Abstract
Cyanide inhibits aerobic metabolism by binding to the binuclear heme center of cytochrome c oxidase (CcOX). Amyl nitrite and sodium nitrite (NaNO2) antagonize cyanide toxicity in part by oxidizing hemoglobin to methemoglobin (mHb), which then scavenges cyanide. mHb generation is thought to be a primary mechanism by which the NO2− ion antagonizes cyanide. On the other hand, NO2− can undergo biotransformation to generate nitric oxide (NO), which may then directly antagonize cyanide inhibition of CcOX. In this study, nitrite-mediated antagonism of cyanide inhibition of oxidative phosphorylation was examined in rat dopaminergic N27 cells. NaNO2 produced a time- and concentration-dependent increase in whole-cell and mitochondrial levels of NO. The NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxy 3-oxide (PTIO) reversed this increase in cellular and mitochondrial NO. NO generated from NaNO2 decreased cellular oxygen consumption and inhibited CcOX activity. PTIO reversed the NO-mediated inhibition, thus providing strong evidence that NO mediates the action of NaNO2. Under similar conditions, KCN (20μM) inhibited cellular state-3 oxygen consumption and CcOX activity. Pretreatment with NaNO2 reversed KCN-mediated inhibition of both oxygen consumption and CcOX activity. The NaNO2 antagonism of cyanide was blocked by pretreatment with the NO scavenger PTIO. It was concluded that NaNO2 antagonizes cyanide inhibition of CcOX by generating of NO, which then interacts directly with the binding of KCN × CcOX to reverse the toxicity. In vivo antagonism of cyanide by NO2− appears to be due to both generation of mHb and direct displacement of cyanide from CcOX by NO.
Keywords: cyanide, sodium nitrite, nitric oxide, cytochrome c oxidase
Cyanide induces a rapid onset of toxicity by binding to the binuclear heme center of cytochrome c oxidase (CcOX), thereby inhibiting mitochondrial respiration (Way, 1984). Toxicity is characterized by histotoxic hypoxia in which tissue oxygen tension is normal but utilization of oxygen via oxidative phosphorylation is blocked, resulting in rapid depletion of ATP and Ca2+ overload in susceptible tissues (Maduh et al., 1991; Zhang et al., 2007a). The central nervous system is highly dependent on aerobic metabolism and is a primary target organ in cyanide toxicity. The histotoxic hypoxia can produce a rapid, catastrophic onset of neurological dysfunction and death can occur within minutes (Way, 1984). Acute exposure can result in immediate fatality or a latent neuropathy that manifests as a Parkinson-like condition (Ballantyne, 1987; Rachinger et al., 2002). Acute exposure to cyanide produces marked changes in central catecholaminergic pathways, and dopaminergic basal ganglia are the most sensitive.
Acute cyanide toxicity is treated with sodium thiosulfate to accelerate biotransformation of cyanide to thiocyanate and an indirect cyanide scavenger, such as amyl nitrite and sodium nitrite (NaNO2) (Way, 1983). Rhodanese, a mitochondrial enzyme localized in liver in high concentration, catalyzes conversion of cyanide to thiocyanate with thiosulfate serving as a sulfane sulfur substrate in the detoxification reaction (Isom and Baskin, 1997). The linkage of rhodanese metabolism to the scavenging action of other antidotes, such as NaNO2, has proven difficult to model in isolated cells. On the other hand, amyl nitrite and NaNO2 promote cyanide detoxification through oxidation of oxy-hemoglobin (Fe2+) to methemoglobin (mHb) (Fe3+) (Keszler et al., 2008; Way, 1984). mHb has a higher binding affinity for cyanide than the CcOX binuclear heme center (Fea3-CuB), thus functioning as a cyanide scavenger to reactivate CcOX. Recommended doses of amyl nitrite and NaNO2 increase basal blood mHb by 7 and 20% of total hemoglobin (Hb), respectively, over 5–20 min (Chen and Rose, 1952; Hall et al., 2009). However, the increase in blood mHb appears to only partly account for the antagonism of cyanide toxicity by nitrites. Blood mHb levels must be increased to ∼15% of total Hb to effectively antagonize cyanide toxicity (Bhattacharya, 2000; van Heijst et al., 1987). Even though amyl nitrite increases blood mHb to only ∼5 to 7% of total Hb, it can function as an effective cyanide antidote (Vick and Froehlich, 1985). Also the nitrite-mediated decrease in cyanide toxicity occurs faster than the observed generation of mHb, indicating that the efficacy of nitrites as antagonists may not be due solely to mHb generation (Holmes and Way, 1982; Way et al., 1984).
The antidotal action of NaNO2 has been attributed in part to generation of nitric oxide (NO). In mice administered isosorbide dinitrate, an NO donor and mHb inducer antagonized cyanide, even when mHb generation was blocked (Sun et al., 1995). Baskin et al. (1996) observed that administration of an NO-releasing compound significantly reduced cyanide toxicity in mice and proposed that NO interacted with a component of cyanide toxicity. NO is a potent vasodilator and neurotransmitter that can be generated from NO2− by either enzymatic or nonenzymatic processes. At neutral pH and 37°C, production of NO from NO2− occurs within seconds (Lundberg et al., 2008). Recent studies indicate that NO can be generated from NO2− via reactions mediated by xanthine oxidase, aldehyde oxidase, or nitrite reductase (Feelisch et al., 2008, Jensen, 2009; Li et al., 2008).
NO2−-mediated antagonism of cyanide may be explained by generation of NO that can alter cyanide binding at CcOX. Both cyanide and NO bind to the binuclear heme center (Torres et al., 1995). NO-mediated inhibition of CcOX is well characterized, and endogenously generated NO is thought to play a physiological role in regulating CcOX activity (Collman et al., 2008; Cooper and Brown, 2008). In cultured cells, exogenous NO antagonized cyanide inhibition of CcOX (Leavesley et al., 2008). The NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP) reversed cyanide-mediated inhibition of cellular respiration and CcOX activity. Antagonism of cyanide by NO was confirmed by showing that the NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxy 3-oxide (PTIO) reversed the antagonism of CcOX inhibition. Recent studies have shown that NO can displace cyanide from ferriheme a3 by a complex interaction with the catalytic cycle of the enzyme in which NO functions as a substrate (Collman et al., 2008; Pearce et al., 2003, 2008).
It is proposed that NO2−-mediated antagonism of cyanide is due in part to generation of NO, which then alters the kinetics of cyanide binding to CcOX to accelerate restoration of oxidative phosphorylation. In this study, the involvement of NO in NO2− antagonism of cyanide inhibition of cellular respiration and CcOX was confirmed by using a selective NO scavenger.
MATERIALS AND METHODS
Materials.
RPMI 1640 media, penicillin/streptomycin, and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY). NaNO2, 2-(carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO), digitonin, 2′, 5′-adenosine diphosphate (ADP), and bovine heart cytochrome c were purchased from Sigma Chemical Company (St Louis, MO). 4-Amino-5-methylamino-2′,7′-difluorofluorescein-diacetate (DAF) and MitoTracker Red chloromethyl-X-rosamine were obtained from Invitrogen (Carlsbad, CA).
N27 cell culture.
The immortalized rat mesencephalic cell line 1RB3N27 (N27) was obtained from Dr A. G. Kanthasamy (Iowa State University, Ames, IA). N27 cells have been used to examine neurotoxic mechanisms in dopaminergic-type cells (Clarkson et al., 1999). The N27 cell line was selected for this study since cyanide-induced changes in cellular respiration and CcOX activity are well characterized in N27 cells (Leavesley et al., 2008; Zhang et al., 2007b). Cells (5 × 104 cells per milliliter) were seeded onto poly-L-lysine–coated dishes containing RPMI media (supplemented with 2 g/l NaHCO3, 10% FBS, and penicillin [100 U/ml]/streptomycin [100 μg/ml], pH 7.2) and cultured at 37°C in 5% CO2 and 95% air. Confluent (1 × 107 cells per milliliter) cultures were obtained after 3–4 days of growth. Cells were passaged at least two times prior to use.
Fluorescence measurement of intracellular and mitochondrial NO.
N27 cells (2 × 105 cells per milliliter) were seeded onto 96-well, poly-L-lysine–coated fluorescence microtiter plates and cultured 1–2 days or until 60–80% confluent. To measure intracellular NO, cells were incubated with the membrane permeant fluorescence NO-sensitive dye 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate (7μM) in RPMI for 40 min at 37°C. After three times rinse, cells were treated with NaNO2 (100–500μM) and/or the NO scavenger carboxy-PTIO (200μM) for 30 min. Whole-cell DAF fluorescence was quantified with the Fusion Universal Microplate Analyzer (PerkinElmer, Waltham, MA) using a 485 and a 535nM filter for measurement of emission fluorescence. DAF fluorescence was normalized by dividing the treatment fluorescence (F) by the control (untreated) fluorescence (F0) and expressed as F/F0.
For measurement of mitochondrial NO, DAF-loaded cells were permeabilized with digitonin in a low-Ca2+ (90nM) buffer (135mM KCl, 10mM NaCl, 5mM pyruvate, 2mM glutamate, 2mM malate, 0.5mM KH2PO4, 1mM MgCl2, 5mM ethylene glycol tetraacetic acid, 1.86mM CaCl2, 10μM digitonin, and 20mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.3) for 1 min at room temperature to remove cytosolic and nuclear-loaded dye (Dedkova et al., 2004). To confirm mitochondrial localization of NO, cells were coloaded with MitoTracker Red (200nM) and DAF prior to permeabilization. Punctate mitochondrial NO fluorescence was confirmed by fluorescence microscopy. To quantify mitochondrial NO, DAF fluorescence was measured using the Fusion Universal Microplate Analyzer, as described above.
To estimate intracellular NO concentrations, standard concentrations of the NO donor (±)-E-4-methyl-2-[(E)-hydroxyimino]-5-nitro-6-methoxy-3-hexeneamide (NOR-1) were used as described by Ueki et al. (2002). An NO standard curve was established by determining the DAF-FM fluorescence in the presence of varying concentrations of NOR-1/NO. N27 cells were loaded with DAF-FM, and then NOR-1 was added to the cell incubation as described above. The standard curve was expressed as DAF-FM fluorescence intensity (relative fluorescence units) versus NO concentration (micromolar).
Cellular oxygen consumption.
Analyses of cellular respiration were conducted as described by Leavesley et al. (2008). N27 cells (1 × 107 cells per milliliter) were harvested in PBS and resuspended in permeabilization buffer (250mM sucrose, 10mM MgCl2, 2mM KH2PO4, 4μM rotenone, 10mM succinate, 0.01% digitonin, 20mM HEPES, pH 7.1). Oxygen consumption was monitored using a Clark-type oxygen electrode (Digital Model 10 Controller, Rank Brothers Ltd, Cambridge, UK). ADP (1mM) was added to the permeabilized cell suspension to initiate state-3 respiration. When the electrode’s voltage due to oxygen (VO2) reached 0.70, KCN (20μM), NaNO2 (200μM), or NaCl (100μM) was added. To examine the effect of NO2− on cyanide-mediated inhibition of oxygen consumption, cells were incubated for 2–3 min with NaNO2 prior to initiation of state-3 respiration and addition of KCN . Oxygen consumption rates were expressed as .
CcOX activity.
Cellular CcOX activity was determined by the microassay method of Chrzanowska-Lightowlers et al. (1993). This assay determines CcOX activity in intact cells as opposed to the traditional spectrophotometric method using homogenated cells/tissues. N27 cells were grown in 96-well microtiter plates and at the time of assay, cells were permeabilized with saponin (0.01%) in order for cytochrome c to access CcOX in mitochondria. In the assay, permeabilized cells were incubated with reduced cytochrome c (100μM) and 3,3-diaminobenzidine (DAB) (4mM). As CcOX oxidizes cytochrome c, DAB re-reduces cytochrome c to generate an oxidized DAB species that is detected at 450 nm. The rate of formation of oxidized DAB is directly proportional to CcOX activity.
Statistical analyses.
Graphical data are depicted as mean ± SEM. Data are considered significantly different from control group at p < 0.05 based on a one-way ANOVA using a Tukey’s multiple comparisons test.
RESULTS
NaNO2-Mediated Increase of Whole-Cell NO Levels
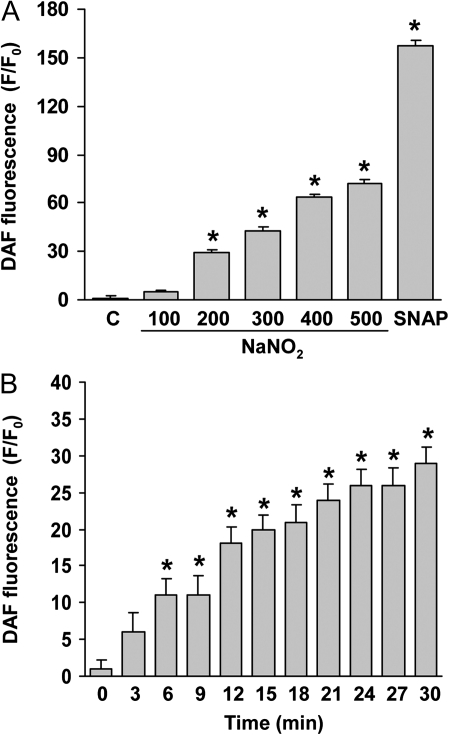
To determine if NaNO2 generated NO, whole-cell NO levels were measured fluorometrically with the NO-sensitive indicator DAF as described by Dedkova et al. (2004). Incubation of N27 cells for 30 min with NaNO2 (100–500μM) produced a concentration-related increase of cellular NO levels as measured by DAF fluorescence (Fig. 1A). To confirm that the DAF fluorescence reflected NO levels, the NO donor SNAP (200μM) was used as a positive control. Generation of cellular NO from NaNO2 was rapid, and a significant increase was noted within 6 min. NO fluorescence continued to increase over a 30-min period (Fig. 1B). It was concluded that under the incubation conditions, NaNO2 increased cellular NO in N27 cells.
FIG. 1.
Generation of NO from NaNO2 in N27 cells. NO-induced DAF fluorescence was measured in N27 cells after treatment with NaNO2. (A) Concentration-dependent production of NO by NaNO2. After loading with DAF-FM, cells were treated with NaNO2 for 20 min. SNAP (200μM) was used as a positive control. (B) Time course of NaNO2-induced NO production. Cells were loaded with DAF-FM and then treated with NaNO2 (200μM) and at the indicated time fluorescence determined. DAF-FM fluorescence was normalized to control (F/F0, F = treatment group fluorescence, F0 = fluorescence of untreated control). Data represent mean ± SEM of six determinations. *p < 0.05, significantly different from control group.
NaNO2-Mediated Increase of Mitochondrial NO
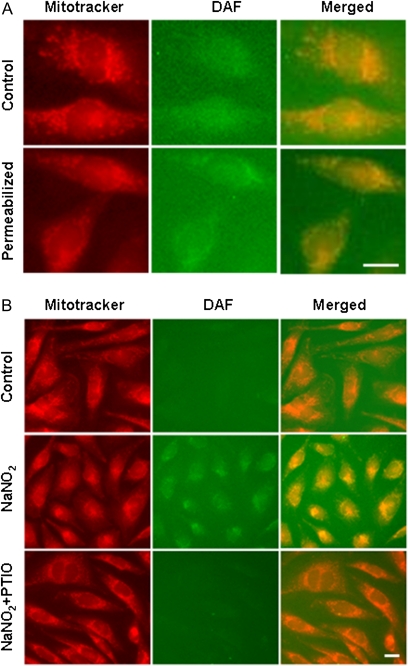
Subcellular localization of NO after treatment with NaNO2 was determined by fluorescence imaging of cells loaded with the NO-sensitive indicator DAF and the mitochondrial dye, MitoTracker Red (Fig. 2). Incubation with 200μM NaNO2 for 30 min increased whole-cell NO levels as shown by a homogenous distribution of NO-induced fluorescence throughout the cell (Fig. 2A). Permeabilization of the plasma membrane by digitonin reduced nuclear and cytosolic NO fluorescence, as reflected by a punctate pattern of yellow coloration upon merging of MitoTracker Red and DAF fluorescence signals. To confirm that the increased DAF fluorescence reflected generation of NO, cells were loaded with the selective NO scavenger carboxy-PTIO, a cell permeant form which does not bind NO2− or cyanide (Bethke et al., 2006; Goldstein et al., 2003). Preincubation with carboxy-PTIO reduced mitochondrial NO to control levels (no NaNO2 exposure), showing that carboxy-PTIO scavenged exogenous NO under the incubation conditions (Fig. 2B).
FIG. 2.
Fluorescence micrographs of NaNO2-mediated increase of mitochondrial NO. (A) Cells were loaded with MitoTracker Red and DAF-FM and then treated with NaNO2 (200μM) for 30 min. Whole cells = cells treated with NaNO2. Permeabilized = cells treated with NaNO2, followed by permeabilization with digitonin. Micrographs are images of MitoTracker Red, DAF, or merged fluorescence. (B) Reduction of nitrite-induced mitochondrial NO by loading cells with PTIO (200μM). Cells were pretreated with PTIO for 10 min and then incubated with NaNO2 for 30 min. Cellular fluorescence was monitored after permeabilization with digitonin. Scale bar = 25 μm.
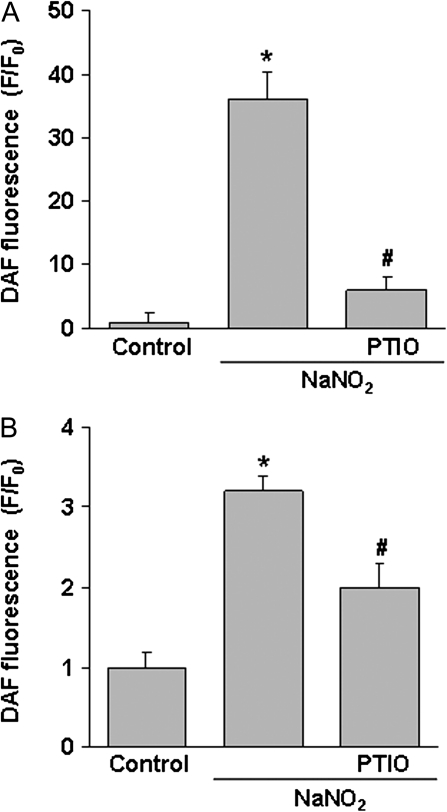
Analysis of whole-cell NO levels showed that carboxy-PTIO significantly reduced nitrite-mediated elevation of NO (Fig. 3A). Permeabilization of cells with digitonin also reduced NO fluorescence intensity as compared to whole-cell levels (Fig. 3B). Permeabilization reduces nuclear and cytosolic DAF levels so that the subsequent fluorescence signal reflects primarily mitochondrial sequestered NO-DAF (Dedkova et al., 2004). Using an NO standard curve, it was estimated that 100μM NaNO2 produced a peak mitochondrial level of ∼54nM NO compared to 16nM NO under control conditions (data not shown). To support the conclusion that NaNO2 elevated mitochondrial NO levels, it was observed that carboxy-PTIO significantly reduced mitochondrial NO in both cell image (Fig. 2) and whole-cell analysis (Fig. 3).
FIG. 3.
Reduction of cellular and mitochondrial NO by PTIO scavenging. After loading with DAF-FM, cells were pretreated with PTIO (200μM) for 10 min and then incubated with NaNO2 (200μM) for 30 min. Fluorescence intensity was determined in (A) whole cells (no digitonin treatment) or (B) mitochondria (permeabilization with digitonin). Data represent the mean ± SEM of six separate determinations. *Significantly different from untreated control group; #significantly different from NaNO2 treatment group. p < 0.05.
Nitrite Antagonism of Cyanide-Inhibited Cellular Respiration
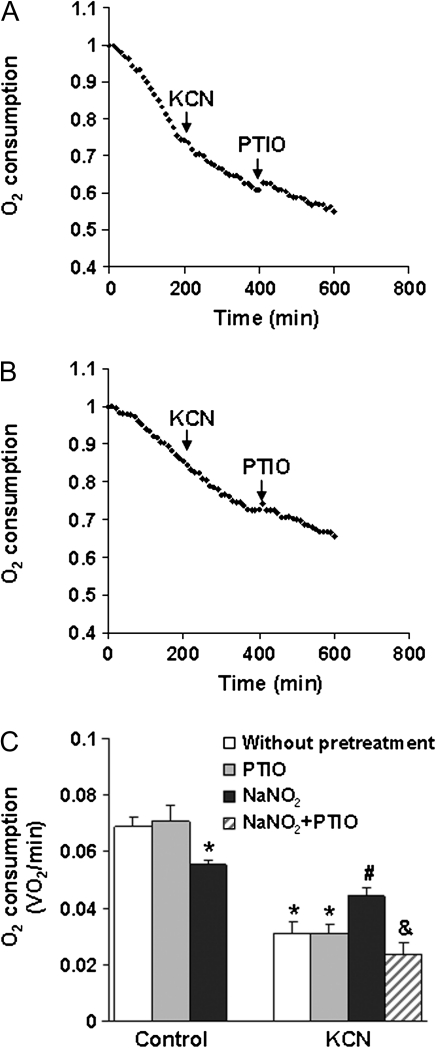
NaNO2 (200μM) alone reduced state-3 oxygen consumption by ∼20% (Fig. 4). On the other hand, 20μM KCN rapidly inhibited state-3 oxygen consumption and reduced respiration by ∼60% (Figs. 4B and 4C). Pretreatment with 20μM NaNO2 inhibited the effect of KCN. Treatment with carboxy-PTIO significantly reversed the antagonism of cyanide inhibition of oxygen consumption by NaNO2, thereby showing that NO scavenging reduced NaNO2-mediated antagonism of cyanide. It was concluded that nitrite-mediated antagonism of cyanide inhibition of cellular respiration was due to increased NO.
FIG. 4.
Effect of NaNO2 on cyanide inhibition of cellular oxygen consumption. Representative oxygen consumption as determined by Clark-type oxygen electrode showing (A) inhibition by KCN (20μM) and lack of effect of PTIO; (B) reversal of KCN inhibition in cells pretreated with NaNO2 (200μM) and reversal of NaNO2 action by pretreatment with PTIO. (C) Effect of PTIO and NaNO2 on cyanide inhibition of oxygen consumption. Cells were pretreated with NaNO2 (200μM) for 10 min and then KCN (20μM) and PTIO (200μM) were added. Data represent the mean ± SEM of three or more experiments. *Significantly different from untreated control group; #significantly different from KCN treatment group; &significantly different from NaNO2 + KCN treatment group. p < 0.05.
Nitrite Antagonism of Cyanide-Inhibited CcOX
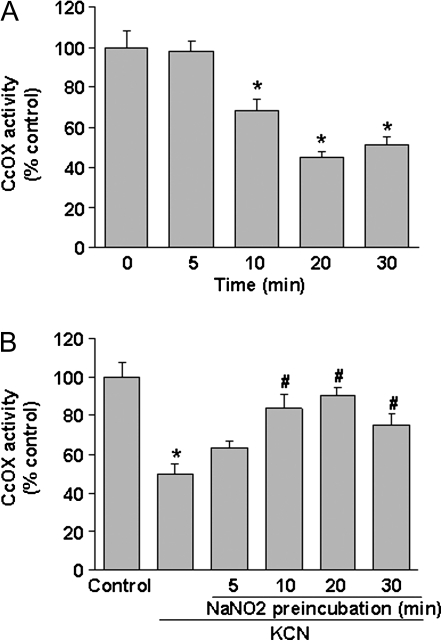
NaNO2 (200μM) produced a time-dependent inhibition of CcOX, which was significant within 10 min and peaked at 20 min (Fig. 5A). Ten minutes after addition of cyanide (20μM), ∼48% inhibition of CcOX was noted (Fig. 5B). Preincubation of the cells for 10–20 min with NaNO2 significantly reduced cyanide inhibition, with a maximum reduction at 20 min. This is similar to the temporal profile of the NaNO2-mediated effect.
FIG. 5.
Nitrite-mediated antagonism of cyanide inhibition of CcOX. (A) Time course of NaNO2-mediated inhibition of CcOX. Cellular CcOX activity was determined at specific times of incubation with NaNO2 (100μM). (B) Antagonism of cyanide inhibition of CcOX by NaNO2. Cells were pretreated with NaNO2 (100μM) for the indicated time, followed by incubation with KCN (20μM) for 10 min and then CcOX activity determined. Data represent mean ± SEM of six determinations. *Significantly different from untreated control group; #significantly different from KCN-treated group. p < 0.05.
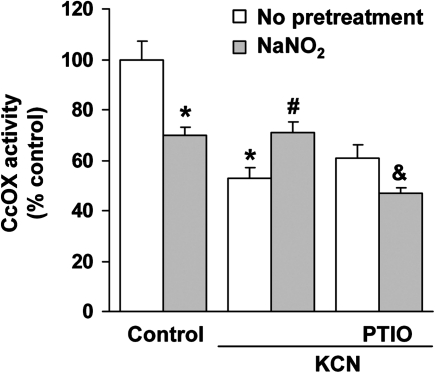
KCN (20μM) reduced cellular CcOX activity by ∼50% as opposed to ∼40% in cells pretreated with PTIO (Fig. 6). The reduction of KCN inhibition can be attributed to scavenging of endogenous NO since PTIO is a selective NO scavenger that does not interact with cyanide (Goldstein et al., 2003). Low-level, endogenously generated NO enhances KCN-mediated inhibition of CcOX (Leavesley et al., 2008). Pretreatment with PTIO reversed the antagonistic action of NaNO2. It appears that NaNO2 generates NO, which then interacts with cyanide binding at the active site of CcOX to reverse the inhibition.
FIG. 6.
Effect of an NO scavenger on NaNO2 antagonism of CcOX inhibition. Cells were pretreated with carboxy-PTIO (200μM) for 10 min, followed by incubation with NaNO2 (200μM) for 10 min, and then KCN (20μM). Data represent mean ± SEM of six determinations. *Significantly different from untreated control group; #significantly different from KCN treatment group; &significantly different from NaNO2 + KCN treatment group. p < 0.05.
DISCUSSION
Cyanide elicits a rapid onset of toxicity by inhibiting mitochondrial CcOX, thereby blocking the terminal step of oxidative phosphorylation. The result is decreased cellular oxygen consumption and marked reduction of ATP synthesis in target organs. Binding of cyanide to CcOX can be reversed, and toxicity antagonized by scavenging free cyanide by antidotes that have a high binding affinity for cyanide (Hall et al., 2009). NaNO2 is a cyanide antidote that generates mHb, an oxidized form of Hb that binds cyanide. However, we postulated that the antidotal action of NaNO2 is not entirely due to binding of cyanide but can be explained in part by generation of NO, which directly alters cyanide inhibition of CcOX.
Cyanide produced a rapid inhibition of CcOX with an medium inhibitory concentration (IC50) = ∼7μM in N27 cells. State-3 oxygen consumption was used as a functional measure of toxicity, and cyanide inhibition of oxygen consumption paralleled inhibition of CcOX. Pretreatment with NaNO2 produced a time- and concentration-related antagonism of cyanide inhibition of CcOX and oxygen consumption. NaNO2 also produced a concentration-related increase of cellular and mitochondrial NO levels, and the antagonism of cyanide was reversed by PTIO, a selective NO scavenger. It was concluded that NaNO2 generates NO, which then interacts with the binding of cyanide at the CcOX binuclear heme center to reverse the inhibition.
Schubert and Brill (1968) provided in vivo evidence for antagonism of cyanide by the nitrite ion. KCN-induced inhibition of CcOX was reversed in mice within 5 min after NaNO2 administration. The antagonism was attributed to mHb formation and subsequent scavenging of cyanide. However, other studies showed that cyanide toxicity can be reversed when tissue NO levels were increased and mHb formation blocked (Sun et al., 1995). In the present study, a cell model devoid of Hb was used to eliminate contributions from mHb scavenging. Nevertheless, NaNO2 reversed cyanide inhibition of CcOX and oxygen uptake, and the antagonism was blocked by PTIO, a compound that is widely used to selectively bind (scavenge) NO in biological tissues (Goldstein et al., 2003). These observations provide strong evidence that the antidotal activity of NaNO2 is mediated in part by NO.
NO can alter binding of cyanide to the CcOX binuclear heme center and change the inhibition kinetics (Leavesley et al., 2008; Pearce et al., 2003). Paradoxically, low endogenous generation of NO by NO synthase (NOS) in mitochondria enhances cyanide inhibition of CcOX (Leavesley et al., 2008), whereas high levels of NO, such as that generated by addition of NaNO2 to cells in culture, antagonizes cyanide. In studies with purified CcOX, incubation with NO appeared to displace cyanide from CcOX to partially reactivate the enzyme (Pearce et al., 2003, 2008). Similarly, in cultured cells, the NO donor SNAP increased mitochondrial NO levels to restore CcOX activity (Leavesley et al., 2008). Based on the present observations, it was concluded that NaNO2 facilitates antagonism of cyanide at CcOX by increasing mitochondrial NO.
The mechanism underlying reversal of cyanide-mediated inhibition of CcOX by NO is complex and varies with intracellular O2 tension, oxidation state of the CcOX binuclear heme center, and concentration of NO relative to cyanide. It has been proposed that NO displaces cyanide from CcOX in which NO does not function as a competitive antagonist rather undergoes oxidation to NO2− by reaction with CcOX to regenerate the active enzyme (Collman, et al., 2008; Pearce et al., 2003, 2008). Formation of NO2− would reduce the inhibition of CcOX by NO. Displacement of cyanide by NO would facilitate O2 binding at CcOX, restoring cellular respiration (Leavesley et al., 2008). The displaced cyanide could either rebind and inhibit CcOX or undergo biotransformation by rhodanese, a mitochondrial matrix enzyme, to generate the nontoxic metabolite thiocyanate (SCN−). This mechanism would explain the well-known antidotal synergism produced by the classic antidote combination of sodium thiosulfate (rhodanese sulfur substrate) and NaNO2 (Hall et al., 2009; Way, 1983). This is an important consideration in design of cyanide antidotes, particularly in the use of cyanide scavengers that do not directly displace cyanide from CcOX.
In addition to antagonism of cyanide via generation of NO, it is possible that the NO2− ion reversed cyanide inhibition by directly altering CcOX-binding kinetics. A previous study showed that high levels of nitrite (millimolar concentrations) can alter CcOX activity (Paitian et al., 1985). In this study, the antagonism of cyanide inhibition of CcOX was observed at low nitrite concentrations (200μM) and was reversed by selective NO scavenging. It is concluded that in the cultured cells, antagonism of cyanide toxicity by NO2− at CcOX is due to generation of NO.
Detoxification of cyanide by NaNO2 is enhanced by sodium thiosulfate and/or 100% oxygen (Isom and Way, 1984; Isom et al., 1982). Sodium thiosulfate serves as a sulfur substrate for metabolism of cyanide to thiocyanate via mitochondrial enzyme rhodanese. Traditionally, the antidotal action of NaNO2 was attributed to oxidization of Hb to mHb, which functions as a cyanide scavenger by binding cyanide with a higher affinity than CcOX (Chen and Rose, 1952). Coadministration of NaNO2 and sodium thiosulfate enhances reactivation of CcOX by decreasing cyanide levels within and outside of mitochondria (Schubert and Brill, 1968). Present results show that NaNO2 also acts as a cyanide antidote by forming NO, which then displaces cyanide from the CcOX-binding site. Interaction of NO and cyanide at the CcOX binuclear heme center alters kinetics of CcOX to favor O2 binding, thus reactivating oxidative phosphorylation (Leavesley et al., 2008).
It is well established that under physiological conditions, NO can be generated from NaNO2. In the presence of oxygen, NO is produced by NOS by converting L-arginine to NO (Lundberg et al., 2008). Under hypoxic conditions, NOS activity decreases and endogenous NO2− becomes the primary source of NO. As pH decreases, the rate of NO formation from NO2− increases (Webb et al., 2004). For instance, Modin et al. (2001) found that NO-mediated relaxation of rat aortic smooth muscle elicited by NO2− increased as pH decreased from 7.4 to 6.6, and generation of NO from NO2− was primarily nonenzymatic. Other reports provide evidence that during ischemia/hypoxia, there is an increased conversion of NO2− to NO in mammalian tissues (Feelisch et al., 2008; Gonzalez et al., 2008). In cyanide toxicity, the histoxic hypoxia and lactic acidosis would likely promote rapid conversion of NO2− to NO.
Cellular levels of NO increased rapidly after addition of NO2− to N27 cells. Under physiological conditions, NO2− is reduced to HNO2, which can readily distribute across cell membranes (Samouilov et al., 2007). In cytosol, NO can also be generated from NO2− by xanthine oxidoreductase or via nonenzymatic protonation (Lundberg et al., 2008). NO generated in the cytosol can readily diffuse into mitochondria to interact with CcOX (Malinski et al., 1993). Several processes in mitochondria can produce NO from nitrite ion, including chemical reduction by nitrite reductase, which involves leakage of electrons from ubiquinol to complex III (Kozlov et al., 1999).
The toxicity of complex fire gas mixtures containing cyanide has been modeled (Chaturvedi, et al., 1995; Levin, 1997), and interestingly, these gases can markedly influence the toxicity of cyanide. When nitrogen dioxide (NO2), a common component of toxic combustion gases, was combined with HCN, an antagonistic toxicological interaction was noted. In the presence of 200 ppm of NO2, the LD50 of cyanide was increased 2.3- to 2.5-fold. It was proposed that NO2 might generate mHb, which would then serve as a cyanide scavenger. However, based on the present results, it is possible that the antagonism produced by inhalation of NO2 may be due to an NO-like action at the CcOX-binding site.
In summary, NaNO2 reversed cyanide inhibition of CcOX in a cell culture model by generating NO. NaNO2 increased both whole-cell and mitochondrial NO levels, and mitochondrial NO likely displaced cyanide from the CcOX binuclear a3 heme site, leading to reactivation of the enzyme. Intracellular conversion of NO2− to NO is accelerated under conditions of histotoxic hypoxia and low pH, both of which occur during cyanide toxicity. It is concluded that in addition to mHb formation, NaNO2 contributes to the intramitochondrial detoxification of cyanide by generating NO, which appears to displace cyanide from the CcOX heme center.
FUNDING
National Institutes of Health (ES04140); Predoctoral Fellowship to H.B.L.
References
- Ballantyne B. Toxicology of cyanides. In: Ballantyne B, Marrs TC, editors. Clinical and Experimental Toxicology of Cyanides. Bristol, UK: Wright; 1987. pp. 41–126. [Google Scholar]
- Baskin SI, Nealley EW, Lempka JC. Cyanide toxicity in mice pretreated with diethylamine nitric oxide complex. Hum. Exp. Toxicol. 1996;15:13–18. doi: 10.1177/096032719601500103. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Libourel IG, Reinohl V, Jones RL. Sodium nitroprusside, cyanide, nitrite, and nitrate break arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta. 2006;223:805–812. doi: 10.1007/s00425-005-0116-9. [DOI] [PubMed] [Google Scholar]
- Bhattacharya R. Antidotes to cyanide poisoning: present status. Indian J. Pharmacol. 2000;32:94–101. [Google Scholar]
- Chaturvedi AK, Sanders DC, Endecott BR, Ritter RM. Exposures to carbon monoxide, hydrogen cyanide and their mixtures: interrelationship between gas exposure concentration, time to incapacitation, carboxyhemoglobin and blood cyanide in rats. J. Appl. Toxicol. 1995;15:357–363. doi: 10.1002/jat.2550150504. [DOI] [PubMed] [Google Scholar]
- Chen KK, Rose CL. Nitrite and thiosulfate therapy in cyanide poisoning. J. Am. Med. Assoc. 1952;149:113–119. doi: 10.1001/jama.1952.02930190015004. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Lightowlers ZMA, Turnbull DM, Lightowlers RN. A microtiter plate assay for cytochrome c oxidase in permeabilized whole cells. Anal. Biochem. 1993;214:45–49. doi: 10.1006/abio.1993.1454. [DOI] [PubMed] [Google Scholar]
- Clarkson ED, Edwards-Prasad J, Freed CR, Prasad KN. Immortalized dopamine neurons: a model to study neurotoxicity and neuroprotection. Proc. Soc. Exp. Biol. Med. 1999;222:157–163. doi: 10.1046/j.1525-1373.1999.d01-126.x. [DOI] [PubMed] [Google Scholar]
- Collman JP, Dey A, Decreau RA, Yang Y, Hosseini A, Solomon EI, Eberspacher TA. Interaction of nitric oxide with a functional model of cytochrome c oxidase. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9892–9896. doi: 10.1073/pnas.0804257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- Dedkova EN, Ji X, Lipsius SL, Blatter LA. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2004;286:C406–C415. doi: 10.1152/ajpcell.00155.2003. [DOI] [PubMed] [Google Scholar]
- Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura FF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J, Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J. Biol. Chem. 2008;283:33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Russo A, Samuni A. Reactions of PTIO and carboxy-PTIO with.NO,.NO2, and O2.- J. Biol. Chem. 2003;278:50949–50955. doi: 10.1074/jbc.M308317200. [DOI] [PubMed] [Google Scholar]
- Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, III, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit. Rev. Toxicol. 2009;39:541–552. doi: 10.1080/10408440802304944. [DOI] [PubMed] [Google Scholar]
- Holmes RK, Way JL. Mechanism of cyanide antagonism by sodium nitrite. Pharmacologist. 1982;24:182. [Google Scholar]
- Isom GE, Baskin SI. Enzymes involved in cyanide metabolism. In: Sipes IG, McQueen CA, Gandolfi AJ, editors. Comprehensive Toxicology: Biotransformation. Vol. 3. New York: Elsevier Science; 1997. pp. 477–488. [Google Scholar]
- Isom GE, Burrows GE, Way JL. Effect of oxygen on the antagonism of cyanide intoxication—cytochrome oxidase, in vivo. Toxicol. Appl. Pharmacol. 1982;65:250–256. doi: 10.1016/0041-008x(82)90007-2. [DOI] [PubMed] [Google Scholar]
- Isom GE, Way JL. Effects of oxygen on the antagonism of cyanide intoxication: cytochrome oxidase, in vitro. Toxicol. Appl. Pharmacol. 1984;74:57–62. doi: 10.1016/0041-008x(84)90269-2. [DOI] [PubMed] [Google Scholar]
- Jensen FB. The role of nitrite in nitric oxide homeostasis: a comparative perspective. Biochim. Biophys. Acta. 2009;1787:841–848. doi: 10.1016/j.bbabio.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J. Biol. Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE. Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol. Sci. 2008;101:101–111. doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- Levin BC. New approaches to toxicity: a seven-gas predictive model and toxicant suppressants. Drug Chem. Toxicol. 1997;20:271–280. doi: 10.3109/01480549709003885. [DOI] [PubMed] [Google Scholar]
- Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J. Biol. Chem. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Maduh EU, Borowitz JL, Isom GE. Cyanide-induced alteration of the adenylate energy pool in a rat neurosecretory cell line. J. Appl. Toxicol. 1991;11:97–101. doi: 10.1002/jat.2550110205. [DOI] [PubMed] [Google Scholar]
- Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem. Biophys. Res. Commun. 1993;193:1076–1082. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- Modin A, Bjorne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol. Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- Paitian NA, Markossian KA, Nalbandyan RM. The effect of nitrite on cytochrome oxidase. Biochem. Biophys. Res. Commun. 1985;133:1104–1111. doi: 10.1016/0006-291x(85)91250-1. [DOI] [PubMed] [Google Scholar]
- Pearce LL, Bominaar EL, Hill BC, Peterson J. Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide: an endogenous antidote to cyanide poisoning? J. Biol. Chem. 2003;278:52139–52145. doi: 10.1074/jbc.M310359200. [DOI] [PubMed] [Google Scholar]
- Pearce LL, Manzano EL, Martinez-Bosch S, Peterson J. Antagonism of nitric oxide toward the inhibition of cytochrome c oxidase by carbon monoxide and cyanide. Chem. Res. Toxicol. 2008;21:2073–2081. doi: 10.1021/tx800140y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachinger J, Fellner FA, Stieglbauer K, Trenkler J. MR changes after acute cyanide intoxication. Am. J. Neuroradiol. 2002;23:1398–1401. [PMC free article] [PubMed] [Google Scholar]
- Samouilov A, Woldman YY, Zweier JL, Khramtsov VV. Magnetic resonance study of the transmembrane nitrite diffusion. Nitric Oxide. 2007;16:362–370. doi: 10.1016/j.niox.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J, Brill WA. Antagonism of experimental cyanide toxicity in relation to the in vivo activity of cytochrome oxidase. J. Pharmacol. Exp. Ther. 1968;162:352–359. [PubMed] [Google Scholar]
- Sun P, Borowitz JL, Kanthasamy AG, Kane MD, Gunasekar PG, Isom GE. Antagonism of cyanide toxicity by isosorbide dinitrate: possible role of nitric oxide. Toxicology. 1995;104:105–111. doi: 10.1016/0300-483x(95)03152-6. [DOI] [PubMed] [Google Scholar]
- Torres J, Darley-Usmar V, Wilson MT. Inhibition of cytochrome c oxidase in turnover by nitric oxide: mechanism and implications for control of respiration. Biochem. J. 1995;312:169–173. doi: 10.1042/bj3120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Nakamura H, Matsumoto K, Tominaga T, Miyake S, Kita Y, Katayama Y, Fukuyama S, Hirasawa Y, Yoshida K, et al. NOR-1: a nitric oxide releasing agent for calibrating low levels of nitric oxide by the chemiluminescence method. Blood Coagul. Fibrinolysis. 2002;13:75–80. doi: 10.1097/00001721-200203000-00001. [DOI] [PubMed] [Google Scholar]
- van Heijst AN, Douze JM, van Kesteren RG, van Bergen JE, van Dijk A. Therapeutic problems in cyanide poisoning. J. Toxicol. Clin. Toxicol. 1987;25:383–398. doi: 10.3109/15563658708992641. [DOI] [PubMed] [Google Scholar]
- Vick JA, Froehlich HL. Studies of cyanide poisoning. Arch. Int. Pharmacodyn. Ther. 1985;273:314–322. [PubMed] [Google Scholar]
- Way JL. Cyanide antagonism. Fundam. Appl. Toxicol. 1983;3:383–386. [PubMed] [Google Scholar]
- Way JL. Cyanide intoxication and its mechanism of antagonism. Annu. Rev. Pharmacol. Toxicol. 1984;24:451–481. doi: 10.1146/annurev.pa.24.040184.002315. [DOI] [PubMed] [Google Scholar]
- Way JL, Sylvester D, Morgan RL, Isom GE, Burrows GE, Tamulinas CB, Way JL. Recent perspectives on the toxicodynamic basis of cyanide antagonism. Fundam. Appl. Toxicol. 1984;4:S231–S239. doi: 10.1016/0272-0590(84)90157-x. [DOI] [PubMed] [Google Scholar]
- Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li L, Liu H, Prabhakaran K, Zhang X, Borowitz JL, Isom GE. HIF-1alpha activation by a redox-sensitive pathway mediates cyanide-induced BNIP3 upregulation and mitochondrial-dependent cell death. Free Radic. Biol. Med. 2007a;43:117–127. doi: 10.1016/j.freeradbiomed.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li L, Prabhakaran K, Zhang L, Leavesley HB, Borowitz JL, Isom GE. Uncoupling protein-2 up-regulation and enhanced cyanide toxicity are mediated by PPARalpha activation and oxidative stress. Toxicol. Appl. Pharmacol. 2007b;223:10–19. doi: 10.1016/j.taap.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]