Abstract
Hepatic inflammation is a common finding during a variety of liver diseases including drug-induced liver toxicity. The inflammatory phenotype can be attributed to the innate immune response generated by Kupffer cells, monocytes, neutrophils, and lymphocytes. The adaptive immune system is also influenced by the innate immune response leading to liver damage. This review summarizes recent advances in specific mechanisms of immune-mediated hepatotoxicity and its application to drug-induced liver injury. Basic mechanisms of activation of lymphocytes, macrophages, and neutrophils and their unique mechanisms of recruitment into the liver vasculature are discussed. In particular, the role of adhesion molecules and various inflammatory mediators in this process are explored. In addition, the authors describe mechanisms of liver cell damage by these inflammatory cells and critically evaluate the functional significance of each cell type for predictive and idiosyncratic drug-induced liver injury. It is expected that continued advances in our understanding of immune mechanisms of liver injury will lead to an earlier detection of the hepatotoxic potential of drugs under development and to an earlier identification of susceptible individuals at risk for predictive and idiosyncratic drug toxicities.
Keywords: adverse drug reactions, hepatotoxicity, immune-mediated liver injury, idiosyncratic liver toxicity, lymphocytes, macrophages, neutrophils, innate immunity
Acute drug-induced liver injury (DILI) is a significant cause of patient morbidity and mortality and continues to be a serious problem during drug development. In addition, the use of many drugs on the market is restricted and requires extensive monitoring due to the potential for idiosyncratic drug-induced liver injury (IDILI). Compared to other liver diseases, IDILI is more likely to develop into fulminant liver failure, accounting for more than 25% of cases in the ICU (Ostapowicz et al., 2002). In contrast to acute DILI, the prediction and prevention of IDILI have been difficult due to the lack of reliable screening methods, the relatively low incidence of occurrence, and the limited knowledge regarding the underlying mechanisms (Lee and Senior, 2005).
Drugs or their reactive metabolites can have marked effects on gene expression and cellular homeostasis in hepatocytes (Boelsterli and Lim, 2007; Jaeschke et al., 2002; Park et al., 2005). In addition, environmental stresses or genetic disposition can affect an individual's susceptibility to drug toxicity (Deng et al., 2009; Andrade et al., 2009). Although many of these mechanisms can directly lead to cell death, there is growing support for the hypothesis that immune cells play a critical role in drug hepatotoxicity. Emerging evidence suggests that, in many cases, the direct effects of drugs on liver cells may be an initiating event for an immune response, which determines the extent of liver damage. It is therefore important to better understand the mechanisms by which cells of the immune system are activated and recruited into the liver during drug hepatotoxicity and how these cells cause hepatocellular injury. This review summarizes current knowledge on basic mechanisms of lymphocyte trafficking in the liver and the importance of various lymphocyte populations for DILI. In addition, the activation and recruitment of macrophages and neutrophils during drug toxicity are discussed and the relative contribution of these phagocytes to liver injury versus hepatic regeneration is explored. Finally, the role of the more recently recognized inflammatory mediator osteopontin (OPN) is highlighted. However, due to the ever increasing complexity of the topic of immune-mediated liver injury mechanisms and some of the controversies, not all aspects of this field can be covered in depth. For additional insight into specific mechanisms of DILI and IDILI, the reader is also referred to other recent reviews (Andrade et al., 2009; Boelsterli and Lim, 2007; Deng et al., 2009; Jaeschke and Bajt, forthcoming; Russmann et al., 2009).
LYMPHOCYTE RECRUITMENT TO THE LIVER
The human liver contains up to 1010 resident lymphocytes (Racanelli and Rehermann, 2006) comprised of B cells, T cells, and natural killer (NK) and natural killer T (NKT) cells. Lymphocyte recruitment increases in response to inflammation and the intrahepatic localization of lymphocytes determines the pattern of disease. DILI is frequently associated with a lymphocytic infiltrate, and the nature and extent of the inflammation determine the progression and severity of the injury. This applies to both the hepatitic type of DILI which is usually histologically indistinguishable from other forms of hepatitis and some forms of cholestatic hepatitis where lymphocytes can be seen surrounding and infiltrating bile ducts. The mechanisms by which drugs activate immune-mediated mechanisms are multiple and often poorly understood, but liver infiltration by effector lymphocytes is a common effector pathway leading to hepatocyte and cholangiocyte destruction and persistent liver injury. Thus, understanding how lymphocytes are recruited and positioned in the liver is critical for the pathogenesis of DILI.
The Hepatic Vasculature: A Unique Environment for Lymphocyte Recruitment
The liver has a unique dual blood supply from the hepatic arteries and portal venous blood that drains the gut. Both hepatic artery and portal vein drain into the hepatic sinusoids and thence into hepatic venules at the center of the hepatic lobule, which connect to form the hepatic veins that drain into the inferior vena cava. Inflammatory responses to infection and injury are characterized by increased lymphocyte binding to and migration across sinusoidal endothelial cells that line the hepatic sinusoidal microvasculature (Adams and Eksteen, 2006). Intravital experiments show that although leukocytes are capable of adhesion and migration across different regions of the hepatic microvasculature, the majority enters the parenchyma via hepatic sinusoids. Hepatic sinusoidal endothelial cells (HSECs) display differences in adhesion molecule expression compared with other endothelial cells including a lack of P-selectin and markedly reduced E-selectin expression (Adams et al., 1996). In contrast, selectins are expressed by inflamed portal vascular endothelium highlighting the heterogeneity within the hepatic microvasculature.
Endothelial Adhesion Molecules Involved in Lymphocyte Recruitment to the Liver
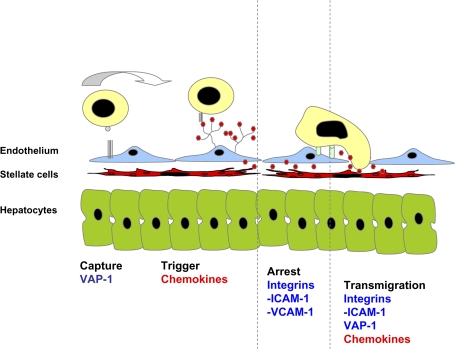
Murine studies confirmed that lymphocyte recruitment within the hepatic sinusoids was selectin independent (Lee and Kubes, 2008), and it was proposed that lymphocyte recruitment was due to “physical trapping.” However, subsequent studies demonstrated that lymphocytes interact with sinusoidal endothelium via adhesion receptors including intercellular adhesion molecular-1 (ICAM-1), which is constitutively expressed on sinusoidal endothelium. ICAM-1–deficient mice demonstrate reduced leukocyte adhesion to hepatic sinusoids. In vitro studies using human HSEC and flow-based adhesion assays show that lymphocyte adhesion to hepatic sinusoids is inhibited by blocking vascular cell adhesion molecule-1 (VCAM-1) and ICAM-1, and VCAM-1 has been shown to mediate lymphocyte capture and adhesion via α4 integrins in vivo (Lalor et al., 2002; Lee and Kubes, 2008; Fig. 1).
FIG. 1.
Lymphocyte adhesion mechanisms in liver sinusoids. Lymphocytes bind to HSECs in a modified version of the classic adhesion cascade model. Selectins, which are involved in capture in other vascular beds, play a minimal role in the liver, and other molecules including VAP-1 may be involved in this stage. Chemokines presented by the endothelial glycocalyx then trigger integrin-mediated adhesion to ICAM-1 and VCAM-1, and transmigration into tissue is driven by multiple poorly understood molecular mechanisms that involve VAP-1, chemokines, and integrins. The HSECs exist in close proximity to stellate cells and hepatocytes.
Nonclassical Endothelial Adhesion Molecules in the Hepatic Sinusoids
The liver sinusoids also express other adhesion receptor including vascular adhesion protein-1 (VAP-1), which mediates lymphocyte recruitment to the liver. VAP-1 is a 170-kDa homodimeric glycoprotein that has semicarbazide-sensitive monoamine oxidase activity. It is expressed constitutively on hepatic sinusoids but largely absent from other noninflamed vessels (Lalor et al., 2002). VAP-1 mediates lymphocyte adhesion to hepatic sinusoidal endothelium in vitro and in vivo (Bonder et al., 2005; Lalor et al., 2002) and has been implicated in the recruitment of lymphocytes to rodent liver transplants (Martelius et al., 2004). The precise role of VAP-1 is unclear. It is heavily sialidated allowing it to mediate brief rolling adhesion. However, our observations using human HSEC monolayers in vitro under flow suggest that liver endothelial cells support little classical rolling and instead VAP-1 may mediate initial tethering interactions and transmigration (see below). In addition, the enzyme activity of VAP-1 may modulate the function of other adhesion molecules because provision of substrate to VAP-1 on hepatic endothelial cells results in nuclear factorκ-β–dependent upregulation of VCAM-1 and ICAM-1 and enhanced lymphocyte adhesion from flow (Lalor et al., 2007). Another adhesion molecule which plays a particular role in the liver is CD44 which has been shown to mediate sequestration of neutrophils in hepatic sinusoid during sepsis as a consequence of increased deposition of serum-derived hyaluronan-associated protein on sinusoidal endothelium (McDonald et al., 2008).
In inflammatory liver disease associated with inflammatory bowel disease, HSECs express the endothelial adhesion molecule MAdCAM-1, which is normally confined to mucosal endothelium in the bowel (Adams and Eksteen, 2006). It plays a critical role in the compartmentalization of lymphocyte trafficking by recruiting gut-specific mucosal lymphocytes. However, in immune-mediated liver diseases that complicate inflammatory bowel disease, MAdCAM-1 can be induced in the liver by as yet unknown factors, with the result that mucosal T cells are recruited to the liver where they drive immune-mediated damage (Grant et al., 2001).
Chemokines Involved in Lymphocyte Recruitment to the Liver
Chemokines secreted by liver cells contribute to the recruitment and compartmentalization of lymphocyte within the liver (Table 1). The CC chemokine receptor (CCR)5 ligands CC chemokine ligand (CCL)3–5 are strongly expressed in portal vascular endothelium where they mediate lymphocyte recruitment in a range of inflammatory diseases including graft versus host disease, immune-mediated liver disease, and graft rejection (Murai et al., 1999). One of the critical events in the progression of injury in hepatitis is infiltration of the hepatic parenchyma, either as a component of active hepatitis in which parenchymal infiltration occurs in the periportal areas or as direct infiltration of hepatic lobules in lobular hepatitis. Progressive liver injury in chronic viral infection and autoimmune disease is associated with strong expression of the CXC chemokine receptor (CXCR)3 ligands CXCL9–11 on sinusoidal endothelium (Shields et al., 1999). These chemokines are not only secreted by endothelium but also by cholangiocytes, hepatocytes, and stellate cells in the inflamed liver (Holt et al., 2008a). The process of transcytosis allows chemokines produced by stellate cells or underlying hepatocytes in the local microenvironment to be transported from the basolateral to the luminal endothelial surface, and chemokines secreted by “upstream” cholangiocytes can be captured by retention in the proteoglycan-rich endothelial glycocalyx (Curbishley et al., 2005). Lymphocytes isolated from inflamed human liver express CXCR3 and migrate to CXCR3 ligands in vitro and undergo transmigration across HSEC (Curbishley et al., 2005), and murine studies suggest that CXCR3 and its ligands play a significant role in recruitment of virus-specific CD8+ T cells to the liver (Hokeness et al., 2007).
TABLE 1.
Expression of Chemokine Receptors on Specific Hepatic Lymphocyte Subpopulations in Humans
 |
Note. Different lymphocyte subsets express distinct combinations of chemokine receptors allowing them to respond to specific signals in inflamed tissues. The table shows the major chemokines and their receptors and expression of receptors on the major lymphocyte subsets in blood. The far right-hand column reports what is currently known about patterns of expression in liver-infiltrating lymphocytes in inflamed liver tissue. Data derived from Boisvert et al. (2003), Shields et al. (1999), Heydtmann et al. (2006), and Eksteen et al. (2006).
Recruitment and Retention of Specific Lymphocyte Subpopulations
Selective recruitment at the level of the endothelium and subsequent compartmentalization within the liver determines the intrahepatic distribution of specific lymphocyte populations. For example, regulatory T cells (Treg) are important for controlling autoreactive T cells and the resolution of inflammation. They maintain stable chronic inflammation and prevent fulminant tissue destruction. The frequency and function of intrahepatic Treg affect the outcome of chronic viral hepatitis and liver injury (Lan et al., 2006). In biliary diseases, CCL28, a chemokine associated with mucosal surfaces in the gut and lung promotes infiltration of bile ducts by CD4+CD25+FoxP3+Treg expressing the CCL28 receptor CCR10 (Eksteen et al., 2006). These tissue-infiltrating Treg use another chemokine receptor CXCR3 to enter the liver via inflamed sinusoidal endothelium (Eksteen et al., 2006). We have recently reported another subset of FoxP3+CD4+Treg within infiltrates in chronically inflamed human liver. These cells also use CXCR3 to enter the liver from blood and then use another chemokine receptor, CCR4, to migrate toward dendritic cells and macrophages within infiltrates in chronic hepatitis.
CXCL16 is an unusual chemokine, which has a transmembrane domain allowing expression on cell surfaces without presentation on the glycocalyx (Wilbanks et al., 2001). The CXCL16 receptor, CXCR6, is expressed on liver-infiltrating T cells (Boisvert et al., 2003; Heydtmann et al., 2005) and permits localization of cells to hepatocytes and biliary cells, which coexpress CXCL16 and VCAM-1 in areas of active hepatitis and liver injury. While the structure of CXCL16 supports adhesion directly, it also acts by promoting activation of β1 integrins, thus enhancing binding to VCAM-1 (Heydtmann et al., 2005). These examples illustrate how signals mediated through chemokine receptors allow different cells to respond to a complex network of signals provided by local interactions between sinusoidal endothelium, stromal, and parenchyma cells and infiltrating leukocytes. The balance between the recruitment and survival of regulatory and effector T cells in the liver may determine the outcome of DILI.
Chemokine-Mediated Exit of Lymphocyte from the Liver
In addition to their role in recruitment, chemokines are also important for exit of effector cells out of tissues via lymphatics to promote resolution of inflammation (Debes et al., 2005). We reported a subset of liver-infiltrating lymphocytes expressing the lymph node homing receptor CCR7 but little L-selectin, which precludes them from entering lymph nodes from blood via high endothelial venules. We suggest that CCR7 is involved in trafficking from the liver via the lymphatics where CCR7 ligands CCL19 and CCL21 are detected in inflamed liver tissue. The presence of CCL19 on the abluminal aspect of lymphatic vessels and CCL21 on sinusoidal endothelium is consistent with a role in promoting exit from tissue into the lymphatics (Grant et al., 2002) and suggests that CCR7 ligands provide a route for CCR7+ lymphocytes to exit the liver to regional lymph nodes, thereby promoting resolution of inflammation (Heydtmann et al., 2006).
The Role of Other Cells in the Hepatic Microenvironment
Studies of lymphocyte trafficking focus on endothelial interactions, but these cells exist in a complex multicellular microenvironment in the hepatic sinusoid and other cells types, and signals will influence lymphocyte recruitment (Holt et al., 2008a). Kupffer cells (KCs), resident liver macrophages, patrol the sinusoids and phagocytose foreign particles, and they also release proinflammatory cytokines that promote leukocyte adhesion. KCs also play a direct role in leukocyte adhesion by trapping leukocytes within the sinusoids and by providing adhesive ligands (Matsuno et al., 2002). Hepatic stellate cells are fibroblasts that act as pericytes surrounding the sinusoidal endothelium. When activated, they are the major mediators of matrix formation in response to injury (Friedman, 2008) and also play a direct role in the inflammatory process (Holt et al., 2008a; Safadi et al., 2004). They secrete chemokines and express cell adhesion molecules important for lymphocyte adhesion such as ICAM-1 and VCAM-1. Moreover, their ability to support lymphocyte adhesion and promote migration suggests that they play a critical role as regulators of postendothelial migration and positioning of cells within the liver parenchyma (Holt et al., 2009). Stellate cells and KCs can be activated by oxidative stress and innate immune signals both of which can be triggered in DILI. Such mechanisms could result in activation of the overlying endothelium, chemokine secretion, and enhanced lymphocyte recruitment to the liver thereby contributing to or amplifying immune-mediated liver damage (Fig. 3).
FIG. 3.
Dual function of sterile inflammation. Drugs or chemicals cause cell injury and necrosis, which results in the release of cellular contents including DNA, RNA, and proteins (HMGB1, HSPs). These DAMPs can cause complement activation or bind to toll-like receptors on neutrophils and macrophages where they trigger cytokine and chemokine formation. These proinflammatory mediators can activate neutrophils and monocytes in blood and recruit them into the liver vasculature. After extravasation into the parenchyma and adherence to target cells, neutrophils generate potent reactive oxygen species, which trigger cell death (cytotoxicity). On the other hand, both macrophages and neutrophils can phagocytose cell debris and digest it (clean-up mission). Whether these phagocytes cause additional tissue damage or mainly remove cell debris depends on the individual drug. If the drug itself effectively causes cell death, a cytotoxic effect of neutrophils is less likely. However, if the drug induces significant stress in many cells but is able to kill only a few, the recruited neutrophils may aggravate the injury by attacking and killing some of the stressed cells. C5a, activated complement factor C5a; HMGB1, high mobility group box-1 protein; HOCl, hypochlorous acid; HSPs, heat shock proteins; TLRs, toll-like receptors.
Once lymphocytes enter the liver interactions with epithelial cells or stromal cells retain cells at sites of inflammation. In addition, in some forms of chronic hepatic inflammation, tertiary lymphoid structures develop within the liver. Neovessels form in the portal tracts, and these show similarities to high endothelial venules in secondary lymphoid tissue. In both animals and humans, they can become organized into “portal tract–associated lymphoid tissue” (Grant et al., 2002; Yoneyama et al., 2001) which contains discreet T and B cell areas and dendritic cells. The development of this tissue provides an environment for ongoing recruitment and retention of lymphocytes within the liver as part of the process of lymphoneogenesis in chronic inflammatory and autoimmune liver diseases.
ROLE OF AUTOIMMUNITY IN DRUG-INDUCED LIVER INJURY
IDILI has been divided into two categories: immune idiosyncrasy and metabolic idiosyncrasy by Zimmerman (1999). In this categorization, in general, metabolic idiosyncrasy is referred to idiosyncrasy based on differences in metabolism of the drug. A more general and fundamental question is what fraction and which specific cases of IDILI are mediated by the adaptive immune system and which are not. At the present time, this is a matter of dispute and the evidence does not provide a definitive answer.
The category of immune idiosyncrasy is based on the characteristics of the liver injury. Drugs that cause IDILI associated with fever, rash, a relatively short period of therapy before the onset of IDILI (1–4 weeks), and a rapid onset on rechallenge fit into the immune idiosyncrasy category. In addition, in many cases, antidrug antibodies or autoantibodies have been detected (Liu and Kaplowitz, 2002). Drugs that cause IDILI that fit this category include halothane and tienilic acid. In contrast, drugs such as isoniazid, ketoconazole, troglitazone, and pyrazinamide cause IDILI that has been classified as metabolic idiosyncrasy. Cases associated with these drugs are not usually associated with the characteristics listed for immune idiosyncrasy, the time to onset is usually longer and more variable, and it may not occur on rechallenge. This categorization appears to be well accepted, and yet there is no example of IDILI in which the idiosyncratic nature can be explained by a polymorphism in a metabolic pathway. Isoniazid hepatotoxicity is slightly more common in patients who are slow acetylators; however, the relative risk is much too small to explain the idiosyncratic nature of isoniazid-induced hepatotoxicity (Huang et al., 2002). Valproate-induced hepatotoxicity is different from the IDILI associated with other drugs, and patients with mitochondrial dysfunction appear to be at increased risk; however, most patients who develop valproate-induced liver toxicity do not appear to have mitochondrial dysfunction (Krahenbuhl et al., 2000). It is possible that many cases of IDILI involve mitochondrial dysfunction (Boelsterli and Lim, 2007; Labbe et al., 2008). Much of the data relevant to this mechanism comes from in vitro studies which may not reproduce the in vivo mechanism of an idiosyncratic reaction. One exception is the delayed toxicity induced by troglitazone and other drugs in mice who are heterozygous for the mitochondrial superoxide dismutase 2 enzyme (Ong et al., 2007). However, investigators at Sankyo have been unable to reproduce these findings (Fujimoto et al., 2009). Involvement of mitochondria and an immune mechanism are not mutually exclusive.
Characteristics of IDILI and Their Implications
The characteristics of IDILI that have been used as evidence that specific reactions are not mediated by the adaptive immune system are also compatible with an immune mechanism. Certainly, the fact that a case of IDILI is not associated with fever, rash, and eosinophilia does not provide significant evidence that a reaction is not immune mediated; there are many examples of immune-mediated pathology that are not associated with these characteristics. The fact that IDILI does not recur rapidly on rechallenge is not typical of an immune-mediated reaction; however, there are several examples of immune-mediated reactions that do not recur rapidly on rechallenge. One clear example is heparin-induced thrombocytopenia, which is mediated by antibodies against the heparin-platelet factor 4 complex and does not usually recur on rechallenge once these antibodies are gone (Warkentin and Kelton, 2001). We have also found that the autoimmunity associated with penicillamine in Brown Norway rats and the autoimmunity associated with propylthiouracil in cats does not occur rapidly on rechallenge (Waldhauser and Uetrecht, 1996). Therefore, lack of immune memory is not clear evidence that a reaction is not immune mediated. One possible reason for the lack of immune memory in an immune-mediated reaction is if the mechanism has a significant autoimmune component.
When the drug responsible for an autoimmune reaction (e.g., procainamide-induced lupus-like syndrome) is stopped, the symptoms usually—but not always—resolve quite rapidly. Given that the reaction is autoimmune in nature and so the autoantigen is still present, the autoreactive T cells involved in the immune response must be either deleted or anergized. This would presumably also eliminate the memory T cells that are responsible for a rapid response on rechallenge. Some drugs such as minocycline, nitrofurantoin, and α-methyldopa are associated with IDILI that clearly resembles idiopathic autoimmune hepatitis (Lawrenson et al., 2000; Liu and Kaplowitz, 2002). The time to onset of this type of IDILI is often quite long; it usually requires more than a year of therapy before the onset of this type of IDILI. It is also interesting to note that many of the drugs associated with IDILI are also associated with a significant incidence of an autoimmune reaction such as a lupus-like syndrome, autoimmune hemolytic anemia, or vasculitis. These drugs include isoniazid (Lawson et al., 2001), minocycline (Lawson et al., 2001), α-methyldopa (Murphy and Kelton, 1988), hydralazine (Batchelor et al., 1980), propylthiouracil (Horton et al., 1989), phenytoin (Benton et al., 1962), statins (Ahmad, 1991), and zafirlukast (Finkel et al.,1999). Therefore, the lack of immune memory and long delay between starting a drug and the onset of IDILI are also consistent with an immune mechanism, especially if it has an autoimmune component. In fact, the long delay in onset, which is a characteristic of drug-induced autoimmunity, would be difficult to explain by any other mechanism.
The long delay in onset of drug-induced autoimmunity is presumably due to the difficulty in overcoming tolerance because the T cells with a high affinity for autoantigens are deleted in the thymus. Some drugs such as minocycline are associated with two types of IDILI: one with a short time to onset and having features of a hypersensitivity reaction and the other an autoimmune reaction with a quite long time to onset (Lawrenson et al., 2000). The time to onset for isoniazid-induced IDILI is quite variable with some cases occurring after a year of therapy. The response to rechallenge is also variable; in many cases, there is no recurrence, and yet there is one report in which the patient developed a fever and a 10-fold increase in aspartate transaminase 4 h after a single rechallenge dose (Maddrey and Boitnott, 1973). It is likely that such variation in characteristics of IDILI from one patient to another reflects mechanistic differences, and in this case, it could be due to differences in the degree of autoimmunity with the cases occurring after a short period of treatment and rapid onset on rechallenge representing a typical adaptive immune response and those cases that occur after a long period of treatment having a significant autoimmune component. It is known that the spectrum of antibodies induced by a drug such as halothane varies from one patient to another with some being antidrug antibodies and others being autoantibodies (Bourdi et al., 1996); therefore, it is not surprising that the type of immune response leading to IDILI might vary from one patient to another. In one study, 50% of patients who developed acute liver failure induced by anti-tuberculosis drugs had autoantibodies, and the incidence was higher in patients with a more gradual onset (Bernal et al., 2007). However, most cases of IDILI that would likely be classified as metabolic idiosyncrasy probably are not associated with the typical autoantibodies that have been used to diagnosis autoimmune hepatitis. It is also true that the diagnosis of autoimmune hepatitis can be difficult, and even many patients with idiopathic autoimmune hepatitis do not have the typical characteristics and serum markers of the disease (Manns and Vogel, 2006).
Involvement of T Helper 17 Cells in Autoimmunity
There is a “new” type of helper T cell, the T helper 17 (Th17) cell, which appears to be a major component of the immune response in autoimmune reactions (Romagnani et al., 2009; Fig. 2). A characteristic cytokine produced by Th17 cells is interleukin (IL)-17, hence the name. If some cases of IDILI have a significant autoimmune component, it would be expected that the patients would have elevated IL-17 levels. In a model of acute holthane hepatotoxicity, anti-IL-17 antibodies attenuated the increase in plasma ALT levels (Kobayashi et al., 2009). We tested the serum from several patients with drug-induced liver failure, and many did, in fact, have elevated IL-17 levels (Li et al., 2009). However, some patients with acetaminophen (APAP)-induced liver failure also had elevated IL-17 levels, and it has been found that other innate immune cells such as NK cells and γδ-T cells also produce IL-17 (Roark et al., 2008). Therefore, we need other methods to probe the involvement of Th17 cells and the role of autoimmunity in the mechanisms of IDILI.
FIG. 2.
Drug-induced classic adaptive immune responses and autoimmune responses. Depending on which helper T cells happen to have the highest affinity, the dominant immune response induced by a drug-modified protein may be a classic adaptive immune response against the drug or an autoimmune response against the protein portion of the modified protein. Th17 cells appear to play a major role in autoimmune responses; however, any immune response is likely to involve a combination of multiple types of T cells and B cells as well as innate immune cells.
In summary, although many cases of IDILI are not generally believed to be mediated by the adaptive immune system, the characteristics that have led to this conclusion are also compatible with an immune mechanism, especially a mechanism that includes an autoimmune component (Fig. 2). In general, it is more difficult to determine if a case of IDILI is immune mediated than it is to demonstrate that it is associated with a single polymorphism in a metabolic pathway. However, polymorphisms in multiple enzymes involved in the metabolic activation, detoxification, and transport of drugs and metabolites can complicate this scenario (Andrade et al., 2009). These mechanisms will be open to debate until we have better data. IDILI is a significant source of patient morbidity and mortality as well as a significant issue for drug development. Therefore, we need better methods to determine the mechanisms of these adverse reactions because without a better mechanistic understanding it is unlikely that we will make significant progress in preventing these serious reactions.
ROLE OF HEPATIC MACROPHAGES IN DRUG-INDUCED LIVER INJURY
The liver contains a large number of various types of innate immune cells, including resident macrophages, KCs, NK cells, and NKT cells. Aside from the resident innate immune cells, other leukocytes, such as neutrophils and monocytes, can be recruited into the liver during inflammation. The pathological roles of NK/NKT cells, neutrophils, and macrophages have been intensively investigated using the murine model of APAP-induced liver injury. However, the exact role of these cells in the pathogenesis of injury remains controversial. Regarding the role of hepatic macrophages, it has been demonstrated that these cells contribute to APAP-induced hepatotoxicity, through the production of pro-inflammatory cytokines and mediators, including tumor necrosis factor (TNF)-α, IL-1β, and nitric oxide (NO) (Laskin et al., 1995; Michael et al., 1999). However, hepatic macrophages also play a hepatoprotective role through the production of cytokines and mediators, such as IL-10–, IL-6–, and IL-18–binding protein, which counteract inflammatory events and promote liver regeneration (Ju et al., 2002). This dichotomy of both pro-toxicant and hepatoprotective functions of hepatic macrophages may be explained by the heterogeneity of these cells. Our recently published study demonstrated the differentiation of resident KC from a population of transient, APAP-induced infiltrating macrophages (IMs) (Holt et al., 2008b).
Identification of Two Distinct Macrophage Populations in the Liver of Mice after APAP Treatment
Our initial flow cytometric analysis of the hepatic nonparenchymal cells isolated from APAP-treated mice revealed two populations of macrophages with differential expression levels of two commonly used macrophage surface markers, F4/80 and CD11b. In addition to the resident KCs (CD11blowF4/80high), the second IM population (CD11bhighF4/80low) appeared within the liver ∼12 h after APAP challenge and remained present until their eventual disappearance by 5 days after APAP challenge. The finding that the IMs were still present in the liver of mice depleted of KC prior to APAP administration confirmed that the IMs represented a distinct population from resident KCs. In contrast, we observed an almost complete absence of the IMs in the liver of mice subjected to bone marrow irradiation 3 days prior to APAP challenge. It has been demonstrated that macrophage chemotactic protein-1 and its receptor CCR2 play a key role in the recruitment of monocytes into inflamed tissues (Kuziel et al., 1997). Our data showed that APAP treatment elicited a significantly decreased number of IMs in the liver of CCR2−/− mice compared with that in WT mice. These results suggest that the IMs represent a bone marrow–derived, circulating monocyte population that infiltrates the liver in response to chemokines released after APAP challenge.
Phenotypic Characterization of IMs
Two major classes of macrophages have been identified, classically activated macrophages (M1) and alternatively activated macrophages (M2). M1 macrophages generate proinflammatory cytokines and bacteriocidal mediators, whereas M2 cells play an important role in the downregulation of inflammation, tissue remodeling, elimination of tissue debris, and apoptotic bodies, as well as the induction of angiogenesis (Fadok et al., 1998; Goerdt and Orfanos, 1999). Our data revealed that the IMs expressed significant levels of a unique set of genes known to be markers of M2 cells, including Ym1, a heparin-binding lectin; Fizz1, a cysteine-rich secreted protein; and arginase-1, which competes with NO synthetase for metabolism of L-arginine. The IMs also expressed matrix metalloproteinase-12 (MMP-12), MMP-9, macrophage galactose N-acetyl-galactosamine–specific C-type lectin 1, and macrophage mannose receptor, all of which have been reported to play important roles in tissue repair processes (Curran et al., 2006; Gill and Parks, 2008; Sato et al., 2005; Sturge et al., 2007). Collectively, this gene expression profile suggested an M2 signature for the IMs, a signature that has been associated with a phenotype involving downregulation of inflammation, high capacity for phagocytosis, and induction of an angiogenesis and tissue repair.
The ability of the IMs to phagocytose apoptotic cells in vitro and in vivo was examined. IMs were isolated and purified from mice 24 h after APAP challenge and co-cultured with viable or apoptotic Jurkat T cells for 90 min. The phagocytic index, a quantitive measure of phagocytosis, for IMs was greater than 30%, similar to that of potent phagocytes reported in the literature (Bijl et al., 2006; Gardai et al., 2005). The in vivo phagocytic ability of the IMs was determined at 24 h following APAP challenge after injection of mice with red fluorescent latex beads and detecting the uptake of beads by the IMs. Similar to the results from the in vitro phagocytosis assay, the IMs demonstrated in vivo phagocytic capacity. Aside from their phagocytic ability, our data demonstrated that IMs could induce apoptosis of neutrophils. After 24 h coculturing of IMs with neutrophils, the number of neutrophils decreased significantly. Among the remaining neutrophils, the percentage of apoptosis increased significantly in the cocultures (67%) compared with that in cultures of neutrophils alone (19%), suggesting that the IMs are potent inducers of neutrophil apoptosis.
Investigation of the Role of IMs in APAP-Induced Liver Injury
To investigate the involvement of the IMs in APAP-induced liver injury, we treated female BALB/cJ WT and CCR2−/− mice with APAP. No significant difference was observed in the degree of APAP-induced hepatotoxicity, as measured by ALT levels at 10 and 24 h following APAP challenge. However, extensive hepatocytes necrosis and tissue hemorrhage were still evident and remained elevated in CCR2−/− mice at 48 and 72 h after APAP treatment, whereas the hepatic damage was diminished dramatically and the liver appeared normal in WT mice by 48 and 72 h (Holt et al., 2008b).
In summary, IMs are recruited into the liver of mice following APAP-induced liver injury. Characterization of the IMs suggests that they share a phenotype with M2 macrophages. In the absence of the IMs, the repair of liver damage following APAP-induced liver injury was delayed in CCR2−/− mice compared with WT mice. The observed IM-mediated induction of neutrophil apoptosis as well as the ability to phagocytose apoptotic cells likely contributes to their role in the resolution of inflammation and promotion of tissue repair following APAP-induced liver injury.
ROLE OF NEUTROPHILS IN DRUG-INDUCED LIVER INJURY
Polymorphonuclear leukocytes (neutrophils) are derived from the bone marrow and are circulating in blood. These cells are critical for host defense function as part of the innate immunity. They are highly mobile and can migrate from the blood into the tissue where they can phagocytose, kill, and digest any microorganism or foreign material. Together with macrophages, neutrophils also remove cell debris after tissue damage. However, during the last two decades, evidence accumulated that neutrophils can also cause additional liver injury in various models including ischemia-reperfusion, endotoxemia, alcoholic hepatitis, obstructive cholestasis, and even DILI (Jaeschke, 2003, 2006; Ramaiah and Jaeschke, 2007).
Neutrophil Accumulation in the Liver Vasculature
Prerequisite for neutrophil-induced liver injury is the accumulation of these leukocytes in hepatic sinusoids, which does not require selectins, integrins, and most other adhesion molecules (Jaeschke, 1997; Lee and Kubes, 2008). The initial recruitment into sinusoids is affected by rheological changes in activated neutrophils and cell swelling of sinusoidal lining cells making it more likely that neutrophils get stuck due to the low shear stress in these capillaries (Jaeschke and Smith, 1997). However, recently it was shown that interactions between CD44 on neutrophils and the extensively expressed hyaluronan on sinusoidal endothelial cells were mainly responsible for neutrophil accumulation in sinusoids during endotoxemia (McDonald et al., 2008). Independent of the specific mechanism of neutrophil accumulation, there is no question that cytokines, such as TNF-α and interleukin-1, activated complement factors, and to a lesser degree, CXC chemokines (KC, MIP-2) are potent activators of neutrophils that trigger their accumulation in sinusoids (Bajt et al., 2001). It is well established that endotoxin and other bacterial products can activate complement, which primes neutrophils for reactive oxygen formation (Jaeschke et al., 1993). Furthermore, endotoxin can directly bind to toll-like receptors on macrophages and induce proinflammatory mediator formation (Beutler et al., 2003). More recently, it was recognized that a number of intracellular proteins, for example, high-mobility group box-1 protein and heat shock proteins, as well as DNA and RNA fragments are also substrates for toll-like receptors and can trigger cytokine formation independent of infectious material (Bianchi, 2007). The so-called endogenous danger-associated molecular patterns (DAMPs) are responsible for the inflammatory response and hepatic neutrophil accumulation after cell necrosis, for example, ischemia-reperfusion injury (Kaczorowski et al., 2009) and APAP hepatotoxicity (Chen et al., 2007; Imaeda et al., 2009; Scaffidi et al., 2002).
Mechanisms of Neutrophil-Mediated Liver Injury
Although neutrophils accumulated in sinusoids are partially activated and primed, they do not cause liver injury (Jaeschke and Smith, 1997). Prerequisite for cytotoxicity is the extravasation and adherence to target cells. Most neutrophils involved in the injury process are extravasating from sinusoids not from postsinusoidal venules (Jaeschke and Smith, 1997). If the endothelium is intact, the extravasation involves integrins on neutrophils and the respective counter-receptor (ICAM-1, VCAM-1) on endothelial cells (Jaeschke, 1997; Ramaiah and Jaeschke, 2007). If the endothelial cells are damaged, adhesion molecules are less critical for the extravasation step but are still required for the adhesion to the target and the triggering of adherence-dependent reactive oxygen formation and degranulation (Jaeschke, 2006). Although some co-culture experiments support the hypothesis that proteases such as elastase and cathepsin G are important for the injury (Jaeschke and Smith, 1997), more direct evidence in vivo supports the conclusion that neutrophils kill through reactive oxygen species (Jaeschke et al., 1999). Neutrophils generate superoxide through NADPH oxidase (NOX-2). The resulting hydrogen peroxide can either directly diffuse into hepatocytes and generate an intracellular oxidant stress (Jaeschke et al., 1999) or myeloperoxidase uses it to generate hypochlorous acid, which also diffuses into target cells as indicated by the detection of chlorotyrosine protein adducts and other modified proteins inside the cells (Gujral et al., 2003, 2004; Hasegawa et al., 2005). Some of the proteases released by neutrophils assist in the extravastion and migration process and are involved in the processing of cytokines (Jaeschke, 2006). Thus, both reactive oxygen species and proteases are critical for a neutrophil-mediated liver injury. Since neutrophils do not attack healthy cells, a signal is required to induce transmigration. CXC chemokines generated by hepatocytes are potent chemoattracts for neutrophils and can trigger neutrophil extravasation (Lentsch et al., 1998). However, other chemotactic mechanisms including direct contact due to the close proximity between neutrophils in sinusoids and the underlying hepatocytes need to be considered (Ito et al., 2006).
Neutrophils in Drug-Induced Liver Injury
While there is general consensus regarding the detrimental role of neutrophils in the pathogenesis of ischemia-reperfusion injury or obstructive cholestasis, there is controversy regarding their role in APAP-induced liver injury, which is the most frequent cause of drug-induced hepatotoxicity and acute liver failure in the United States and many other countries. APAP overdose causes reactive metabolite formation, GSH depletion, and mitochondrial oxidant stress, which contributes directly to the mitochondrial membrane permeability transition pore opening and collapse of the membrane potential and indirectly, through release of intermembrane proteins, to nuclear DNA damage (Jaeschke and Bajt, 2006). The resulting necrotic cell death leads to the release of DAMPs, which triggers cytokine formation and neutrophils accumulation (Chen et al., 2007; Imaeda et al., 2009; Scaffidi et al., 2002).
Depletion of neutrophils with an anti-Gr-1 antibody 24 h before APAP administration protected against APAP-induced liver injury (Ishida et al., 2006; Liu et al., 2006). These findings lead to the conclusion that neutrophils contribute to the aggravation of liver injury (Ishida et al., 2006; Liu et al., 2006). However, treatment with the same antibody 2 h after APAP administration did not protect (Cover et al., 2006). Further evidence against a role of neutrophils came from experiments using CD18 antibodies (Lawson et al., 2000) or inhibitors of NOX-2 (Cover et al., 2006). In addition, mice deficient in a functional NOX-2 (James et al., 2003), ICAM-1 (Cover et al., 2006), CD18 (Jaeschke et al., 2009), or elastase (Bajt and Jaeschke, unpublished observation) were not protected. Also, circulating neutrophils do not show increased expression of CD11b/CD18 or priming for reactive oxygen formation during APAP hepatotoxicity (Jaeschke et al., 2009; Lawson et al., 2000). Moreover, neutrophils appear to be mainly localized in sinusoids away from the area of necrosis (Cover et al., 2006). Given that all the above mentioned interventions are effective in models where neutrophils are contributing to the injury process, together these experiments strongly suggest that neutrophils are not a critical factor in APAP-induced liver injury. How can we explain the beneficial effects of the neutropenia-inducing antibody (Ishida et al., 2006; Liu et al., 2006)? When given as a pretreatment for 24 h, the neutrophils removed by the antibody end up in capillaries such as liver sinusoids. KCs will remove these antibody-tagged cells by phagocytosis and get activated in the process. The mediators generated by KCs induce a number of proinflammatory and stress genes in hepatocytes, which has a preconditioning effect that renders the hepatocytes potentially more resistant to APAP overdose (Jaeschke and Liu, 2007). Although the exact mechanism of protection by Gr-1 has not been elucidated, the fact that certain genes are upregulated that have been shown to protect against APAP hepatotoxicity makes these experiments at least difficult to interpret (Jaeschke and Liu, 2007).
In summary, neutrophils are readily activated by cytokines and other inflammatory mediators, they accumulate mainly in sinusoids, and after receiving a chemotactic signal, can extravasate and attack distressed hepatocytes (Fig. 3). Generation of reactive oxygen species by neutrophils, especially hypochlorous acid, triggers an intracellular oxidant stress in the target cell and causes cell death. The pathogenic role of neutrophils is well established in a number of liver diseases (Jaeschke, 2003, 2006; Ramaiah and Jaeschke, 2007). However, evidence for the involvement in DILI is limited to a few examples such as α-naphthylisothiocyanate (Dahm et al., 1991) and halothane (You et al., 2006). In the case of APAP hepatotoxicity, the vast majority of experiments do not support a relevant contribution of neutrophils to the liver injury in murine models. However, these results require confirmation in the human pathophysiology before this therapeutic target can be dismissed for APAP overdose patients.
ROLE OF OPN IN HEPATIC INFLAMMATION
OPN Structure and Function
OPN also described frequently in literature as secreted phosphoprotein 1 is an acidic member of the small integrin-binding ligand N-linked glycoprotein family of proteins (Denhardt et al., 2001a,b; Ramaiah and Rittling, 2007a,b). One of the major structural and functional OPN domains observed includes arginine-glycine-aspartic (RGD) cell-binding domain similar to that present in many extracellular matrix proteins which is considered critical to binding with integrins. Another integrin-binding site present on OPN includes SVVYGLR domain in human OPN and SLAYGLR in rat and mouse which is reported to bind to α9/β1 and α4/β1 integrins (Ramaiah and Rittling, 2007a,b). Integrins are known to bind to the N-terminal thrombin cleaved fragment of OPN which contains the above mentioned RGD and the SVVYGLR domain. N-terminal thrombin cleaved fragment of OPN is known to be mediated by the thrombin cleavage site present on OPN. These OPN integrin-binding sites appear to be important based on the reported role of OPN in several inflammatory events involving multiple target organs such as kidney (glomerular nephritis and puromycin-induced nephrotoxicity) and liver (CCl4-induced hepatotoxicity). While such a role for OPN in target organ inflammation has been reported, the molecular basis by which such events are mediated is currently not clear.
Role of OPN in Inflammatory Cell Recruitment during Drug-Induced Liver Injury
The role of OPN in hepatic inflammation has been extensively studied with macrophages, wherein it is shown that OPN binds to macrophage integrins and is chemotactic for macrophages in vivo (Giachelli et al., 1998; Kawashima et al., 1999; Ramaiah and Rittling, 2007a,b). When a chemotactic peptide (formyl-methionyl-leucyl-phenylalanine) is injected, macrophages appear to accumulate at the injection site and administration of neutralizing antibody to OPN reduced this macrophage accumulation by 60% (Giachelli et al., 1998), providing strong evidence that OPN mediates at least in part the chemotactic response of macrophages. In addition to the role of OPN in attracting macrophages in a variety of pathologic situations such as myocardial necrosis (Murry et al., 1994), pulmonary fibrosis, and interstitial monocyte infiltration in the kidney (Ramaiah and Rittling, 2007b), there are examples of OPN-mediated infiltration of macrophages within the liver. In the liver, it is known that OPN is expressed in activated KCs in response to CCl4 treatment (Kawashima et al., 1999). Also, significant macrophage infiltration was reported following treatment of rats with heat-killed Propionibacterium acnes (Wang et al., 2000). These examples point to the fact that OPN is expressed at sites of injury and acts as a chemoattractant for macrophages.
While there is evidence for the correlation between hepatic OPN expression and macrophage infiltration, the cells expressing OPN within the liver is not clear. In a comparison of liver injury following bile duct ligation (BDL) and CCl4 treatment, Lorena et al. (2006) identified biliary epithelial cells to be the main source of OPN in both normal and BDL-treated mice. However, in CCl4-treated mice, OPN expression was associated with infiltrating ED-1–positive macrophages adjacent to the necrotic areas. In a model of chemical-induced sclerosing cholangitis, OPN was extensively expressed in proliferating cholangiocytes and in hepatocytes surrounding the portal tract (Fickert et al., 2006). Although the OPN expression correlated with neutrophil infiltration, neither OPN-nor TNF receptor-1–deficient mice showed reduced inflammation or attenuated fibrosis in this model (Fickert et al., forthcoming). Autoimmune hepatitis and viral hepatitis are other examples of hepatic inflammatory liver diseases, wherein increased neutrophil and lymphocyte (NKT and T cell) infiltration is evident. The role of OPN has been identified in a concanavalin A (Con A)–induced hepatitis mouse model (Diao et al., 2004), wherein OPN-deficient mice were reported to exhibit significantly lower liver injury when compared to their wild-type counterparts. In this study, NKT cells were shown to be important for the occurrence of Con A–induced hepatic injury. Also, neutrophils were identified to be important contributors for hepatic inflammation in this model. Both lymphocytes and neutrophils appeared to be attracted toward the cleaved form of OPN. The authors in this study tested the hypothesis that the integrin-binding sites on OPN such as SLAYGLR shows affinity towards the β1 and α4 integrins on neutrophils. Mice treated with a neutralizing antibody specific for a cryptic epitope of OPN were protected against Con A–induced liver injury (Diao et al., 2004). These findings show that NKT cells secrete cleaved OPN, which enhances NKT cell activation and causes recruitment of neutrophils and their activation during the course of Con A–induced hepatitis (Diao et al., 2004).
Hepatic OPN Induction in Alcoholic Liver Disease
Alcoholic liver disease (ALD) exemplifies the role of hepatic inflammation as a cause for associated liver pathology. In this disease, heavy alcohol consumption results in an inflammatory pathologic stage termed steatohepatitis (Apte et al., 2005), which can develop from hepatic steatosis. Steatohepatitis in preclinical rodent models and in human patients is usually characterized by hepatic fat accumulation, neutrophil infiltration, and neutrophil-mediated parenchymal injury. We tested the hypothesis of whether OPN has a role in the mediation of hepatic neutrophilic inflammation during alcoholic steatohepatitis. In a preclinical rodent model of steatohepatitis, male Sprague-Dawley rats were fed ethanol-containing Lieber-DeCarli liquid diet for 6 weeks followed by endotoxin injection (Apte et al., 2005). In this study, the significantly higher neutrophilic inflammation, necrosis, and liver injury strongly correlated with increased levels of hepatic and circulating levels of OPN (Apte et al., 2005). In addition, the thrombin cleaved OPN which is considered the functionally active form was also much higher in the liver in this rodent model. Further confirmation of the role of OPN in mediating hepatic inflammation during ALD was tested by employing neutralizing antibodies in this model, wherein the hepatic inflammation was significantly decreased (Ramaiah and Rittling, 2007a). Consistent with rodent models, a clinical study has revealed higher hepatic OPN gene expression in human alcoholic hepatitis (Seth et al., 2006). Clearly, these findings implicate the role of OPN in attracting neutrophils into the liver during ethanol ingestion facilitating the pathogenesis of alcoholic steatohepatitis.
Role of OPN in Female Gender Sensitivity in Alcoholic Liver Disease
As mentioned above, OPN mediates hepatic neutrophil infiltration making rats more susceptible to steatohepatitis. If this hypothesis is correct, it is likely that there is higher OPN induction in a female rodent model of ALD. Females are known to be more susceptible to ALD than males. However, the precise mechanism for such increased susceptibility of females to ALD is not completely understood. Similar to the OPN induction and association with hepatic inflammation in a male rodent model of ALD, our results showed higher hepatic expression of OPN to be the likely reason for higher and early hepatic neutrophil infiltration making females more susceptible to ALD (Banerjee et al., 2006, 2008).
To understand the molecular basis for increased neutrophil infiltration in female rats, we hypothesized that OPN-mediated hepatic neutrophil infiltration is a result of signaling by the N-terminal integrin-binding motif (SLAYGLR) of OPN through its receptor α9β1 (VLA9) and α4β1 (VLA4) on neutrophils. When compared to males, females in the ALD group showed higher expression of β1 integrins (both α4β1 and α9β1). Also, neutralizing antibodies against OPN showed not only decreased hepatic neutrophil infiltration but also decreased expression of these integrins. We also demonstrated binding of OPN to α4β1 and α9β1 integrins using immunoprecipitation experiments. Additional confirmation was obtained by Boyden chamber assays. Using antibodies directed against the α4 or the β1 subunits or the OPN-SLAYGLR sequence, a significantly decreased neutrophilic migration in vitro was noted (Banerjee et al., 2008)
Role of OPN in Nonalcoholic Fatty Liver Disease
Similar to ALD, nonalcoholic fatty liver disease (NAFLD) is an important disease that leads to hepatic inflammation (Sahai et al., 2004). The prevalence of hepatic injury in the United States due to NAFLD has significantly increased over time, and a fraction of the DILI population appears to have the biochemical picture of NAFLD. While several mechanisms for the causation of hepatic inflammation have been discussed, OPN has been identified as an important cytokine whose expression is increased early in the course of the disease in an experimental dietary rodent model of nonalcoholic steatohepatitis. It was reported that the upregulation of OPN correlates with hepatic inflammation and occurs prior to hepatic cirrhosis. The reduced liver injury and inflammation in OPN knockout mice suggests an important of OPN expression in the NAFLD model (Sahai et al., 2004).
In summary, several studies have investigated the role of OPN during hepatic macrophage, lymphocyte, and neutrophilic infiltration. The mechanisms by which OPN mediates hepatic inflammation can be partly attributed to the synthesis and secretion of OPN by a variety of immune and nonimmune cells and its interactions through its integrin-binding RGD and non-RGD sequences. In rodent models of alcoholic and non-ALD, the expression of OPN is increased in response to inflammation within the liver, and alteration of OPN function is thought to occur by cleaved OPN. The confirmation of the role of OPN using knockout mice and neutralizing antibodies against OPN integrin-binding sites have revealed a significant proinflammatory role of OPN in hepatic inflammation. However, complete mechanisms that facilitate inflammatory cell infiltration into liver by OPN are not completely understood. In addition, the specificity of OPN-mediated attraction of inflammatory cells will need further testing before the scientific community accepts OPN to be a major chemoattracant for leukocytes in hepatic inflammation. Potentially, inhibition of OPN by a variety of approaches can be employed to derive therapeutic benefits in various hepatic inflammatory states, in addition to its likely usefulness to assess inflammatory components of DILI.
PERSPECTIVES
Based on accumulating evidence in the literature on the role of immune cells in hepatic pathology, it is clear that immune responses and associated autoimmunity can play an important role in both predictive (acute) and idiosyncratic DILI. There appears to be increased weight of evidence for a role of lymphocytes, macrophages, and neutrophils in the mechanisms of liver injury and regeneration in most but not all cases of DILI. What are the implications and path forward for some of these observations? Because DILI is a significant burden for drug development in addition to morbidity and mortality, a preclinical in vivo model to incorporate inflammatory cells to assess immune responses may be critical to address all potential aspects of DILI (Fig. 4). In addition, blood biomarkers to monitor the function of lymphocytes, macrophages, and neutrophils within the liver should be established so that DILI can be potentially identified early in the development of drugs. Such biomarkers need to be tested in appropriate in vivo models before applied in the clinic. With ever-increasing regulatory oversight, pharmaceutical companies are addressing the issue of DILI more rigorously now than ever before. Screening and managing DILI in the preclinical and clinical settings are challenging, and there is an urgent need for predictive tools to characterize both acute DILI and especially IDILI, at various stages of drug development.
FIG. 4.
DILI: future strategies to incorporate in vivo models and biomarkers to address immune cell mechanisms.
FUNDING
National Institutes of Health (AA014257 to D.H.A., ES012914 to C.J., AA016316 to S.K.R., and AA012916 and DK070195 to H.J.); European Commission (QLG1-CT-1999-00295 to D.H.A.); the Medical Research Council (G0300101 to D.H.A.); the Canadian Institutes for Health Research (to J.U.).
Acknowledgments
This article is based on a symposium entitled “The Role of Inflammation during Metabolic Liver Disease and Drug-induced Liver Toxicity” presented at the 48th Annual Meeting of the Society of Toxicology, March 2009, Baltimore, MD.
References
- Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat. Rev. Immunol. 2006;6:244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- Adams DH, Hubscher SG, Fisher NC, Williams A, Robinson M. Expression of E-selectin and E-selectin ligands in human liver inflammation. Hepatology. 1996;24:533–538. doi: 10.1002/hep.510240311. [DOI] [PubMed] [Google Scholar]
- Ahmad S. Lovastatin-induced lupus erythematosus. Arch. Intern. Med. 1991;151:1667–1668. [PubMed] [Google Scholar]
- Andrade RJ, Robles M, Ulzurrun E, Lucena MI. Drug-induced liver injury: insights from genetic studies. Pharmacogenomics. 2009;10:1467–1487. doi: 10.2217/pgs.09.111. [DOI] [PubMed] [Google Scholar]
- Apte UM, Banerjee A, McRee R, Wellberg E, Ramaiah SK. Role of osteopontin in hepatic neutrophil infiltration during alcoholic steatohepatitis. Toxicol. Appl. Pharmacol. 2005;207:25–38. doi: 10.1016/j.taap.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Apte UM, Smith R, III, Ramaiah SK. Higher neutrophil infiltration mediated by osteopontin is the likely contributing factor for increased susceptibility of females to alcoholic liver disease. J. Pathol. 2006;208:473–485. doi: 10.1002/path.1917. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Lee J-H, Ramaiah SK. Interaction of osteopontin with neutrophil α4β1 and α9β1 integrins in a rodent model of alcoholic liver disease. Toxicol. Appl. Pharmacol. 2008;233:238–246. doi: 10.1016/j.taap.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Batchelor JR, Welsh KI, Tinoco RM, Dollery CT, Hughes GRV, Bernstein R, Ryan P, Naish PF, Aber GM, Bing RF, et al. Hydralazine-induced systemic lupus erythematosus: influence of HLA-DR and sex on susceptibility. Lancet. 1980;1:1107–1109. doi: 10.1016/s0140-6736(80)91554-8. [DOI] [PubMed] [Google Scholar]
- Benton JW, Tynes B, Register HB, Alford C, Holley HL. Systemic lupus erythematosus occurring during anticonvulsive drug therapy. J. Am. Med. Assoc. 1962;180:115–118. [Google Scholar]
- Bernal W, Ma Y, Smith HM, Portmann B, Wendon J, Vergani D. The significance of autoantibodies and immunoglobulins in acute liver failure: a cohort study. J. Hepatol. 2007;47:664–670. doi: 10.1016/j.jhep.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 2003;74:479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bijl M, Reefman E, Horst G, Limburg PC, Kallenberg CG. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus: correlates with decreased serum levels of complement. Ann. Rheum. Dis. 2006;65:57–63. doi: 10.1136/ard.2005.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelsterli UA, Lim PL. Mitochondrial abnormalities—a link to idiosyncratic drug hepatotoxicity? Toxicol. Appl. Pharmacol. 2007;220:92–107. doi: 10.1016/j.taap.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J. Hepatol. 2003;38:67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Bonder CS, Norman MU, Swain MG, Zbytnuik LD, Yamanouchi J, Santamaria P, Ajuebor M, Salmi M, Jalkanen S, Kubes P. Rules of recruitment for Th1 and Th2 lymphocytes in inflamed liver: a role for alpha-4 integrin and vascular adhesion protein-1. Immunity. 2005;23:153–163. doi: 10.1016/j.immuni.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Chen W, Peter RM, Martin JL, Buters JT, Nelson SD, Pohl LR. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem. Res. Toxicol. 1996;9:1159–1166. doi: 10.1021/tx960083q. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am. J. Pathol. 2005;167:887–899. doi: 10.1016/S0002-9440(10)62060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JN, Winter DC, Bouchier-Hayes D. Biological fate and clinical implications of arginine metabolism in tissue healing. Wound Repair Regen. 2006;14:376–386. doi: 10.1111/j.1743-6109.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- Dahm LJ, Schultze AE, Roth RA. An antibody to neutrophils attenuates alpha-naphthylisothiocyanate-induced liver injury. J. Pharmacol. Exp. Ther. 1991;256:412–420. [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Luyendyk JP, Ganey PE, Roth RA. Inflammatory stress and idiosyncratic hepatotoxicity: hints from animal models. Pharmacol. Rev. 2009;61:262–282. doi: 10.1124/pr.109.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt DT, Giachelli C, Rittling SR. Role of osteopontin in cellular signalling and toxicant injury. Annu. Rev. Pharmacol. Toxicol. 2001a;41:723–749. doi: 10.1146/annurev.pharmtox.41.1.723. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Noda M, O'Regan AW, Pavlin D. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Invest. 2001b;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D, Segawa T, Maeda M, Hamuro J, Nakayama T, et al. Osteopontin as a mediator of NKT cell function in T-cell mediated liver disease. Immunity. 2004;21:539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LS, Adams DH. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J. Immunol. 2006;177:593–603. doi: 10.4049/jimmunol.177.1.593. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Stöger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, Jaeschke H, Zatloukal K, Denk H, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am. J. Pathol. 2006;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Thueringer A, Moustafa T, Silbert D, Gumhold J, Tsybrovskyy O, Lebofsky M, Jaeschke H, Denk H, Trauner M (Forthcoming) Role of osteopontin and tumor necrosis factor alpha receptor-1 in xenobiotic-induced cholangitis and biliary fibrosis in mice. Lab. Invest. doi: 10.1038/labinvest.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel TH, Hunter DJ, Paisley JE, Finkel RS, Larsen GL. Drug-induced lupus in a child after treatment with zafirlukast (Accolate) J. Allergy Clin. Immunol. 1999;103:533–534. doi: 10.1016/s0091-6749(99)70482-3. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol. Pathol. 2009;37:193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J. Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int. J. Biochem. Cell. Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, Wu L, Hübscher SG, Adams DH. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am. J. Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G243–G252. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G760–G767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Hardie D, Shields PL, Faint J, Buckley CD, Campbell JJ, Salmon M, Adams DH. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J. Immunol. 2006;177:729–738. doi: 10.4049/jimmunol.177.1.729. [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- Hokeness KL, Deweerd ES, Munks MW, Lewis CA, Gladue RP, Salazar-Mather TP. CXCR3-dependent recruitment of antigen-specific T lymphocytes to the liver during murine cytomegalovirus infection. J. Virol. 2007;81:1241–1250. doi: 10.1128/JVI.01937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AP, Haughton EL, Lalor PF, Filer A, Buckley CD, Adams DH. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology. 2009;136:705–714. doi: 10.1053/j.gastro.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Holt AP, Salmon M, Buckley CD, Adams DH. Immune interactions in hepatic fibrosis. Clin. Liver Dis. 2008a;12:861–882. doi: 10.1016/j.cld.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 2008b;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RC, Sheppard MC, Emery P. Propylthiouracil-induced systemic lupus erythematosus. Lancet. 1989;2:568. doi: 10.1016/s0140-6736(89)90696-x. [DOI] [PubMed] [Google Scholar]
- Huang YS, Chern HD, Su WJ, Wu JC, Lai SL, Yang SY, Chang FY, Lee SD. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology. 2002;35:883–889. doi: 10.1053/jhep.2002.32102. [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur. J. Immunol. 2006;36:1028–1038. doi: 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- Ito Y, Abril ER, Bethea NW, McCuskey MK, Cover C, Jaeschke H, McCuskey RS. Mechanisms and pathophysiological implications of sinusoidal endothelial cell gap formation following treatment with galactosamine/endotoxin in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G211–G218. doi: 10.1152/ajpgi.00312.2005. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Cellular adhesion molecules: regulation and functional significance in the pathogenesis of liver diseases. Am. J. Physiol. 1997;273:G602–G611. doi: 10.1152/ajpgi.1997.273.3.G602. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Mechanisms of acetaminophen hepatotoxicity. In Comprehensive Toxicology (C. A. McQueen, Ed.), Volume X: Hepatic Toxicology (R. A. Roth and P. Ganey, Eds.), Elsevier, Oxford. (Forthcoming) [Google Scholar]
- Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am. J. Physiol. 1993;264:G801–G809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Ho YS, Fisher MA, Lawson JA, Farhood A. Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: importance of an intracellular oxidant stress. Hepatology. 1999;29:443–450. doi: 10.1002/hep.510290222. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology. 2007;45:1588–1589. doi: 10.1002/hep.21549. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J. Leukoc. Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Bajt ML. (2009). Role of caspases in modulating the inflammatory response after acetaminophen hepatotoxicity. Hepatology 50 (Suppl.), 1163A–1164A (Abstract) [Google Scholar]
- James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic. Res. 2003;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem. Res. Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- Kaczorowski DJ, Tsung A, Billiar TR. Innate immune mechanisms in ischemia/reperfusion. Front. Biosci. (Elite Ed) 2009;1:91–98. doi: 10.2741/E10. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Mochida S, Matsui A, YouLuTuZ Y, Ishikawa K, Toshima K, Yamanobe F, Inao M, Ikeda H, et al. Expression of osteopontin in Kupffer cells and hepatic macrophages and stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem. Biophys. Res. Commun. 1999;256:527–531. doi: 10.1006/bbrc.1999.0372. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Kobayashi M, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Halothane-induced liver injury is mediated by interleukin-17 in mice. Toxicol. Sci. 2009;111:302–310. doi: 10.1093/toxsci/kfp165. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl S, Brandner S, Kleinle S, Liechti S, Straumann D. Mitochondrial diseases represent a risk factor for valproate-induced fulminant liver failure. Liver. 2000;20:346–348. doi: 10.1034/j.1600-0676.2000.020004346.x. [DOI] [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam. Clin. Pharmacol. 2008;22:335–353. doi: 10.1111/j.1472-8206.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J. Immunol. 2002;169:983–992. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Sun PJ, Weston CJ, Martin-Santos A, Wakelam MJ, Adams DH. Activation of vascular adhesion protein-1 on liver endothelium results in an NF-kappaB-dependent increase in lymphocyte adhesion. Hepatology. 2007;45:465–474. doi: 10.1002/hep.21497. [DOI] [PubMed] [Google Scholar]
- Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, Chuang YH, Nakamura T, Saito S, Shimoda S, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729–737. doi: 10.1002/hep.21123. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–1050. [PubMed] [Google Scholar]
- Lawrenson RA, Seaman HE, Sundstrom A, Williams TJ, Farmer RD. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23:333–349. doi: 10.2165/00002018-200023040-00006. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol. Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Lawson TM, Amos N, Bulgen D, Williams BD. Minocycline-induced lupus: clinical features and response to rechallenge. Rheumatology (Oxford) 2001;40:329–335. doi: 10.1093/rheumatology/40.3.329. [DOI] [PubMed] [Google Scholar]
- Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol. Pathol. 2005;33:155–164. doi: 10.1080/01926230590522356. [DOI] [PubMed] [Google Scholar]
- Lee WY, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. J. Hepatol. 2008;48:504–512. doi: 10.1016/j.jhep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu X, Liu F, Cai P, Sanders C, Lee WM, Uetrecht J. Cytokine and autoantibody patterns in acute liver failure. J. Immunotoxicol. 2009 doi: 10.3109/15476910903501748. Advance access published on December 29, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin. Liver Dis. 2002;6:467–486. doi: 10.1016/s1089-3261(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Lorena D, Darby IA, Gadeau AP, Leen LL, Rittling S, Porto LC, Rosenbaum J, Desmoulière A. Osteopontin expression in normal and fibrotic liver: altered liver healing in osteopontin-deficient mice. J.Hepatol. 2006;44:383–390. doi: 10.1016/j.jhep.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Maddrey WC, Boitnott JK. Isoniazid hepatitis. Ann. Intern. Med. 1973;79:1–12. doi: 10.7326/0003-4819-79-1-1. [DOI] [PubMed] [Google Scholar]
- Manns MP, Vogel A. Autoimmune hepatitis, from mechanisms to therapy. Hepatology. 2006;43:S132–S144. doi: 10.1002/hep.21059. [DOI] [PubMed] [Google Scholar]
- Martelius T, Salaspuro V, Salmi M, Krogerus L, Hockerstedt K, Jalkanen S, Lautenschlager I. Blockade of vascular adhesion protein-1 inhibits lymphocyte infiltration in rat liver allograft rejection. Am. J. Pathol. 2004;165:1993–2001. doi: 10.1016/S0002-9440(10)63250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Nomiyama H, Yoneyama H, Uwatoku R. Kupffer cell-mediated recruitment of dendritic cells to the liver crucial for a host defense. Dev. Immunol. 2002;9:143–149. doi: 10.1080/1044667031000137610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, McAvoy EF, Lam F, Gill V, de la Motte C, Savani RC, Kubes P. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J. Exp. Med. 2008;205:915–927. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–195. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, Asakura H, Matsushima K. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J. Clin. Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WG, Kelton JG. Methyldopa-induced autoantibodies against red blood cells. Blood Rev. 1988;2:36–42. doi: 10.1016/0268-960x(88)90006-9. [DOI] [PubMed] [Google Scholar]
- Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am. J. Pathol. 1994;145:1450–1462. [PMC free article] [PubMed] [Google Scholar]
- Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol. Sci. 2007;97:205–213. doi: 10.1093/toxsci/kfl180. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Park BK, Kitteringham NR, Maggs JL, Pirmohamed M, Williams DP. The role of metabolic activation in drug-induced hepatotoxicity. Annu. Rev. Pharmacol. Toxicol. 2005;45:177–202. doi: 10.1146/annurev.pharmtox.45.120403.100058. [DOI] [PubMed] [Google Scholar]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(Suppl. 1):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol. Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in liver inflammation, toxicity and carcinogenesis. Toxicol. Sci. 2007a;103:4–13. doi: 10.1093/toxsci/kfm246. [DOI] [PubMed] [Google Scholar]
- Ramaiah SK, Rittling S. Role of osteopontin in regulating hepatic inflammatory responses and toxic liver injury. Expert Opin. Drug Metab. Toxicol. 2007b;3:519–526. doi: 10.1517/17425225.3.4.519. [DOI] [PubMed] [Google Scholar]
- Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr. Opin. Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S, Maggi E, Liotta F, Cosmi L, Annunziato F. Properties and origin of human Th17 cells. Mol. Immunol. 2009;47:3–7. doi: 10.1016/j.molimm.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Russmann S, Kullak-Ublick GA, Grattagliano I. Current concepts of mechanisms in drug-induced hepatotoxicity. Curr. Med. Chem. 2009;16:3041–3053. doi: 10.2174/092986709788803097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai A, Malladi P, Melin-Aldana H, Green RM, Whitington PF. Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G264–G273. doi: 10.1152/ajpgi.00002.2004. [DOI] [PubMed] [Google Scholar]
- Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, Friedman SL. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870–882. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- Sato K, Imai Y, Higashi N, Kumamoto Y, Onami TM, Hedrick SM, Irimura T. Lack of antigen-specific tissue remodeling in mice deficient in the macrophage galactose-type calcium-type lectin 1/CD301a. Blood. 2005;106:207–215. doi: 10.1182/blood-2004-12-4943. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Seth D, Gorrell MD, Cordoba S, McCaughan GW, Haber PS. Intrahepatic gene expression in human alcoholic hepatitis. J. Hepatol. 2006;45:306–320. doi: 10.1016/j.jhep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- Sturge J, Todd SK, Kogianni G, McCarthy A, Isacke CM. Mannose receptor regulation of macrophage cell migration. J. Leukoc. Biol. 2007;82:585–593. doi: 10.1189/jlb.0107053. [DOI] [PubMed] [Google Scholar]
- Waldhauser L, Uetrecht J. Antibodies to myeloperoxidase in propylthiouracil-induced autoimmune disease in the cat. Toxicology. 1996;114:155–162. doi: 10.1016/s0300-483x(96)03476-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mochida S, Kawashima R, Inao M, Matsui A, YouLuTuZ Y, Nagoshi S, Uede T, Fujiwara K. Increased expression of osteopontin in activated Kupffer cells and hepatic macrophages during macrophage migration in Propionibacterium acnes-treated rat liver. J. Gastroenterol. 2000;35:696–701. doi: 10.1007/s005350070049. [DOI] [PubMed] [Google Scholar]
- Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N. Engl. J. Med. 2001;344:1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- Wilbanks A, Zondlo SC, Murphy K, Mak S, Soler D, Langdon P, Andrew DP, Wu L, Briskin M. Expression cloning of the strl33/bonzo/tymstr ligand reveals elements of cc, cxc, and cx3c chemokines. J. Immunol. 2001;166:5145–5154. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]
- Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, Hasegawa G, Naito M, Asakura H, Matsushima K. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 2001;193:35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- Zimmerman H. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. [Google Scholar]