natriuretic peptide (NT-proBNP)) and renal function (creatinine) were determined. In urine samples microalbiminuria and creatinine was determined. The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease (MDRD) formula. All markers were analysed in the hospital laboratory.
Histology
For apoptosis detection, we stained for caspase-3 and TUNEL. Formalin fixed paraffin sections were incubated with an antibody that specifically recognises the active form of caspase-3 as previously reported.8,9 TUNEL staining was performed as previously described.8 For quantitative analysis, active caspase-3 positive cells or TUNEL-positive cells were counted in six random fields per section (80 to 120 cells per field) at high-power magnification (40x).
Statistics
Results are reported as mean ± standard error of the mean (SEM) or otherwise if stated. Because of small samples sizes, comparisons of differences between groups were analysed by the Wilcoxon or Friedman tests when appropriate. The correlation between aortic cross clamp time and the rate of apoptosis in ventricular tissue was assessed using Spearman’s coefficient. A p value of <0.05 was considered significant. All analyses were performed using SPSS version 16.0 software (SPSS, Chicago, IL, USA).
Results
Patient characteristics
We included ten patients undergoing elective CABG surgery with CPB and cardioplegic arrest for two- and three-vessel disease. All patients were male, and their age ranged from 39 to 73 years. Two patients had two-vessel disease, and eight patients had three-vessel disease. Baseline characteristics are summarised in table 2. Mean duration of the complete procedure was 222±19 minutes with a mean perfusion time of 80±80 minutes and a mean aortic cross clamp time of 46±5 minutes. All patients recovered well from their surgery. No rethoracotomies for postoperative bleeding were performed. Postoperative atrial fibrillation was seen in two patients. Average stay after surgery at the intensive care unit was 1.2 days. Patients were discharged from the hospital at a mean of eight days after surgery.
Table 2.
Baseline characteristics.
| n=10(average±SEM) | |
|---|---|
| General | |
| Age (years) | 60±3.4 |
| Sex (% male) | 100 |
| Race (Caucasian, %) | 100 |
| No. of diseased vessels | 2.8±0.1 |
| Height (cm) | 177±1.8 |
| Weight (kg) | 85.1±2.7 |
| BMI (kg/mm2) | 27±0.5 |
| Blood pressure (mmHg) | 146/73±6/3 |
| Heart rate (beats/min) | 70±5 |
| Medical history | |
| Previous myocardial infarction (%) | 20 |
| Previous CABG surgery (%) | 0 |
| Previous PCI (%) | 20 |
| Diabetes mellitus (%) | 40 |
| History of hypertension (%) | 70 |
| Obesity (%) | 80 |
| History of hypercholesterolemia (%) | 20 |
| Smoking (%) | 10 |
| Family history* | 40 |
| Renal dysfunction | 0 |
| Medication | |
| β-blocker (%) | 100 |
| ACE inhibitor (%) | 20 |
| Statin (%) | 80 |
| Diuretic (%) | 10 |
| Nitrate (%) | 20 |
| Calcium antagonist (%) | 30 |
| AT1-antagonist (%) | 30 |
| Acetylsalicylic acid (%) | 100 |
| Other anticoagulants (%) | 10 |
SEM=standard error of the mean, BMI=body mass index, CABG=coronary artery bypass graft surgery, PCI=percutaneous coronary intervention, AT1-antagonist=type I angiotensin II receptor antagonist. *A positive family history was defined by the presence of a composite endpoint (myocardial infarction, revascularisation, chronic heart failure, stroke and death) in first-degree family members before the age of 50 (males) or 60 (females).
Apoptosis in atria and ventricles
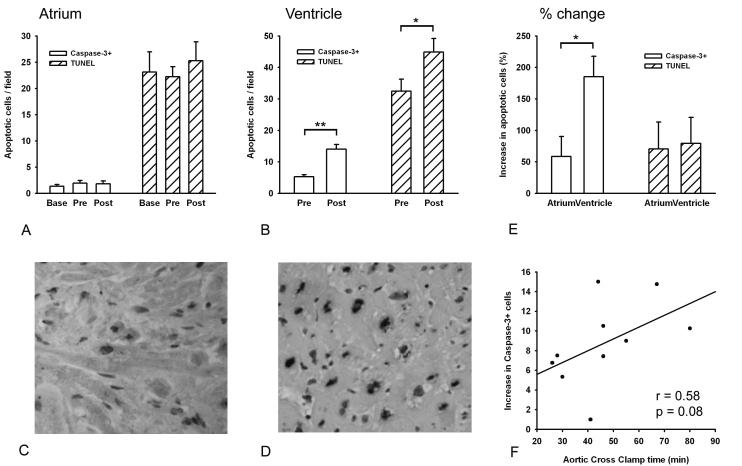
Figures 2A and B show the rate of apoptosis in the right atrial appendage and left ventricular wall as determined by caspase-3 and TUNEL staining. In the left ventricle, significantly more caspase-3 positive cells were counted in the biopsies taken after aortic cross clamp release compared with the number of caspase-3 positive cells found in the biopsies taken before the start of CPB (5.3±0.6 vs. 14.0±1.5 cells per microscopic field, p<0.01). Similar results were obtained from TUNEL staining (32.5±4.0 vs. 44.9±4.6 cells per microscopic field, p<0.05). In the atria, the number of caspase-3 positive cells was not altered during surgery (1.4±0.3 vs. 1.9±0.5 vs. 1.8±0.6 cells per microscopic field; p=NS). This was confirmed by TUNEL staining (23.1±4.1 vs. 22.2±2.1 vs. 25.3±3.8 cells per microscopic field; p=NS). Correlation between aortic cross clamp time and the change in ventricular caspase-3 positive cells showed a trend towards a positive correlation with an r of 0.58 (p=0.08; figure 2F). Similar results were obtained from the TUNEL staining (data not shown).
Figure 2.
Number of apoptotic cells during cardiopulmonary bypass. (A) Number of caspase-3+ and TUNEL+ cells in the atria. (B) Number of caspase-3+ and TUNEL+ cells in the ventricles. (C and D) Immunohistological examples of caspase-3+ staining in atria (C) and ventricles (D). (E) Percentage change in caspase-3+ and TUNEL+ cells during cardiopulmonary bypass in atria and ventricles. (F) Linear regression of aortic cross clamp time and change in caspase-3+ cells in the ventricles. Base=baseline: atrial biopsy taken after opening chest, pre=biopsies taken during placement of the venous cannula (for atrial biopsy) and just after going on cardiopulmonary bypass (for ventricular biopsy), post=biopsies taken 10 minutes after aortic cross clamp release. * p<0.05, ** p<0.01.
Cardiac enzymes and renal function
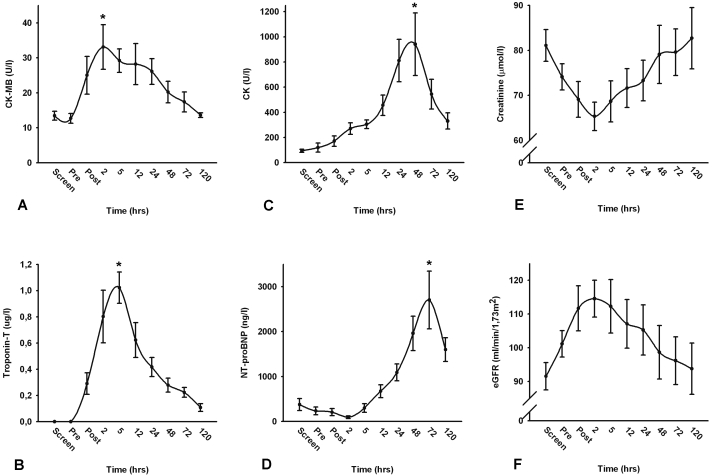
CPB was associated with a transient small, but significant increase in CK, CK-MB, troponin-T and NT-proBNP levels (all: p<0.05). CK-MB levels and troponin-T levels reached their maximum after two and five hours, respectively, and returned to baseline within 120 hours post-surgery (percentage change: 128±38% and 102±13%, respectively; figures 3A and B). CK levels peaked at 48 hours post-surgery (percentage change 1091±374%). Maximum NT-proBNP levels were found at 72 hours post-CABG (percentage change 1308±372%; figures 3C and D). Serum creatinine levels decreased shortly after the procedure and returned to baseline after 120 hours. Accordingly, eGFR levels were higher at two hours post-surgery with a return to baseline at day 5 (figures 3E and F). Other measurements showed no or very little fluctuation in time.
Figure 3.
Time course of markers of myocardial ischaemia and renal function. (A) Time course of creatinine kinase-myoglobin (CK-MB) pre- and post-surgery. (B) Time course of troponin-T pre- and post-surgery. (C) Time course of creatinine kinase (CK) pre- and post-surgery. (D) Development of NT-pro-BNP pre- and post-surgery. E) Time course of creatinine pre- and post-surgery. (F) Time course of eGFR (estimated glomerular filtration rate) pre- and post-surgery. Screen=values during screening, pre=values directly before surgery, post=values during closing Chest. * p<0.05 vs. screening values.
Discussion
We demonstrate that CABG surgery with CPB and cardioplegic arrest causes an increased rate of apoptosis in ventricular but not in atrial myocardial tissue. Thus, it seems that ventricular myocardium is more vulnerable to cardioplegic arrest. In addition, our data suggest that studies with pharmacological interventions aimed at salvaging apoptosis during cardioplegic arrest should use ventricular biopsies rather than atrial biopsies.
Different studies use either atrial or ventricular tissue to show apoptosis,10-12 but no comparison has been made. Our study shows clear differences in the rate of apoptosis between ventricular and atrial myocardial tissue. Furthermore, manipulating myocardial tissue, for example with forceps, induces apoptosis. In our study, care was taken to only use biopsies of non-manipulated tissue to avoid induction of apoptosis by manipulation.
Apoptosis has been considered to be one of the mechanisms of cardiomyocyte loss during CPB and cardioplegic arrest, such as in CABG surgery.13,14 Apoptosis during CPB and cardioplegic arrest can be triggered by several mechanisms, including ischaemia-reperfusion injury14 and release of cytokines and inflammatory factors.15 Longer aortic cross clamp time correlates, as shown in this and other studies, with an increase in number of apoptotic cells.16 This increased loss of cells might contribute to an impaired contractility of the Heart.16 Several strategies are being tested to ensure a lower rate of apoptosis, by reducing aortic cross clamp time as much as possible, or using cytoprotective agents against ischaemia-reperfusion injury.17,18
Prevention of apoptosis during CPB: proposal for an intervention trial
We postulate that apoptosis during CABG surgery is a maladaptive effect of CPB and cardioplegic arrest and might be amendable for intervention. Under normal circumstances erythropoietin (EPO) and its receptors (EPOR) have a relatively low expression in non-haematopoietic tissue.19 However, expression of EPO and EPOR is rapidly increased in response to hypoxia and increased reactive oxygen species.20,21 Numerous studies have shown that EPO has cytoprotective effects22-24 and EPOR signalling pathways are associated with cell survival.25-28 Since EPO reduces apoptosis, we postulate that EPO administration may have salutary effects on the heart in the setting of cardiac surgery employing CPB and cardioplegic arrest. We therefore started a prospective, randomised clinical trial in which patients are randomised to EPO (60,000 IU) or placebo (clinical trials protocol ID NCT00524901) before cardiac surgery employing CPB and cardioplegic arrest, which aims to prove the anti-apoptotic effects of EPO. Patients are included according to the inclusion and exclusion criteria summarised in table 1.
Conclusions
We conclude that CABG surgery with CPB and cardioplegic arrest is associated with an elevated rate of apoptosis in ventricular tissue. Ventricular tissue may be more sensitive to detect changes than atrial tissue, and may be more useful to investigate the protective effects of therapeutic interventions. We speculate that the observed increase in apoptosis may be amendable to anti-ischaemic and antiapoptotic agents. EPO may be useful in this respect, as it has established antiapoptotic effects and the EPOR is expressed in the Heart.
Acknowledgements
RAdB is supported by Netherlands Heart Foundation (2007T046). BDW is supported by the Netherlands Organisation for Scientific Research (920-03-404). DJvV and AAV are clinical established investigators from the Netherlands Heart Foundation (D97-017 and 2006T037 respectively).
References
- 1.Alexiou K, Kappert U, Staroske A, Joskowiak D, Wilbring M, Matschke K, et al. Coronary surgery for acute coronary syndrome: which determinants of outcome remain? Clin Res Cardiol. 2008;97:601-8. [DOI] [PubMed] [Google Scholar]
- 2.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461-70. [DOI] [PubMed] [Google Scholar]
- 3.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075-81. [DOI] [PubMed] [Google Scholar]
- 4.Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid Redox Signal. 2006;8:2111-24. [DOI] [PubMed] [Google Scholar]
- 5.Sabbah HN, Sharov VG, Goldstein S. Cell death, tissue hypoxia and the progression of heart failure. Heart Fail Rev. 2000;5:131-8. [DOI] [PubMed] [Google Scholar]
- 6.Alexiou K, Kappert U, Staroske A, Joskowiak D, Wilbring M, Matschke K, et al. Coronary surgery for acute coronary syndrome: which determinants of outcome remain? Clin Res Cardiol. 2008;97:601-8. [DOI] [PubMed] [Google Scholar]
- 7.Morariu AM, Loef BG, Aarts LP, Rietman GW, Rakhorst G, van Oeveren W, et al. Dexamethasone: benefit and prejudice for patients undergoing on-pump coronary artery bypass grafting: a study on myocardial, pulmonary, renal, intestinal, and hepatic injury. Chest. 2005;128:2677-87. [DOI] [PubMed] [Google Scholar]
- 8.de Boer RA, van Veldhuisen DJ, van der Wijk J, Brouwer RM, de Jonge N, Cole GM, et al. Additional use of immunostaining for active caspase 3 and cleaved actin and PARP fragments to detect apoptosis in patients with chronic heart failure. J Card Fail. 2000;6:330-7. [DOI] [PubMed] [Google Scholar]
- 9.Beller CJ, Horvath E, Kosse J, Becker A, Radovits T, Krempien R, et al. Opposite effects of vascular irradiation on inflammatory response and apoptosis induction in the vessel wall layers via the peroxynitrite-poly(ADP-ribose) polymerase pathway. Clin Res Cardiol. 2007;96:8-16. [DOI] [PubMed] [Google Scholar]
- 10.Fischer UM, Tossios P, Huebner A, Geissler HJ, Bloch W, Mehlhorn U. Myocardial apoptosis prevention by radical scavenging in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2004;128:103-8. [DOI] [PubMed] [Google Scholar]
- 11.Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, et al. N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2003;126:1513-20. [DOI] [PubMed] [Google Scholar]
- 12.Mehlhorn U, Krahwinkel A, Geissler HJ, LaRosee K, Fischer UM, Klass O, et al. Nitrotyrosine and 8-isoprostane formation indicate free radical-mediated injury in hearts of patients subjected to cardioplegia. J Thorac Cardiovasc Surg. 2003;125:178-83. [DOI] [PubMed] [Google Scholar]
- 13.Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease--a novel therapeutic target? FASEB J.. 2002;16:135-46. [DOI] [PubMed] [Google Scholar]
- 14.Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, Possati G, et al. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardiothorac Surg. 2004;25:304-11. [DOI] [PubMed] [Google Scholar]
- 15.Poulsen TD, Andersen LW, Steinbruchel D, Gotze JP, Jorgensen OS, Olsen NV. Two Large Preoperative Doses of Erythropoietin Do Not Reduce the Systemic Inflammatory Response to Cardiac Surgery. J Cardiothorac Vasc Anesth. 2008;23:316-23. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt JP, Schroder J, Schunkert H, Birnbaum DE, Aebert H. Role of apoptosis in myocardial stunning after open heart surgery. Ann Thorac Surg. 2002;73:1229-35. [DOI] [PubMed] [Google Scholar]
- 17.Shalaby A, Rinne T, Jarvinen O, Saraste A, Laurikka J, Porkkala H, et al. Initial results of a clinical study: adenosine enhanced cardioprotection and its effect on cardiomyocytes apoptosis during coronary artery bypass grafting. Eur J Cardiothorac Surg. 2008;33:639-44. [DOI] [PubMed] [Google Scholar]
- 18.Flesch M, Knipp S, Kessler G, Geissler HJ, Massoudy P, Wilhelm H, et al. ARTA: AT1-receptor blocker therapy in patients undergoing coronary artery bypass grafting. Clin Res Cardiol. 2009;98:33-43. [DOI] [PubMed] [Google Scholar]
- 19.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484-94. [DOI] [PubMed] [Google Scholar]
- 20.Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J Cereb Blood Flow Metab. 2002;22:503-14. [DOI] [PubMed] [Google Scholar]
- 21.Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11:863-71. [DOI] [PubMed] [Google Scholar]
- 22.Lipsic E, Schoemaker RG, van der Meer P, Voors AA, van Veldhuisen DJ, van Gilst WH. Protective effects of erythropoietin in cardiac ischemia: from bench to bedside. J Am Coll Cardiol. 2006;48:2161-7. [DOI] [PubMed] [Google Scholar]
- 23.Lipsic E, van der Meer P, Voors AA, Westenbrink BD, van den Heuvel AF, de Boer HC, et al. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20:135-41. [DOI] [PubMed] [Google Scholar]
- 24.Haljan G, Maitland A, Buchan A, Arora RC, King M, Haigh J, et al. The Erythropoietin NeuroProtective Effect: Assessment in CABG Surgery (TENPEAKS). A Randomized, Double-Blind, Placebo Controlled, Proof-of-Concept Clinical Trial. Stroke. 2009, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973-9. [DOI] [PubMed] [Google Scholar]
- 26.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, et al. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308:990-4. [DOI] [PubMed] [Google Scholar]
- 27.Hanlon PR, Fu P, Wright GL, Steenbergen C, Arcasoy MO, Murphy E. Mechanisms of erythropoietin-mediated cardioprotection during ischemia-reperfusion injury: role of protein kinase C and phosphatidylinositol 3-kinase signaling. FASEB J. 2005;19:1323-5. [DOI] [PubMed] [Google Scholar]
- 28.Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol. 2005;100:397-403. [DOI] [PubMed] [Google Scholar]