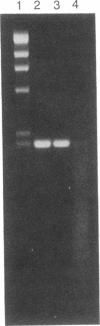
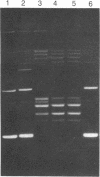
Abstract
Extensive digestion of the covalent intermediate between DNA and Saccharomyces cerevisiae DNA topoisomerase I with trypsin yields a 7-amino acid peptide covalently linked to DNA. Direct sequencing of the DNA-linked peptide identifies Tyr-727 as the active site tyrosine that forms an O4-phosphotyrosine bond with DNA when the enzyme cleaves a DNA phosphodiester bond. Site-directed mutagenesis of the cloned yeast TOP1 gene encoding the enzyme confirms the essentiality of Tyr-727 for the relaxation of supercoiled DNA by the enzyme. From amino acid sequence homology, Tyr-771 and -773 are readily identified as the active site tyrosines of Schizosaccharomyces pombe and human DNA topoisomerase I, respectively. Sequence comparison and site-directed mutagenesis also implicate Tyr-274 of vaccinia virus DNA topoisomerase as the active site residue. There appears to be a 70-amino acid domain near the carboxyl terminus of eukaryotic DNA topoisomerase I and vaccinia topoisomerase, within which the active site tyrosine resides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh T., Ishii K., Suzuki Y., Ikegami Y., Kusunoki Y., Takemoto Y., Okada K. Characterization of a mammalian mutant with a camptothecin-resistant DNA topoisomerase I. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W. R., Ressner E. C., Kates J., Patzke J. V. A DNA nicking-closing enzyme encapsidated in vaccinia virus: partial purification and properties. Proc Natl Acad Sci U S A. 1977 May;74(5):1841–1845. doi: 10.1073/pnas.74.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti M. A., Wang J. C. Expression of yeast DNA topoisomerase I can complement a conditional-lethal DNA topoisomerase I mutation in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8971–8975. doi: 10.1073/pnas.84.24.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S. J., DiNardo S., Voelkel-Meiman K., Sternglanz R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. 1987 Mar 26-Apr 1Nature. 326(6111):414–416. doi: 10.1038/326414a0. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Cozzarelli N. R. A sign inversion mechanism for enzymatic supercoiling of DNA. Science. 1979 Nov 30;206(4422):1081–1083. doi: 10.1126/science.227059. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Evidence for an intermediate with a single-strand break in the reaction catalyzed by the DNA untwisting enzyme. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3488–3491. doi: 10.1073/pnas.73.10.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Dietrich F. S., Fink G. R. Mitotic recombination in the rDNA of S. cerevisiae is suppressed by the combined action of DNA topoisomerases I and II. Cell. 1988 Nov 4;55(3):413–425. doi: 10.1016/0092-8674(88)90027-x. [DOI] [PubMed] [Google Scholar]
- D'Arpa P., Machlin P. S., Ratrie H., 3rd, Rothfield N. F., Cleveland D. W., Earnshaw W. C. cDNA cloning of human DNA topoisomerase I: catalytic activity of a 67.7-kDa carboxyl-terminal fragment. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2543–2547. doi: 10.1073/pnas.85.8.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Eng W. K., Faucette L., Johnson R. K., Sternglanz R. Evidence that DNA topoisomerase I is necessary for the cytotoxic effects of camptothecin. Mol Pharmacol. 1988 Dec;34(6):755–760. [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G. N., Wang J. C. Supercoiling of intracellular DNA can occur in eukaryotic cells. Cell. 1988 Dec 2;55(5):849–856. doi: 10.1016/0092-8674(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Goto T., Laipis P., Wang J. C. The purification and characterization of DNA topoisomerases I and II of the yeast Saccharomyces cerevisiae. J Biol Chem. 1984 Aug 25;259(16):10422–10429. [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987 Apr 15;262(11):5339–5344. [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Keller W., Müller U., Eicken I., Wendel I., Zentgraf H. Biochemical and ultrastructural analysis of SV40 chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):227–244. doi: 10.1101/sqb.1978.042.01.025. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss J., Wang J. C. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddou P., Schmidt U., Knippers R., Richter A. Monoclonal antibodies neutralizing mammalian DNA topoisomerase I activity. Eur J Biochem. 1988 Nov 15;177(3):523–529. doi: 10.1111/j.1432-1033.1988.tb14404.x. [DOI] [PubMed] [Google Scholar]
- Ross W. E. DNA topoisomerases as targets for cancer therapy. Biochem Pharmacol. 1985 Dec 15;34(24):4191–4195. doi: 10.1016/0006-2952(85)90273-4. [DOI] [PubMed] [Google Scholar]
- Shaffer R., Traktman P. Vaccinia virus encapsidates a novel topoisomerase with the properties of a eucaryotic type I enzyme. J Biol Chem. 1987 Jul 5;262(19):9309–9315. [PubMed] [Google Scholar]
- Shuman S., Golder M., Moss B. Characterization of vaccinia virus DNA topoisomerase I expressed in Escherichia coli. J Biol Chem. 1988 Nov 5;263(31):16401–16407. [PubMed] [Google Scholar]
- Shuman S., Moss B. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7478–7482. doi: 10.1073/pnas.84.21.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewey K. M., Chen G. L., Nelson E. M., Liu L. F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182–9187. [PubMed] [Google Scholar]
- Thrash C., Bankier A. T., Barrell B. G., Sternglanz R. Cloning, characterization, and sequence of the yeast DNA topoisomerase I gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4374–4378. doi: 10.1073/pnas.82.13.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Morino K., Uzawa S., Shiozaki K., Yanagida M. Cloning and sequencing of Schizosaccharomyces pombe DNA topoisomerase I gene, and effect of gene disruption. Nucleic Acids Res. 1987 Dec 10;15(23):9727–9739. doi: 10.1093/nar/15.23.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Ohkura H., Adachi Y., Morino K., Shiozaki K., Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987 Sep 11;50(6):917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P. DNA topoisomerases: enzymes that control DNA conformation. Curr Top Microbiol Immunol. 1985;114:19–102. doi: 10.1007/978-3-642-70227-3_2. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Recent studies of DNA topoisomerases. Biochim Biophys Acta. 1987 Jun 6;909(1):1–9. doi: 10.1016/0167-4781(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Worland S. T., Wang J. C. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J Biol Chem. 1989 Mar 15;264(8):4412–4416. [PubMed] [Google Scholar]
- Zhang H., Wang J. C., Liu L. F. Involvement of DNA topoisomerase I in transcription of human ribosomal RNA genes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1060–1064. doi: 10.1073/pnas.85.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumstein L., Wang J. C. Probing the structural domains and function in vivo of Escherichia coli DNA topoisomerase I by mutagenesis. J Mol Biol. 1986 Oct 5;191(3):333–340. doi: 10.1016/0022-2836(86)90130-0. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Silberman L., Estey E. Intercalator-induced, topoisomerase II-mediated DNA cleavage and its modification by antineoplastic antimetabolites. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1041–1047. doi: 10.1016/0360-3016(86)90222-1. [DOI] [PubMed] [Google Scholar]