Abstract
Background
Perianal fistulas are a debilitating manifestation of Crohn's disease (CD) in the pediatric population and present a management challenge. The aims of this study were to describe our experience using EUS to guide management of perianal CD (PCD) in a pediatric population, and determine whether using EUS to monitor healing after seton placement improves outcomes.
Methods
Retrospective study of two cohorts: pediatric subjects with PCD who underwent EUS and pediatric subjects who underwent seton placement between 2002 and 2007.
Results
25 children underwent a total of 42 EUS procedures. Of 28 EUSs performed to evaluate suspected perianal disease, complex fistulizing disease was identified in 15 (54%). Setons were placed after most EUSs demonstrating complex fistulizing disease and after none demonstrating superficial or no fistulizing disease. Of 14 EUSs performed to monitor healing around a seton, 7 (50%) demonstrated persistent peri-seton inflammation. Setons were more often left in place after an EUS revealing persistent inflammation (86% vs 0%), and the patients were more likely to have a biologic initiated or changed (57% versus 0%). Amongst all subjects who underwent seton placement, time from seton removal to recurrence was longer for those followed by EUS compared to those followed by physical exam only; however, we were not powered to test for statistical significance.
Conclusion
EUS to guide the combined medical and surgical management of PCD is feasible in the pediatric population. Larger prospective studies are needed to determine if EUS-directed management improves outcomes in pediatric patients with PCD.
Keywords: Crohn Disease, fistula, pediatrics, endosonography
Perianal lesions are a common and often debilitating manifestation of Crohn's disease (CD) in the pediatric population.1-7 The clinical manifestations of perianal Crohn's disease (PCD) can range from asymptomatic skin tags and fissures to draining fistulas, abscesses, and anal stenosis. Between 15 and 49% of pediatric patients with CD have perianal involvement and 8-15% develop perianal fistulas.1, 2, 4, 5
Treatment of perianal fistulas consists of medical therapy with a combination of antibiotics, immunomodulators and/or biologic agents with or without a surgical intervention, such as abscess drainage or seton placement. The advent of anti-tumor necrosis alpha (anti-TNF) therapy such as infliximab has dramatically improved the medical treatment for PCD by reducing fistula drainage in 68% of patients and inducing complete closure of fistulas in 58%.8 The ACCENT II trial demonstrated infliximab efficacy for maintaining fistula closure in adult patients with PCD; however, only 38% of patients maintained a complete response at the 54-week endpoint. Additionally, 12% of patients in the treatment arm developed new abscesses (less than the 17% in the placebo arm).9 This loss of response in the majority of patients and the development of new abscess in some patients may indicate that medical therapy alone is not optimal for long term resolution of perianal lesions.
Many experts feel that combining a surgical exam under anesthesia (EUA) and seton placement with biologic therapy produces the best long term outcomes by preventing premature closure of the cutaneous opening and allowing for fistula healing from the inside-out. This concept was supported by a retrospective study showing better initial response, lower recurrence rate, and longer time to recurrence in patients who had an EUA and seton placement prior to infliximab therapy, compared to those receiving infliximab alone.10 It is not known which patients are best served by seton placement and when is the proper timing of seton removal.
Rectal endoscopic ultrasound (EUS) is an effective modality for assessing PCD lesions.11-14 EUS may also be used to assess the degree of active inflammation surrounding a fistula drained by a seton. A randomized pilot study by our group suggests that using EUS to guide the combination medical and surgical therapy for PCD improves outcomes in adult patients.14
It is not known whether EUS can be used effectively in a pediatric population to assess perianal lesions, guide management, and potentially improve outcomes. The aims of this study were 1) to describe our pediatric experience using EUS to evaluate and guide management of PCD, and 2) to determine whether using EUS to monitor healing after seton placement improves time to recurrence of drainage once the seton is removed. Our a priori hypothesis was that the use of EUS to monitor fistula healing after seton placement would lead to longer disease-free intervals after seton removal.
Materials and Methods
Subject Identification
We conducted a retrospective study of two cohorts from the Monroe Carell, Jr. Children's Hospital at Vanderbilt. To review our experience with rectal EUS for CD, the first cohort (Cohort 1) included all patients under 18 years of age followed in the Pediatric Gastroenterology Clinic for CD who underwent EUS between Jan 1, 2002 and December 31, 2007. Subjects were identified by cross-referencing patients with an International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification diagnosis code for CD (555.xx) against those with a Current Procedural Terminology (CPT) code for rectal EUS (45391 or 45341).
Next, to compare outcomes of subjects followed with and without EUS after seton placement, the second cohort (Cohort 2) included all patients under 18 years of age followed in the Pediatric Gastroenterology Clinic for CD who underwent seton placement between Jan 1, 2002 and December 31, 2007. Subjects were identified by cross-referencing patients with a CD ICD-9 code against patients with a CPT code for any perianal procedure (46020, 46040, 46045, 46050, 46060, 46080, 45990, or 45910). Due to the gradual introduction of rectal EUS for the evaluation of PCD in children over this time period at our institution, and due to different provider treatment philosophies, some patients underwent EUS to monitor fistula healing around setons, while others were followed by exam only. For comparison, subjects were dichotomized into those who had their seton followed-up by at least one EUS to monitor healing (EUS-directed care) and those who were not followed with EUS (standard care).
Chart Review
Patient information was abstracted from the electronic medical record by a single investigator (M.R.), using a standardized chart abstraction form. Data were collected for each EUS procedure including type of sedation, indication, findings, and the post-EUS management. For both cohorts, baseline patient information collected included age at first EUS or seton placement, gender, medications, other anatomic involvement of disease, and prior history of perianal disease. For Cohort 2, biologic use was divided into three categories: biologic use for greater than 2 months prior to seton placement, biologic use from 2 months prior to seton placement to one month following seton placement, and no biologic around seton placement. We reasoned that if a biologic was started within one month of seton placement, then the decision to start the biologic was likely made at the time of seton placement and not influenced by subsequent follow-up EUS findings.
Endoscopic Ultrasound
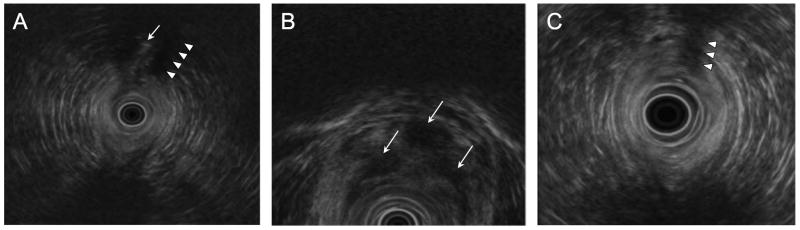
All rectal EUS procedures were performed by a single investigator (D.S.) using a 5-20 MHz radial scanning echoendoscope and then an electronic 5-7 MHz biplane probe (Olympus GF-UM 160, Melville, N.Y.). The instruments were advanced to just beyond the rectum and imaging was performed on a slow scope withdrawal. On imaging, an active fistula appears as hyperechoic beads (air) within a hypoechoic (inflammatory) tract (Figure 1).
Figure 1.
Representative series of EUS images from a 15-year-old boy with CD who presented with purulent drainage from the base of his left hemiscrotum unresponsive to azathioprine and metronidazole. (A) Baseline EUS revealed an echodense fistulous tract (arrow) flanked by wide areas of echolucency (arrowheads) indicating significant peri-tract inflammation. (B) Follow-up EUS four months after seton placement and after two doses of infliximab demonstrated persistent peri-seton inflammation (arrows). The seton was left in place, and (C) repeat EUS after 3 months and two additional doses of infliximab showed decreased peri-seton inflammation, as evidenced by an increased heterogeneity of the echotexture surrounding the seton (arrowheads). The seton was removed. Except for scant recurrence of drainage six months later, which responded to increased infliximab frequency, the patient remained well through 16 months of follow-up.
Primary Outcomes
For Cohort 1, we described the post-EUS management based on EUS indication and findings. Post EUS management was described separately for those undergoing EUS for suspected perianal disease or to confirm healing of a prior fistula and for those undergoing EUS for seton follow-up as the decision trees are very different for these two groups (e.g. a subject without a seton in place cannot have a seton removed).
For Cohort 2, we compared EUS-directed care and standard care in all patients who underwent seton placement, and the primary outcome was time to recurrence of drainage from seton removal. Secondary outcomes for this group included duration of seton placement, and frequency of initiation or change in biologic therapy while the seton was in place. For this analysis, if a patient had two setons placed at different times during the follow-up period, then only data from the first seton placement and subsequent time to recurrence were used.
Statistical Analysis
Baseline characteristics were reported as frequencies and percents, or means and standard deviations. The time from removal of seton to recurrence of disease was presented using Kaplan-Meier curves. The frequency of the addition or change of biologic therapy was compared between groups using Fisher's Exact Test. Since all subjects with a seton placed had it removed, the duration of seton placement was compared between groups using the non-parametric Wilcoxon Rank Sum Test. For all comparisons, the small sample size precluded multivariable analyses due to risk of over-fitting the model. Statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL) and R 2.6.0.15
Ethical Considerations
The study was approved by the Vanderbilt University Medical Center Institutional Review Board. No identifying patient information was recorded during chart abstraction.
Results
Cohort 1: EUS to Guide Management
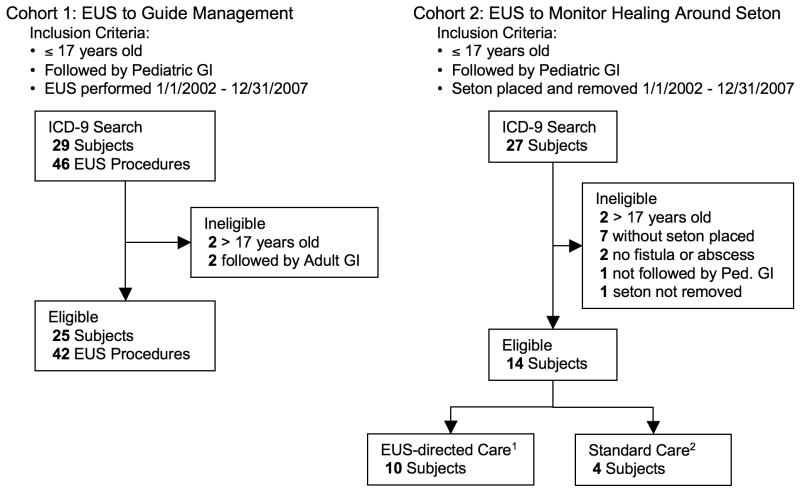
ICD9 and CPT code search revealed 46 EUS procedures on 29 subjects. After chart abstraction, 4 patients did not meet inclusion criteria and their EUS procedures were excluded from analysis yielding 42 EUS procedures on 25 subjects for analysis (Figure 2).
Figure 2.
Flow diagram of the selection of patients in each cohort. Cohort 1 comprised all patients who underwent EUS for PCD and was assembled to describe our experience using EUS to guide the management of pediatric patients with PCD. Cohort 2 comprised all patients who underwent seton placement and was assembled to determine if using EUS to monitor healing around a seton improves outcome. 1EUS-directed Care – subjects underwent at least one EUS following seton placement to monitor healing; 2Standard care – subjects followed by physical exam only following seton placement.
Fifteen subjects (60%) underwent a single EUS, 5 (20%) underwent two EUSs, and 5 (20%) subjects underwent 3 or more EUSs. Conscious sedation was used in 24 (57%) and monitored anesthesia care in 18 (43%) of 42 procedures. Table 1 details the baseline characteristics of Cohort 1 subjects at their first EUS procedure.
Table 1.
Cohort 1: Patient baseline characteristics at first Rectal EUS (n=25)
| Variable | n (%) or mean ± SD |
|---|---|
| Age, y | 14 ± 2.5 |
| Male sex | 21 (84) |
| Prior perianal disease (> 6 months) | 10 (40) |
| Other anatomic involvement | |
| Small intestine only | 3 (12) |
| Colonic only | 4 (16) |
| Small intestine and colonic | 17 (68) |
| Ileo-anal Pouch | 1 (4) |
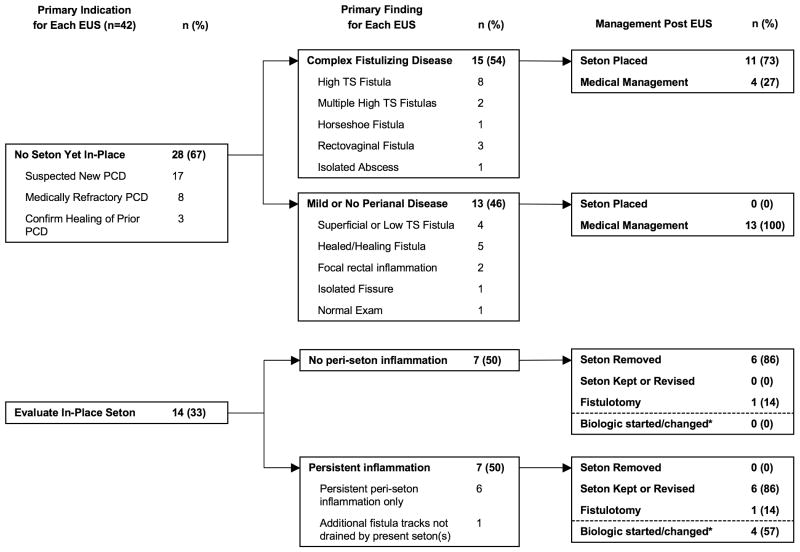
Figure 3 outlines the findings of EUS procedures based on initial indication, followed by subsequent management based on EUS findings. The two most common indications for EUS were suspected new PCD (40%) and evaluation of an in-place seton (33%).
Figure 3.
Flow diagram of indications, findings, and post-procedure management for all EUS procedures. Non-bold numbers for subcategories add up to the bold number for each corresponding main category. TS, trans-sphincteric. *Incidence of starting or changing a biologic is reported independent of the surgical management post-EUS.
There were 28 EUS procedures performed on subjects for evaluation of suspected PCD or to confirm healing of known PCD. Of these, complex fistulizing disease was identified in 15 (54%). Complex fistulizing disease was defined as a deep abscess, single or multiple high trans-sphincteric fistula(s), rectovaginal or horseshoe fistula. A seton was placed after most EUS's demonstrating complex fistulizing disease (73%), and no setons were placed after EUSs demonstrating superficial or no fistulizing disease (Figure 3).
Fourteen EUSs were performed to monitor healing after seton placement, and 7 (50%) demonstrated persistent peri-seton inflammation or undrained tracts (A representative case is shown in Figure 1). In 6 of 7 instances of persistent peri-seton inflammation, the seton was left in place to allow continued or altered medical therapy to improve healing. Conversely, the seton was promptly removed following 6 of 7 EUSs demonstrating no or little residual inflammation. Also, following an EUS revealing persistent peri-seton inflammation or undrained tracts, patients were more often started on a first or different biologic therapy (Figure 3).
In order to evaluate the value of EUS for identifying patients who do not require a surgical intervention, we analyzed the clinical course of 10 subjects who underwent EUS for evaluation of suspected PCD, but no complex fistulizing disease was identified, and, thus, were not referred for EUA. Of these 10 subjects, 9 (90%) were free of drainage and did not require any perianal surgical intervention after a mean follow-up of 1.3 years. The remaining subject underwent a fistulotomy with an outside local adult surgeon 13 weeks after his EUS.
Cohort 2: EUS to Monitor Healing Around Seton
ICD9 and CPT code search identified 27 subjects with CD who had a perianal surgical procedure. After chart abstraction, 13 patients did not meet inclusion criteria and were excluded from analysis, yielding 14 subjects less than 18 years of age, followed in the Pediatric GI Clinic, who had a seton placed for PCD (Figure 2).
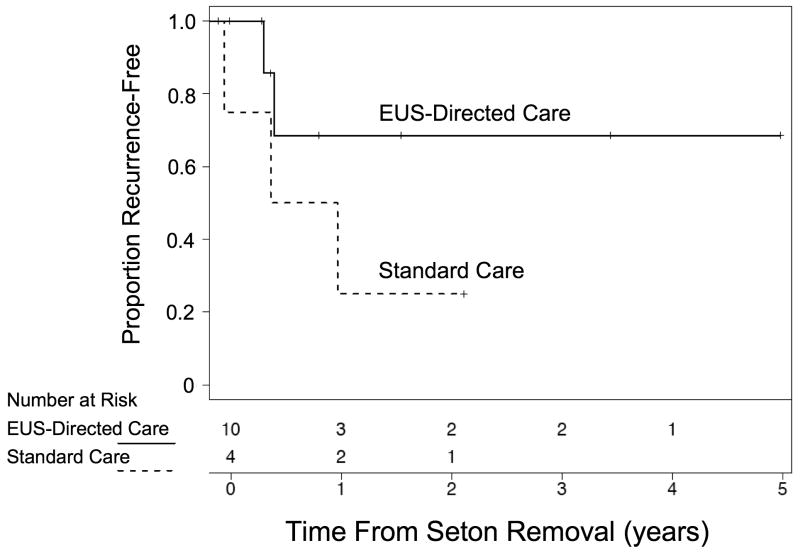
Ten subjects had at least one EUS performed to monitor healing after seton placement (EUS-directed care) and 4 subjects were only followed by physical exam alone (standard care.) Baseline characteristics of Cohort 2 subjects at the time of seton placement are presented in Table 2. Time from seton removal to recurrence of drainage was longer in the EUS-directed care group compared to the standard care group (Figure 4); however, given the small number of subjects, we were not powered to test for significance. In subjects with at least one year of follow-up after seton removal, 3 of 5 in the EUS-directed care group remained free of recurrence versus 1 of 4 in the standard care group. Two subjects in the EUS directed care group remained recurrence free for over three years. Median duration of seton placement was 28 weeks (range 15-70 weeks) in the EUS –directed care group and 25 weeks (range 10-108 weeks) in the standard care group (P=0.8). If the outlier of 108 weeks in the standard care group is excluded, however, duration of seton placement in the remaining 3 patients ranged between 10-28 weeks. Five subjects (50%) in the EUS-directed care group initiated or changed biologics prior to seton removal versus zero in the standard care group (P=0.2).
Table 2.
Cohort 2: Patient baseline characteristics at seton placement
| EUS-Directed Care (n=10) | Standard Care (n=4) | P value | |
|---|---|---|---|
| Age, y, mean±SD | 13.5±2.4 | 14.4±2.0 | 0.5 |
| Male sex, n (%) | 9 (90) | 3 (75) | 0.5 |
| Prior perianal disease (> 6 months) | 5 (50) | 2 (50) | 1.0 |
| Other anatomic involvement | |||
| Small intestine only | 3 (30) | 0 (0) | 0.4 |
| Colonic only | 2 (20) | 2 (50) | |
| Small intestine and colonic | 5 (50) | 2 (50) | |
| Biologic | |||
| > 2 months treatment | 1 (10) | 1 (25) | 0.4 |
| Started at time of seton* | 5 (50) | 2 (50) | |
| None | 4 (40) | 1 (25) | |
| Thiopurine | 10 (100) | 4 (100) | 1.0 |
| Antibiotics | 9 (90) | 2 (50) | 0.2 |
| Baseline EUS obtained | 6 (60) | 3 (75) | 1.0 |
Biologic started between 2 months prior and one month after seton placement.
Figure 4.
Kaplan-Meier plot of the proportion of subjects free from recurrence versus time from seton removal. Subjects monitored by EUS (EUS-directed care) while the seton was in place are compared to those monitored by physical exam alone (standard care).
Discussion
We describe our 5-year experience performing EUS on pediatric patients to guide management of PCD. While the use of rectal EUS for the evaluation and management PCD in children has been reported in abstract form by our group and one other16, 17, to our knowledge, this is the first investigation in the published literature. We demonstrate that EUS is feasible in the pediatric population, and, in our center, guided which patients were referred for surgical evaluation and seton placement, as well as the timing of seton removal. EUS also accurately identified patients who did not require surgical referral. In our small cohort of patients who underwent seton placement, recurrence-free time after seton removal was longer in the group followed by EUS; however, we were not powered to do a statistical comparison.
A recent prospective, multicenter, observational study demonstrated that 10% of children newly diagnosed with CD have perianal fistulas and/or abscesses by exam, and, with treatment, 71% of those patients experienced resolution of perianal disease within 12 months after diagnosis while the remaining 29% had chronic recurrent lesions.1 Nearly half of these patients underwent a surgical intervention. The authors did not describe the indications for surgery or stratify outcomes by those who did and did not undergo a surgical procedure. Our study complements this important recent study by describing a strategy to proactively determine which patients may benefit from a surgical intervention to optimize the effectiveness of medical therapy.
There are several explanations why using EUS to guide medical and surgical management following seton placement might prolong time to recurrence once the seton is removed. By MRI, a significant portion of patients with PCD with cessation of drainage by exam on infliximab still have persistent active subcutaneous fistulous tracts.18, 19 In the Accent II trial of infliximab for maintenance of CD fistulas, 62% of subjects receiving placebo after responding to the infliximab induction regimen experienced a recurrence of fistulas or required a change in medical therapy.9 It may be that the drug was withdrawn prior to complete quiescence of sub-clinical inflammation. In adults, our group has demonstrated that a clinical protocol of infliximab, antibiotics, seton placement when appropriate, and serial EUS examinations led to long-term cessation of drainage in 76% of subjects. This study was conducted prior to the realization of the benefit of long-term maintenance infliximab therapy, and a subset of subjects were actually taken off infliximab. The seven subjects who had complete resolution of inflammation by EUS and were taken off infliximab remained drainage-free after a median 47 weeks of follow-up.12 Thus, EUS may determine the appropriate timing of seton removal by demonstrating resolution of peri-seton inflammation which can not be detected by physical exam alone.
One might expect that if EUS identified patients with persistent peri-seton inflammation who required continued seton drainage, then duration of seton placement would be longer in the EUS-directed care group. In our study, median seton duration was 3-weeks longer in the EUS-directed care group. (If the outlier patient with seton duration of 108 weeks is removed from the standard care group, the difference is greater). While this difference was not statistically significant, it is consistent with the notion that EUS identifies patients who require continued seton drainage while medical therapy is allowed more time to heal inflammation. The difference may not be larger than that observed because EUS may also identify certain patients with rapid resolution of peri-seton inflammation in whom setons could be confidently removed earlier than they might with standard care.
Another explanation why serial EUS may improve time to recurrence is by directing “step-ups” in medical therapy. Anti-TNF therapy, including infliximab and adalimumab, are the most effective medical treatment for PCD and are the only therapies shown in large randomized controlled trials to completely heal fistulas and maintain fistula closure.8, 9, 20, 21 Despite this known efficacy, in our experience, parents are often reticent to have their children start infliximab, primarily due to the rare incidence of hepatosplenic T-cell lymphoma in young patients.22 This clinical observation, is corroborated by a study showing that parents of children with inflammatory bowel disease (IBD) estimated a higher risk or lymphoma and lower efficacy than adult patients with IBD.23 Interestingly, in our study, there was a higher incidence of starting or changing a biologic therapy in subjects who had an EUS showing persistent peri-seton inflammation compared to those with EUS showing no residual inflammation. Furthermore, of subjects with setons, more patients started or changed a biologic during the course of seton therapy in the EUS-directed care group than in the standard care group. One explanation for this finding is that EUS may provide the objective evidence parents need to see that conservative therapy is not effecting healing and anti-TNF therapy is warranted. The concepts of EUS detecting persistent inflammation around a seton and directing “step-ups” in medical therapy are exemplified in the findings and management of the subject in Figure 1.
Pelvic MRI is an alternative imaging modality for evaluating PCD and has been used in pediatric patients.13, 18, 19, 24-26 While the use of MRI to guide surgical therapy of PCD has not specifically been studied, MRI has been shown to detect active fistula inflammation on infliximab therapy in the setting of apparent clinical remission18, 19 MRI, EUS, and EUA all have about 90% accuracy in characterizing PCD lesions, and this accuracy increases to 100% when any two modalities are combined.13 Initial evaluation with imaging may preclude the need for a more invasive surgical EUA in select patients, as was seen in a subset of subjects in this study. Given the availability of EUS at our institution, we did not perform enough pelvic MRIs for PCD to compare the two modalities in this study. The choice of imaging modality depends on institutional resources and expertise. In younger children, sedation is usually necessary for both EUS and MRI. EUS has the advantage that it may be done at the same time as colonoscopy and is in the hands of the gastroenterologist.
There are several limitations to this study that should be noted. First, it is a retrospective study, which by its design is subject to potential bias. By performing a comprehensive ICD9 and CPT code search, we sought to limit selection bias by capturing most if not all of the patients that met inclusion criteria during the study period. Additionally, the investigator reviewing the charts (M.R.) was not blinded to the outcome and expectation bias may have been introduced. A structured chart abstraction form was used to limit this risk.
While we found a prolonged time to recurrence of fistula drainage from seton removal in the group receiving follow-up EUSs, the small number or patients preclude statistical testing of this difference. Also, due to the retrospective design, the risk of confounding exists. The outcome difference observed may be explained by infliximab use at baseline, or colonic involvement of disease. Similar numbers of patients were receiving infliximab within one month of seton placement making this variable an unlikely confounder. With regard to colonic involvement, all four patients in the standard care group had colonic involvement compared to seven of ten patients in the EUS-directed care group. Given the risk of poorer fistula healing in the presence of colonic inflammation27-29, this imbalance may have favored the EUS-directed care group.
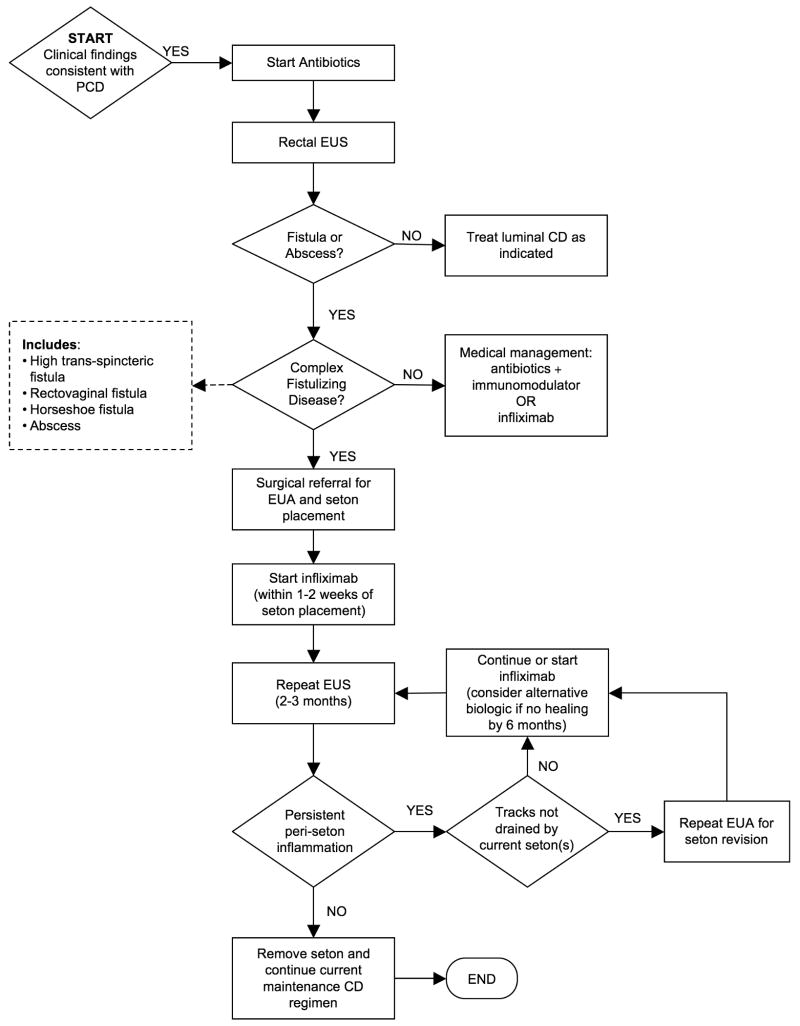
Our approach to the management of children with suspected PCD is diagrammed in the algorithm in Figure 5. We first obtain a baseline EUS and colonoscopy to evaluate for complex fistulizing disease and colonic inflammation. In patients with superficial fistulas only (not traversing the sphincter complex) we would defer surgical exam under anesthesia and treat conservatively with antibiotics initially (in addition to their other CD medications). Patients with abscesses or complex fistulizing disease identified on EUS are referred to our surgeons for EUA and seton placement. When complex fistulizing disease is identified, we recommend infliximab with an antibiotic; however, some parents wish to try conservative therapy with antibiotic and an immunomodulator. Once a seton is placed, we aim for serial EUS evaluations every 2-3 months to evaluate peri-seton inflammation, and, depending on the findings, change medical management or remove the seton.
Figure 5.
Suggested algorithm for using EUS to guide the medical and surgical management of PCD in children. (While we use EUS at our institutions, pelvic MRI could also be used depending on institutional resources and expertise.)
In conclusion, the use of rectal EUS to guide the combined medical and surgical management of PCD is feasible in the pediatric population. Larger prospective studies are needed to determine if EUS-directed management improves outcomes in pediatric patients with PCD.
Acknowledgments
Statistical support was funded through the Vanderbilt Digestive Disease Research Center, NIH Grant DK058404. Dr. Rosen is supported through NIH Training Grant T32DK07673.
Support: Statistical support funded through the Vanderbilt Digestive Disease Research Center, NIH Grant DK058404. Dr. Rosen is supported through NIH Training Grant T32DK07673.
Footnotes
Financial Disclosure: The authors have no financial affiliations to disclose.
Contributor Information
Michael J. Rosen, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Vanderbilt University School of Medicine, Nashville, TN.
Dedrick E. Moulton, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Vanderbilt University School of Medicine, Nashville, TN.
Tatsuki Koyama, Department of Biostatistics, Vanderbilt University School of Medicine, Nashville, TN.
Walter M. Morgan, III, Department of Pediatric Surgery, Vanderbilt University School of Medicine, Nashville, TN.
Stephen E. Morrow, Department of Pediatric Surgery, Vanderbilt University School of Medicine, Nashville, TN.
Alan J. Herline, Department of Surgery, Vanderbilt University School of Medicine, Nashville, TN.
Roberta L. Muldoon, Department of Surgery, Vanderbilt University School of Medicine, Nashville, TN.
Paul E. Wise, Department of Surgery, Vanderbilt University School of Medicine, Nashville, TN.
D. Brent Polk, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Vanderbilt University School of Medicine, Nashville, TN.
David A. Schwartz, Inflammatory Bowel Disease Center, Vanderbilt University School of Medicine, Nashville, TN.
References
- 1.Keljo DJ, Markowitz J, Langton C, et al. Course and treatment of perianal disease in children newly diagnosed with Crohn's disease. Inflamm Bowel Dis. 2009;15:383–7. doi: 10.1002/ibd.20767. [DOI] [PubMed] [Google Scholar]
- 2.Burbige EJ, Huang SH, Bayless TM. Clinical manifestations of Crohn's disease in children and adolescents. Pediatrics. 1975;55:866–71. [PubMed] [Google Scholar]
- 3.Mamula P, Markowitz JE, Baldassano RN. Inflammatory bowel disease in early childhood and adolescence: special considerations. Gastroenterol Clin North Am. 2003;32:967–95. viii. doi: 10.1016/s0889-8553(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 4.Kugathasan S, Judd RH, Hoffmann RG, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr. 2003;143:525–31. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz J, Daum F, Aiges H, et al. Perianal disease in children and adolescents with Crohn's disease. Gastroenterology. 1984;86:829–33. [PubMed] [Google Scholar]
- 6.Markowitz J, Grancher K, Rosa J, et al. Highly destructive perianal disease in children with Crohn's disease. J Pediatr Gastroenterol Nutr. 1995;21:149–53. doi: 10.1097/00005176-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Palder SB, Shandling B, Bilik R, et al. Perianal complications of pediatric Crohn's disease. J Pediatr Surg. 1991;26:513–5. doi: 10.1016/0022-3468(91)90694-o. [DOI] [PubMed] [Google Scholar]
- 8.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 9.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–85. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 10.Regueiro M, Mardini H. Treatment of perianal fistulizing Crohn's disease with infliximab alone or as an adjunct to exam under anesthesia with seton placement. Inflamm Bowel Dis. 2003;9:98–103. doi: 10.1097/00054725-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Caprioli F, Losco A, Vigano C, et al. Computer-assisted evaluation of perianal fistula activity by means of anal ultrasound in patients with Crohn's disease. Am J Gastroenterol. 2006;101:1551–8. doi: 10.1111/j.1572-0241.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz DA, White CM, Wise PE, et al. Use of endoscopic ultrasound to guide combination medical and surgical therapy for patients with Crohn's perianal fistulas. Inflamm Bowel Dis. 2005;11:727–32. doi: 10.1097/01.mib.0000172811.57242.18. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz DA, Wiersema MJ, Dudiak KM, et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology. 2001;121:1064–72. doi: 10.1053/gast.2001.28676. [DOI] [PubMed] [Google Scholar]
- 14.Spradlin NM, Wise PE, Herline AJ, et al. A randomized prospective trial of endoscopic ultrasound to guide combination medical and surgical treatment for Crohn's perianal fistulas. Am J Gastroenterol. 2008;103:2527–35. doi: 10.1111/j.1572-0241.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- 15.TEAM RDC. A language and environment for statistical computing. 2.6.0. Vienna, Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 16.Flomenhoft DR, Mardini HE, De Villiers WJ et al. Rectal EUS is a Useful Imaging Modality in Children. Gastrointestinal Endoscopy. 2007;65:AB117. [Google Scholar]
- 17.Rosen MJ, Moulton D, Little C, et al. The Use of Endoscopic Ultrasound in Pediatric Patients with Perianal Crohn's Disease. Gastroenterology. 2007;132:M2066. [Google Scholar]
- 18.Bell SJ, Halligan S, Windsor AC, et al. Response of fistulating Crohn's disease to infliximab treatment assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2003;17:387–93. doi: 10.1046/j.1365-2036.2003.01427.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Assche G, Vanbeckevoort D, Bielen D, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn's disease. Am J Gastroenterol. 2003;98:332–9. doi: 10.1111/j.1572-0241.2003.07241.x. [DOI] [PubMed] [Google Scholar]
- 20.Colombel JF, Schwartz DA, Sandborn WJ, et al. Adalimumab for the treatment of fistulas in patients with Crohn's disease. Gut. 2009 doi: 10.1136/gut.2008.159251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Rosh JR, Gross T, Mamula P, et al. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn's disease: a cautionary tale? Inflamm Bowel Dis. 2007;13:1024–30. doi: 10.1002/ibd.20169. [DOI] [PubMed] [Google Scholar]
- 23.Siegel CA, Levy LC, Mackenzie TA, et al. Patient perceptions of the risks and benefits of infliximab for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1–6. doi: 10.1002/ibd.20283. [DOI] [PubMed] [Google Scholar]
- 24.Haggett PJ, Moore NR, Shearman JD, et al. Pelvic and perineal complications of Crohn's disease: assessment using magnetic resonance imaging. Gut. 1995;36:407–10. doi: 10.1136/gut.36.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koelbel G, Schmiedl U, Majer MC, et al. Diagnosis of fistulae and sinus tracts in patients with Crohn disease: value of MR imaging. AJR Am J Roentgenol. 1989;152:999–1003. doi: 10.2214/ajr.152.5.999. [DOI] [PubMed] [Google Scholar]
- 26.Essary B, Kim J, Anupindi S, et al. Pelvic MRI in children with Crohn disease and suspected perianal involvement. Pediatr Radiol. 2007;37:201–8. doi: 10.1007/s00247-006-0372-2. [DOI] [PubMed] [Google Scholar]
- 27.Levien DH, Surrell J, Mazier WP. Surgical treatment of anorectal fistula in patients with Crohn's disease. Surg Gynecol Obstet. 1989;169:133–6. [PubMed] [Google Scholar]
- 28.Nordgren S, Fasth S, Hulten L. Anal fistulas in Crohn's disease: incidence and outcome of surgical treatment. Int J Colorectal Dis. 1992;7:214–8. doi: 10.1007/BF00341224. [DOI] [PubMed] [Google Scholar]
- 29.Radcliffe AG, Ritchie JK, Hawley PR, et al. Anovaginal and rectovaginal fistulas in Crohn's disease. Dis Colon Rectum. 1988;31:94–9. doi: 10.1007/BF02562636. [DOI] [PubMed] [Google Scholar]