Abstract
Yellow fever virus (YFV) continues to cause outbreaks of disease in endemic areas where vaccine is underutilized. Due to the effectiveness of the vaccine, antiviral development solely for the treatment of YFV is not feasible, but antivirals that are effective in the treatment of related viral diseases may be characterized for potential use against YFV as a secondary indication disease. 2′-C-methylcytidine (2′-C-MeC), a compound active against hepatitis C virus, was found to have activity against the 17D vaccine strain of YFV in cell culture (EC90 = 0.32 μg/ml, SI = 141). This compound was effective when added as late as 16 h after virus challenge of Vero cells. When administered to YFV-infected hamsters 4 hours prior to virus challenge at a dose as low as 80 mg/kg/d, 2′-C-MeC was effective in significantly improving survival and other disease parameters (weight change, serum ALT, and liver virus titers). Disease was improved when compound was administered beginning as late as 3 days post virus infection. Broadly active antiviral compounds, such as 2′-C-MeC, represent potential for the development of compounds active against related viruses for the treatment of YFV.
1. Introduction
Yellow fever virus (YFV) is a member of the flavivirus family. Transmitted by mosquitoes, this blood-borne pathogen is endemic to Africa and South America. Epidemics occur in endemic areas with an average attack rate of 5% and an annual estimated 24-240 thousand people infected (Monath, 2006). Disease caused by this virus may be highly lethal with fatality rates up to 50% as compared to lower fatality rates with other flavivirus infections (Tomori, 2004; Tuboi et al., 2007). A highly effective vaccine is available for the prevention of YFV, although it is underutilized in many endemic countries (Monath, 2006). Aside from more widespread use of the YFV vaccine, antiviral therapies for the treatment of YFV disease would be important in cases where vaccination may not be recommended, such as in pregnant women or in immunodeficient individuals, or in cases during unexpected outbreak, or cases occurring in areas where heath care is not readily accessible. There are currently no approved antiviral therapies for the treatment of YFV. Rare severe adverse events have also been reported in association with vaccination (Domingo and Niedrig, 2009; Hayes, 2007), representing another possible need for treatment.
While development of an antiviral specifically for the treatment of YFV (as well as many other acute viral diseases) is not feasible, an alternative approach is to evaluate compounds that are used clinically to treat related viral disease for efficacy against YFV under the Federal Drug Administrations (FDA) “two animal rule” (Code of Federal Regulations, Title 21, Volume 5, Section 314.610, Revised April 1, 2009: taken from FDA website www.fda.gov). Hepatitis C virus (HCV), a related flavivirus that also targets the liver, would be a likely candidate as many compounds active against HCV are also active, at least in cell culture, against YFV (Buckwold et al., 2007; Fogt et al., 2008; Yin et al., 2009).
Historically, work to develop YFV antiviral therapies was hampered by lack of an appropriate small animal model of disease. Primates replicate many of the disease parameters observed in the natural infection of man, although the disease progresses more rapidly and is highly lethal in primate models (Monath et al., 1981). Disease in mouse models usually requires adapted virus and generally includes encephalopathy (Charlier et al., 2004), which is a rare occurrence in human infection. Recently, a mouse model of YFV visceral disease was developed in mice, although this model requires the use of mice lacking interferon receptors or the STAT 1 signaling molecule (Meier et al., 2009). The hamster model of YFV infection, which utilizes an adapted Jimenez virus strain, manifests many disease parameters observed in human disease, including hepatic dysfunction and necrosis, elevated aminotransferase levels, bilirubinemia, and lymphoid hyperplasia in the spleen, which provide different parameters for use in antiviral studies (Julander et al., 2007b; Monath, 2008; Sbrana et al., 2006; Tesh et al., 2001; Xiao et al., 2001).
Broad-spectrum antivirals, such as interferon, ribavirin, T-705, and T-1106, have shown efficacy in the hamster model (Julander et al., 2007a; Julander et al., 2007b; Julander et al., 2009; Sbrana et al., 2004). These compounds were effective in improving survival, reducing liver virus titer, and reducing serum alanine aminotransferase (ALT) levels in YFV-infected animals. African green monkeys treated with interferon had reduced viremia and serum ALT as compared with untreated controls (Monath, 2008), which was similar to the effect of interferon observed in the hamster model (Julander et al., 2007b). Ribavirin therapy in a primate model, however, was more variable with reduction of serum ALT in one study and survival rates from 0-50% in several studies, depending on challenge dose of virus and dose of ribavirin administered (Monath, 2008). The dose of 20 mg/kg/d used in one primate study (with 0-13% survival in treated animals) was ineffective in the hamster model, and doses of 50-75 mg/kg/d were required to consistently reduce disease parameters in a statistically significant manner (Julander et al., 2007b) and unpublished data). Better results were observed in the primate model after treatment with 120 mg/kg/d of ribavirin (50% survival) (Monath, 2008). It appears that the hamster model is fairly predictive of efficacy of compounds in primate models, but the primate model seems to be more severe and difficult to treat.
The nucleoside analog, 2′-C-Methylcytidine (2′-C-MeC), as well as derivatives and prodrugs of this compound have been shown to be active in vitro and in vivo against hepatitis C virus (HCV) in various models of HCV infection (Carroll and Olsen, 2006; Stuyver et al., 2006; Toniutto et al., 2007). One mechanism of PSI-6130, a β-D-2′-deoxy-2′-fluro derivative of 2′-C-MeC, involves the inhibition of the HCV viral polymerase through chain termination (Murakami et al., 2007; Murakami et al., 2008). Combinations of 2′-C-MeC derivatives with interferon and/or ribavirin were synergistic in a HCV replicon model (Bassit et al., 2008), which was interesting in light of antagonism between 2′-C-MeC and ribavirin in a separate study (Coelmont et al., 2006). Efficacy of 2′-C-MeC has also been observed in a cell culture model of foot-and-mouth disease virus, a picornavirus (Goris et al., 2007), demonstrating broad-spectrum activity of this compound. Valopicitabine, a 3′-O-l-valinyl ester derivative of 2′-C-MeC, is active against several RNA viruses in cell culture (Pierra et al., 2006). This derivative was evaluated in HCV-infected patients during clinical trials (Liu-Young and Kozal, 2008), however severe gastrointestinal side-effects were observed in a dose-dependent manner, resulting in the termination of clinical progression, despite efficacy in improving HCV disease parameters.
During routine antiviral screening in our laboratory, 2′-C-MeC was found to have activity against YFV in cell culture. The β-D-2′-deoxy-2′-fluoro derivative of this compound was previously shown to inhibit HCV, as well as YFV and several other flaviviruses, in cell culture (Stuyver et al., 2006). This report details the activity of 2′-C-MeC in vitro as well as in the hamster model of YFV disease.
2. Materials and methods
2.1. Animals
Female Syrian golden hamsters with an average weight of 100 g were used. After a 24-hour quarantine period and 7-day acclimation period, animals were randomly assigned to cages and individually marked with ear tags. All work with these animals was performed in the Biosafety Level 3 (BSL-3) area of the AAALAC-accredited Laboratory Animal Research Center (LARC) at Utah State University (USU).
2.2. Test articles
2′-C-MeC was obtained as a powder (C. K. Chu, University of Georgia, Athens, GA) and was prepared in sterile saline. Ribavirin was provided by ICN Pharmaceuticals, Inc. (Costa Mesa, CA), and was also prepared in saline. Compounds in solution were prepared just prior to initial administration and were stored at 4°C. Interferon alfacon-1 (IFN alfacon-1, infergen), a consensus-type interferon, was provided by InterMune Inc. (Brisbane, CA, USA) as an aqueous solution.
2.3. Virus
The hamster-adapted Jimenez strain of YFV was obtained as a generous gift from Dr. Robert B. Tesh (University of Texas Medical Branch, Galveston, TX), and prepared as previously described (Julander et al., 2007b). The 17D YFV vaccine strain stock, obtained from the American Type Culture Collection (ATCC, Manassas, VA) (V525-001-522) was amplified after two passages in MA-104 African green monkey kidney cells (ATCC) after initial amplification in chick embryos.
2.4. Evaluation of 2′-C-Methylcytidine in cell culture
The antiviral activity of 2′-C-MeC was evaluated in Vero African green monkey kidney cells (ATCC) by cytopathic effect (CPE) inhibition assays, as determined by visual (microscopic) examination of the cells, increase of neutral red (NR) dye uptake into cells, and virus yield reduction (VYR) following previously described methods (Reed and Muench, 1938; Sidwell and Huffman, 1971). Eight concentrations of the compound were evaluated against the 17D strain of YFV in 96-well flat-bottomed microplates containing Vero cell monolayers. Compounds were added 5 to 10 min prior to the addition of virus. Virus was added at an approximate multiplicity of infection (MOI) of 0.0001 (∼32 50% cell culture infectious doses (CCID50/ml)), which results in 80-100% CPE in virus control wells after incubation for 6 days. After incubation, plates were read visually, and CPE was evaluated and quantified. For NR uptake, dye in medium (0.034% in medium) was added to plates for 2 h, after which the dye was eluted from the cells and absorbed dye was quantified using an ELISA plate reader at 560 nm.
The VYR assay included an eight-concentration assay as above, after which CPE and cytotoxicity were recorded by visual inspection after 6 days of incubation. Test wells with the same concentration of compound were pooled and virus present in these samples was titrated on Vero cells to determine the 90% effective concentration (EC90), or the concentration necessary to reduce virus titer by 1-log10.
Antiviral activity was expressed as the 50% (EC50) or EC90, and a selective index (SI) value was obtained by dividing the 50% cytotoxicity concentration (CC50), obtained from uninfected cells treated with compound, by the EC90 value (Sidwell et al., 2007).
Time of addition studies were also conducted in which 2′-C-MeC was added at 0, 4, 8, 12, or 16 h after virus challenge of Vero cells in 12-well flat-bottomed cell culture plates. Compound was added at 50, 25, 12, 6, and 3 μg/ml. Supernatants from each well were collected at 24 h after virus challenge and analyzed for infectious virus, which corresponded with the first round of virus release.
2.5. Experimental design for animal studies
Hamsters were randomly assigned to groups, and 10-15 compound-treated and 10-20 placebo-treated animals were included in each. Toxicity controls, consisting of 3 animals per group, were included to determine if there was any apparent toxicity associated with treatment. Normal control animals were also included. A concentration of 102 CCID50 of Jimenez YFV was prepared in minimal essential media, which is an approximately 90% lethal dose (LD90) of virus. Hamsters were injected i.p. with 0.1 ml of the virus preparation. Animals were treated twice daily (bid) for 4-7 days, with treatments administered12 h apart. Mortality was observed daily, and weight change between 3 and 6 days post-virus inoculation (dpi) was determined. Serum was taken 6 dpi for quantification of serum ALT. Liver virus titer was evaluated on 5 dpi. Ribavirin, prepared in saline at a dose of 50 mg/kg/d, was used as a positive control compound, and saline was used as placebo control.
In the first experiment, a simple range finding study was conducted to determine the effective dose of 2′-C-MeC in hamsters infected with YFV. Follow-up studies were conducted to determine minimal effective dose and length of time after virus challenge drug could be effectively initiated (2 though 4 dpi).
2.6. Serum alanine (ALT) and aspartate aminotransferase (AST) assays
Serum was collected antemortem by ocular sinus collection from all of the animals in each group. ALT (SGPT) reagent (Teco Diagnostics, Anaheim, CA) was used, and the protocol was altered for use in 96-well flat-bottomed microplates as previously described (Julander et al., 2007a). The aminotransferase concentrations were determined per manufacturer's instructions.
2.7. Titration of virus from livers
Vero cells were cultured in 96-well flat-bottomed microplates one day before use. Liver samples were homogenized in cell culture medium and serial dilutions from 10-1 to 10-8 were added to microplates with semi-confluent Vero cells. Plates were incubated at 37°C for 9 days, after which the cells were observed microscopically for virus cytopathic effect (CPE). The observed titer in Vero cells, calculated by endpoint dilution (Reed and Muench, 1938), was adjusted based on weight of tissue prior to homogenization.
2.8. Statistical analysis
Survival data were analyzed using the Wilcoxon Log-rank survival analysis, and all other statistical analyses were done using one-way ANOVA using a Newman-Keuls multiple comparison test (Prism 5, GraphPad Software, San Diego, CA).
3. Results
3.1. Cell culture studies
The nucleoside analog 2′-C-MeC was tested for activity against YFV in cell culture in 8-concentration assays. The compound was 50% effective in inhibiting viral CPE in the low μg/ml range (Table 1). Activity was similar by visual and neutral red dye uptake assay methods, yielding comparable EC50 values. A virus yield assay was conducted to determine the EC90, or the amount of compound needed to reduce virus titer 10-fold (one log10). An average EC90 of 0.7 ± 0.3 μg/ml was determined from 3 separate studies, which yielded a selective index (SI) of 31. 2′-C-MeC displayed moderately high activity in cell culture, which warranted further studies in the hamster model of YFV infection. Toxicity was observed in uninfected Vero cells treated with 2′-C-MeC, with an average 50% cytotoxic concentration (CC50) of 22 ± 3.5 μg/ml (Table 1). Interferon alfacon-1 (infergen) was included in the cell culture studies as a positive control (Table 1), which showed activity at concentrations that were consistent with previous studies (Julander et al., 2009).
Table 1.
Effect of 2′-C-MeC or interferon alfacon-1 (infergen) on yellow fever virus (17D) infection of confluent Vero cell monolayersa assayed by visual inspection, neutral red uptake assay, or virus yield reduction assay.
| Compound | Units | EC50b (vis) c | EC50 (NR) d | EC90e (VYR) f | CC50g (NR) | SI h |
|---|---|---|---|---|---|---|
| 2′-C-MeC | μg/ml | 2.5 ± 0.7 | 2.1 ± 0.3 | 0.7 ± 0.3 | 22.0 ± 3.5 | 31 |
| Interferon alfacon-1 | ng/ml | 0.011 ± 0.001 | 0.011 ± 0.01 | 0.016 ± 0.09 | >10 ± 0.0 | >625 |
Vero monolayers infected with 102.6 50% cell culture infectious doses of 17D strain YFV. Each assay was conducted at least three times and results are expressed as mean ± standard deviation.
50% effective concentration, or amount of drug required to protect 50% of cells from virus cytopathic effect (CPE).
Visual inspection of cells.
Neutral red dye uptake assay.
90% effective concentration, or amount of drug required to reduce virus yield by 10-fold (1 log10).
Virus yield reduction assay.
Cytotoxic concentration of drug resulting in inhibition of growth in 50% of treated stationary monolayer cells.
Selectivity index determined by dividing the CC50 (NR) by the EC90.
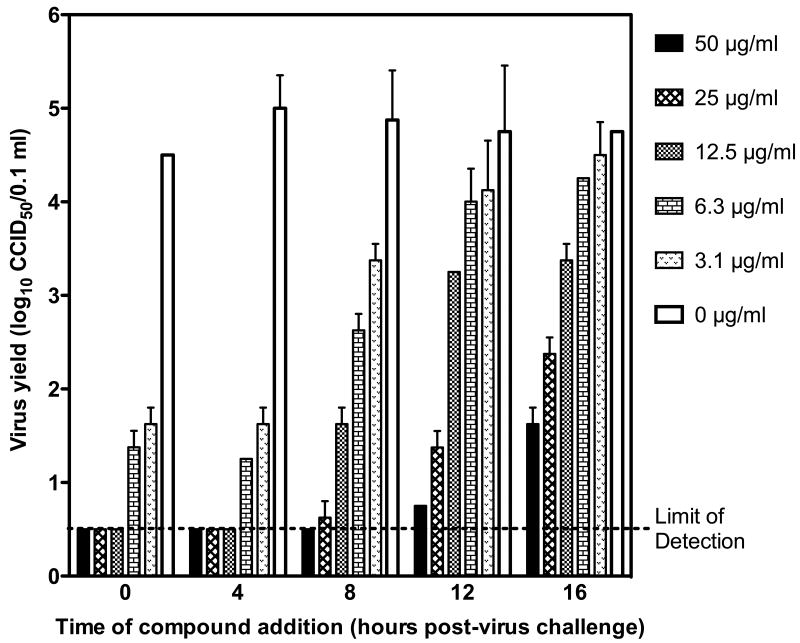
Time of addition studies were conducted with 2′-C-MeC being added at various times after virus challenge of Vero cells, which demonstrated activity against YFV even when added to infected cells 16 hours after virus challenge (Figure 1). The EC90 of this compound increased in a time-dependent manner. Compound added at 0 or 4 hours after virus challenge had similar EC90 values of 0.34 and 0.27 μg/ml, respectively, which increased in a time-dependent manner to 2.4, 7.8, and 8.9 μg/ml when compound was added 8, 12, or 16 h after virus challenge, respectively.
Figure 1.
Effects of 2′-C-MeC on YFV yields from Vero cells when compound was added to cells at various times relative to virus challenge. Supernatant was obtained at 24 hours after virus addition, and virus was quantified by infectious cell culture assay. A concentration- and time-dependent decrease in virus was observed.
3.2. In vivo studies
An initial dose determination study was conducted in the hamster model of YFV disease. A high dose of 120 mg/kg/d was selected based on an effective dose of 800 mg/kg valopicitabine in man (Neyts, 2006), and two half-log lower concentrations of 37 and 12 mg/kg/d were also included. Compound was administered beginning 4 hours prior to virus challenge to hamsters through intraperitoneal (i.p.) injection every 12 hours for 7 days.
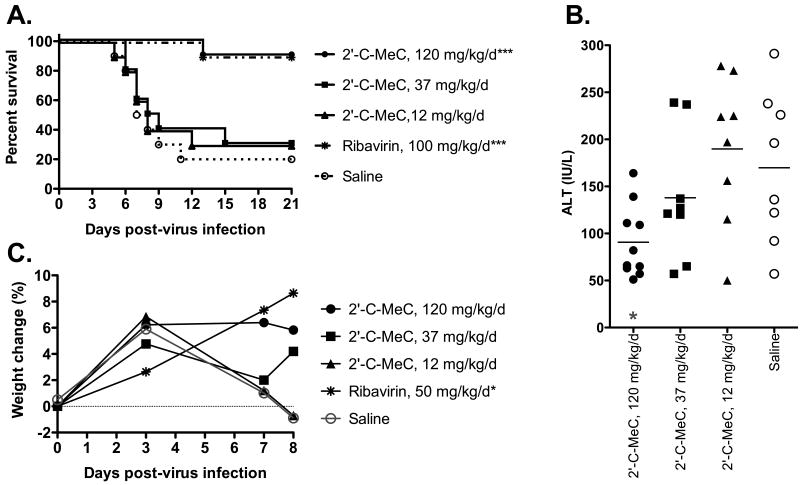
Treatment with the highest tested dose of 120 mg/kg/d of 2′-C-MeC resulted in significant protection of hamsters infected with YFV (Figure 2A). Ninety percent of hamsters treated with this concentration survived, which was the same for animals treated with the positive control compound, ribavirin. Significant reduction in serum ALT on 6 dpi was also observed in YFV-infected hamsters treated with 120 mg/kg/d of 2′-C-MeC (Figure 2B). Weight change was measured on 0, 3, 7, and 8 dpi and a trend towards improvement in weight change was observed in animals treated with high dose 2′-C-MeC (Figure 2C). No apparent toxicity was observed, as determined by significant weight loss or mortality in sham-infected, 2′-C-MeC-treated animals at any of the doses tested (data not shown).
Figure 2.
Effects of pre-virus exposure treatment initiation with 2′-C-MeC or ribavirin on YFV infection in hamsters. Treatment was initiated -4 h and continued bid for 8 days. Disease parameters include: A) survival, B) serum ALT on 6 dpi, and C) percent body weight change. ***P<0.001, *P<0.05, as compared with placebo.
Treatment with concentrations of 37 and 12 mg/kg/d did not improve any measured disease parameters, which were similar to those of placebo-treated infection control animals (Figure 2). A non-significant improvement in the mean day to death (8.3 ± 3.1 days vs 7.5 ± 1.9 days in placebo-treated animals) and in serum ALT levels (Figure 2B) of animals treated with 37 mg/kg/d of 2′-C-MeC suggested that there was some slight effect occurring after treatment with this dose, and that a dose between 120 and 37 mg/kg/d may be active.
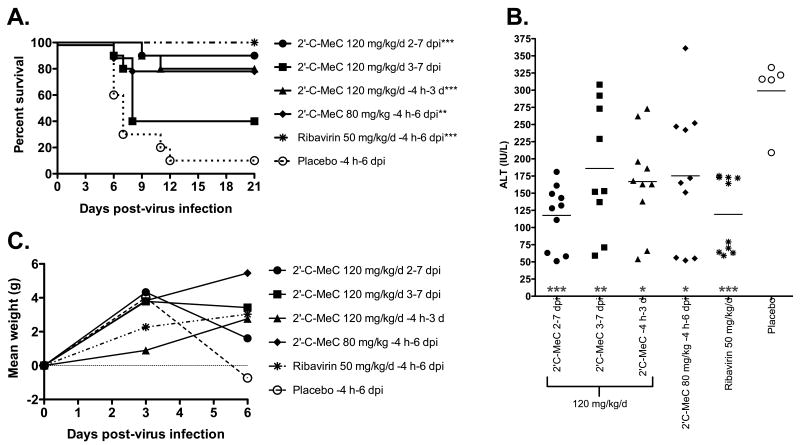
Efficacy of 2′-C-MeC administered beginning -4 h at a dose of 120 mg/kg/d was verified in a follow-up study. Similar results were observed, including significant improvement in survival and serum ALT on 6 dpi (Figure 3A and B, respectively). Weight change was recorded between 3 and 6 dpi, but despite measurement at an earlier time-point of 6 dpi, as compared with 7 dpi from the first experiment, around 50% of the animals from the placebo control group had succumbed to illness. Therefore, weight change was not significantly improved, despite a trend towards improvement in 2′-C-MeC-treated animals (Figure 3B). Liver virus titer was significantly reduced on 4 dpi in animals treated with 120 mg/kg/d of 2′-C-MeC as compared with placebo treatment, which was similar to the reduction in liver titer from animals treated with 50 mg/kg/d of ribavirin given along the same schedule (Figure 3D). Only 1 of 5 animals in the 2′-C-MeC-treated group had a titer above the level of detection, as compared with 2 of 5 and 5 of 5 in ribavirin-treated or placebo-treated groups, respectively. Animals treated with the positive control compound, ribavirin, initiated -4 h significantly improved survival and liver virus titer with accompanying non-significant improvements in weight change and serum ALT (Figure 3).
Figure 3.
Effects of post-virus exposure treatment initiation of hamsters with 2′-C-MeC (120 mg/kg/d) and ribavirin (50 mg/kg/d) on YFV infection in hamsters. Parameters include: A) survival, B) serum ALT on 6 dpi, C) percent body weight change, or D) liver virus titers on 4 dpi. ***P<0.001, **P<0.01, as compared with placebo.
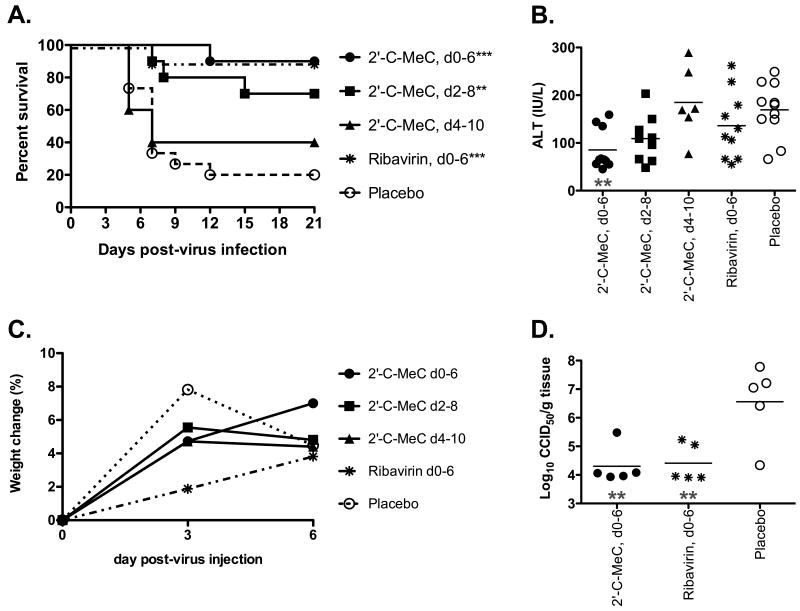
The effect of delayed treatment initiation beginning at various times after virus challenge on 2′-C-MeC activity was also investigated. Animals were treated beginning 2 or 4 days after virus challenge, which was compared with 2′-C-MeC or placebo treatment beginning -4 h (as described above). Significant improvement in survival, as compared with placebo treated animals, was observed when treatment was initiated 2 dpi (Figure 3A). A nonspecific improvement was also observed in serum ALT levels on 6 dpi from animals treated beginning 2 dpi (Figure 3B). Treatment initiated 4 dpi did not have any effect on disease parameters, although there was a slight trend towards improvement in survival (Figure 3).
With efficacy observed when treatment was initiated 2 but not 4 dpi, it was necessary to determine the effect of treatment beginning 3 dpi. An experiment was conducted which included treatment beginning 2 dpi as a control. A non-significant improvement (P<0.066) was observed in the survival rate of animals treated beginning 3 dpi, which resulted in survival of 40% as compared with 10% in placebo-treated controls (Figure 4A). Serum ALT levels were significantly reduced with initiation of treatment on 3 dpi (Figure 4B), further suggesting a beneficial effect. As in the previous experiment, treatment beginning 2 dpi resulted in significant improvement in survival (Figure 4A). Serum ALT levels were also significantly improved (Figure 4B), which was not observed in the previous experiment (Figure 3B).
Figure 4.
Effects of treatment with 2′-C-MeC or ribavirin initiated 4 h before infection, or 2 or 3 dpi on YFV infection in hamsters. Parameters include: A) survival, B) serum ALT on 6 dpi, and C) percent body weight change. ***P<0.001, **P<0.01, *P<0.05, as compared with placebo.
To determine the effect of 2′-C-MeC when given bid at 120 mg/kg/d for a shorter treatment duration, compound was administered bid from -4 h to 3 dpi (8 treatments total). A significant improvement in survival of YFV-infected hamsters was observed (Figure 4D). Serum ALT was also significantly reduced, accompanied by a trend towards weight gain (Figure 4B and C, respectively). Improvements in disease were similar to improvements observed after 7-day treatment, although the improvements tended to be less robust.
An intermediate dose of 80 mg/kg/d of 2′-C-MeC was also included in this study to better determine a minimal effective dose. Treatment with this dose resulted in a significant improvement in survival (Figure 4A) and serum ALT (Figure 4B), as well as a trend towards improved weight change (Figure 4C). Taken together with the dose-finding study, these results give an extrapolated 50% effective dose (EC50) for 2′-C-MeC of 52 mg/kg/d.
4. Discussion
The nucleoside analog 2′-C-MeC and its derivatives have been shown to have activity against several RNA viruses, including efficacy in the treatment of HCV in people (Clark et al., 2005; Goris et al., 2007; Murakami et al., 2008; Neyts, 2006; Pierra et al., 2005; Shannon et al., 1981; Stuyver et al., 2006). The present study demonstrates activity of this compound in Vero cells infected with YFV as well as in a hamster model of YFV infection and disease, further demonstrating the broad-spectrum activity of this compound. The compound appeared to be well tolerated, with no obvious signs of toxicity, and cell culture studies showed the toxic concentration to be 10-30-fold higher than effective concentrations.
Treatment reduced viral titers in cell culture up to 16 h after virus challenge, suggesting inhibition of later viral processes. This compound inhibits the viral polymerase of HCV (Murakami et al., 2008), and likely interacts directly with the viral polymerase of the related flavivirus, YFV. The flaviviral polymerase is an important target for the development of antiviral therapies (Keller et al., 2006), and compounds targeting this enzyme are potentially useful clinically either as monotherapies or in combination with other effective compounds.
Activity was observed after therapeutic treatment of infected hamsters with 2′-C-MeC. Treatment initiated as late as 3 days after virus infection was effective in improving disease parameters. At this time, virus is present in the liver, serum, and spleen of hamsters, with peak titers occurring 1-2 days later (Julander et al., 2007b; Tesh et al., 2001). The disease phenotype is similar to that in man (Monath, 2008; Monath et al., 1981), suggesting effective treatment in human patients infected with YFV would need to be initiated early during the course of infection, perhaps just after the onset of fever. The efficacy of 2′-C-MeC was shown to be similar to ribavirin in regards to effective dose, treatment delay and improvement of disease parameters.
Activity was seen at a dose comparable to doses effective in the treatment of people infected with HCV, suggesting potential for use of the compound in the treatment of YFV in man. No apparent toxicity was observed with treatment, which would be expected with short-term treatment with this compound. HCV causes a chronic disease, whereas YFV causes an acute disease. Treatment of patients for several weeks with valopicitabine resulted in severe gastrointestinal side effects (Lawitz et al., 2007), which eventually halted clinical trials. Treatment of an acute viral disease such as YFV would likely preclude such side effects associated with long-term treatment, so development of 2′-C-MeC and its derivatives for the treatment of acute flaviviral diseases may be an alternative treatment indication. It is unlikely that valopicitabine will be used clinically for the treatment of HCV, but other less toxic derivatives may ultimately be used. The hamster model of YFV infection would be useful in testing active derivatives of 2′-C-MeC, as well as other compounds that are or may be used clinically in the treatment of HCV infection. While clinical development of a compound solely for the purpose of treating YFV is not feasible, it may be possible to broaden the indication of compounds used in the treatment of other viral diseases.
It appears as though 2′-C-MeC is a broad-spectrum inhibitor of flaviviruses in cell culture as well as in animal models and man. With therapeutic activity and reduction of infectious virus, this compound could have potential utility in the treatment of human disease. While vaccination remains the most important means of YFV control, there is a need for the development of a broad-spectrum antiviral compound for the treatment of YFV and other acute flaviviral infections. The efficacy of 2′-C-MeC in the treatment of YFV in a hamster model warrants further investigation in this regard, and future research might include mechanistic studies, efficacy against other acute flaviviruses, and evaluation of 2′-C-MeC derivatives.
Acknowledgments
We thank Robert Tesh for providing the Jimenez hamster-adapted YFV strain. We also thank Garret Child and Deanna Larson for their work in the animal and cell culture labs. This work was supported by contract #N01-AI-30048 from the Virology Branch, NIAD, NIH and #N01-AI-30063 (awarded to Southern Research Institute) from the Division of Microbiology and Infectious Diseases, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassit L, Grier J, Bennett M, Schinazi RF. Combinations of 2′-C-methylcytidine analogues with interferon-alpha2b and triple combination with ribavirin in the hepatitis C virus replicon system. Antivir Chem Chemother. 2008;19:25–31. doi: 10.1177/095632020801900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwold VE, Wei J, Huang Z, Huang C, Nalca A, Wells J, Russell J, Collins B, Ptak R, Lang W, Scribner C, Blanchett D, Alessi T, Langecker P. Antiviral activity of CHO-SS cell-derived human omega interferon and other human interferons against HCV RNA replicons and related viruses. Antiviral Res. 2007;73:118–125. doi: 10.1016/j.antiviral.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Carroll SS, Olsen DB. Nucleoside analog inhibitors of hepatitis C virus replication. Infect Disord Drug Targets. 2006;6:17–29. doi: 10.2174/187152606776056698. [DOI] [PubMed] [Google Scholar]
- Charlier N, Leyssen P, De Clercq E, Neyts J. Rodent models for the study of therapy against flavivirus infections. Antiviral Res. 2004;63:67–77. doi: 10.1016/j.antiviral.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Clark JL, Hollecker L, Mason JC, Stuyver LJ, Tharnish PM, Lostia S, Mcbrayer TR, Schinazi RF, Watanabe KA, Otto MJ, Furman PA, Stec WJ, Patterson SE, Pankiewicz KW. Design, synthesis, and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-methylcytidine, a potent inhibitor of hepatitis C virus replication. J Med Chem. 2005;48:5504–5508. doi: 10.1021/jm0502788. [DOI] [PubMed] [Google Scholar]
- Coelmont L, Paeshuyse J, Windisch MP, De Clercq E, Bartenschlager R, Neyts J. Ribavirin antagonizes the in vitro anti-hepatitis C virus activity of 2′-C-methylcytidine, the active component of valopicitabine. Antimicrob Agents Chemother. 2006;50:3444–3446. doi: 10.1128/AAC.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo C, Niedrig M. Safety of 17D derived yellow fever vaccines. Expert Opin Drug Saf. 2009;8:211–221. doi: 10.1517/14740330902808086. [DOI] [PubMed] [Google Scholar]
- Fogt J, Januszczyk P, Framski G, Onishi T, Izawa K, De Clercq E, Neyts J, Boryski J. Synthesis and antiviral activity of novel derivatives of 2′-beta-C-methylcytidine. Nucleic Acids Symp Ser (Oxf) 2008:605–606. doi: 10.1093/nass/nrn306. [DOI] [PubMed] [Google Scholar]
- Goris N, De Palma A, Toussaint JF, Musch I, Neyts J, De Clercq K. 2′-C-methylcytidine as a potent and selective inhibitor of the replication of foot-and-mouth disease virus. Antiviral Res. 2007;73:161–168. doi: 10.1016/j.antiviral.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Hayes EB. Acute viscerotropic disease following vaccination against yellow fever. Trans R Soc Trop Med Hyg. 2007;101:967–971. doi: 10.1016/j.trstmh.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Julander JG, Furuta Y, Shafer K, Sidwell RW. Activity of T-1106 in a hamster model of yellow Fever virus infection. Antimicrob Agents Chemother. 2007a;51:1962–1966. doi: 10.1128/AAC.01494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Morrey JD, Blatt LM, Shafer K, Sidwell RW. Comparison of the inhibitory effects of interferon alfacon-1 and ribavirin on yellow fever virus infection in a hamster model. Antiviral Res. 2007b;73:140–146. doi: 10.1016/j.antiviral.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Shafer K, Smee DF, Morrey JD, Furuta Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob Agents Chemother. 2009;53:202–209. doi: 10.1128/AAC.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TH, Chen YL, Knox JE, Lim SP, Ma NL, Patel SJ, Sampath A, Wang QY, Yin Z, Vasudevan SG. Finding new medicines for flaviviral targets. Novartis Found Symp. 2006;277:102–114. discussion 114-109, 251-103. [PubMed] [Google Scholar]
- Lawitz E, Nguyen T, Younes Z, Santoro J, Gitlin N, Mceniry D, Chasen R, Goff J, Dieterich D, Knox S, Kleber K, Belanger B, Brown NA. Clearance of HCV RNA with valopicitabine (NM) plus peg-interferon in treatment-naive patients with HCV-1 infection: results at 24 and 48 weeks. J Hepatol. 2007;46:S9. [Google Scholar]
- Liu-Young G, Kozal MJ. Hepatitis C protease and polymerase inhibitors in development. AIDS Patient Care STDS. 2008;22:449–457. doi: 10.1089/apc.2007.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 2009;5:e1000614. doi: 10.1371/journal.ppat.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP. Yellow fever as an endemic/epidemic disease and priorities for vaccination. Bull Soc Pathol Exot. 2006;99:341–347. [PubMed] [Google Scholar]
- Monath TP. Treatment of yellow fever. Antiviral Res. 2008;78:116–124. doi: 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Monath TP, Brinker KR, Chandler FW, Kemp GE, Cropp CB. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am J Trop Med Hyg. 1981;30:431–443. doi: 10.4269/ajtmh.1981.30.431. [DOI] [PubMed] [Google Scholar]
- Murakami E, Bao H, Ramesh M, Mcbrayer TR, Whitaker T, Micolochick Steuer HM, Schinazi RF, Stuyver LJ, Obikhod A, Otto MJ, Furman PA. Mechanism of activation of beta-D-2′-deoxy-2′-fluoro-2′-c-methylcytidine and inhibition of hepatitis C virus NS5B RNA polymerase. Antimicrob Agents Chemother. 2007;51:503–509. doi: 10.1128/AAC.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami E, Niu C, Bao H, Micolochick Steuer HM, Whitaker T, Nachman T, Sofia MA, Wang P, Otto MJ, Furman PA. The mechanism of action of beta-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine involves a second metabolic pathway leading to beta-D-2′-deoxy-2′-fluoro-2′-C-methyluridine 5′-triphosphate, a potent inhibitor of the hepatitis C virus RNA-dependent RNA polymerase. Antimicrob Agents Chemother. 2008;52:458–464. doi: 10.1128/AAC.01184-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyts J. Selective inhibitors of hepatitis C virus replication. Antiviral Res. 2006;71:363–371. doi: 10.1016/j.antiviral.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Pierra C, Amador A, Benzaria S, Cretton-Scott E, D'amours M, Mao J, Mathieu S, Moussa A, Bridges EG, Standring DN, Sommadossi JP, Storer R, Gosselin G. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J Med Chem. 2006;49:6614–6620. doi: 10.1021/jm0603623. [DOI] [PubMed] [Google Scholar]
- Pierra C, Benzaria S, Amador A, Moussa A, Mathieu S, Storer R, Gosselin G. Nm 283, an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. Nucleosides Nucleotides Nucleic Acids. 2005;24:767–770. doi: 10.1081/ncn-200060112. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench CH. A simple method of estimating fifty percent endpoint. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Sbrana E, Xiao SY, Guzman H, Ye M, Travassos Da Rosa AP, Tesh RB. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am J Trop Med Hyg. 2004;71:306–312. [PubMed] [Google Scholar]
- Sbrana E, Xiao SY, Popov VL, Newman PC, Tesh RB. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus) III. Clinical laboratory values. Am J Trop Med Hyg. 2006;74:1084–1089. [PubMed] [Google Scholar]
- Shannon WM, Arnett G, Westbrook L, Shealy YF, O'dell CA, Brockman RW. Evaluation of carbodine, the carbocyclic analog of cytidine, and related carbocyclic analogs of pyrimidine nucleosides for antiviral activity against human influenza Type A viruses. Antimicrob Agents Chemother. 1981;20:769–776. doi: 10.1128/aac.20.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Barnard DL, Day CW, Smee DF, Bailey KW, Wong MH, Morrey JD, Furuta Y. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother. 2007;51:845–851. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Huffman JH. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971;22:797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuyver LJ, Mcbrayer TR, Tharnish PM, Clark J, Hollecker L, Lostia S, Nachman T, Grier J, Bennett MA, Xie MY, Schinazi RF, Morrey JD, Julander JG, Furman PA, Otto MJ. Inhibition of hepatitis C replicon RNA synthesis by beta-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine: a specific inhibitor of hepatitis C virus replication. Antivir Chem Chemother. 2006;17:79–87. doi: 10.1177/095632020601700203. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Guzman H, Da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J Infect Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- Tomori O. Yellow fever: the recurring plague. Crit Rev Clin Lab Sci. 2004;41:391–427. doi: 10.1080/10408360490497474. [DOI] [PubMed] [Google Scholar]
- Toniutto P, Fabris C, Bitetto D, Fornasiere E, Rapetti R, Pirisi M. Valopicitabine dihydrochloride:a specific polymerase inhibitor of hepatitis C virus. Curr Opin Investig Drugs. 2007;8:150–158. [PubMed] [Google Scholar]
- Tuboi SH, Costa ZG, Da Costa Vasconcelos PF, Hatch D. Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998-2002. Trans R Soc Trop Med Hyg. 2007;101:169–175. doi: 10.1016/j.trstmh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Xiao SY, Zhang H, Guzman H, Tesh RB. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J Infect Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]
- Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, Kondreddi RR, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JY, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi PY. An adenosine nucleoside inhibitor of dengue virus. Proc Natl Acad Sci U S A. 2009;106:20435–20439. doi: 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]