Abstract
In spite of a wealth of information on feed and nutrition in cattle, there little is published of what they actually can taste. Here, we attempt to remedy some of this deficiency by presenting recordings of the chorda tympani proper nerve of young Holstein calves during stimulation of approximately 30 compounds. Hierarchical cluster analysis of 46 single taste fibers separated 4 fiber clusters: N (salt best), H (sour best), and 2 clusters, which could not be related to any human taste quality. The N fibers responded best to LiCl, NaCl, urea, monosodium glutamate, and KCl, whereas the H fibers responded strongly to citric and ascorbic acid. Interestingly, propionic and butyric acid stimulated best the 3rd cluster, whereas the 4th cluster responded best to denatonium benzoate and only to a small extent to quinine hydrochloride. Sweeteners stimulated moderately all clusters. Beginning with the largest response to sweet, the order between the responses was: acesulfame-K, saccharin, D-phenylalanine, glycine, sucrose, fructose, erythritol, cyclamate, and lactose. Alitame, aspartame, and super-aspartame evoked no or little responses. Three and 5 M ethanol stimulated all clusters. Comparison with taste fibers in other species suggests that the taste world of cattle is quite different from other species’.
Keywords: cattle, chorda tympani, coding, electrophysiology, single fibers, taste
Introduction
In spite of a wealth of information on feed and nutrition in cattle, there little is published of what they actually can taste. This is surprising considering the fact that in cattle as in humans, a functioning sense of taste is vital. Cattle are also equipped with at least as many taste buds (Davies et al. 1979; Miller 1986) and taste fibers as humans do (Kitchell 1963; Okamura 1996). In spite of this, to our knowledge, there are only 5 studies of taste nerve activity in cattle and they all are multifiber recordings. One showed actual multifiber responses to NaCl, sucrose, acetic acid, and quinine hydrochloride (QHCl) recorded as impulses from the chorda tympani (CT) nerve (Bell 1959). Later Kitchell (1963) presented summated CT responses to the same compounds. Bernard (1964) published summated responses from both CT and glossopharyngeal (NG) nerves to the above compounds and to KCl, fructose, glucose, xylose, Na-saccharin, HCl, propionic acid, and quinine sulfate. Finally, in 1989, we presented summated recordings from the whole CT (Hård af Segerstad and Hellekant 1989a) and NG nerves (Hård af Segerstad and Hellekant 1989b).
The data in these 2 last studies corroborated the previous studies but added also data on the response to the sweeteners: acesulfame-K, aspartame, galactose, glycine, lactose, maltose, monellin, thaumatin, and xylitol. Our CT study showed that glycine and xylitol gave large responses, the carbohydrates and acesulfame-K moderate responses, and aspartame, which, at that time was considered as a potential sweetener of animal feed, gave a small response and then only in some animals. Finally, monellin and thaumatin elicited no response at all. Interestingly, the responses to sweeteners expressed in percent of the response to NaCl were larger in the NG nerve than in the CT (Hård af Segerstad and Hellekant 1989b).
The scarcity of published taste studies and the overwhelming economical importance of cattle prompted this study. It presents results of electrophysiological recordings from the CT during stimulation with some 30 compounds. Our selection of stimuli was guided by choices in earlier studies. Its aims are not only to present single CT taste fibers recordings from a species, whose sense of taste is of great importance when one considers its economical value but also to relate the results with earlier taste fiber data from some other mammalian species.
Materials and methods
Twelve males, 1- to 2-week-old Holstein bull calves were used. They were initially anesthetized with intramuscularly injection of xylazin (Rompun, 1 mg/kg body weight). The anesthesia was maintained with intravenous sodium pentobarbital at concentrations of 40 mg/mL as needed. Body temperature, heart, and respiratory rates were monitored continuously. The right CT was dissected free between the lingual nerve and the tympanic bulla, where the CT was cut to record impulses from the taste buds on the tongue.
Stimuli
The solutions and their concentrations are presented in Table 1. Three concerns guided our choice of stimuli. First of all, we included representatives of all 4 basic taste qualities, as humans perceive them. Second, we included for comparison compounds used in other species. Third, we wanted to obtain taste nerve recordings of compounds, which may be or can be used in calf feed formula and diet.
Table 1.
Stimuli used in electrophysiological recordings
| Compound | Concentration |
| NaCl | 70 mM |
| LiCl | 70 mM |
| KCl | 0.1 M |
| MSG (monosodium glutamate) | 70 mM |
| Urea | 1 M |
| Citric acid | 20 mM |
| Ascorbic acid | 40 mM |
| Butyric acid | 10 mM |
| Propionic acid | 20 mM |
| QHCl | 10 mM |
| DB | 5 mM |
| Caffeine | 3% (0.15 M) |
| Neohesperidin dihydrochalcone | 0.49 mM |
| Alitame | 0.3 mM |
| Aspartame | 10 mM |
| Super-aspartame | 0.11 mM |
| SC-45647 | 0.1 mM |
| Sucrose | 0.3 M |
| Fructose | 0.3 M |
| Glucose | 0.5 M |
| Lactose | 0.6 M |
| D-Tryptophan | 30 mM |
| Stevioside | 0.87 mM |
| Suosan | 1.1 mM |
| Xylitol | 0.75 M |
| Cyclamate | 20 mM |
| Ace-K | 6 mM |
| D-phenylalanine | 0.1 M |
| Saccharin | 3.2 mM |
| Glycine | 0.4 M |
| Erythritol | 0.5 M |
| Ethanol | 3 M |
| Ethanol | 5 M |
With regard to stimuli and concentration used, our previous summated recording and behavioral studies influenced the choice so that we knew that concentrations and stimuli chosen would elicit a taste nerve response (Hård af Segerstad and Hellekant 1989a, 1989b; Hellekant et al. 1994). Our Taste-O-matic limits the number of stimuli that can be used in one single cycle to <32. Because of the time it takes to “reload” the Taste-O-matic, this limitation plays a larger role in recordings from single fibers, which are more fragile than the whole nerve.
The stimuli were delivered for 10 s over the anterior part of the tongue by a computerized system. The stimuli were dissolved in artificial saliva, which also was used as a rinsing solution for 40 s between stimulations. For rinsing between stimulations, we used a special bovine saliva containing: 0.5 g NaCl, 1.5 g KCl, 14.1 g NaHCO3, 1.2 g KHCO3, 0.1 g CaCl2, 0.8 g MgCl2, 4.3 g KHPO4, and 5.5 g K2HPO4 dissolved in H2O to 2 L. The stimuli and rinses were maintained and delivered at constant temperature (Hellekant and Roberts 1995).
The order between stimuli was quasirandomized so that no 2 stimuli followed within the same taste quality. Generally, the order of stimuli was the same from one experiment to the other, but if doubt arose that stimulation was insufficient, it was repeated.
Recording technique
Nerve impulses were recorded with a PAR 113 amplifier, monitored over a loudspeaker and an oscilloscope, and fed into a recorder (Gould ES 1000) and into an IBM computer via a DAS-Keithley interface. For whole-nerve recordings, the nerve impulses were processed by a smoothed absolute value circuit integrator and changed to a DC potential whose amplitude was related to the nerve impulse frequency, here called the summated response. This signal and a code related to each tastant on the tongue were fed to the computer. The summated response was sampled before, during, and after stimulation and displayed on a monitor. The computer also controlled the stimulation times and the order of stimuli.
For single-fiber recordings, the nerve was desheathed and teased into fine strands. Each strand was tested for nerve activity by being placed on a silver wire electrode held by a micromanipulator. The indifferent electrode was positioned in nearby tissue. The activity of a filament was recorded with a PAR 113 amplifier, monitored over a loudspeaker, shown on an oscilloscope, and fed into a recorder (Gould ES 1000). If there was no response in the filament, it was discarded. If there were too many fibers, the filament was divided into finer filaments until either the nerve impulses could be discriminated or had disappeared. We used an impulse-amplitude analyzer with adjustable upper and lower levels to discriminate between impulses. The nerve impulses and the 2 levels of the impulse-amplitude analyzer were visible on the oscilloscope. When a nerve impulse exceeded the lower but not the upper level it triggered a pulse, which also was displayed on the oscilloscope. It also lit up the pulse that was counted on the oscilloscope. The upper level allowed us to exclude impulses, which exceeded the window, for example, artifacts.
If the recording was deemed reliable, we ran a test set of stimuli consisting of NaCl, sucrose, QHCl, and citric acid. Consistent spike amplitude and shape of impulses, a steady frequency of spontaneous activity, and consistent responses to repeated stimulation with the same stimuli were used as criteria for a reliable recording. When these criteria were satisfied, we ran the whole sequence of stimuli.
The pulses and a digital code related to each tastant on the tongue were then sent to a computer. Custom-made software sampled the response before, during, and after stimulation and displayed it on a second monitor. The computer controlled delivery of stimulus, times for stimulation, order between stimuli and stored intervals between pulses, together with the chemical name of the presented stimulus. The stimulus identity, stimulation order, level of nerve activity before and during each stimulation, maximum impulse activity of the response, and time for each stimulation were continuously printed out during the experiment.
Data analysis
Due to the fact that every nerve impulse is recorded on a continues trail of paper by the Gould recorder, we can go back and assess the validity of each fiber recording. This will reveal for example, if a second fiber becomes active during a sequence of stimulation. This feature is particular important in this study because the taste fibers did not fall in categories related to human taste qualities. The measure for a single-fiber response was the number of impulses during the first 5 s of stimulation minus the number of impulses recorded during 5 s of the prestimulus period. A fiber was considered to be responsive to a stimulus if the nerve impulse rate during the first 5 s of stimulation was more than 2 times the standard deviation (SD) of the activity of the fiber during the rinsing.
Cluster and of stimulus relationship were performed with the statistical package SYSTAT for Macintosh, version 5.2. We measured intercluster similarity using Pearson’s correlation coefficients, and cluster analysis proceeded according to the average linkage method. The responses of the fibers belonging to the same cluster were first evaluated by 2-way analysis of variance on ranked data. This was followed by pairwise comparison of stimuli using Fisher’s least significant differences. Probability less than 0.05 was considered to be significant, compare (Frank et al. 1988). To visualize similarities between different compounds, we used multidimensional scaling (MDS) analysis (SYSTAT for Macintosh). MDS computes coordinates of points in a multidimensional space where each point represents a particular stimulus. As a result of MDS, we have a map where the closeness between points reflects similarities between stimuli. We have used this method and criterion in many studies, for example, (Hellekant and Roberts 1995; Danilova et al. 2002).
Results
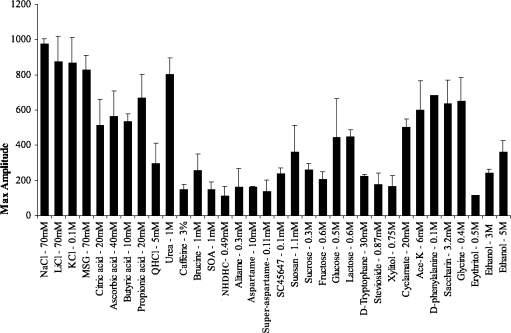
Figure 1 presents a plot of averaged summated responses recorded from the whole CT nerve of 4 animals. It is evident that the responses to the stimuli chosen varied. One striking feature is the large responses to the salts. Another one is the poor response to most of the sweeteners with the exceptions of D-phenylalanine, glycine, cyclamate, acesulfame-K, and saccharin.
Figure 1.
Averaged responses of the summated CT nerve recording from 4 calves. The stimuli have been arranged in the general order of salty, sour, bitter, and sweet, as judged by humans. The amplitudes of the columns are measured in arbitrary units and represent maximum responses.
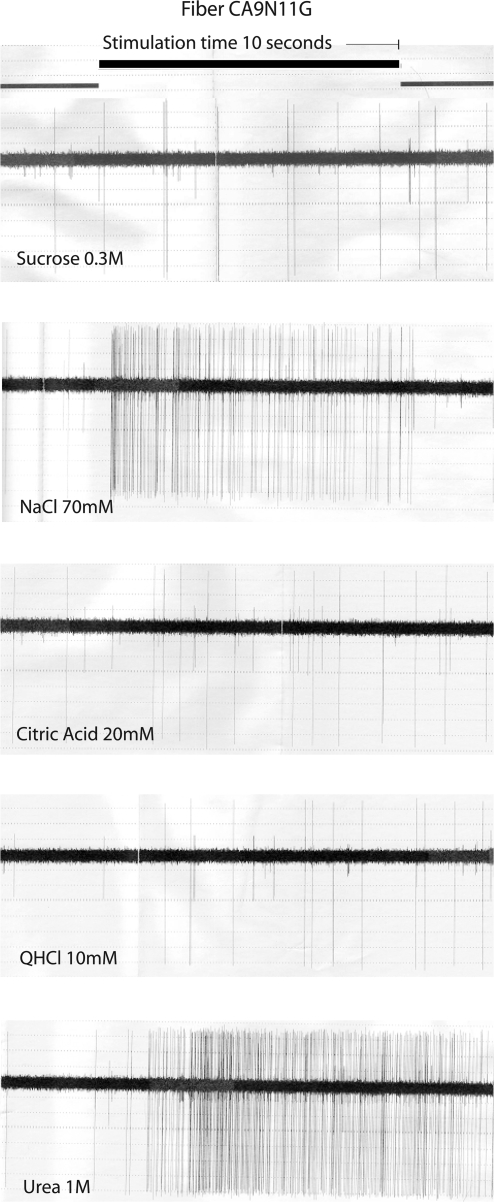
Figure 2 shows large action potentials from the recording of nerve filament CA9N11G to our standards throughout the experiments: sucrose, NaCl, citric acid, and QHCl. The bottom of Figure 2 shows an example of the response to urea in this filament. Of interest here is perhaps that the delay between onset of stimulation and nerve response was quite different for NaCl and urea. The fiber was quite selective in its responses because it responded basically only to salts. The bubble graph in the next figure, Figure 3, shows this.
Figure 2.
Recordings of a CT filament, single fiber CA9N11G during stimulation with sucrose, NaCl, citric acid, QHCl, and urea. Onset and offset of stimuli are shown as change of the top horizontal line. Stimulation time was 10 s, and the stimulation was preceded and followed by a flow of artificial saliva over the tongue for 40 s. Of interest is the difference in latency of response between NaCl and urea.
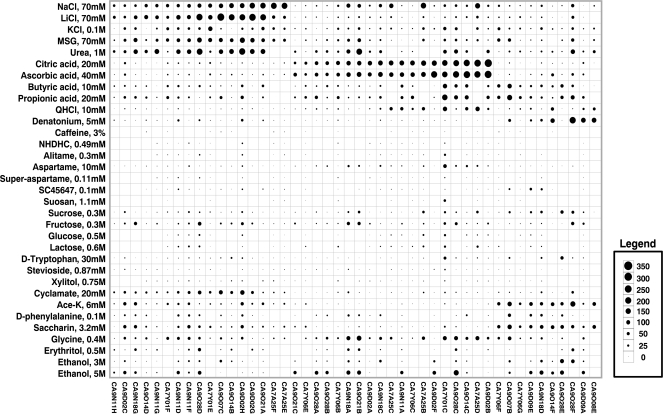
Figure 3.
An overview of the response profiles of 46 CT taste fibers of calves. The area of the circles represents impulse frequency over the first 5 s of stimulation. Absence of a mark shows that data are missing. The stimuli were arranged along the short axis in order of stimuli: salty, sour, bitter, and sweet. The fibers were arranged along the longer axis in order of increasing responses to: NaCl, citric acid, and QHCl.
Figure 3 displays the responses of 46 single fibers as a bubble chart. Each fiber has a unique label, so that they can be identified in the following. The area of the circles in the square formed by the intersection of stimulus and fiber represents impulse frequency over the first 5 s of stimulation. Absence of a mark shows that data are missing. The stimuli were generally arranged along the short axis from the top in order of salty, sour, bitter, sweet, and ethanol, whereas the fibers were arranged along the longer axis in order of best response to the salts, acids, bitter, and sweet compounds.
Figure 3 shows 3 interesting features. First, there is more or less an overlap between the fibers responding to the salts, monosodium glutamate (MSG), and urea. Second, the fibers responding to ascorbic and citric acid and those to butyric and propionic acid generally form 2 different groups, although there is some overlap between some fibers. This also seems be the case for QHCl and denatonium benzoate (DB), although on the whole each stimulated a separate group of fibers. Perhaps most striking (especially in comparison with data from other species), the fibers responding to compounds that humans classify as sweet did not form one single group or cluster.
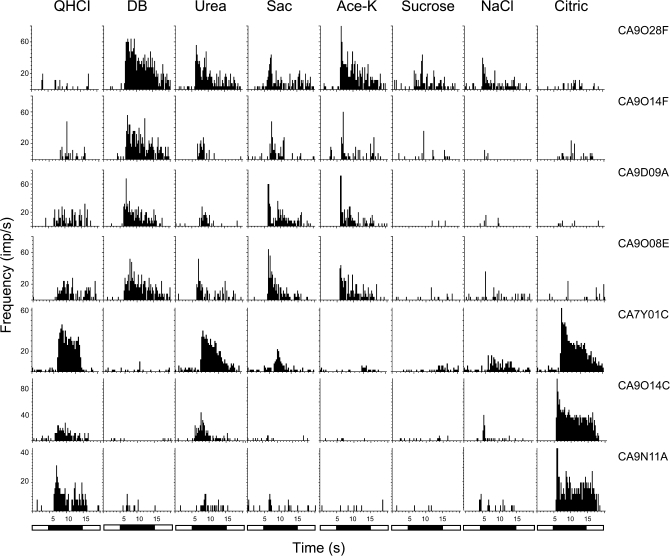
The temporal pattern
Figure 4 presents the temporal pattern or time intensity function of 7 of these taste fibers. The impulse activity of each fiber is displayed during 5 s of rinsing with artificial saliva, followed by 10 s of stimulation and then by 5 s of renewed rinsing to remove the stimulus. The stimuli listed on top of the graph are QHCl, DB, urea, saccharin, acesulfame-K, sucrose, NaCl, and citric acid. Two of the fibers originate from the same animal, the rest from different animals. The main purpose of the plot is to demonstrate that the temporal profile, such as rate of adaptation of response, varied from stimulus to stimulus. This is not made evident in any other Figure. Some of the features in Figure 3 are demonstrated again, such as a poor correlation between a response to QHCl and DB or between acesulfame-K and sucrose or a high between saccharin and acesulfame-K.
Figure 4.
Recordings of the temporal intensity or profile of 7 CT fibers before, during, and after stimulation are shown. The impulse activity of each fiber is displayed during 5 s of rinsing with artificial saliva, followed by 10 s of stimulation and then by 5 s of renewed rinsing with the artificial saliva. The vertical traces show the response to each stimulus listed on top of the graph: QHCl, DB, urea, caffeine, saccharin, acesulfame-K, sucrose, NaCl, and citric acid. Some of the features mentioned in Figure 1 are shown. Thus, it is evident that a response to QHCl is poorly correlated with the response to denatonium or a response to acesulfame-K with the response to sucrose.
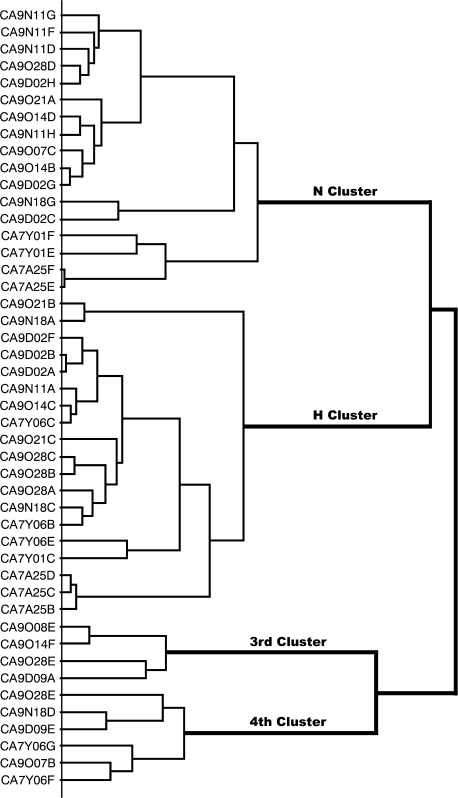
Hierarchical cluster analysis
Figure 5 shows that the cluster analysis distinguished 2 major clusters, the N and H clusters and 2 smaller clusters, which we were unable to label in the usual way as Q and S fiber clusters because they did not contain fibers that responded mainly to bitter or sweet compounds. Therefore, we labeled them the 3rd and 4th cluster. The identity of the fiber is listed on the left side using the same label of the fibers as in Figures 3 and 4.
Figure 5.
Results of hierarchical cluster analysis of the response profile of 46 CT fibers. Intercluster similarity was measured with the Pearson correlation coefficient, and the cluster analysis proceeded according to the average linking method. The identity of the fiber is listed on the left side and response category on the basis of its best response to the 4 basic solutions is entered on the right side. The analysis distinguished 2 major clusters consisting of 17 N fibers and 19 H fibers and 2 clusters that could not be categorized according to human taste qualities. N stands for NaCl and H for citric acid.
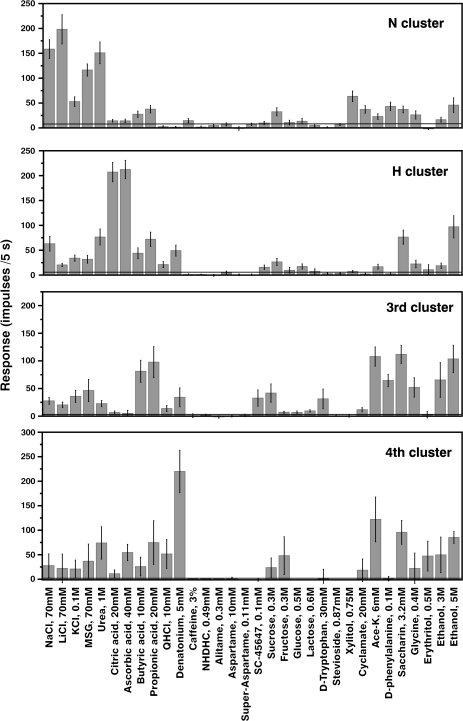
Average response profiles of each fiber cluster
Figure 6 shows the average response profiles of the above 4 clusters. The stimuli are listed along the x axis in the same order as in Figure 3, whereas the average impulse activity, measured over 5 s, is plotted along the y axis. The error bars illustrate the standard error of mean of these averages. The horizontal lines show the 2 SD of the averaged spontaneous level of impulse activity before stimulation. In the following, we will present each cluster.
Figure 6.
Average response profiles of from the top the N cluster, the H cluster, the 3rd and 4th clusters. The error bars show the SE. The horizontal line drawn in each plot illustrates 2 SD of the averaged spontaneous activity.
N cluster. Seventeen N fibers formed a large N cluster. It was characterized by strong responses to NaCl and LiCl but also to MSG and urea. These fibers did not respond or responded poorly to acids or bitter compounds. Some of the compounds that taste sweet to humans stimulated also these fibers. Thus, xylitol, D-phenylalanine, acesulfame-K, and saccharin gave evident responses.
H cluster. Nineteen fibers formed a large H cluster in which citric and ascorbic acid elicited the best responses. In contrast, butyric and propionic acids gave small responses in the H cluster. Instead their responses were distributed over all clusters with the emphasis on fibers in the 3rd cluster.
The 3rd and 4th clusters. The 3rd cluster contains 4 fibers and the 4th cluster contains 6 fibers. In general, these 2 clusters responded to the same stimuli with the exception that the 4th cluster showed a large response to DB absent in the 3rd cluster.
With regard to compounds, sweet to humans, none of the clusters contained taste fibers that were uniquely responsive to sweeteners, although the sweet compounds evoked the largest response in the 3rd and 4th clusters. Three and 5 M ethanol seem to be good stimuli in cattle as judged by their significant responses in all clusters.
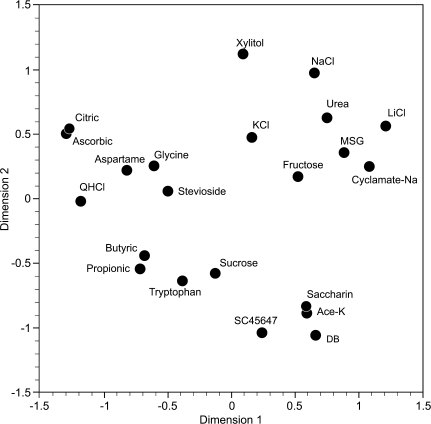
MDS analysis
The result of the analysis of 22 of the stimuli is plotted in Figure 7. The plot shows that the stimuli did not group according to primate taste qualities because compounds that taste sweet to humans did not form a tight group. Instead the sweet compounds are distributed among all stimuli. Furthermore, butyric and propionic acid form a pair far away from citric and ascorbic acid. On the other hand, the results suggest that the taste of urea, generally considered bitter, is more similar to those of the salts than to QHCl and DB.
Figure 7.
The 2D space, showing location of 22 taste stimuli, was obtained from the MDS analysis. The distribution was calculated with Pearson correlation coefficient between stimuli across 46 CT fibers. The value of stress is 0.057. The plot shows that the stimuli did not group according to human taste qualities. For example, the compounds that to humans share the quality of being sweet do not form one group but are distributed among all the other stimuli; citric and ascorbic acid are located away from the other 2 acids, butyric and propionic acid; QHCl is distant from DB; urea in the vicinity of NaCl and LiCl.
Discussion
The hierarchical cluster analysis revealed 4 clusters with the clusters responding best to salts and acids as most populous (38% and 39%, respectively). There was no designated fiber cluster responding to stimuli representing only one of the human taste qualities, for example, only sweet or bitter. Instead the salts, the acids, the bitter compounds and the sweeteners used gave a response in all 4 clusters. Furthermore, stimuli that to human represent the same taste quality and in primates stimulate the same taste fibers, stimulated in cattle different fibers. In sour taste, butyric and propionic acids, which stimulated a different cluster than did ascorbic and citric acid, are examples of this. In bitter taste, several of the fibers responding to DB did not respond to QHCl and vice versa. Finally, there was no designated group of fibers responding to sweet and very few of the fibers responded to the high-potency sweeteners. In short, the analyses gave results that showed little or no similarity with findings in primates or any other mammalian species, except perhaps pig. This, and evolutionary, hedonic and coding in taste will be discussed in the following.
One important question is how well does an array of single fibers represent the more than thousand fibers in a whole nerve. For example, is it possible that the absence of an S and Q cluster (sweet or quinine best fibers) may be due to potential imbalance in sampling the fibers? In attempt to address this concern, we looked at the relationship between the amplitude of the summated recordings from the whole CT and the total number of impulses in all single fibers to each stimulus. Our assumption was that if the fibers in our single-fiber array did not represent the general distribution of taste fibers, the correlation between the results of the 2 recording techniques would be poor. A comparison between the 2 sets of data did not support this assumption. The Pearson correlation coefficient was 0.76, with P < 0.0001. The result does not support the suspicion that our methods to isolate single taste fibers favored one particular fiber type.
Comparison with taste fibers in other species
The fact that only 2 taste fiber clusters could be related to the generally found N, H, Q, and S fiber clusters is unique to cattle. Thus, the literature in general identifies 4 clusters in the dominant animal model, rodents, represented by mouse, rat, and hamster. In rats, the general consensus is that there are N, H, and Q fibers, but very few S fibers in its CT. This is also true for its NG nerve. On the other hand, the greater superficial petrosal nerve of rat has a large number of sweet sensitive taste fibers as suggested by summated nerve recordings (Nejad 1986; Harada et al. 1997). In the mouse, all 4 types (N, H, Q, and S) have been identified (Ninomiya et al. 1984, 1999; Yoshida et al. 2006, 2009). Similarly, in the hamster all the generally occurring N, H, Q, and S fibers clusters have been identified (Frank et al. 1988; Danilova, Hellekant, Tinti, and Nofre 1998).
We have earlier published single taste fiber data from the domestic pig. Because calf and pig are grouped in the same order, Artiodactyla, there may be more similarities between them in the composition of taste fibers than between cattle and rodents. In pig, we used essentially the same taste stimuli as here and identified M, H, Q, and S clusters (Danilova et al. 1999; Hellekant and Danilova 1999). The M cluster is equivalent to the N cluster here but was labeled M because the responses to MSG were larger than to NaCl (see Figure 6, Hellekant and Danilova 1999). The H cluster gave a solid response to acids and very little to any other stimulus. The Q cluster was less defined and responded well only to QHCl out of the bitter stimuli, DB, caffeine, and sucrose octaacetate. With regard to the pig’s S cluster, the responses to sweeteners were distributed over all fiber clusters. Consequently, it is arguable whether there is a distinct S cluster in the pig (Figure 6 in Danilova et al. 1999). The general conclusion of the data in pig is that although its taste fibers could be clustered according to human taste qualities, these clusters were considerably less distinct than in primates and more similar to those in cattle.
Single taste fibers of the nonhuman primates, the common marmoset, Callithrix jacchus jacchus; rhesus monkey, Macaca mulatta; cynomolgus, Macaca fascicularis and chimpanzee, Pan troglodytes display clusters of taste fibers whose grouping and sensitivity to tastants fit better with human taste qualities than in any other species studied. The fit becomes better the closer related the primate is with humans and is best in the chimpanzee where the clusters are congruent with human taste qualities (Brouwer et al. 1983; Hellekant, Danilova, and Ninomiya 1997; Hellekant, Danilova, Roberts, and Ninomiya 1997; Hellekant, Ninomiya, and Danilova 1997; Danilova, Hellekant, Roberts, et al. 1998; Hellekant et al. 1998; Danilova et al. 2002; Jin et al. 2003; Wang et al. 2009). From this it is evident that the taste fibers of cattle showed the largest differences in the comparison with primates.
The sense of taste in cattle seen in evolutionary perspective
Cattle are strict herbivores. Consequently, their sense of taste must evolve in constant communication with the plants they feed on because as the plants evolve, so must the herbivore. Here, we attempt to apply this perspective on our taste fiber data.
Plants have low concentrations of Na and ruminants exist on a precarious mineral balance (Bell 1963). The fact that during rumination cattle produce approximately 150 L/24 h of saliva, which has a high content of Na, adds importance to the need to identify and ingest Na. Seen from this perspective, the existence of a dedicated group of salt sensing fibers makes sense (Bell 1963; Bell and Sly 1980).
Figures 3, 6, and 7 show that citric and ascorbic acid gave a response in taste fibers, which are different from those responding to butyric and propionic acid. This may not be too unexpected because ascorbic and citric acid enter through the feed, whereas short-chain volatile fatty acids, such as butyric and propionic acids, are produced in the rumen during fermentation and are part of the gases expelled into oral cavity during eructation. There they will most likely come in contact with taste buds. It is not unlikely that ability to discriminate between organic acids and short-chain volatile fatty acids is important. The separation of their taste to different taste fibers may facilitate this.
Grazing cattle encounters many different species of vegetation. Many of these plants produce compounds that are toxic. Consequently, cattle must possess a well-developed ability to detect toxic compounds. In other species, a close connection between toxicity and bitter taste has been postulated. Because the bitter taste quality is dependent upon presence of T2R receptors on the type II taste bud cells, distribution of their information among many taste fibers may facilitate the ability to identify toxic compounds. On the other hand, there are no evolutionary reasons to develop the ability taste compounds, which are broken down in the rumen. This could explain why compounds that are bitter to human such as caffeine or DB gave little or no response here.
As shown in Figures 1, 3, and 6, the sweet compounds elicited a moderate and for some compounds no or a small response in the CT nerve. It is not surprising that aspartame, alitame, super-aspartame, and neohesperidin dihydrochalcone did not elicit a response and SC 45647, cyclamate, and erythritol elicited small responses because they were discovered or developed for their sweetness to humans. Consequently, there is no evolutionary pressure to develop tasting ability for these compounds. On the other hand, acesulfame-K, saccharin, and xylitol, which also are artificial, elicited significant responses in one or more fiber clusters and were moderately preferred by calves in 2-bottle tests (Hellekant et al. 1994). Cattle share this with other mammals, which generally prefer these compounds.
Finally, the finding that sweet tasting carbohydrates, sucrose, glucose, fructose, and lactose elicited no or small responses is perhaps not unexpected, considering they are normally not constituents of the cattle diet. On the other hand, as mentioned in the introduction, we have earlier recorded from both the CT and NG nerves in calves (Hård af Segerstad and Hellekant 1989a, 1989b) and found that the NG was more sensitive to sweet. The summated responses to sweeteners were considerably larger in the NG nerve than in the CT. In some animals, the phasic responses to sucrose and fructose were almost 3 times larger than that of NaCl. This suggests that the NG nerve is major route for sweet in cattle.
Relation between the taste fiber clusters with preference or rejection
In primates and hamster, studies suggest that the hedonic value of a tastant can be related to the ratio of the S and Q fiber response the compound elicits (Hellekant, Danilova, and Ninomiya 1997; Danilova, Hellekant, Tinti, and Nofre 1998; Hellekant et al. 1998; Danilova et al. 2002; Jin et al. 2003; Danilova and Hellekant 2004, 2006; Wang et al. 2009). This suggests that one can use the ratio between the response in Q and S fibers as a measure of a compound’s attractiveness. However, one condition for a successful use of this method is that the array of taste stimuli contains both compounds that are highly preferred and strongly rejected as otherwise distinct S and Q clusters cannot be identified.
We have earlier recorded the preference of 4- to 16-weeks calves for xylitol, acesulfame-K, saccharin, and glycine in addition to the 4 carbohydrates used here (Hellekant et al. 1994). The results showed that sucrose, fructose, and glycine reached an intake level of 90%, whereas glucose, lactose, saccharin, acesulfame-K hovered between 75% and 80%. However, there is nothing in the distribution of taste fiber responses in Figure 3 that distinguishes the response to these compounds from those of the significantly less preferred, such as xylitol. Furthermore, as shown in Figure 6, no fiber cluster displayed a decisive sensitivity to sucrose, fructose, and glycine. We also included erythritol and ethanol, for which we had anecdotal evidence that they are strongly preferred by cattle. However, our cluster analysis did not identify a separate taste fiber cluster linked to them.
Interesting is the similarity in single N fiber pattern between NaCl, MSG, and urea, which suggests that they have a similar taste to cattle. Because cattle like NaCl, it is possible that the N fibers substitute for the S fibers in primates as the cluster that triggers intake in cattle. The over all conclusion here is that until we are able to use compounds strongly preferred or rejected as stimuli in single-fiber recordings, cluster analysis cannot be used to identify unknown compounds as either strongly preferred or rejected by calves.
The influence of species differences on hypothesis of coding in taste
The presence of the species differences in taste has been stressed by several earlier investigators coming from the veterinary side (Baldwin et al. 1959; Bell 1959; Bell and Williams 1959; Bell et al. 1959; Kare 1960, 1961, 1971; Bernard and Kare 1961; Kare and Ficken 1963; Kitchell 1963; Bernard 1964; Bell and Kitchell 1966; Goatcher and Church 1969, 1970a, 1970b, 1970c, 1971).
These earlier conclusions support the results here because the taste fiber data in Figure 3 have little similarity with similarly plotted data from other species, especially from higher primates, for example, (Hellekant, Danilova, and Ninomiya 1997; Hellekant, Ninomiya, and Danilova 1997). We suggest that species differences in taste strongly contribute to the difficulties of linking activity in animal taste fibers with the human taste qualities and subsequent attempt to solve how taste is coded from the periphery. If cattle were the only species from which we had single taste fiber recordings, we would never have discovered how strong the relationship is between taste fiber clusters and taste qualities. In contrast, primate data demonstrate a strong link between fiber clusters and human taste qualities.
To summarize, the organization of single taste fibers of cattle has little similarity with the organization in other species. The closest similarity was observed with pigs. The organization and response to compounds in cattle taste fibers present a picture that cannot be related to human taste qualities or primate taste fiber data. This is not caused by inability to taste by cattle.
Funding
This work was supported by grant [R01 DC006016] from the National Institutes of Health and Duluth Medical Research Institute to G.H..
Acknowledgments
This study was approved by the Institutional Animal Care and Use Committee. The authors have no conflict of interests.
References
- Baldwin BA, Bell FR, Kitchell RL. Gustatory nerve impulses in ruminant ungulates. J Physiol (Lond) 1959;146:14P–15P. [Google Scholar]
- Bell FR. The sense of taste in domesticated animals. Vet Rec. 1959;71:1071–1079. [Google Scholar]
- Bell FR. The variation in taste thresholds of ruminants associated with sodium depletion. In: Zotterman Y, editor. Olfaction and taste I. Oxford: Pergamon Press; 1963. pp. 299–307. [Google Scholar]
- Bell FR, Kitchell RL. Taste reception in the goat, sheep and calf. J Physiol. 1966;183:145–151. doi: 10.1113/jphysiol.1966.sp007856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell FR, Sly J. The olfactory discrimination of salt by cattle. In: van der Starre H, editor. Proceedings of the Seventh International Symposium on Olfaction and Taste and the fourth Congress of the European Chemoreception Research Organization; 1980 July 22–25 July; London. Washington (DC): IRL Press Ltd: 1980. pp. 343–347. [Google Scholar]
- Bell FR, Williams HI. Threshold values for taste in monozygotic twin calves. Nature. 1959;183:345. doi: 10.1038/183345a0. [DOI] [PubMed] [Google Scholar]
- Bell GH, Davidson JN, Scarborough H. Sense of taste (gustation), in textbook of physiology and biochemistry. 4th ed. Edinburgh (UK): E. & S. Livingstone Ltd; 1959. p. 1065. [Google Scholar]
- Bernard RA. An electrophysiological study of taste reception in peripheral nerves of the calf. Am J Physiol. 1964;206:827–835. doi: 10.1152/ajplegacy.1964.206.4.827. [DOI] [PubMed] [Google Scholar]
- Brouwer JN, Glaser D, Hard Af Segerstad C, Hellekant G, Ninomiya Y, Van der Wel H. The sweetness-inducing effect of miraculin; behavioural and neurophysiological experiments in the rhesus monkey Macaca mulatta. J Physiol. 1983;337:221–240. doi: 10.1113/jphysiol.1983.sp014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova V, Danilov Y, Roberts T, Tinti J-M, Nofre C, Hellekant G. The sense of taste in a new world monkey, the common marmoset: recordings from the chorda tympani and glossopharyngeal nerves. J Neurophysiol. 2002;88:579–594. doi: 10.1152/jn.2002.88.2.579. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. Sense of taste in a new world monkey, the common marmoset. II. Link between behavior and nerve activity. J Neurophysiol. 2004;92:1067–1076. doi: 10.1152/jn.01183.2003. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. Elucidating coding of taste qualities with the taste modifier miraculin in the common marmoset. Brain Res Bull. 2006;68:315–321. doi: 10.1016/j.brainresbull.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G, Roberts R, Tinti JM, Nofre C. Behavioral and single chorda tympani taste fiber responses in the common marmoset, Callithrix jacchus jacchus. Ann N Y Acad Sci. 1998;855:160–164. doi: 10.1111/j.1749-6632.1998.tb10559.x. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G, Tinti JM, Nofre C. Gustatory responses of the hamster Mesocricetus auratus to various compounds considered sweet by humans. J Neurophysiol. 1998;80:2102–2112. doi: 10.1152/jn.1998.80.4.2102. [DOI] [PubMed] [Google Scholar]
- Danilova V, Roberts T, Hellekant G. Responses of single taste fibers and whole chorda tympani and glossopharyngeal nerve in the domestic pig, Sus scrofa. Chem Senses. 1999;24:301–316. doi: 10.1093/chemse/24.3.301. [DOI] [PubMed] [Google Scholar]
- Davies RO, Kare MR, Cagan RH. Distribution of taste buds on fungiform and circumvallate papillae of bovine tongue. Anat Rec. 1979;195:443–446. doi: 10.1002/ar.1091950304. [DOI] [PubMed] [Google Scholar]
- Frank ME, Bieber SL, Smith DV. The organization of taste sensibilities in hamster chorda tympani nerve fibers. J Gen Physiol. 1988;91:861–896. doi: 10.1085/jgp.91.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goatcher WD, Church DC. The sense of taste in sheep. Feedstuffs. 1969;41:32. [Google Scholar]
- Goatcher WD, Church DC. Taste responses in ruminants. I. Reactions of sheep to sugars, saccharin, ethanol and salts. J Anim Sci. 1970a;30:777–783. doi: 10.2527/jas1970.305777x. [DOI] [PubMed] [Google Scholar]
- Goatcher WD, Church DC. Taste responses in ruminants. III. Reactions of pygmy goats, normal goats, sheep and cattle to sucrose and sodium chloride. J Anim Sci. 1970b;31:364–372. doi: 10.2527/jas1970.312364x. [DOI] [PubMed] [Google Scholar]
- Goatcher WD, Church DC. Taste responses in ruminants. IV. Reactions of pygmy goats, normal goats, sheep and cattle to acetic acid and quinine hydrochloride. J Anim Sci. 1970c;31:373–382. doi: 10.2527/jas1970.312373x. [DOI] [PubMed] [Google Scholar]
- Goatcher WD, Church DC. Review of some nutritional aspects of the sense of taste. 1971 doi: 10.2527/jas1970.315973x. Technical paper 2926, Oregon Agr Exp Station. 973–981. [DOI] [PubMed] [Google Scholar]
- Harada S, Yamamoto T, Yamaguchi K, Kasahara Y. Different characteristics of gustatory responses between the greater superficial petrosal and chorda tympani nerves in the rat. Chem Senses. 1997;22:133–140. doi: 10.1093/chemse/22.2.133. [DOI] [PubMed] [Google Scholar]
- Hård af Segerstad C, Hellekant G. The sweet taste in the calf. I. Chorda tympani proper nerve responses to taste stimulation of the tongue. Physiol Behav. 1989a;45:633–638. doi: 10.1016/0031-9384(89)90084-x. [DOI] [PubMed] [Google Scholar]
- Hård af Segerstad C, Hellekant G. The sweet taste in the calf. II. Glossopharyngeal nerve responses to taste stimulation of the tongue. Physiol Behav. 1989b;45:1043–1047. doi: 10.1016/0031-9384(89)90235-7. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Danilova V. Taste in domestic pig, Sus scrofa. J Anim Physiol Anim Nutr. 1999;63:139–144. [Google Scholar]
- Hellekant G, Danilova V, Ninomiya Y. Primate sense of taste: behavioral and single chorda tympani and glossopharyngeal nerve fiber recordings in the rhesus monkey, Macaca mulatta. J Neurophysiol. 1997;77:978–993. doi: 10.1152/jn.1997.77.2.978. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Danilova V, Roberts T, Ninomiya Y. The taste of ethanol in a primate model: I. Chorda tympani nerve response in Macaca mulatta. Alcohol. 1997;14:473–484. doi: 10.1016/s0741-8329(96)00215-7. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Hard af Segerstad C, Roberts TW. Sweet taste in the calf: III. Behavioral responses to sweeteners. Physiol Behav. 1994;56:555–562. doi: 10.1016/0031-9384(94)90301-8. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Ninomiya Y, Danilova V. Taste in chimpanzees II: single chorda tympani fibers. Physiol Behav. 1997;61:829–841. doi: 10.1016/s0031-9384(96)00562-8. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Ninomiya Y, Danilova V. Taste in chimpanzees III: labeled line coding in sweet taste. Physiol Behav. 1998;65:191–200. doi: 10.1016/s0031-9384(97)00532-5. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Roberts TW. Whole nerve and single fiber recordings in non-human primates. In: Spielman AI, Brand JG, editors. Experimental cell biology of taste and olfaction: current techniques and protocols. Boca Raton (FL): CRC Press; 1995. pp. 277–290. [Google Scholar]
- Jin Z, Danilova V, Assadi-Porter FM, Markley JL, Hellekant G. Monkey electrophysiological and human psychophysical responses to mutants of the sweet protein brazzein: delineating brazzein sweetness. Chem Senses. 2003;28:491–498. doi: 10.1093/chemse/28.6.491. [DOI] [PubMed] [Google Scholar]
- Kare M. Comparative study of taste. In: Beidler LM, editor. Taste. Berlin (Germany): Springer-Verlag; 1971. pp. 278–290. [Google Scholar]
- Kare MR. Senses of animals differ from man's. Farm Res. 1960;26:8–9. [Google Scholar]
- Kare MR. Comparative aspects of the sense of taste. In: Kare MR, Halpern BP, editors. Physiological and behavioral aspects of taste. Chicago: University of Chicago Press; 1961. pp. 6–15. [Google Scholar]
- Kare MR, Ficken MS. Comparative studies on the sense of taste. In: Zotterman Y, editor. Olfaction and taste I. Oxford: Pergamon Press; 1963. pp. 285–297. [Google Scholar]
- Kitchell RL. Comparative anatomical and physiological studies of gustatory mechanisms. London: Pergamon Press; 1963. [Google Scholar]
- Miller IJ. Variation in human fungiform taste bud densities among regions and subjects. Anat Rec. 1986;216:474–482. doi: 10.1002/ar.1092160404. [DOI] [PubMed] [Google Scholar]
- Nejad M. The neural activities of the greater superficial petrosal nerve of the rat in response to chemical stimulation of the palate. Chem Senses. 1986;11:283–293. [Google Scholar]
- Ninomiya Y, Imoto T, Sugimura T. Sweet taste responses of mouse chorda tympani neurons: existence of gurmarin-sensitive and -insensitive receptor components. J Neurophysiol. 1999;81:3087–3091. doi: 10.1152/jn.1999.81.6.3087. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Mizukoshi T, Higashi T, Katsukawa H, Funakoshi M. Gustatory neural responses in three different strains of mice. Brain Res. 1984;302:305–314. doi: 10.1016/0006-8993(84)90244-0. [DOI] [PubMed] [Google Scholar]
- Okamura R. [Studies on total nerve fibers of chorda tympani nerve in man] Nippon Jibiinkoka Gakkai Kaiho. 1996;99:28–37. doi: 10.3950/jibiinkoka.99.28. [DOI] [PubMed] [Google Scholar]
- Wang Y, Danilova V, Cragin T, Roberts TW, Koposov A, Hellekant G. The sweet taste quality is linked to a cluster of taste fibers in primates: lactisole diminishes preference and responses to sweet in S fibers (sweet best) chorda tympani fibers of M. fascicularis monkey. BMC Physiol. 2009;9:1. doi: 10.1186/1472-6793-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, et al. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol. 2009;587:4425–4439. doi: 10.1113/jphysiol.2009.175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y. Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol. 2006;96(6):3088–3095. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]