Abstract
1,3-Butadiene (BD) is a known rodent and human carcinogen that is metabolized mainly by P450 2E1 to three epoxides, 1,2-epoxy-3-butene (EB), 1,2:3,4-diepoxybutane (DEB), and 1,2-epoxy-3,4-butanediol. The individual epoxides vary up to 200-fold in their mutagenic potency, with DEB being the most mutagenic metabolite. It is important to understand the internal formation of the individual epoxides to assign the relative risk for each metabolite and to understand the molecular mechanisms responsible for extensive species differences in carcinogenicity. This study presents a comprehensive exposure-response for the formation of the DEB-specific N,N-(2,3-dihydroxy-1,4-butadiyl)valine (pyr-Val) in mice and rats. Using nano-ultra high pressure liquid chromatography-tandem-mass spectrometry allowed analysis of pyr-Val in mice and rats exposed to BD as low as 0.1 and 0.5 ppm BD, respectively, and demonstrated significant differences in the amounts and exposure-response of pyr-Val formation. Mice formed 10- to 60-fold more pyr-Val compared to rats at similar exposures. The formation of pyr-Val increased with exposures, and the formation was most efficient with regard to formation per parts per million BD at low exposures. While formation at higher exposures appeared linear in mice, in rats formation saturated at exposures ≥ 200 ppm for 10 days. In rats, amounts of pyr-Val were lower after 20 days than after 10 days of exposure, suggesting that the lifespan of rat erythrocytes may be shortened following exposure to BD. This research supports the hypothesis that the lower susceptibility of rats to BD-induced carcinogenesis results from greatly reduced formation of DEB following exposure to BD.
Keywords: butadiene diepoxide, molecular dosimetry, N-terminal valine adducts
1,3-Butadiene (BD) is an important industrial chemical and indirect alkylating agent that people are exposed to during the production of synthetic rubber and resins (Himmelstein et al., 1997) and as a byproduct of incomplete combustion in cigarette smoke, auto exhaust, and fossil fuels (Agency for Toxic Substances and Disease Registry [ATSDR], 1993). BD has been recently rated the cigarette constituent with the highest cancer risk index (Fowles and Dybing, 2003). Epidemiologic studies have shown an increased incidence of leukemia in workers exposed to BD in synthetic rubber production and an increase in lymphohematopoietic cancers in BD production workers that was not exposure related (International Agency for Research on Cancer [IARC], 2008). Most agencies (ATSDR, National Toxicology Program [NTP], and U.S. Environmental Protection Agency [U.S. EPA]) have classified BD as a human carcinogen based on evidence from epidemiologic and animal studies (ATSDR, 1993; IARC, 2008; NTP, 2005; U.S. EPA, 2002). BD is a multispecies multisite carcinogen in rodents, with mice being a much more sensitive species than rats (IARC, 2008). The complexity of BD carcinogenesis largely has been attributed to species-dependent differences in BD metabolism.
BD is metabolized to several epoxide metabolites that are known to bind to DNA and proteins. For BD risk assessment, the mode of action is assumed to be that of a DNA-reactive compound and the corresponding key events have been listed by Preston (2007) based on the U.S. EPA guidelines for risk assessment. Formation of promutagenic DNA adducts that subsequently lead to mutations represent the causal link between exposure and tumor development (Preston, 2007). BD-derived epoxides differ in their mutagenic potency up to 200-fold with DEB being the most mutagenic metabolite (Meng et al., 2007; Walker et al., 2009). To improve the scientific basis of BD risk assessment, it is important to have detailed knowledge of the internal formation of each potentially mutagenic metabolite and its dependence on species, gender, and exposure concentrations.
While not causally linked with carcinogenesis, protein adducts have been widely used as surrogate biomarkers for internal exposure and DNA adduct formation. Protein adducts and especially N-terminal valine adducts of hemoglobin are well suited to study carcinogen metabolism across species for the following reasons (Boysen et al., 2007a; Törnqvist et al., 2002): First, they are good surrogate biomarkers for the internal formation of reactive metabolites. Second, in molecular epidemiology studies, blood specimens are more easily obtained than tissues. Third, they are not removed by enzymatic repair systems like DNA adducts. Fourth, they accumulate over the lifetime of the erythrocytes, which is about 45, 63, and 120 days for mice, rats, and humans, respectively. Fifth, since they represent cumulative exposure prior to sampling, the time of sample collection is less critical. Lastly, the N-terminal valine is freely accessible to alkylating agents in all species, allowing interspecies comparisons relative to metabolism and cancer risk. Therefore, N-terminal valine adducts are valuable biomarkers to study the internal formation of the reactive metabolites of BD-derived epoxides.
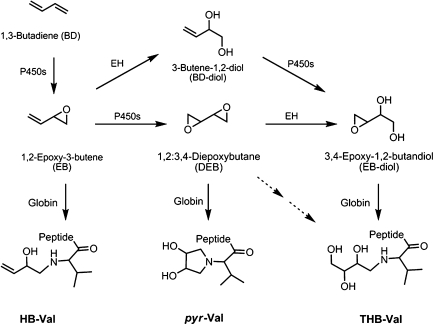
BD is metabolized primarily by P450 2E1 to 1,2-epoxybutene (EB), 1,2:3,4-diepoxybutane (DEB), and 3,4-epoxy-1,2-butanediol (EB-diol) (Himmelstein et al., 1997) that produce various hemoglobin adducts shown in Figure 1. EB produces N-(2-hydroxy-3-buten-1-yl)-valine (HB-Val), and DEB forms N,N-(2,3-dihydroxy-1,4-butadiyl)-valine (pyr-Val) and 2,3,4-trihydroxybutyl-valine (THB-Val). The later adduct is also formed from EB-diol and is present in unexposed animals and humans from endogenous sources (Koc et al., 1999). THB-Val and HB-Val adducts can be readily measured using the modified Edman degradation followed by liquid-liquid extraction and analysis by gas chromatography-mass spectrometry (GC-MS or GC-MS/MS) (Booth et al., 2004; Powley et al., 2005; Törnqvist et al., 1986). Unfortunately, the presence of a tertiary amine makes pyr-Val unsuitable for analysis by Edman degradation. Therefore, a proteomics approach was established for the analysis of pyr-Val in rodents (Boysen et al., 2004; Kautiainen et al., 2000). This method utilized trypsin hydrolysis and immunoaffinity (IA) purification prior to quantitation of the N-terminal pyr-Val (1–7) peptide by liquid chromatography-tandem-mass spectrometry (LC-MS/MS). We report herein a comprehensive exposure-response of pyr-Val formation in male and female mice and rats that had been exposed to various concentrations of BD by inhalation. Exposures ranged from 0.1 to 625 ppm for 10 days and from 1.0 to 62.5 ppm for 20 days.
FIG. 1.
BD metabolism and formation of N-terminal valine adducts. The numbers in EB-diol are based on the metabolism and are not in accordance with IUPAC nomenclature.
MATERIALS AND METHODS
Materials.
Trypsin (biotin-agarose, from bovine pancreas) was purchased from Sigma-Aldrich (St Louis, MO). All reagents and solvents were ACS grade or higher. Centricon-3 filters were obtained from Amicon Inc. (Beverly, MA), and Microspin filter tubes (regenerated cellulose, 0.2 μm) were from Alltech Associates Inc. (Deerfield, IL). Polyclonal antibodies against pyr-Val (1–11) were raised by Anaspec (San Jose, CA) using a standard peptide synthesized as described previously (Jayaraj et al., 2003).
Animals and exposures.
The exposures were performed at the Lovelace Respiratory Research Institute (Albuquerque, NM) according to protocols approved by the Institutional Animal Care and Use Committee. B6C3F1 mice (male and female) and F344 rats (male and female) were exposed by inhalation to 0.1, 0.5, 1, 1.5, 6.25, 62.5, 200, or 625 ppm BD for 10 days (2 weeks, 5 day/week, and 6 h/day). In addition, mice and rats were exposed to 1, 6.25, or 62.5 ppm BD for 20 days (4 weeks, 5 day/week, and 6 h/day). The animals were exposed to BD using multitiered whole-body exposure chambers (H-2000; Lab Products, Aberdeen, MD). The total volume of the H-2000 chamber is 1.7 m3. The flow rate through each chamber was maintained at 15 ± 2 air changes per hour. All chamber supply air was high efficiency particulate air-filtered before being introduced into the chamber supply system. Exposures were conducted using BD gas delivered to each chamber from compressed gas cylinders. Flow from each cylinder was controlled via a rotameter. Rodents in one chamber received filtered air only as a control group, and rodents in other chambers received BD. Each exposure was operated for 6 h + T90 (time to reach 90% of the target concentration) per exposure day. T90 values for each exposure level were determined prior to the start of exposures.
Vapor concentrations in exposure chambers were monitored by two independent methods. A MIRAN infrared spectrometer was used periodically to monitor chamber vapor concentration in real time via infrared absorbance. The MIRAN infrared spectrometer is a single-beam variable filter infrared spectrometer, and the gas cell parameters, wavelength, and path length were adjusted for optimal operation. In the backup quantification of BD in the atmospheres, grab samples were collected three times daily during each exposure day, and the samples were analyzed by GC. BD atmospheres were compared to five-point calibration curves that spanned the exposure range required to measure with accuracy the lower and upper ends of the BD exposure concentrations used in rodent inhalation studies. At no time did the BD exposure atmosphere concentration exceed the upper calibration concentration. Analysis of BD exposure atmospheres by GC and a flame ionization detector revealed no impurities, attesting to both the stability of the exposure atmosphere and the lack of detectable impurities in the reference BD (certified as > 99% pure).
At the end of the last exposure, animals were killed by exsanguination under CO2 anesthesia and blood samples were collected by cardiac puncture within 2 h of the last exposure. Red blood cells were isolated, washed twice with 0.9% saline, diluted 2× in distilled water, and stored at −80°C before extraction of globin.
Quantitation of pyr-Val.
Amounts of pyr-Val were measured based on a procedure described by Boysen et al. (2004) using peptide standards that had been quantified accurately as described by Bordeerat et al. (2009). In brief, globin isolation was performed according to Mowrer et al. (1986). Globin samples of 10–50 mg (depending on the species and the exposures) were dissolved in 1.5 ml of 0.1M NH4HCO3 and 2 pmol of internal standard [2H3]N,N-(2,3-dihydroxy-1,4-butadiyl)-valine ([2H3]pyr-Val) (1–11) peptide was added. Samples were digested for 24 h at 37°C with 50–100 μl of trypsin-biotin-agarose suspension (washed in advance twice with 0.1M NH4HCO3). After Centricon-3 filtration, samples were dried and redissolved in 600-μl PBS buffer, loaded on IA columns, and left capped for 1 h. After extensive washing with distilled water (5 × 7 ml) and elution in 3 ml of 5% formic acid, followed by sample drying, filtration on Microspin filters, and final drying, samples were stored at −20°C until analysis by nano-ultra high pressure liquid chromatography-tandem-mass spectrometry (nano-UPLC-MS/MS).
Liquid chromatography and mass spectrometry.
The quantitative analysis of the pyr-Val and [2H3]pyr-Val by nano-LC-MS/MS was performed with an nano-UPLC (Waters, Milford, MA) coupled to a TSQ-Quantum Ultra triple quad mass analyzer (ThermoFinnigan, San Jose, CA). The system utilized a 2.0 × 20–mm Symmetry C18, 5-μm column (Waters) as a “trap column” for samples loading at 15 μl/min 15mM ammonium formate-0.7% formic acid for 1 min. After sample loading, the flow rate was reduced to 1.2 μl/min and the column exit flow was directed to an “analytical column” consisting of a 100 μm × 100–mm BEH C18 UPLC column (Waters). pyr-Val and [2H3]-pyr-Val were eluted with a linear gradient of 5% acetonitrile/15mM ammonium formate-0.7% formic acid for 2 min then to 70% acetonitrile/15mM ammonium formate-0.7% formic acid for 10 min at a flow rate of 1.2 μl/min. The retention times for the analyte and internal standard were determined with authentic standards, and pyr-Val and [2H3]pyr-Val were detected in selected reaction monitoring mode, monitoring the transitions of the double-charged ions m/z 417.25–158.25 and m/z 418.75–158.75, respectively. The electrospray conditions were spray voltage of 1800 V and capillary temperature of 240°C. Collision energy was 28 V.
Statistical analysis.
Statistical analyses were performed using Microsoft Excel spreadsheet analysis tools. The amounts of pyr-Val adduct formation are reported as average and SD (mean ± SD) for each animal group. In addition, the interanimal variability was determined by calculating the coefficient of variation (CV). It was calculated as the mean of amount pyr-Val divided by SD and reported as a percentage. Comparisons of gender differences in adduct levels were made by a Student’s t-test (two-sample unequal variances). The analyses were performed separately for mice and rats. A significant difference was defined by p < 0.05.
RESULTS
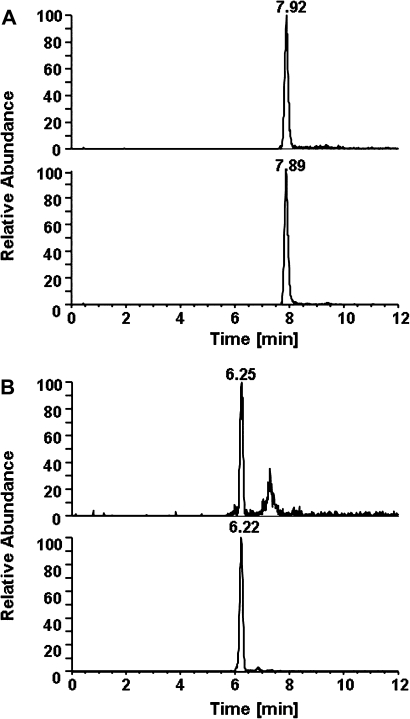
The peptide method was used for analysis of pyr-Val in mice and rats exposed to low concentrations of BD. Using nano-UPLC-MS/MS instead of capillary-LC-MS/MS increased sensitivity (The limit of detection was 1 fmol and the limit of quantitation was 4 fmol on column) and allowed detection of pyr-Val in mice and rats exposed to BD as low as 0.1 and 0.5 ppm, respectively. The amounts of pyr-Val in rats exposed to 0.1 ppm were below the limit of detection. Overall, in mice, the CV within each group was < 25% (except for the 0.1 ppm exposures), while in rats, larger CVs were observed. However, positive controls consisting of control globin spiked with authentic 1–11 peptide standards from both species and had CV < 10%, demonstrating the accuracy and interday and intraday reproducibility of the assay (data not shown). The larger CV in rats suggests larger interanimal variability in rats than in mice. The amounts of pyr-Val formed in mice and rats are presented in Table 1 and representative ion chromatograms for pyr-Val in mice and rats exposed to 1 ppm BD for 10 days are shown in Figure 2.
TABLE 1.
Amounts of pyr-Val Adducts (picomoles per gram of globin) in Mice and Rats after Inhalation Exposures to BD
| B6C3F1 mice |
F344 rats |
|||||||||
| Duration | Gender | BD exposure (ppm) | n | Mean ± SD | %CV | Adducts/ppm BD | n | Mean ± SD | %CV | Adducts/ppm BD |
| 10 days | Females | 0 | 11 | ND | 4 | ND | ||||
| 0.1 | 5 | 2.1 ± 1.3 | 62 | 21 | 5 | ND | ||||
| 0.5 | 5 | 10.5 ± 2.5 | 24 | 21 | 6 | 0.7 ± 0.1 | 10 | 1.4 | ||
| 1 | 5 | 20.2 ± 2.0 | 10 | 20 | 5 | 2.0 ± 0.2 | 11 | 2.0 | ||
| 1.5 | 3 | 38.2 ± 2.2 | 6 | 26 | 6 | 3.4 ± 0.2 | 6 | 2.3 | ||
| 6.25 | 5 | 75.8 ± 10 | 13 | 12 | 5 | 9.1 ± 0.9 | 9 | 1.7 | ||
| 62.5 | 5 | 419 ± 63 | 15 | 6.7 | 8 | 47 ± 8.3 | 18 | 0.7 | ||
| 200 | 4 | 739 ± 99 | 13 | 3.7 | 10 | 120 ± 36 | 30 | 0.6 | ||
| 625 | 7 | 1532 ± 362 | 24 | 2.5 | 4 | 119 ± 14 | 12 | 0.2 | ||
| Males | 0 | 6 | ND | 6 | ND | |||||
| 0.1 | 5 | 3.1 ± 1.1 | 35 | 31 | 5 | ND | ||||
| 0.5 | 5 | 15.4 ± 2.0 | 13 | 31 | 5 | 0.6 ± 0.3 | 47 | 1.2 | ||
| 1.5 | 5 | 49.8 ± 8.6 | 17 | 33 | 5 | 1.5 ± 1.0 | 62 | 1.0 | ||
| 200 | 5 | 859 ± 126 | 15 | 4.3 | 10 | 119 ± 25 | 21 | 0.6 | ||
| 625 | 5 | 1980 ± 195 | 10 | 3.2 | 8 | 125 ± 33 | 27 | 0.2 | ||
| 20 days | Females | 0 | 3 | ND | 5 | ND | ||||
| 1 | 3 | 53.7 ± 9.5 | 18 | 54 | 6 | 4.1 ± 1.5 | 37 | 4.1 | ||
| 6.25 | 7 | 290 ± 58 | 20 | 46 | 6 | 9.9 ± 1.7 | 17 | 1.5 | ||
| 62.5 | 6 | 1571 ± 456 | 29 | 25 | 6 | 29.4 ± 12 | 42 | 0.5 | ||
| Males | 0 | ND | 5 | ND | ||||||
| 1 | 6 | 65.6 ± 21 | 32 | 66 | 6 | 3.3 ± 1.7 | 52 | 3.3 | ||
| 6.25 | 6 | 379 ± 34 | 9 | 61 | 6 | 7.1 ± 1.6 | 23 | 1.1 | ||
| 62.5 | 6 | 1385 ± 227 | 16 | 22 | 6 | 21.5 ± 8.3 | 35 | 0.3 | ||
Note. ND, not detected.
FIG. 2.
Ion chromatograms of pyr-Val from a B6C3F1 mouse (A) and a F344 rat (B) exposed to 1 ppm BD for 10 days. Shown are the ion transitions of the doubly charged analyte (m/z 417.3–158.3, top) and internal standard peptides (m/z 418.8–158.7, bottom).
Exposure-Response of pyr-Val Formation in Mice
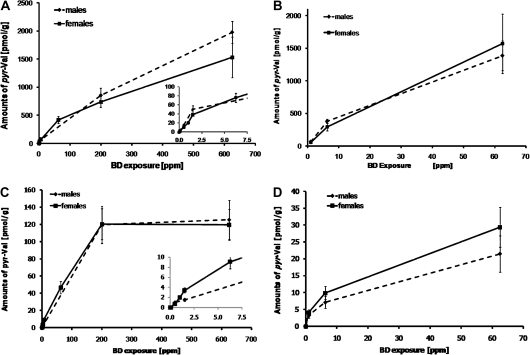
The exposure-responses of pyr-Val formation in male and female B6C3F1 mice are plotted in Figures 3A and 3B. The formation of pyr-Val increased with exposure, and the slopes of formation were steepest at exposures ≤ 1.5 ppm (Fig. 3A, insert) independent of exposure duration (10 or 20 days). Formation of pyr-Val in mice also increased with prolonged exposure and was 3.4 ± 0.7–fold higher after 20 days compared to 10 days of exposure (Table 1). There were no significant gender differences in mice at the exposure concentrations and durations studied.
FIG. 3.
Exposure-response of pyr-Val in male (solid lines) and female (dashed lines) B6C3F1 mice and F344 rats. (A) Mice were exposed to BD for 10 days. (B) Mice were exposed to BD for 20 days. (C) Rats were exposed to BD for 10 days. (D) Rats were exposed to BD for 20 days. Rodents were exposed to BD by inhalation, and globin was obtained and analyzed for pyr-Val as described in the “Material and Methods” section. Inserts in (A) and (C) show exposure-responses at exposures below 7.5 ppm BD.
Exposure-Response of pyr-Val Formation in Rats
The exposure-responses of pyr-Val formation in male and female F344 rats are plotted in Figures 3C and 3D. In female rats exposed for 10 days, progressive decreases in the slope for formation of pyr-Val were found at exposures between 1.5 and 62.5 ppm BD (β < 1) with a plateau in formation found at exposures of 200 ppm BD and above (Fig. 3C). Similar changes in slope may also occur after 10 days of exposures in male rats, but there are currently insufficient data points to determine the slope at low BD exposures. After 20 days of exposure, progressive decreases in slope were observed between exposures to 1, 6.25, and 62.5 ppm BD (β < 1) (Fig. 3D). In contrast to the findings in mice, there were no significant differences between 10- and 20-day exposures to ≤ 6.25 ppm BD in the formation of pyr-Val in rats (Table 1). Surprisingly, in female rats, the amounts of pyr-Val were significantly lower after 20 days compared to 10 days of exposure (Table 1). At lower exposures, amounts of pyr-Val were higher after 20 days compared to 10 days of exposure, but the number of data points was insufficient for establishing a biphasic dose-response over time.
Formation of pyr-Val in Mice Compared to Rats
After 10 days of exposure, female mice formed 10 ± 2.5-fold more DEB, measured as pyr-Val formation, than rats at similar exposures. After 20 days of exposure, mice had increasingly more pyr-Val than rats. With increasing exposure concentrations for 10 days, the decreases in slope for pyr-Val were most apparent at exposures above 1.5 and 6.25 ppm BD in mice and rats, respectively. The exposure-response curve reached a plateau in rats at exposures of 200 ppm or greater, while it increased more linearly in mice (Fig. 3, Table 1).
DISCUSSION
Determination of the molecular dose of DEB is critical for understanding the mechanisms of BD carcinogenesis and mutagenesis because in vitro DEB is ∼100 and 200-fold more mutagenic than most of the stereochemical forms of EB and EB-diol, respectively (Himmelstein et al., 1997). The analyses of HB-Val and THB-Val have greatly enhanced our knowledge of BD metabolism across species. The first data on the formation of DEB in rats and mice after inhalation exposures were reported by Boysen et al. (2004). In that communication, the DEB-specific pyr-Val adduct was determined in mice and rats exposed to three different concentrations of BD. Comparisons of pyr-Val to previous measurements of HB-Val and THB-Val adducts suggest that DEB may indeed be the most important BD-derived metabolite for inducing mutations (Boysen et al., 2007a).
The current investigation greatly extends our knowledge by providing data on the formation of pyr-Val from a comprehensive series of exposures to BD in mice and rats. B6C3F1 mice and F344 rats were exposed by inhalation to concentrations of BD ranging from 0.1 to 625 ppm. For perspective, ambient BD concentrations in BD monomer and polymer plant work places have ranged from 0.3 to 3 ppm (IARC, 2008). Cigarette smoke and side stream tobacco smoke contains as much as 169 and 42 ppm BD, respectively, as determined by real-time analysis of exhaled breath condensate (Gordon et al., 2002). Therefore, the low exposures applied herein are representative of occupational- and smoking-related human exposures and should be more informative than previous data from higher exposures of BD. The recently established peptide assay allowed analysis of pyr-Val after exposures known to induce tumors in rodents and down to those encountered in occupational settings. As expected, the amount of pyr-Val increases with exposure in both species. However, significant species differences in the magnitude and patterns of exposure-response for pyr-Val formation were observed.
From the limited data available previously, it was suggested that formation of pyr-Val was highest at low exposures and that extrapolation of the high exposure data would not go through zero. To investigate the formation of DEB at low exposures in detail, several exposures were carried out with BD concentrations below 3 ppm, which was the lowest concentration previously analyzed for pyr-Val. The presented exposure-response curves for pyr-Val clearly establish a change in the slope at exposures below 1.5 and 6.25 ppm in mice and rats, respectively (see inserts in Figs. 3A and 3C). In mice, exposures below 1.5 ppm BD (10 days) produced about 21 pmol pyr-Val/g globin/ppm BD, while at exposures greater than 1.5 ppm, this rate decreased as much as 10-fold at higher exposures, reaching 2.5 pmol pyr-Val/g globin/ppm BD at 625 ppm (Table 1).
Previously, it had been noted that the amounts of pyr-Val were similar in F344 rats exposed to 62.5 ppm BD for 20 days compared to Crl:CD rats exposed to 1000 ppm for 90 days (Boysen et al., 2007a; Swenberg et al., 2008), suggesting saturation for pyr-Val formation in rats. This earlier evidence, however, was confounded by the fact that different strains and duration of exposures were combined for interpretation. The data reported herein show that the amount of pyr-Val in F344 rats reaches a plateau at exposures of 200 ppm BD and above for 10 days (Fig. 3C). The saturation curve for pyr-Val in rats is not surprising since formation of EB-diol has been shown to reach a plateau at exposures greater than 62.5 ppm BD for 20 days, using THB-Val and THB-guanine adducts as biomarkers (Koc et al., 1999; Swenberg et al., 2001).
Direct comparisons of pyr-Val to DNA cross-link adduct formation can be made since animals from the same exposures have been studied. Tretyakova and colleagues determined the presence of N7-guanine-N7-guanine [1,4-bis(guan-7-yl)-2,3-butanediol (bisN7-Gua-BD-diol)] and N7-guanine-N1-adenine [1-(guan-7-yl)-4-(aden-1-yl)-2,3-butanediol (N7-Gua-N1-Ade-BD-diol)] in liver, lung, kidney, brain, and thymus of rats and mice after 10-day inhalation exposures to 6.25, 62.5, and 625 ppm BD (Goggin et al., 2007, 2008, 2009). Based on DNA-DNA cross-links, mice form ∼10-fold more DEB than rats. The formation of DNA-DNA cross-links was highest in liver. Thus, both pyr-Val and DNA-DNA cross-links show that mice form much more DEB than rats. There were some differences, however, between the two biomarkers. The formation of pyr-Val saturated in rats at 200 ppm BD and higher, while the DNA-DNA cross-links saturated at 62.5 ppm BD, similar to our previous data on THB-Gua (Koc et al., 1999). Furthermore, pyr-Val did not show a gender difference, while DNA-DNA cross-links were twice as high in liver of female rats and mice compared to males.
In rats, there was a decrease in the amounts of pyr-Val after 20 days of BD exposure to 62.5 ppm compared to that measured after 10 days of exposure. Female rats exposed to 6.25 ppm BD for 10 or 20 days had similar amounts of pyr-Val (Table 1). In both cases, we expected greater amounts of pyr-Val in rats exposed for 20 days. The reason for this unexpected finding is not clear. It is possible that exposure of erythrocytes to DEB cross-links proteins or otherwise damages the cell surface, leading to increased scavenging by the spleen. Walker et al. (1992) showed that rat erythrocytes had a shortened lifespan following high exposures to ethylene oxide. This was thought to be due to a decreased lifespan of the older erythrocytes, reducing the molecular dose of ethylene oxide globin adducts in the cells exposed for the longest period, plus greater release of juvenile erythrocytes from the bone marrow. The similarity of pyr-Val amounts measured in the 20-day F344 rats exposed to 62.5 ppm BD and the 90-day 1000 ppm BD exposures in Sprague-Dawley rats previously reported (Boysen et al., 2004, 2007a) might be due to a combination of increased erythrocyte scavenging, dilution by increased hematopoiesis, and saturation of metabolism at high concentrations of BD. Unfortunately, the exposure regime of the present study was not designed to investigate potential effects of exposure duration on the formation of pyr-Val or the lifespan of erythrocytes. It was expected that pyr-Val adducts would be higher after 20 days of exposure due to the additional exposure time. In contrast, the present data suggest that the lifespan of highly exposed rat erythrocytes may be shorter than 63 days. No such effect was seen in mice. To our knowledge, no data have been reported in the literature on the lifespan of erythrocytes in BD-exposed mice or rats. Formation of HB-Val and THB-Val increases linearly up to 3 weeks in ICR female mice exposed to 500 and 1000 ppm BD (Lee et al., 2005).
In the current study of pyr-Val formation, the exposures reported herein were designed to address potential gender differences in BD metabolism. At exposures longer in duration (90 days) and higher concentrations (1000 ppm BD), formation of HB-Val, pyr-Val, and THB-Val were reported to be 1.6-, 3-, and 2-fold higher in female than in male rats, respectively (Boysen et al., 2004; Swenberg et al., 2000). In the present study, there were no statistically significant gender differences in formation of pyr-Val in rats or mice. Therefore, if there is a gender difference in formation of DEB, it may be only important at high concentrations (> 625 ppm), after prolonged (90 days) exposures, or in certain strains (e.g., Sprague-Dawley rats).
In addition to reaching a plateau, the exposure-response in rats also shows evidence for decreases in slopes for pyr-Val formation similar to the ones observed in mice at exposures above 6.25 ppm. The mechanism behind these complex response curves is not fully understood. It may be the result of overlapping enzyme activities. P450 2E1 and P450 2A6 are involved in BD metabolism to EB, with P450 2E1 being the high-affinity low-capacity isozyme and the P450 2A6 the low-affinity high-capacity isozyme (Krause and Elfarra, 1997). The subsequent conversion of EB to DEB is catalyzed by P450 2E1 (Krause and Elfarra, 1997) and potentially also by P450 3A4 (Seaton et al., 1995). In rat liver microsomes, the formation of DEB showed a change in slope similar to the response for pyr-Val, while the formation was linear in mouse liver microsomes. In addition, epoxide hydrolase and alcohol dehydrogenase compete for the BD metabolites and influence the half-life of free DEB (Kemper et al., 1998). Lastly, inhibition of P450 2E1 by controlled phosphorylation (Oesch-Bartlomowicz et al., 1998) or by covalent binding of reactive metabolites to the active site similar to tert-butyl acetylenes has been proposed (Blobaum et al., 2002; Boysen et al., 2007b). Such hypotheses are supported by the observation that p-nitrophenol hydroxylation activity (specific P450 2E1 substrate) is reduced in microsomes isolated from BD-diol–treated mice (Kemper et al., 1998). Future research on mechanisms responsible for the regulation of BD metabolism are needed to better understand the species and dose dependence of BD mutagenesis and carcinogenesis.
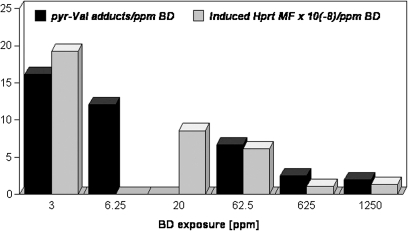
Earlier, metabolite-specific Hprt mutation studies in mice, and comparisons between biomarkers of exposure and effect in mice exposed to BD or its epoxy intermediates, suggested that the mutagenicity of BD at relatively low exposure concentrations (3–62.5 ppm) could be attributed to DEB (Meng et al., 2001; Walker and Meng, 2000b). For example, previous comparisons between the efficiency of formation of several hemoglobin/DNA adducts and the efficiency of Hprt mutant T-cell induction showed poor correlations between EB-specific adducts and Hprt mutant frequencies but positive correlations between THB-guanine adducts (derived from DEB and EB-diol) in lung and mutant frequencies in T cells of BD-exposed mice (Meng et al., 2001). Figure 4 compares the efficiency of pyr-Val formation with the efficiency of Hprt mutant T-cell induction in female mice following exposures of 3–1250 ppm BD for 10 days and shows the strongest correlation to date between in vivo formation of an exposure marker for a specific BD epoxide intermediate (pyr-Val) and mutagenic responses following BD exposures. Additional analyses are needed to fill gaps in the pyr-Val and Hprt mutant frequency data for mice, rats, and humans, but these preliminary data suggest that in vivo amounts of this DEB-specific hemoglobin adduct may be a highly predictive biomarker for BD-induced mutations and cancer risk.
FIG. 4.
Efficiency of pyr-Val adduct formation (picomoles per gram of globin per parts per million of BD) and Hprt mutation induction in splenic T cells from female B6C3F1 mice following inhalation exposure to BD. Mice were exposed to BD for 10 days (5 day/week and 6 h/day) and then groups of control and BD-exposed animals were necropsied 2 h or 4 weeks after the cessation of exposures for respective measurements of pyr-Val and Hprt mutant frequency (MF). pyr-Val data are from Boysen et al. (2004) and current work; Hprt MF data are from an earlier report (Meng et al., 2001; Walker and Meng, 2000b; Walker et al., 2009). Data for pyr-Val adducts at 20 ppm BD and Hprt MFs at 6.25 ppm are currently not available.
In summary, the results presented clearly demonstrate the complexity of BD metabolism, including formation of DEB in mice and rats. Therefore, quantitative data on DEB formation cannot be simply translated to human exposures without obtaining accurate measures of the metabolic rate in humans. This can now be accomplished with similar assays on human globin. We have analyzed over 300 human samples for the presence of pyr-Val (data not shown) and are currently performing similar assays for HB-Val and THB-Val. At exposures between 0.1 and 1.0 ppm, it appears that humans form ∼10% of the pyr-Val formed by rats, which in turn form about 10% of the pyr-Val formed by mice. Thus, the mouse produces ∼100-fold more pyr-Val per parts per million BD than humans. Fred et al. (2008) recently suggested conversion of individual adduct values into EB equivalents to determine the carcinogenic and mutagenic potency of the individual epoxides. When all data on BD-derived hemoglobin adducts in mice, rats, and humans are available, such conversion will quantitatively demonstrate differences in mutagenic and carcinogenic potency of BD and its epoxide metabolites. It will also be possible to construct comparisons of the exposure-response for biomarkers of exposure (hemoglobin adducts) and biomarkers of effect (mutations), including the determination of exposures that do not result in increases in mutations over the normal background (Swenberg et al., 2008). These data will be very helpful in science-based risk assessment.
FUNDING
National Institutes of Health (1 R01 ES012689 and 5 P30-ES10126 to J.A.S.); Health Effects Institute (agreements 99-5 and 05-12 to V.E.W.); American Chemistry Council. The Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000 (to G.B.).
Acknowledgments
The authors acknowledge the technical assistance of Dr Valeriy Afonin for the isolation of all the globin samples and the editorial assistance of Ms Patricia Upton in formatting and editing this manuscript.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for 1,3-butadiene. 1993. U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [PubMed] [Google Scholar]
- Blobaum AL, Kent UM, Alworth WL, Hollenberg PF. Mechanism-based inactivation of cytochromes P450 2E1 and 2E1 T303A by tert-butyl acetylenes: characterization of reactive intermediate adducts to the heme and apoprotein. Chem. Res. Toxicol. 2002;15:1561–1571. doi: 10.1021/tx020052x. [DOI] [PubMed] [Google Scholar]
- Booth ED, Kilgour JD, Watson WP. Dose responses for the formation of hemoglobin adducts and urinary metabolites in rats and mice exposed by inhalation to low concentrations of 1,3-[2,3-14C]-butadiene. Chem. Biol. Interact. 2004;147:213–232. doi: 10.1016/j.cbi.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Bordeerat NK, Georgieva NI, Klapper DG, Collins LB, Cross TJ, Borchers CH, Swenberg JA, Boysen G. Accurate quantitation of standard peptides used for quantitative proteomics. Proteomics. 2009;9:3939–3944. doi: 10.1002/pmic.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boysen G, Georgieva NI, Upton PB, Jayaraj K, Li Y, Walker VE, Swenberg JA. Analysis of diepoxide-specific cyclic N-terminal globin adducts in mice and rats after inhalation exposure to 1,3-butadiene. Cancer Res. 2004;64:8517–8520. doi: 10.1158/0008-5472.CAN-04-3184. [DOI] [PubMed] [Google Scholar]
- Boysen G, Georgieva NI, Upton PB, Walker VE, Swenberg JA. N-terminal globin adducts as biomarkers for formation of butadiene derived epoxides. Chem. Biol. Interact. 2007a;166:84–92. doi: 10.1016/j.cbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Boysen G, Scarlett CO, Temple B, Combs TP, Brooks NL, Borchers CH, Swenberg JA. Identification of covalent modifications in P450 2E1 by 1,2-epoxy-3-butene in vitro. Chem. Biol. Interact. 2007b;166:170–175. doi: 10.1016/j.cbi.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fred C, Tornqvist M, Granath F. Evaluation of cancer tests of 1,3-butadiene using internal dose, genotoxic potency, and a multiplicative risk model. Cancer Res. 2008;68:8014–8021. doi: 10.1158/0008-5472.CAN-08-0334. [DOI] [PubMed] [Google Scholar]
- Goggin M, Anderson C, Park S, Swenberg J, Walker V, Tretyakova N. Quantitative high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry analysis of the adenine-guanine cross-links of 1,2,3,4-diepoxybutane in tissues of butadiene-exposed B6C3F1 mice. Chem. Res. Toxicol. 2008;21:1163–1170. doi: 10.1021/tx800051y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin M, Loeber R, Park S, Walker V, Wickliffe J, Tretyakova N. HPLC-ESI+-MS/MS analysis of N7-guanine-N7-guanine DNA cross-links in tissues of mice exposed to 1,3-butadiene. Chem. Res. Toxicol. 2007;20:839–847. doi: 10.1021/tx700020q. [DOI] [PubMed] [Google Scholar]
- Goggin M, Swenberg JA, Walker VE, Tretyakova N. Molecular dosimetry of 1,2,3,4-diepoxybutane-induced DNA-DNA cross-links in B6C3F1 mice and F344 rats exposed to 1,3-butadiene by inhalation. Cancer Res. 2009;69:2479–2486. doi: 10.1158/0008-5472.CAN-08-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SM, Wallace LA, Brinkman MC, Callahan PJ, Kenny DV. Volatile organic compounds as breath biomarkers for active and passive smoking. Environ. Health Perspect. 2002;110:689–698. doi: 10.1289/ehp.02110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelstein MW, Acquavella JF, Recio L, Medinsky MA, Bond JA. Toxicology and epidemiology of 1,3-butadiene. Crit. Rev. Toxicol. 1997;27:1–108. doi: 10.3109/10408449709037482. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) 1,3-Butadiene, Ethylene Oxide and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide), 2008. Vol. 97. World Health Organization, Lyon, France. [Google Scholar]
- Jayaraj K, Georgieva NI, Gold A, Sangaiah R, Koc H, Klapper DG, Ball LM, Reddy AP, Swenberg JA. Synthesis and characterization of peptides containing a cyclic Val adduct of diepoxybutane, a possible biomarker of human exposure to butadiene. Chem. Res. Toxicol. 2003;16:637–643. doi: 10.1021/tx020099i. [DOI] [PubMed] [Google Scholar]
- Kautiainen A, Fred C, Rydberg P, Törnqvist M. A liquid chromatography tandem mass spectrometric method for in vivo dose monitoring of diepoxybutane, a metabolite of butadiene. Rapid Commun. Mass Spectrom. 2000;14:1848–1853. doi: 10.1002/1097-0231(20001015)14:19<1848::AID-RCM106>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kemper RA, Elfarra AA, Myers SR. Metabolism of 3-butene-1,2-diol in B6C3F1 mice. Evidence for involvement of alcohol dehydrogenase and cytochrome p450. Drug Metab. Dispo. 1998;26:914–920. [PubMed] [Google Scholar]
- Koc H, Tretyakova NY, Walker VE, Henderson RF, Swenberg JA. Molecular dosimetry of N-7 guanine adduct formation in mice and rats exposed to 1,3-butadiene. Chem. Res. Toxicol. 1999;12:566–574. doi: 10.1021/tx980265f. [DOI] [PubMed] [Google Scholar]
- Krause RJ, Elfarra AA. Oxidation of butadiene monoxide to meso- and (±)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch. Biochem. Biophys. 1997;337:176–184. doi: 10.1006/abbi.1996.9781. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kang HS, Han DH. Ratios of N-(2,3,4-trihydroxybutyl) valine and N-(2-hydroxy-3-butenyl) valine formed hemoglobin adducts in female mice inhalation exposure with 1,3-butadiene. Toxicol. Ind. Health. 2005;21:15–20. doi: 10.1191/0748233705th210oa. [DOI] [PubMed] [Google Scholar]
- Meng Q, Henderson RF, Long L, Blair L, Walker DM, Upton PB, Swenberg JA, Walker VE. Mutagenicity at the Hprt locus in T cells of female mice following inhalation exposures to low levels of 1,3-butadiene. Chem. Biol. Interact. 2001;135–136:343–361. doi: 10.1016/s0009-2797(01)00222-8. [DOI] [PubMed] [Google Scholar]
- Meng Q, Redetzke DL, Hackfeld LC, Hodge RP, Walker DM, Walker VE. Mutagenicity of stereochemical configurations of 1,2-epoxybutene and 1,2:3,4-diepoxybutane in human lymphblastoid cells. Chem. Biol. Interact. 2007;166:207–218. doi: 10.1016/j.cbi.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Mowrer J, Törnqvist M, Jensen S, Ehrenberg L. Modified Edman degradation applied to hemoglobin for monitoring occupational exposure to alkylating agents. Toxicol. Environ. Chem. 1986;11:215–231. [Google Scholar]
- National Toxicology Program (NTP) Report on Carcinogens, Eleventh Edition. Washington, DC: U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program; 2005. [Google Scholar]
- Oesch-Bartlomowicz B, Padma PR, Becker R, Richter B, Hengstler JG, Freeman JE, Wolf CR, Oesch F. Differential modulation of CYP2E1 activity by cAMP-dependent protein kinase upon Ser129 replacement. Exp. Cell Res. 1998;242:294–302. doi: 10.1006/excr.1998.4120. [DOI] [PubMed] [Google Scholar]
- Powley MW, Li Y, Upton PB, Walker VE, Swenberg JA. Quantification of DNA and hemoglobin adducts of 3,4-epoxy-1,2-butanediol in rodents exposed to 3-butene-1,2-diol. Carcinogenesis. 2005;26:1573–1580. doi: 10.1093/carcin/bgi119. [DOI] [PubMed] [Google Scholar]
- Preston RJ. Cancer risk assessment for 1,3-butadiene: data integration opportunities. Chem. Biol. Interact. 2007;166:150–155. doi: 10.1016/j.cbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Seaton MJ, Follansbee MH, Bond JA. Oxidation of 1,2-epoxy-3-butene to 1,2:3,4-diepoxybutane by cDNA-expressed human cytochromes P450 2E1 and 3A4 and human, mouse and rat liver microsomes. Carcinogenesis. 1995;16:2287–2293. doi: 10.1093/carcin/16.10.2287. [DOI] [PubMed] [Google Scholar]
- Swenberg JA, Christova-Gueorguieva NI, Upton PB, Ranasinghe A, Scheller N, Wu KY, Hayes R. 1,3-Butadiene: Cancer, Mutations, and Adducts. Part V: Hemoglobin Adducts as Biomarkers of 1,3-Butadiene Exposure and Metabolism. Cambridge, MA: Health Effects Institute; 2000. [PubMed] [Google Scholar]
- Swenberg JA, Fryar-Tita E, Jeong YC, Boysen G, Starr T, Walker VE, Albertini RJ. Biomarkers in toxicology and risk assessment: informing critical dose-response relationships. Chem. Res. Toxicol. 2008;21:253–265. doi: 10.1021/tx700408t. [DOI] [PubMed] [Google Scholar]
- Swenberg JA, Koc H, Upton PB, Georgieva N, Ranasinghe A, Walker VE, Henderson R. Using DNA and hemoglobin adducts to improve the risk assessment of butadiene. Chem. Biol. Interact. 2001;135–136:387–403. doi: 10.1016/s0009-2797(01)00221-6. [DOI] [PubMed] [Google Scholar]
- Törnqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- Törnqvist M, Mowrer J, Jensen S, Ehrenberg L. Monitoring of environmental cancer initiators through hemoglobin adducts by a modified Edman degradation method. Anal. Biochem. 1986;154:255–266. doi: 10.1016/0003-2697(86)90524-5. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Health Assessment of 1,3-Butadiene. Washington, DC: National Center for Environmental Assessment; 2002. [Google Scholar]
- Walker VE, MacNeela JP, Swenberg JA, Turner MJ, Fennell TR. Molecular dosimetry of ethylene oxide: formation and persistence of 7-(2-hydroxyethyl)valine in hemoglobin following repeated exposures of rats and mice. Cancer Res. 1992;52:4320–4327. [PubMed] [Google Scholar]
- Walker VE, Meng Q. 1,3-Butadiene: cancer, mutations, and adducts. Part III: in vivo mutation of the endogenous hprt genes of mice and rats by 1,3-butadiene and its metabolites. Res. Rep. Health Eff. Inst. 2000a:89–139. [PubMed] [Google Scholar]
- Walker VE, Meng Q. In Vivo Mutation of the Endogenous hprt Genes of Mice and Rats by 1,3-Butadiene and its Metabolites. Boston, MA: Health Effects Institute; 2000b. [PubMed] [Google Scholar]
- Walker VE, Walker DM, Meng Q, McDonald JD, Scott BR, Bauer MJ, Seilkop SK, Claffey DJ, Upton PB, Powley MW, et al. Genotoxicity of 1,3-Butadiene and its Epoxy Intermediates. Montpelier, VT: Health Effects Institute, Capital City Press; 2009. [PubMed] [Google Scholar]