Abstract
The effects of Triton X-100 on the olfactory epithelium (OE) of adult zebrafish were examined to study neuronal turnover in this model system. Fish were killed at various time points after detergent application and stained with hematoxylin and eosin to examine olfactory structures, immunocytochemistry to examine cell types, or DiI to examine connections to the olfactory bulb. A significant decrease in epithelial thickness of treated sides was observed 1-day posttreatment. Epithelium thickness recovered by 5 days. The most significant reduction in the OE following Triton X-100 treatment corresponded to the region of supporting cells and mature olfactory sensory neurons. Labeling for all neurons with anti-Hu and for the 3 sensory neuron subtypes of the zebrafish OE (ciliated, microvillous, and crypt neurons) diminished 1 day after lesion and returned by 5 days posttreatment. Retrograde labeling from the olfactory bulb showed that the majority of mature olfactory sensory neurons disappeared in 1 day and reappeared by 5 days after treatment. Anti-proliferating cell nuclear antigen was used to show mitotic activity, and after chemical lesion, there was an increase in proliferation in specific regions of the OE. Thus, chemical ablation causes temporary reduction with swift regeneration of the OE occurring within a week.
Keywords: chemical lesion, immunocytochemistry, neuronal turnover, olfactory sensory neurons, teleost
Introduction
The olfactory epithelium (OE) is renowned for its neuronal turnover, with olfactory sensory neurons continually replaced, both in response to injury and under physiological conditions (Farbman 2000). The OE is made of mature and immature olfactory sensory neurons, basal cells, and supporting cells. Immature neurons and basal cells make up approximately 37% of the total cell population in mammals (Mackay-Sim and Kittel 1990), and cells increase in maturity as they proceed apically (Graziadei and Metcalf 1971). An olfactory sensory neuron is considered mature when it expresses olfactory marker protein (Farbman and Margolis 1980) and possesses a dendritic knob (Hinds and McNelly 1981). The life span of the olfactory sensory neuron can vary from as short as 1 month (Graziadei and Monti Graziadei 1979), 3 months in mice kept in normal laboratory settings (Mackay-Sim and Kittel 1990), to 12 months in a pure air setting (Hinds et al. 1984).
The zebrafish olfactory system presents a tractable model for examining neural regeneration for many reasons. First, fish are notable in their abilities to regenerate neural structures both naturally and in response to injury (Zippel 1993; Otteson and Hitchcock 2003; Becker CG and Becker T 2008). Second, there are many similarities in cell types and organization between zebrafish and higher vertebrates (Byrd and Brunjes 1995). Third, the accessibility of olfactory structures and its manageable number of cells make it well suited for experimental analysis.
The organization of the zebrafish olfactory system is similar to that of other teleosts. There is a pair of olfactory organs open to the environment and a pair of olfactory bulbs found at the rostral part of the brain. The peripheral olfactory rosettes, so called in fish because of their unique structure, are composed of several lamellae that project off of a single, central raphe. The rosettes contain both nonsensory and sensory regions. The sensory epithelium is found generally in the central portions of the rosettes, which includes the medial extent of each lamella (Byrd and Brunjes 1995; Barth et al. 1996; Weth et al. 1996). This is a pseudostratified, columnar epithelium with supporting cells, basal cells, and sensory neurons (Byrd and Brunjes 1995). There are 3 types of olfactory sensory neurons found in the zebrafish: ciliated, microvillous, and crypt neurons (Hansen and Zeiske 1998; Hamdani and Doving 2007). There is no clear pattern of arrangement of neurons around the OE (Hansen and Zeiske 1998), and they are packed very closely together with supporting cells (Byrd and Brunjes 1995). As in other animals, the proliferative zone of the zebrafish OE is the basal layer, as determined using the thymidine analog bromodeoxyuridine (Byrd and Brunjes 2001; Oehlmann et al. 2004) and an antibody to proliferating cell nuclear antigen (PCNA; Bravo and Macdonald-Bravo 1987). Specifics of neuronal turnover in this species are unknown.
A variety of methods have been used in many animal species to investigate the ability of the OE to recover from damage. There is a history of using chemicals to lesion the olfactory organs in rodents, as the exposed nature of the OE makes it susceptible to damage by externally applied agents. In some studies, rodents were subjected to intranasal irrigation with zinc sulfate (Burd 1993; Herzog and Otto 1999), which is directly toxic to the OE. This treatment is followed immediately by periods of epithelial and axonal degeneration, with incomplete but extensive recovery of the OE after 30 days. The detergent Triton X-100 (Nadi et al. 1981; Baker et al. 1983; Cummings et al. 2000) and methyl bromide inhalation (Schwob et al. 1999) have also been used in rodents to degrade the OE and show similar effects. These types of analyses are sparse in other models, such as teleosts, although Triton X-100 and zinc sulfate have been applied to the olfactory organs of catfish (Cancalon 1982, 1983a). At low doses, Triton X-100 solubilizes receptor proteins and only the sensory region is affected in catfish. At high doses, both sensory and nonsensory regions are affected. The apical portions of the OE are destroyed, and regeneration does not occur until after approximately 2 months recovery. The effects of zinc sulfate on the catfish OE are similar to the results of the high dosage of Triton X-100.
Despite its popularity as a model, OE regeneration studies have not been done in zebrafish. In the current study, zebrafish were subjected to intranasal irrigation with the detergent Triton X-100 in order to determine the timing and extent of OE regeneration. These findings offer a new tool of study in a highly regenerative model.
Materials and methods
Adult zebrafish, Danio rerio, approximately 4–5 cm in length, aged at least 5 months, and obtained from a commercial vendor were used. They were maintained in 28.5 °C aerated tanks of conditioned freshwater and fed freshwater flake food twice daily. All experimental and animal care protocols were approved by the Institutional Animal Care and Use Committee.
Chemical lesions
Fish were anesthetized with tricaine (0.03% MS222; Sigma) until they no longer responded to tail pinch. Fish were removed from water during the chemical application period. To ensure that there would be no leakage of the chemical to the control naris, petroleum jelly was placed between the 2 nares. A pulled glass capillary tube was placed through the skin opening of the naris and approximately 1 μL of a solution of 0.7% Triton X-100 and 0.15% methylene blue in 0.1 M phosphate buffered saline (PBS) was applied to right naris. The solution remained in contact with the naris for 2 min during which fish were placed on ice to maintain the proper level of anesthesia. Afterward, fish were moved to a recovery tank where detergent and anesthesia were washed away as the fish began swimming. The left naris was untreated as an internal control to allow for comparison. Fish were killed immediately or 1–5, 7, or 14 days later. For the vehicle-treated control group, right nares were treated with a solution of methylene blue (0.15%) and PBS only and killed after 1 or 5 days. Unoperated control animals and internal control (left) sides of treated animals received no treatment.
Tissue processing
Fish were euthanized by overdosing in 0.03% MS222 until cessation of opercular movement. They were perfused transcardially with 4% paraformaldehyde or immersed in Bouin's fixative and fixed for 2 h at room temperature or 20 h at 4 °C. Heads were decalcified with an acid (RDO; Apex Engineering Corporation Products) for 1.5 h at room temperature or with 20% ethylenediaminetetraacetic acid for 36 h at 4 °C, dehydrated through an ascending ethanol series, and embedded in paraffin. Sections at 10 or 20 μm were adhered to positively charged slides and left at 37 °C for 24 h.
Quantitative analysis of epithelial thickness
Slides containing semi-serial horizontal sections of tissue were stained with hematoxylin and eosin following standard protocols. Spot Advanced digital camera software was used for quantitative analyses. OE depth was measured from basement membrane to apical surface, not including cilia length, at ×100. Three measurements were taken from every other lamella in the rosette and averaged for that section. Data from each semi-serial horizontal section were averaged for a mean epithelial thickness of each olfactory organ. The percent difference in thickness between the 2 olfactory organs of each animal was calculated in order to compare the treated side with the internal control side. Unpaired t-tests and analysis of variance (ANOVA) with the Tukey test for multiple comparisons were performed for analysis of changes in epithelial thickness. P < 0.05 was considered significant. N = 3 for each time point.
Immunocytochemistry
Paraffin-embedded sections were dewaxed with xylenes, rehydrated with descending ethanol solutions, and subjected to antigen retrieval for 10 min in 10 mM citrate solution at 95 °C. Slides were then blocked for 1 h with 1% bovine serum albumin in PBS and then incubated in primary antibody for 2 h at room temperature or 24 h at 4 °C. Antibodies used were monoclonal α-Hu (1:100; Molecular Probes), polyclonal α-S100 (1:1000; Dako), polyclonal α-calretinin (1:1000; Santa Cruz), polyclonal α-Gα s/olf (1:1000; Santa Cruz), or monoclonal α-PCNA (1:1000; Sigma). Slides were then rinsed with PBS, incubated in biotinylated secondary antibody diluted in blocking solution (1:100; Dako) for 1 h at room temperature, immersed in an avidin–biotin peroxidase solution (ABC Vectastain Elite, Vector Labs) for an hour, rinsed in PBS followed by 0.5 M Tris-buffered saline, and then reacted with diaminobenzidine (DAB kit, Vector Labs) for visualization.
Densitometry
Image J software was employed to analyze levels of immunoreactive labeling of α-Hu immunocytochemistry. Images taken at ×10 were converted to 8-bit gray scale, and average gray values in the area of interest were calculated. Gray values were converted to optical density (OD) by the following formula: OD = log (intensity of background/intensity of area of interest). For each group, mean OD of each region of interest was calculated by averaging the values from each of the 3 animals. Because α-Hu labeling is found throughout the sensory epithelium, the area containing the entire epithelium was traced and the OD was measured from that area. This was done at 2 different depths of the olfactory organ for each animal to account for potential differences in populations found at different depths of the rosette. Three animals were measured from unoperated control, 1 and 5 days post Triton X-100, and 1 and 5 days post-vehicle treatment groups. Paired t-tests were used to compare treated and untreated sides within groups and ANOVA with the Tukey test allowed comparisons between groups. P < 0.05 was considered significant. OD was used for these analyses rather than cell counts because it was thought to be more accurate due to the closely packed nature of the labeled cells.
Counts of mitotic profiles
For evaluation of α-PCNA immunoreactivity, labeled profile counts were performed on 3 regions of the olfactory rosette of unoperated and treated fish: the trough OE, side OE, and nonsensory epithelium regions. The trough OE area is defined as the portion of the OE along the central raphe. The side OE is defined as the medial extent of the lamellae. The nonsensory epithelium is the lateral extent of the lamellae. The total number of immunoreactive profiles in an area of 25 × 50 μm was counted from 3 different trough OE regions, 3 different side OE regions, and 3 different nonsensory epithelium regions from a horizontal section using a grid reticle. This was done for 3 unoperated control animals and 3 treated animals at each of the following time points post Triton X-100 lesion: 1, 2, 3, 4, and 5 days. ANOVA with the Tukey test was used for group comparisons. P < 0.05 was considered significant.
Retrograde tract tracing with DiI
Fish were euthanized with 0.03% MS222 and perfused transcardially with 4% paraformaldehyde. Heads were fixed overnight in the fixative and then dissected to reveal the olfactory bulbs. Approximately 1 μL of a 5 mg/mL solution of DiI (Invitrogen) in dimethylformamide was pressure injected into both olfactory bulbs, the heads were returned to the fixative solution, and the dye was allowed to transport for 5 days at 37 °C. Olfactory rosettes were dissected, mounted in agar, and viewed with a Zeiss LSM510 confocal microscope.
Results
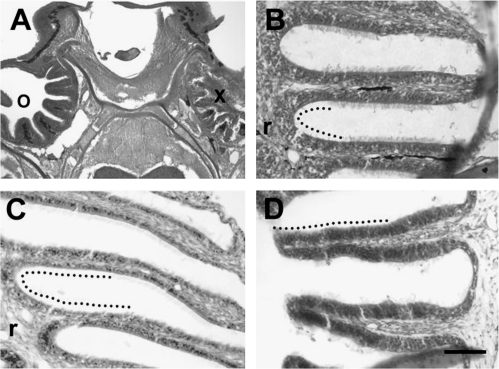
The effect of Triton X-100 application on the OE of zebrafish was considerable but transient. Various morphological changes were observed in hematoxylin and eosin-stained sections of tissue following application of Triton X-100 to the right rosette of adult zebrafish. Immediately after detergent application, the lamellae were severely disrupted and cellular debris littered the nasal cavity (Figure 1A). The contralateral rosette, which was not exposed to Triton X-100 and served as an internal control, had the typical morphology of the zebrafish OE. The effects on the treated side appeared to be specific to the chemical lesion because vehicle-treated control animals had no observable changes in structure of the rosette (Figure 1B). Six of 12 animals showed a noticeable thinning of the OE 1 day following Triton X-100 application (Figure 1C). The remaining animals exhibited fusion of the rosette as described in detail below. There is thinning of the nonsensory area of lamellae, and the long cilia, characteristic of nonsensory cells, were present in only scattered areas of all 6 nonfused rosettes. The epithelial structure appeared to return to near-normal conditions by 5 days after the chemical lesion (Figure 1D).
Figure 1.
Analysis of hematoxylin and eosin-stained horizontal sections through the whole head showed obvious morphological changes in the OE following Triton X-100 treatment. (A) Immediately after Triton X-100 was applied to the olfactory organ, the integrity of the right OE (x) was drastically compromised, with cellular debris visible throughout the nasal cavity. The left olfactory organ was not affected (o), and the multiple lamellae that make up the rosette were present and intact. (B) Vehicle-treated olfactory organs showed no alterations in lamellar morphology or epithelial structure 1 day after application. An example of an area of sensory epithelium adjacent to the central raphe (r) is shown with a dotted line. (C) One day following Triton X-100 application, the depth from the apical surface to the basal membrane of the OE appeared reduced. A dotted line marks an area of OE, which includes troughs adjacent to the central raphe (r) and the medial portion of lamellae. (D) By 5 days posttreatment, the epithelium no longer appeared thinned. The sensory epithelium, indicated by a dotted line, is found in this section on the portion of lamellae medial to the central raphe (to the left in the micrograph but not present in this section). Scale bar = 200 μm for A, 50 μm for B–D.
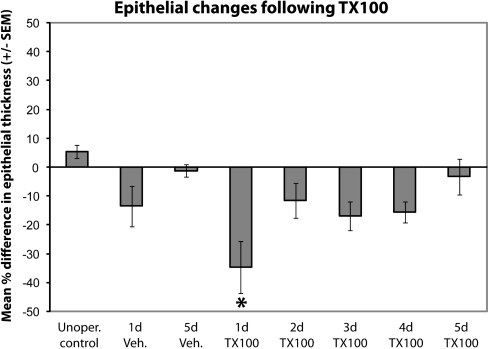
Measurements of epithelial depth were made and compared between unoperated control, vehicle-treated control, and Triton X-100-treated fish. Unoperated control animals had a 5% (±2%) difference between the left and right rosettes. Vehicle-treated control animals had a reduction in OE thickness of 14% (±7%) at 1 day and 1% (±2%) at 5 days, which was not significant but noticeable at the earlier time point. Animals processed 1 day after Triton X-100 application had sensory epithelium in the treated rosette that was significantly thinner than the internal control, a reduction of 35% (±9%). This difference steadily decreased over time, and measurements of OE depth were back to control levels (−3 ± 6%) by approximately 5 days (Figure 2). T-test analyses revealed a significant decrease in thickness on the treated side compared with the untreated side of the 1-day group (P = 0.04). Significant differences were not noted for unoperated control (P = 0.35), vehicle-treated control (P = 0.34 at 1 day; P = 0.88 at 5 days), 2 day (P = 0.28), 3 day (P = 0.06), 4 day (P = 0.22), or 5 day groups (P = 0.70). ANOVA confirmed that the percent difference in thickness between the internal control and treated OE of the 1 day postdetergent application group was different from unoperated control and 5 days postlesion groups (P = 0.01). Because the 2-day, 3-day, and 4-day–treated groups were not statistically different from any group, this likely suggests a gradual recovery where these time points represent intermediate stages between lesioned and recovered epithelia.
Figure 2.
Quantitative analysis of olfactory epithelial thickness following Triton X-100 application allowed comparison of epithelial changes. The percent difference in thickness of the OE between the internal control and treated sides of the 1 day post-detergent application group was confirmed by ANOVA to be different from unoperated control, 5 days post-vehicle, and 5 days post-lesion groups. For each group n = 3 and P < 0.05 was considered significant.
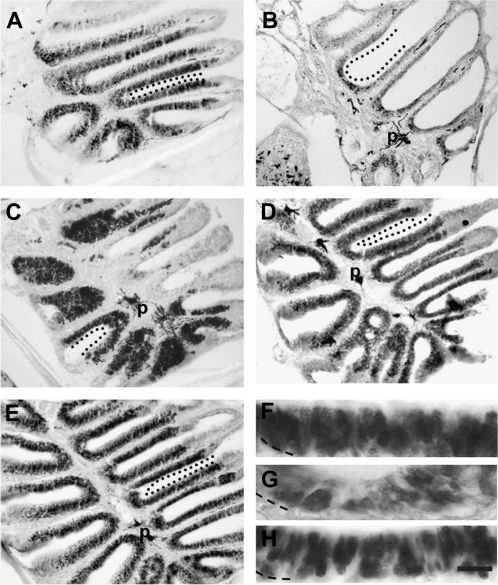
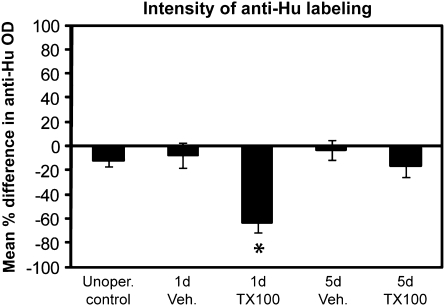
In order to determine whether the recovery in epithelial depth was due to recovery of neurons, an antibody to Hu, a marker of mature and immature neurons (Marusich et al. 1994; Byrd and Brunjes 2001), was used at different stages after ablation. In control animals, α-Hu labeling was found heavily throughout the sensory regions of the olfactory rosette (Figure 3A,F). One day following chemical lesion, α-Hu labeling identified few neurons (Figure 3B,G). By 5 days, α-Hu levels of the treated side were close to normal levels (Figure 3C,H). Immunocytochemistry of animals viewed 7 (Figure 3D) and 14 days (Figure 3E) postablation showed α-Hu levels remained steady over time. Percent differences in OD levels between treated and untreated sides were calculated (Figure 4), and statistical analyses were performed. Paired t-tests revealed that there was no significant difference in α-Hu immunoreactivity between the left (0.14 ± 0.03) and right (0.12 ± 0.02) sides of unoperated animals (P = 0.104), meaning there is no inherent difference in anti-Hu labeling and therefore neuronal content between the left and right rosette. Also, there was no significant effect of vehicle treatment on anti-Hu labeling at 1 day (P = 0.35) and 5 days (P = 0.48) postapplication. There was a significant decrease in α-Hu immunoreactivity on the treated side (0.03 ± 0.01) compared with the control side (0.08 ± 0.02) 1-day post-detergent application (P = 0.038), but there was no significant difference 5 days later (P = 0.109) when treated (0.11 ± 0.01) and control (0.13 ± 0.01) sides were compared. ANOVA results confirmed that there was a significant decrease in the population of neurons after detergent application that was recovered by 5 days because the percent difference in OD at 1 day (−63.2 ± 5.5%) was significantly different from unoperated controls (−11.7 ± 5.5%) and 5-day (−16.3 ± 9.1%) groups (P = 0.0005).
Figure 3.
Examination of distribution of mature and immature neurons using α-Hu analysis. (A) Heavy labeling for α-Hu was present in the sensory regions of the control rosette. (B) One day following chemical ablation of the OE, there were few α-Hu immunoreactive profiles in the olfactory rosette. (C) By 5 days after Triton X-100 treatment, the amount of α-Hu labeling had increased dramatically throughout the sensory areas of the rosette. Seven (D) and 14 (E) days after detergent treatment, Hu-positive profiles populated the entire OE. Control (F), 1d (G), and 5d (H) α-Hu labeled OEs at higher magnification show cellular distribution of the antibody label. Basement membrane is marked with a dashed line. Demarcated with dotted line (A–E) are areas of sensory epithelium along lamellae. p = pigment of the lamina propria in the central raphe and is not indicative of antibody labeling. Scale bar = 100 μm for A–E and 10 μm for F–H.
Figure 4.
Quantitative analysis of α-Hu OD allowed evaluation of changes to numbers of neurons present in epithelium. Percent differences in OD levels between treated and untreated nares of 3 animals from each group were calculated, and ANOVA was employed to confirm differences (P < 0.05 was considered significant). There was a significant difference in OD 1 day post Triton X-100 compared with unoperated controls and 5 day groups.
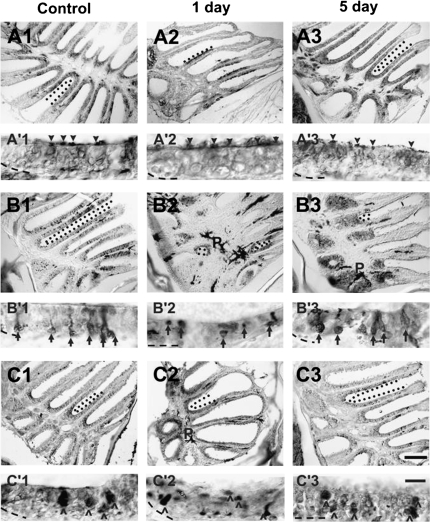
To investigate whether the 3 different neuron subtypes of the zebrafish OE had differential responses to the procedure, antibodies were used to recognize each. Because epithelial thickness was most affected 1 day after Triton X-100 application and returned to normal levels by 5 days posttreatment, these time points were examined. Antibody to Gα s/olf identified ciliated olfactory sensory neurons (Figure 5A1–3, A′1–3) (Jones 1990; Belanger et al. 2003; Hansen et al. 2003; Wakabayashi and Ichikawa 2008). Antibodies to calcium-binding proteins, calretinin (Figure 5B1–3, B′1–3) and S100 (Figure 5C1–3, C′1–3), were used to label microvillous and crypt neurons, respectively, as previously shown in zebrafish (Germana et al. 2007). In all 3 cases, labeling of the antibody was decreased in rosettes processed 1 day after Triton X-100 compared with unoperated and internal controls but returned to near control levels by 5 days posttreatment. In control tissue, Gα s/olf labeled throughout the sensory epithelium of the olfactory rosette. At higher magnification, it is apparent that the antibody strongly labeled dendritic knobs and cilia (Figure 5A′1, arrowheads) and also was present diffusely throughout the depth of the sensory epithelium, excluding the area right above the lamina propria, which would be the region of the basal cells. In tissue from the 1-day post Triton X-100 group, there were few areas of the rosette that labeled with Gα s/olf (Figure 5A2), and in those areas, dendritic knobs were stained (Figure 5A′2, arrowheads), but the epithelium underneath labeled only faintly with the antibody. By 5 days, the labeling for ciliated neurons was again found throughout the sensory areas of the rosette (Figure 5A3), and the dendritic knobs and cilia as well as the epithelium underneath were labeled by the antibody (Figure 5A′3, arrowheads), again except for the region right above the lamina propria. Labeling for microvillous neurons using antibody to calretinin was also found throughout the sensory epithelium in control tissue (Figure 5B1), and α-calretinin stained cell somas as well as processes (Figure 5B′1, arrows). In the tissue of 1-day post-lesion animals, α-calretinin labeled a few areas of cells but was largely absent throughout the organ. In the areas that contained label, it appeared as though cell bodies were labeled, but there were no processes off of the soma visible as was seen in control tissue (Figure 5A′2, arrows). By 5 days, though the structure of the rosette was not completely recovered, there were large areas of α-calretinin labeling (Figure 5B3), and cell bodies as well as their dendritic knobs were labeled as in control tissue (Figure 5B′3, arrows). Similarly, α-S100 labeling was found throughout the sensory epithelium (Figure 5C1), and labeled large round cells ′(Figure 5C′1, ^), which is the morphology expected of crypt neurons. In the 1 day group, the cell bodies labeled by S100 antibody appeared smaller and flattened compared with control and were found at various levels of the epithelium (Figure 5C′2, ^), and staining levels were decreased throughout the rosette (Figure 5C2). These may be crypt neurons with altered morphology. The somas labeled in the 5-day group were rounder and bigger (Figure 5C′3, ^), similar to control, and once again found throughout the sensory portions of the olfactory rosette (Figure 5C3).
Figure 5.
Antibodies were used to characterize the effects of chemical ablation on specific cell types of the zebrafish OE. (A) Anti-Gα s/olf in control epithelia (A1) revealed immunoreactive profiles throughout the sensory region of the rosette. Dendritic knobs and cilia were distinctly labeled (A′1, arrowheads), and there was staining found diffusely through the depth of the epithelium except the basal region. One day after Triton X-100 application, there were fewer Gα s/olf -positive areas in the epithelium (A2), but where labeling occurred, the dendritic knobs and cilia of ciliated neurons were marked (A′2) with only faint labeling of the epithelium underneath. Five days after chemical lesion (A3), however, α-Gα s/olf staining had returned to normal levels and pattern (A′3). (B) Anti-calretinin labeling in control olfactory organs identified discrete cell bodies and their dendritic processes (B′1, arrows) scattered throughout the OE (B1). Immunoreactivity to this cell marker was greatly diminished 1 day following Triton X-100 treatment (B2), and those cells labeled appeared to be without dendritic knobs (B′2). Labeling to anti-calretinin was increased by 5 days after treatment (B3), and the bodies that were labeled also had obvious processes (B′3). (C) Anti-S100 labeled large round cells (^ in C′1), the crypt neurons, scattered around the sensory regions in the OE of control rosettes (C1). S100-immunoreactivity showed an overall decrease 1 day after treatment (C2), and the bodies were smaller and flattened compared with control (C′2). Immunoreactivity recovered to near control levels (C3) and morphology (C′3) 5 days after lesioning. Demarcated with dotted lines are examples of areas where labeling was present within the sensory epithelium. p = pigment of the lamina propria in the central raphe and is not indicative of antibody labeling. Basement membrane is marked with a dashed line. Scale bar = 100 μm for A1–3, B1–3, C1–3 and 10 μm for A′1–3, B′1–3, C′1–3.
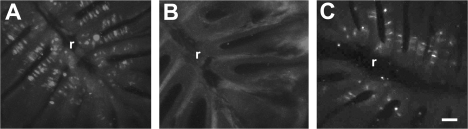
In order to confirm that the loss of cell-specific labeling at 1 day following detergent application and the return of labeling by 5 days after treatment were due to the loss and recovery of mature olfactory sensory neurons, retrograde tract tracing was performed. DiI was applied to the olfactory bulbs and allowed to transport to the OE. Only those cells in the OE with axonal connections to the olfactory bulb were labeled. Control animals had numerous DiI-labeled cells throughout the rosette (Figure 6A). Fish examined 1 day after Triton X-100 application showed few, if any, labeled cells, indicating a loss of mature neurons with axons to the bulb (Figure 6B). Recovery was evident in the 5-day group (Figure 6C), where numerous DiI-labeled cells were present.
Figure 6.
Retrograde labeling from the olfactory bulb using DiI showed numerous mature olfactory sensory neurons throughout the rosette of control animals (A). There was little or no DiI labeling in the rosettes of animals examined 1 day after Triton X-100 treatment (B), but there were numerous DiI-labeled cells 5 days after detergent application (C). r = central raphe. Scale bar = 50 μm for all.
Recovery of the OE after chemical treatment likely involves increased proliferation of stem cells that replenish the ablated cells. To compare mitotic activity following Triton X-100 exposure, antibody to PCNA was used. PCNA immunoreactivity was at high levels in discrete regions of the rosettes of unoperated controls and in the internal control sides of treated animals: the troughs of the OE adjacent to the central raphe and the nonsensory epithelium on the lateral extent of the lamellae had numerous mitotic profiles throughout the depth of the epithelium (Figure 7A). The areas of OE on the medial extent of the lamellae, which we call the side OE, had few labeled cells, and these were restricted to the basal region of the epithelium. This labeling pattern is analogous to BrdU labeling of zebrafish OE reported in a study by Oehlmann et al. (2004). Immediately after Triton X-100 application, the OE showed significant degradation, with loss of most of the apical region of the epithelium, and almost all remaining cells were stained with anti-PCNA (Figure 7B). Analysis at higher magnification allowed comparison of proliferation patterns. The trough OE region of unoperated animals had numerous labeled cells throughout the depth of the epithelium (Figure 7C1). One day after detergent treatment, this region was thinner and almost every cell present was positive for anti-PCNA (Figure 7C2), whereas 5 days after treatment, the trough OE appeared similar to controls (Figure 7C3). The side OE region had few PCNA-positive cells in unoperated controls, and the labeled cells were present only in the basal region of the epithelium (Figure 7D1). This region of the 1-day group was notably different, with anti-PCNA labeling present in almost every cell remaining in the thinned epithelium (Figure 7D2). By 5 days after Triton X-100 treatment, the side OE was back to control levels of mitotic activity (Figure 7D3). The nonsensory epithelium showed fairly high levels of anti-PCNA in the control (Figure 7E1), 1 day (Figure 7E2), and 5 day (Figure 7E3) groups.
Figure 7.
Anti-PCNA-immunoreactivity was observed in unoperated animals (A) in the basal region of the OE throughout the rosette, with higher levels of staining in the troughs between lamellae (longer arrow) as well as in the nonsensory regions (arrowhead). The side OE on the medial extent of the lamellae (shorter arrow) had less labeling. (B) Immediately after Triton X-100 application, significant damage to the apical regions of the epithelium throughout the rosette was apparent. Almost all the remaining cells were anti-PCNA positive. Higher magnification images of the trough OE (C), side OE (D), and nonsensory (E) regions of unoperated controls (C1, D1, E1), 1-day treated (C2, D2, E2), and 5-day treated (C3, D3, E3) animals revealed different patterns of cell proliferation. Control animals had numerous PCNA-positive profiles throughout the trough region (C1), few in the side OE region (D1), and many in the nonsensory region (E1). One day after detergent treatment, the epithelium was thinner, but the remaining cells in all 3 regions were heavily labeled for anti-PCNA (C2, D2, E2). Five days after detergent application, epithelial thickness returned and the pattern of antibody labeling was similar to controls (C3, D3, E3). Basement membrane is marked with a dashed line. Scale bar = 100 μm for A, B and 10 μm for C1–E3.
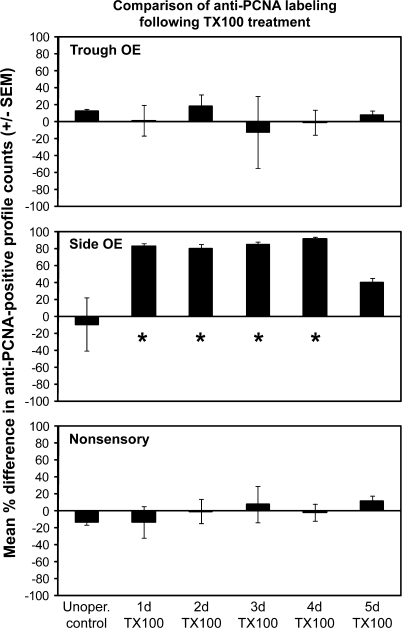
Counts of anti-PCNA–immunoreactive mitotic profiles in control animals revealed that the trough OE and nonsensory regions have higher levels of mitotic activity than the side OE. In a 25 × 50 μm area, unoperated controls had an average of 24.2 ± 1.7 (left side) and 27.0 ± 1.3 (right side) labeled profiles in the trough OE, an average of 18.4 ± 2.3 (left side) and 14.9 ± 1.0 (right side) labeled profiles in the nonsensory region, and an average of 3.5 ± 1.1 (left side) and 3.5 ± 1.2 (right side) labeled profiles in the side OE. There was no difference between the left (internal control) and right (treated) sides at any of the time points (Figure 8). The side OE region, however, showed a significant increase in the percent difference in anti-PCNA labeling in the 1-day, 2-day, 3-day, and 4-day–treated animals (Figure 8) compared with unoperated controls and the 5 day treated group.
Figure 8.
Quantitative analysis of anti-PCNA allowed comparison of mitotic activity in unoperated animals and animals allowed to survive for various time periods following Triton X-100 treatment. The percent differences in average number of anti-PCNA labeled profiles in a 25 × 50 μm region of the trough OE, side OE, and nonsensory regions of right and left olfactory organs were compared with ANOVA. There was a significant difference in the side OE region at 1d, 2d, 3d, and 4d postdetergent application. For each group n = 3 and P < 0.05 was considered significant.
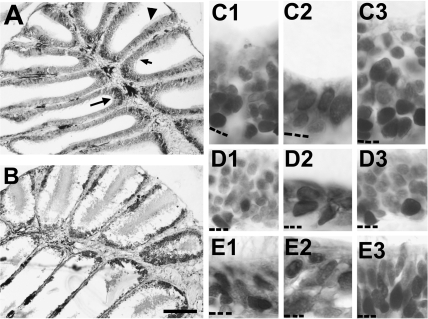
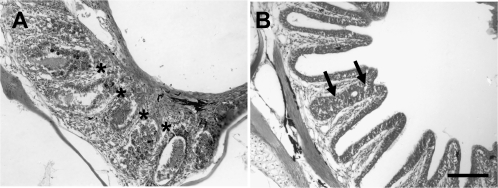
There were other morphological changes that were frequently observed when the rosette was exposed to Triton X-100. In some animals, the lamellae were completely fused together (Figure 9A) and the epithelium was altered. This was observed in 6 of 12 fish examined at 1-day postlesioning and 1 of 6 at 2 days following detergent application. This type of severe fusion was not apparent at later time points. None of the fish examined 3 days (n = 3), 4 days (n = 4), or 5 days (n = 12) after Triton X-100 showed this abnormality. However, there were instances of partial fusion between adjacent lamellae observed between 3 and 5 days after treatment (Figure 9B).
Figure 9.
(A) Some additional morphological abnormalities were sometimes observed following Triton X-100 treatment. At survival times of 1 or 2 days, changes to the structure of the olfactory rosette were observed occasionally, such as complete fusion of lamellae. Although lamellar morphology was destroyed, remnants of olfactory tissue were still visible (asterisks). (B) Sometimes seen at later time points was fusion between 2 adjacent lamellae (arrows). Scale bar = 100 μm for both.
Discussion
Intranasal irrigation with Triton X-100 causes severe and immediate effects on the OE of adult zebrafish. The detergent solubilizes cell membranes, and cellular contents flood the nasal cavity and are presumably washed away. One day after detergent exposure, 2 morphologies result. Either the OE is significantly reduced in thickness or the lamellae are fused. Both morphologies show the presence of few mature neurons, as indicated by the low levels of staining for antibodies and retrograde tract tracing. The labeling that remained most likely indicates cells that did not get exposed to sufficient amounts of detergent rather than cells that have already grown into mature neurons after lesioning occurred. Our method of application involved applying a bolus of the detergent and letting it remain in contact with the rosette for 2 min before returning the fish to water, which flushed the detergent away. During the time of exposure, gravity would cause the detergent to settle into the rosette and allow for variable levels of exposure to the chemical. The nonsensory epithelium also can be affected and appears thinner with cilia present in only a few regions.
We used antibodies to identify the cell types present at various time points following detergent application. The cell markers that we used have been used extensively in other applications. Labeling for the neuronal marker anti-Hu is sparse in animals examined 1 day following detergent exposure but is back to control levels by 5 days postablation. Because our analysis examined the OD of anti-Hu labeling, we cannot distinguish between the possibility that the recovery of labeling for the neuronal marker was due to changes in cell size or in cell number. Immunocytochemistry using anti-Gα s/olf, anti-calretinin, and anti-S100 demonstrated that ciliated, microvillous, and crypt cells (respectively) each return to the OE, suggesting a return to normal functioning. Our retrograde labeling from the olfactory bulb with DiI supports the conclusion that there is loss and recovery of mature olfactory sensory neurons in the 5 days following Triton X-100 exposure. The results of this analysis show that cells with connections to the bulb are not present 1 day after detergent exposure, but they return by 5 days after treatment. This supports the idea that the olfactory sensory neurons do indeed die and are replaced instead of the alternate explanation that the cells changed their phenotype and temporarily lost antigenicity for neuronal markers.
Olfactory sensory neuron turnover is well studied in mammals. The rate of physiological OE turnover was originally traced using tritiated thymidine (3H-TdR) in mice. Labeled cells are initially clustered at the basal membrane before moving apically to near the epithelial surface over the next 20 days (Graziadei and Monti Graziadei 1979). The 2 types of progenitors found at the basal portion of the OE are globose basal cells (GBCs) and horizontal basal cells (HBCs) (Mackay-Sim and Kittel 1991; Schwartz Levey et al. 1991; Murdoch and Roskams 2007). The HBCs sit lower in the basal membrane than the GBCs and label with different markers (Schwartz Levey et al. 1991). In another autoradiographic study of mouse OE following bulbectomy, it was shown that after 3H-TdR pulsing, both GBCs and HBCs pick up the label, but only labeled GBCs migrate apicalward from the basement membrane, and these cells eventually populate the receptor cell layer (Schwartz Levey et al. 1991). This suggests that GBCs are the ones that can be induced to divide after cell death of their targets and their progeny can move up to the area populated by mature neurons to replace lost cells. Indeed, Calof and Chikaraishi (1989) describe a type of immediate neuronal precursor cell that is morphologically distinct from basal cells, which, under appropriate conditions, can be induced to migrate apicalward and divide into 2 neuronal daughters. Additionally, there is evidence that when neurogenesis occurs, it is to maintain the population of neurons that have not completely matured rather than directly replacing mature cells (Walker et al. 1990). These immature neurons are lacking dendrites and thus are not yet functionally mature sensory cells but could quickly replace mature olfactory sensory neurons when needed.
Although not examined in as much detail in fish, it is likely that similar processes occur. For example, Cancalon (1983b) has shown that there are waves of olfactory sensory neuron genesis after axonal lesion of the garfish. The main phase of regeneration constituted 50–70% of the original population of axons and did not start regenerating until 12 days after axonal lesion. A more rapid phase of regeneration, approximately 3–5% of the original population, occurred at a rate 7 times faster than the main phase. The rapid phase of fibers comes from the population of immature neurons growing at the time of injury and capable of quickly maturing. The time course observed by Cancalon is on a different scale than in our study most likely due to the fact that the garfish is substantially larger than zebrafish, and rates were based on growth of axons not by the reconstitution of the OE as in this study.
Our observation that the OE is thinned and not eliminated following detergent application suggests that the deeper layers, containing immature and basal cells, remain after this treatment. This is supported by our finding that immediately after detergent exposure almost all remaining cells are labeled with anti-PCNA. Our finding that anti-Hu is still present 1 day following Triton X-100 application, although at a significantly reduced level, is also consistent with the idea that immature and basal cells remain. Because anti-Hu labels mature and immature neurons (Marusich et al. 1994), and we have shown a loss of mature cells with our DiI experiment, the remaining Hu-immunoreactive cells may be immature neurons or the few mature neurons that were spared. There may even be additional immature neurons not revealed by anti-Hu labeling if they have recently formed and are not expressing Hu protein yet, although this is less likely because anti-Hu has been shown to identify even neuronal precursors (Adolf et al. 2006; Grandel et al. 2006). The consequence of leaving basal and immature cells is that they are available to replenish the epithelium with new sensory cells. We have shown that in the adult zebrafish, this can be done rapidly compared with the time frame reported for mammals. For example, in a similar study using Triton X-100 intranasal application in mice, one week after the chemical lesion turbinates are thinner due to a loss of olfactory receptor neurons and epithelial tissue (Cummings et al. 2000). By 3 weeks post-chemical lesion, some newly formed cells appear, and by 6 weeks, regular patterning returns. Likewise, the main phase of axon regeneration in the garfish did not occur until 12 days postinjury (Cancalon 1983b). It is possible that the gross anatomy of the zebrafish makes it well suited for regenerating injured populations. The length of the olfactory nerve in garfish is approximately 20 cm (Easton 1971), considerably longer than that of the zebrafish, in which the olfactory nerve is approximately 250 μm long (Iqbal T, unpublished data). Thus, just by having a shorter distance to extend axons to their target, zebrafish olfactory sensory neurons are more quickly reestablished. An olfactory sensory neuron is mature when it has contact with the olfactory bulb and has extended a dendrite. It seems possible that the populations of cells in our experiments are replaced so rapidly because there is a population of immature cells unaffected by the detergent that need only extend a dendrite and contact the bulb to be mature.
Our examination of mitotic activity in the OE showed that the epithelium recovers, at least in part, due to increased proliferation of cells. The pattern of proliferating cells in unoperated control animals is consistent with previous reports that used bromodeoxyuridine (Byrd and Brunjes 2001; Oehlmann et al. 2004). There are higher levels of cell genesis occurring in the trough regions of the OE (which Oehlmann et al. [2004] term the “curves” at the apex of lamellae) and in the border between the sensory and nonsensory epithelium than in the side OE area. Damage of the OE with detergent alters this pattern by causing an increase in proliferation in cells of the side OE. This analysis supports the idea that basal cells of the OE replenish the damaged cells by increasing their cell division and forming new cells. The trough OE and nonsensory regions do not show a significant increase in proliferation above control levels, possibly due to the high levels they normally exhibit. Although we did not examine basal cells directly, due to lack of sufficient markers, we show that there is increased proliferation following Triton X-100 treatment. Immediately after exposure to the detergent, much of the OE is destroyed. The remaining cells are almost all immunoreactive for anti-PCNA, suggesting that they are proliferating cells. Thus, a likely explanation is that basal cells replenish the damaged epithelium.
Using our methods of estimating epithelial depth, we found some variability in thickness of the OE of right and left olfactory organs, evidenced by the 5% difference between the 2 sides of unoperated control rosettes. This likely represents natural variability and differences in sampling regions, and it is not statistically significant. Thus, in order for significant differences to be detected in the treated animals, they had to be above the normal range of variance. Our 1-day vehicle-treated control animals had a 14% reduction in OE thickness, which was noticeable but not significant. The reason for this effect is unknown, but there may be an influence of the methylene blue that we used in order to confirm the location of application. Methylene blue has been shown to have neurotoxic effects on the central nervous system (Vutskits et al. 2008). This dye is a nitric oxide synthesis inhibitor (Mayer et al. 1993), and application of a nitric oxide blocker inhibits electro-olfactogram recordings (Zielinski et al. 1996). It is also known that the teleost OE uses nitric oxide (Singru et al. 2003) and that this molecule can regulate OE neurogenesis and neuronal differentiation (Sulz et al. 2009). Thus, the dye used our vehicle may have a slight effect on our sham animals. The effects of the detergent, however, were much more severe.
Although we did not examine physiological responses of the detergent-treated olfactory organs, our results support earlier studies of loss and regaining of behavioral responses following chemical lesion. For example, after administering 1% zinc sulfate to rat pup olfactory epithelia, no behavioral responses were observed after 1 day, but the majority of animals responded 5 days afterward (Stewart et al. 1983). In studies performed in the frog (Adamek et al. 1984) with a lower concentration of detergent flushed over epithelia, the cilia of sensory areas were removed but cells remained intact. Odor-evoked responses were found to occur as early as 18 h after treatment. A study by Cancalon (1983a) showed that detergent application to catfish OE severs cilia and microvilli and regeneration occurred within 4 days. Electrophysiological activity was depleted after detergent application but returned to 50–60% of normal activity levels after just 2 h, suggesting some regeneration of receptors found in cilia. Although it is likely that cilia observed in some instances 1 day after treatment in our study represent regions that were not destroyed by the detergent, these studies support the prediction that the cells that remained after the application of Triton X-100 to the zebrafish OE may have been able to rapidly express cilia after damage.
In conclusion, chemical ablation of the zebrafish olfactory organ using 0.7% Triton X-100 offers a new tool for regeneration and reinnervation studies. Although application of detergent to the OE significantly reduces epithelial thickness, it also appears that Triton X-100 can affect rosette structure. It is unclear whether the fused morphology observed in a few OEs sectioned 1 to 2 days after detergent treatment was due to collapsing of the lamellar structure after connective tissue degeneration or the fusion of disrupted cellular membranes. However, it does indicate another interesting regenerative ability of zebrafish OE; because none of the OEs examined at later time points displayed fused morphology, one can speculate that rosette morphology can recover from Triton X-100 damage as well. The swift thickening of the OE described in this study is possible due to the amount of immature cells and progenitors unharmed after Triton X-100 application. Our Triton X-100 treatment paradigm kills mature neurons and supporting cells of the apical layers but does not penetrate further during the time it is left on the OE before being washed away when the fish is returned to water. This leaves a pool of immediate precursors able to quickly differentiate into the different neurons as well as immature neurons that need only develop a dendrite to become mature neurons. This study provides a foundation for future work on regenerative abilities in this important model system.
Funding
This work was supported by the National Institutes of Health [DC04262 to C.B.J.].
Acknowledgments
We would like to thank the Western Michigan University neuroscience journal club and the manuscript reviewers for helpful comments. We are grateful to T. Paskin for performing the DiI labeling and R. Franklin and J. Bergman for technical assistance.
References
- Adamek GD, Gesteland RC, Mair RG, Oakley B. Transduction physiology of olfactory receptor cilia. Brain Res. 1984;310:87–97. doi: 10.1016/0006-8993(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Adolf B, Chapouton P, Lam CS, Topp S. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;295:278–293. doi: 10.1016/j.ydbio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, Justice NJ, Ngai J. Asynchronous onset of odorant receptor expression in the developing zebrafish olfactory system. Neuron. 1996;16:23–34. doi: 10.1016/s0896-6273(00)80020-3. [DOI] [PubMed] [Google Scholar]
- Becker CG, Becker T. Adult zebrafish as a model for successful central nervous system regeneration. Restor Neurol Neurosci. 2008;26:71–80. [PubMed] [Google Scholar]
- Belanger RM, Smith CM, Corkum LD, Zielinski BS. Morphology and histochemistry of the peripheral olfactory organ in the round goby, Neogobius melanostomus (Teleostei: Gobiidae) J Morphol. 2003;257:62–71. doi: 10.1002/jmor.10106. [DOI] [PubMed] [Google Scholar]
- Bravo R, Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replications sites. J Cell Biol. 1987;105:1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd GD. Morphological study of the effects of intranasal zinc sulfate irrigation on the mouse olfactory epithelium and olfactory bulb. Microsc Res Tech. 1993;24:195–213. doi: 10.1002/jemt.1070240302. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. Organization of the olfactory systems in the adult zebrafish: histological, immunohistochemical, and quantitative analysis. J Comp Neurol. 1995;358:247–259. doi: 10.1002/cne.903580207. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 2001;105:793–801. doi: 10.1016/s0306-4522(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Cancalon P. Degeneration and regeneration of olfactory cells induced by ZnSO4 and other chemicals. Tissue Cell. 1982;14:717–733. doi: 10.1016/0040-8166(82)90061-1. [DOI] [PubMed] [Google Scholar]
- Cancalon P. Influence of a detergent on the catfish olfactory mucosa. Tissue Cell. 1983a;15:245–258. doi: 10.1016/0040-8166(83)90020-4. [DOI] [PubMed] [Google Scholar]
- Cancalon P. Regeneration of three populations of olfactory axons as a function of temperature. Dev Brain Res. 1983b;9:265–278. doi: 10.1016/0165-3806(83)90024-x. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Emge DK, Small SL, Margolis FL. Pattern of olfactory bulb innervations returns after recovery from reversible peripheral deafferentation. J Comp Neurol. 2000;421:363–373. [PubMed] [Google Scholar]
- Easton DM. Garfish olfactory nerve: easily accessible source of numerous long, homogeneous, nonmyelinated axons. Science. 1971;172:952–955. doi: 10.1126/science.172.3986.952. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Cell biology of olfactory epithelium. In: Finger TE, Silver WL, Restrepo D, editors. The neurobiology of taste and smell. Vol. 2. New York: Wiley-Liss; 2000. pp. 131–158. [Google Scholar]
- Farbman AI, Margolis FL. Olfactory marker protein during ontogeny: immunohistochemical localization. Dev Biol. 1980;74:205–215. doi: 10.1016/0012-1606(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Germana A, Paruta S, Germana GP, Ochoa-Erena FJ, Montalbano G, Cobo J, Vega JA. Differential distribution of S100 protein and calretinin in mechanosensory and chemosensory cells of adult zebrafish (Danio rerio) Brain Res. 2007;1162:48–55. doi: 10.1016/j.brainres.2007.05.070. [DOI] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Metcalf JF. Autoradiographic and ultrastructural observations on the frog's olfactory mucosa. Cell Tissue Res. 1971;116:305–318. doi: 10.1007/BF00330630. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Hamdani EH, Doving KB. The functional organization of the fish olfactory system. Prog Neurobiol. 2007;82:80–86. doi: 10.1016/j.pneurobio.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Hansen A, Rolen SH, Anderson K, Morita Y, Caprio J, Finger TE. Correlation between olfactory receptor cell type and function in channel catfish. J Neurosci. 2003;23:9328–9339. doi: 10.1523/JNEUROSCI.23-28-09328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Zeiske E. The peripheral olfactory organ of the zebrafish, Danio rerio: an ultrastructural study. Chem Senses. 1998;23:39–48. doi: 10.1093/chemse/23.1.39. [DOI] [PubMed] [Google Scholar]
- Herzog C, Otto T. Regeneration of olfactory receptor neurons following chemical lesion: time course and enhancement with growth factor administration. Brain Res. 1999;849:155–161. doi: 10.1016/s0006-8993(99)02075-2. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL, McNelly NA. An autoradiographic study of the mouse olfactory epithelium: evidence for long-lived receptors. Anat Rec. 1984;210:375–383. doi: 10.1002/ar.1092100213. [DOI] [PubMed] [Google Scholar]
- Hinds JW, McNelly NA. Aging in the rat olfactory system: correlation of changes in the olfactory epithelium and olfactory bulb. J Comp Neurol. 1981;203:441–453. doi: 10.1002/cne.902030308. [DOI] [PubMed] [Google Scholar]
- Jones DT. Distribution of the stimulatory GTP-binding proteins, Gs and Golf, within the olfactory neuroepithelium. Chem Senses. 1990;15:333–340. [Google Scholar]
- Mackay-Sim A, Kittel PW. On the life span of olfactory receptor neurons. Eur J Neurosci. 1990;3:209–215. doi: 10.1111/j.1460-9568.1991.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Kittel PW. Cell dynamics in the adult mouse olfactory epithelium: a quantitative autoradiographic study. J Neurosci. 1991;11:979–983. doi: 10.1523/JNEUROSCI.11-04-00979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol. 1993;45:367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J Mol Histol. 2007;38:581–599. doi: 10.1007/s10735-007-9141-2. [DOI] [PubMed] [Google Scholar]
- Nadi NS, Head R, Grillo M, Hempstead J, Grannot-Reisfeld N, Margolis FL. Chemical deafferentation of the olfactory bulb: plasticity of the levels of tyrosine hydroxylase, dopamine and norepinephrine. Brain Res. 1981;213:365–371. doi: 10.1016/0006-8993(81)90241-9. [DOI] [PubMed] [Google Scholar]
- Oehlmann VD, Berger S, Sterner C, Korsching SI. Zebrafish beta tubulin 1 expression is limited to the nervous system throughout development, and in the adult brain is restricted to a subset of proliferative regions. Gene Expr Patterns. 2004;4:191–198. doi: 10.1016/j.modgep.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–936. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- Schwartz Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991;11:3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Youngetob SL, Ring G, Iwema CL, Mezza RC. Reinnervation of the rat olfactory bulb after methyl bromide-induced lesion: timing and extent of reinnervation. J Comp Neurol. 1999;412:439–457. doi: 10.1002/(sici)1096-9861(19990927)412:3<439::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Singru PS, Sakharkar AJ, Subhedar N. Neuronal nitric oxide synthase in the olfactory system of an adult teleost fish Oreochromis mossambicus. Brain Res. 2003;977:157–168. doi: 10.1016/s0006-8993(03)02626-x. [DOI] [PubMed] [Google Scholar]
- Sulz L, Astorga G, Bellette B, Iturriaga R, Mackay-Sim A, Bacigalupo J. Nitric oxide regulates neurogenesis in the adult olfactory epithelium in vitro. Nitric Oxide. 2009;20:238–252. doi: 10.1016/j.niox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Stewart WB, Greer CA, Teicher MH. The effect of intranasal zinc sulfate treatment on odor-mediated behavior and on odor-induced metabolic activity on the olfactory bulbs of neonatal rats. Brain Res. 1983;284:247–259. doi: 10.1016/0165-3806(83)90009-3. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Briner A, Klauser P, Gascon E, Dayer AG, Kiss JZ, Muller D, Licker MJ, Morel DR. Adverse effects of methylene blue on the central nervous system. Anesthesiology. 2008;108:684–692. doi: 10.1097/ALN.0b013e3181684be4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Ichikawa M. Localization of G protein alpha subunits and morphology of receptor neurons in olfactory and vomeronasal epithelia in Reeve's turtle, Geoclemys reevesii. Zool Sci. 2008;25:178–187. doi: 10.2108/zsj.25.178. [DOI] [PubMed] [Google Scholar]
- Walker DG, Breipohl W, Simon-Taha A, Lincoln D, Lobie PE, Aragon JG. Cell dynamics and maturation within the olfactory epithelium proper of the mouse—a morphometric analysis. Chem Senses. 1990;15:741–753. [Google Scholar]
- Weth F, Nadler W, Korsching S. Nested expression domains for odorant receptors in zebrafish olfactory epithelium. Proc Natl Acad Sci U S A. 1996;93:13321–13326. doi: 10.1073/pnas.93.23.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BS, Osahan JK, Hara TJ, Hosseini M, Wong E. Nitric oxide synthase in the olfactory mucosa of the larval sea lamprey (Petromyzon marinus) J Comp Neurol. 1996;365:18–26. doi: 10.1002/(SICI)1096-9861(19960129)365:1<18::AID-CNE2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Zippel HP. Regeneration in the peripheral and the central olfactory system: a review of morphological, physiological and behavioral aspects. J Hirnforsch. 1993;34:207–229. [PubMed] [Google Scholar]