Abstract
The ability to identify odors is dependent on the spatial mapping of odorant receptors onto fixed positions within the olfactory bulb. In elderly adults, odor identification and discrimination is often impaired. The objective of this study was to determine if there are age-related changes in odorant receptor mapping. We studied 8 groups of mice ranging in age from 2 weeks to 2.5 years and mapped the projection of P2 odorant receptors onto targeted glomeruli within medial and lateral domains of the olfactory bulb. A total of 60 mice were used to measure the number of P2 glomeruli, bulb length, the position of each glomerulus, and the amount of P2 axons targeting each glomerulus. We found that over 70% of olfactory bulbs contained multiple P2 glomeruli, bulb length increased 42% between the ages of 2 and 13 weeks, and the position of P2 glomeruli shifted with bulb growth. In most cases, targeted glomeruli were either completely or partially filled with P2 axons. In some cases, targeting was diffuse, with glomeruli receiving only a few stray P2-labeled axons. The frequency of diffuse targeting was rare (<4%) in adult mice 3–6 months in age. However, significant increases in diffuse targeting were observed in older mice, reaching 10% at 1 year and 22% at 2 years of age. These findings suggest that odorant receptor mapping becomes more disrupted in old age and could account for impaired olfactory function in elderly adults.
Keywords: aging, glomeruli, olfactory bulb, olfactory discrimination, receptor mapping
Introduction
Many sensory systems project a topographical map of their receptors onto the brain. These maps help to encode information about the external stimulus environment. In the olfactory system, subsets of odorant receptors project to localized regions in the olfactory bulb terminating in discrete glomeruli (Ressler et al. 1994; Mombaerts et al. 1996). This spatial mapping of odorant receptors onto the olfactory bulb provides an anatomical substrate for the coding of odor quality and odor discrimination (Sullivan et al. 1995).
During early development, the targeting of P2 odorant receptors and their projections are relatively scattered and diffuse (Royal and Key 1999). As mice mature, P2 axons converge onto discrete glomeruli located within restricted domains of the olfactory bulb. The convergence and localization of P2 odorant receptors onto targeted glomeruli have been described in a number of studies (Ressler et al. 1994; Sullivan et al. 1995; Mombaerts et al. 1996). Age-related changes in the olfactory system have been primarily associated with neuronal death (Hinds and McNelly 1981; Conley et al. 2003) as well as environmental and other health-related issues (Elsner 2001a; Rawson 2006). Changes in olfactory function are commonly reported in elderly adult patient populations (Doty et al. 1984; Elsner 2001b; Murphy et al. 2002), although the underlying cause for this loss of function has not been identified. It is possible that diffuse axon targeting, and alterations in odorant mapping, occur during the aging processes, just as they do during early development and growth periods (Royal and Key 1999). Do P2 axons continue to target the same glomerulus while the olfactory bulb is growing and increasing in size? Do P2 glomeruli shift or move within the bulb as it increases in length? In this study, we investigated the mapping of P2 axons onto targeted glomeruli in mice at different stages of growth, from 2 weeks to 2.5 years of age. We included age groups representing development and growth (2, 5, 13, and 26 weeks) as well as those representing elderly adults (1, 1.5, 2, 2.5 years of age). We were particularly interested in the elderly adult group and if changes in the precision or location of odor maps could provide some insight into a possible underlying cause of olfactory dysfunction commonly reported in elderly adult populations (Stevens and Cain 1987; Cain and Stevens 1989).
Several methods have been established for mapping P2 glomeruli within the olfactory bulb (Johnson and Leon 2000; Schaefer et al. 2001). Most mapping studies have used relatively young animals. This is the first study to examine and compare P2 odorant mapping in a wide range of age groups.
Materials and methods
Animals
In this study, we used homozygous P2-IRES-tau lacZ mice that were bred and raised in our mouse facility. This strain of mice has been extensively used to map the projection of the P2 odorant receptor onto targeted glomeruli in the olfactory bulb. A total of 60 male and female mice were assigned to 8 different age groups ranging from 2 weeks to 2.5 years (2, 5, 13, 26, 52, 78, 104, and 130 weeks). Table 1 lists the number of mice and average body weight for each group. We used 15 mice for the 5-week age group, 7 females and 8 males. This made it possible for us to compare our data with that reported by Schaefer and his colleagues (Schaefer et al. 2001) who established the methods we used for measuring the coordinate positions of P2 glomeruli. We included 8 male mice in our study so that we could test for sex-related differences in P2 mapping. Several of the mice assigned to the 2.0- and 2.5-year age groups died before reaching the designated age time points (survival rate was only 50% at the 1.5-year age point). This required reassignment of additional mice to these older age groups. Mouse breeding and experimental procedures were all performed under protocols approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Table 1.
Number of mice, age groups, and body weight
| N | Age group |
Body weight (g) |
||
| Group | Weeks | Mean ± standard deviation | Range | |
| 6 | 2 Weeks | 2 | 8.0 ± 0.9 | 6.9–9.1 |
| 15 | 5 Weeks | 5 | 19.8 ± 3.1 | 14.4–25.9 |
| 7 | 3 Months | 13 | 28.1 ± 4.1 | 22.3–33.5 |
| 5 | 6 Months | 26 | 29.7 ± 2.3 | 27.0–33.6 |
| 6 | 1.0 Year | 52 | 31.3 ± 2.4 | 27.7–33.6 |
| 7 | 1.5 Years | 78 | 33.0 ± 3.7 | 25.6–36.4 |
| 8 | 2.0 Years | 104 | 30.0 ± 6.6 | 21.8–40.2 |
| 6 | 2.5 Years | 130 | 33.7 ± 7.5 | 28.1–49.0 |
Tissue processing
Mice were injected with sodium pentobarbital (80 mg/kg) and administered an intracardiac perfusion of saline followed by 4% paraformaldehyde. The head was removed by rapid decapitation, decalcified with 0.3 M ethylenediaminetetraacetic acid, cryoprotected with 30% sucrose. The olfactory bulbs were sectioned with the skull intact, and tissue blocks were oriented relative to the long axis of the head. Heads were blocked by cutting through the middle of the cerebral cortex perpendicular to the dorsal surface of the skull. Serial coronal sections, 15 or 30 μm in thickness, were cut on a cryostat beginning with the nasal cavity through the olfactory bulbs and into cortex. Tissue sections were mounted on glass slides and stained with X-Gal (blue stain) to label the P2 odorant receptor axons and their terminal endings in glomeruli. Neutral red was used as a counterstain to highlight the cellular morphology of the bulb.
Measurements
To map the coordinates of P2 glomeruli, we used a method developed and validated by Schaefer and colleagues (Schaefer et al. 2001). Measurements were made from digitized images taken of the complete set of coronal sections beginning with the anterior (rostral) to the posterior (caudal) limits of each olfactory bulb. Images (2560 × 2048 pixels) were captured using Nikon ACT 1 software and a DXM-1200 digital camera. Image J software and the glomerular analysis plug-in module (version 2.2), written specifically for mapping individual glomeruli, were used to make distance and angle measurements from the digital images (Schaefer et al. 2001; Salcedo et al. 2005). A calibration slide with a 1000 μm reticule was used to determine the pixel resolution (1000 μm = 566 pixels, ∼2 μm/pixel). Images of the left olfactory bulbs were flipped horizontally so that when making angle measurements, the medial side of the bulb was always on the left side of the image (Figure 1).
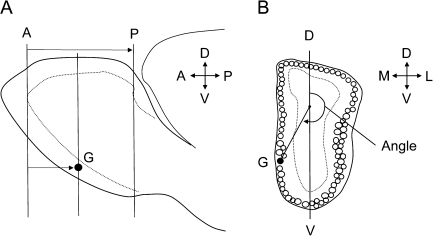
Figure 1.
Diagram illustrating the method used to measure the position of P2 glomeruli. (A) Lateral view of the olfactory bulb showing lines representing coronal sections passing through the plane of the anterior reference section (A), posterior reference section (P), and section containing the glomerulus (G). The AP distance defines the olfactory bulb length and AG distance the position of the glomerulus. (B) Diagram of a coronal section of the olfactory bulb passing through a glomerulus G. A point one-third the distance along a line (D–V) passing though the dorsal and ventral glomerular layers, and a vector extending to the center of the glomerulus G, was used to measure the angle that defines the position of the glomerulus within a section. Both AG distance and angle were used to determine the coordinate position of each glomerulus (see Materials and methods for details). M, medial; L, lateral; D, dorsal; V, ventral.
Every bulb section containing a P2-labeled glomerulus was identified, imaged, and then used to measure and record the exact location of each P2 glomerulus. P2 glomeruli were classified as GF if P2-stained axons “filled” the glomerulus (>90% stained blue). Glomeruli “partially” filled (10–90%) were classified as GP, and those with “trace” amounts of P2 staining (<10%) were labeled GT. For the 5-week age group, cross-sectional area measurements were obtained for all GF glomeruli. For both the lateral and the medial region (domains) of each bulb, the glomerulus with the largest cross-sectional area was assigned the classification GFmax. Specific bulb sections were identified as reference points and subsequently used to make distance measurements. The anterior reference section, A, was defined as the first section in which the mitral cell and external plexiform layer was present. The posterior reference section, P, was defined as the first section in which the granular cusp of the anterior olfactory nucleus (AOB) was present. Glomerular sections, G, were identified for all sections containing a P2-positive glomerulus. AG distances were measured from the anterior bulb reference section (A) to each section containing P2 glomeruli (G) and were calculated by multiplying section thickness by the number of sections from A to G (see Figure 1A). For example, a glomerulus located 40 sections from the anterior section A would have an AG distance of 600 μm (40 × 15 = 600 μm). In addition to the AG distances, for each P2 glomerulus, a corresponding measurement of bulb length (AP distance) was recorded. Within bulb sections, the position of each P2 glomerulus was defined by a vector drawn from a central reference point (one-third the distance along a line bisecting the bulb and passing from the dorsal to ventral surface of the glomerular layer) through the glomerulus (Figure 1B). Then the angle from the dorsal segment to the glomerulus vector was measured. Images of the left olfactory bulb were flipped horizontally so that the medial side of the bulb was always on the left side. Using this system, all angle measurement of <180 degrees located glomerluli on the lateral side of the bulb (lateral domain) and >180 degrees on the medial side (medial domain). We also measured the area (square micron) occupied by each glomerulus, allowing us to identify the largest P2 glomerulus (GFmax). This was useful in cases where multiple glomeruli were found within the same domain (on the same side of the bulb). Mice were excluded from data analysis if there were damaged or missing tissue sections, if it was not possible to clearly identify the A and P reference sections, or if the AP distances for the right and left bulbs differed by more than 10% (e.g., left bulb AP distance − right bulb AP distance/largest AP distance > ±0.10).
Statistical methods
Data were analyzed using SPSS statistical software and SigmaPlot. For comparing P2 mapping data, we performed analyses of variance and used the Tukey honestly significant difference post hoc test for all multiple comparisons. Significant differences were reported at the P < 0.05 level. Confidence intervals (95% confidence interval) were calculated to define regions or domains in which there is a 95% likelihood of finding a P2 glomerulus.
Results
Number, size, and coordinate positions of P2 glomeruli
We first determined the number, size, and position of all GF glomeruli present in the olfactory bulbs of mice in the 5-week age group. This group contained the largest number of mice (N = 15) and represented 30 olfactory bulbs and 60 domains (medial and lateral for each bulb). In all 30 olfactory bulbs examined, there was at least 1 GF glomerulus present in both the medial and the lateral domain (all 60 domains). GFs located on the lateral side of the olfactory bulb (Figure 2A) had an AG distance of 640 ± 165 μm (mean ± standard deviation). These glomeruli were located anterior to those on the medial side, 1287 ± 260 μm. In the majority of cases, multiple GFs (Figure 2B) were found within a single region or domain within the olfactory bulb. Table 2 gives the number and frequency for single, double, and triple occurrences of GF glomeruli within each of the 2 domains. In most cases, there were at least 2, and sometimes 3, GF glomeruli. The most common occurrence was 2 GFs on the lateral side and 2 on the medial side, for a total of 4 GFs in each olfactory bulb.
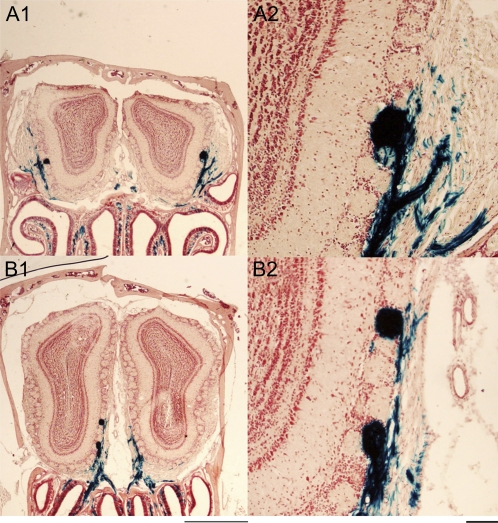
Figure 2.
Coronal sections of olfactory bulbs illustrating location of GF (full) glomeruli. (A1) In anterior sections, P2 glomeruli (round structures, dark blue stain) are found in lateral regions (domains) of the bulb. In more posterior sections, (B1) glomeruli are located on the medial side. Examples of single and double occurrences of GF glomeruli are shown at higher magnification, (A2 and B2). Sections stained with X-Gal and neutral red. Calibration bars: 1000 μm for A1and B1, 100 μm for A2 and B2.
Table 2.
Number and percentage of olfactory bulbs in which single or multiple P2 glomeruli were found
| Glomeruli found | Lateral domain | Medial domain |
| None | 0 (0%) | 0 (0%) |
| Only 1 | 8 (27%) | 6 (20%) |
| Only 2 | 18 (60%) | 17 (57%) |
| Only 3 | 4 (13%) | 7 (23%) |
| 4 or more | 0 (0%) | 0 (0%) |
Data from 5-week-old mice, N = 30 bulbs.
To study the relationship between each of the GF glomeruli within a given domain, glomeruli were subdivided according to their anterior–posterior and dorsal–ventral location. The most anterior and dorsal glomerulus was labeled GF1, the next 1 encountered GF2, and subsequent glomeruli GF3, GF4, etc. The average values and 95% confidence limits for AG distance, area, and diameter measurements for all 106 GF glomeruli are shown in Table 3. In most cases, GF1 and GF2 glomeruli were located in the same or adjacent coronal sections. GF3 glomeruli were on average located farther away (±75–150 μm), although this distance is relatively small when compared with the diameter of a typical GF glomerulus (100 μm). Although GF1 and GF2 glomeruli tended to be similar in size (area), GF3 glomeruli (third GF within a domain) were typically smaller in size (Table 3).
Table 3.
AG distance, area, and diameter measurements for the first, second, and third P2 glomerulus located within the lateral or medial domain
| Parameter | Glomerulus | Lateral domain |
Medial domain |
||||
| N | Mean ± SEM | 95% CI | N | Mean ± SEM | 95% CI | ||
| AG distance (μm) | GF1 | 30 | 618 ± 28 | 561–675 | 30 | 1261 ± 48 | 1164–1358 |
| GF2 | 18 | 647 ± 36 | 574–720 | 17 | 1286 ± 64 | 1158–1415 | |
| GF3 | 4 | 720 ± 77 | 565–875 | 7 | 1457 ± 100 | 1257–1657 | |
| Area (μm2) | GF1 | 30 | 8394 ± 638 | 7112–9677 | 30 | 9640 ± 695 | 8244–11036 |
| GF2 | 18 | 7474 ± 824 | 5819–9130 | 17 | 7015 ± 924 | 5160–8869 | |
| GF3 | 4 | 4794 ± 1748 | 1282–8306 | 7 | 6380 ± 1440 | 3490–9270 | |
| Diametera (μm) | GF1 | 30 | 100 ± 4 | 92–109 | 30 | 108 ± 4 | 99–117 |
| GF2 | 18 | 96 ± 5 | 85–107 | 17 | 91 ± 6 | 79–103 | |
| GF3 | 4 | 75 ± 11 | 52–98 | 7 | 90 ± 9 | 71–108 | |
Data are from 5-week-old mice, N = 30 bulbs. SEM, standard error of the mean; CI, confidence interval.
Diameter values were derived from area measurements.
Mapping the location of P2 glomeruli using cylindrical coordinates
We used a cylindrical coordinate system to map AG distances and angle measurements for each GF glomerulus and then made comparisons between the right and left olfactory bulbs as well as the lateral and medial domains within each bulb (Table 4). Analysis of our GF1, GF2, and GF3 data demonstrated that there were size differences in GF glomeruli. Previous mapping studies reported coordinate positions for only the large volume glomeruli (Schaefer et al. 2001). In this study, we used the GF area measurements to identify the largest glomerulus, GFmax, in each domain (2 GFmax glomeruli per bulb). We then compared the coordinate positions for GFmax glomeruli in the left and right olfactory bulbs (Table 4). There were no significant differences between the left and right olfactory bulbs for GFmax positions in either the lateral (654 μm, 147 degrees vs. 640 μm, 148 degrees) or the medial (1288 μm, 205 degrees vs. 1286 μm, 204 degrees) domains. In this study, we included both female and male mice and demonstrated sex differences for GFmax mapping as shown in Table 5. We found that the AG distances in both the lateral and the medial domains for female mice (735 and 1412 μm) were significantly larger than for males (557 and 1178 μm). However, the position within a bulb section, as defined by the angle measurement, was the same for both male and female mice. There was no difference in the size (area measurements) of GFmax glomeruli between female and male mice.
Table 4.
Coordinates for lateral and medial P2 glomeruli (right vs. left bulb)
| Left bulb |
Right bulb |
P value | Combined |
95% Confidence interval |
|||||
| Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Lower | Upper | ||
| Lateral | |||||||||
| AG distance (μm) | 654 ± 183 | 15 | 626 ± 150 | 15 | 0.650 | 640 ± 165 | 30 | 577 | 703 |
| Angle (degrees) | 147 ± 6 | 15 | 148 ± 9 | 15 | 0.705 | 148 ± 8 | 30 | 145 | 151 |
| Area (μm2) | 8683 ± 3113 | 15 | 10 740 ± 3125 | 15 | 0.082 | 9712 ± 3238 | 30 | 8545 | 10 878 |
| Medial | |||||||||
| AG distance (μm) | 1288 ± 287 | 15 | 1286 ± 254 | 15 | 0.984 | 1287 ± 266 | 30 | 1186 | 1388 |
| Angle (degrees) | 205 ± 3 | 15 | 204 ± 5 | 15 | 0.671 | 205 ± 4 | 30 | 203 | 206 |
| Area (μm2) | 10 642 ± 3033 | 15 | 11 494 ± 3206 | 15 | 0.461 | 11 068 ± 3097 | 30 | 9901 | 12 235 |
| AP distance (μm) | 1884 ± 162 | 15 | 1876 ± 130 | 15 | 0.882 | 1880 ± 144 | 30 | 1825 | 1935 |
Data are from 5-week-old mice, N = 30 bulbs. Only the largest glomerulus (GFmax) in each of the 2 domains (lateral and medial) was used in calculating coordinates for this table. SD, standard deviation.
Table 5.
Coordinates for lateral and medial P2 glomeruli in 5-week-old mice (female vs. male)
| Female |
Male |
P value | Combined |
95% Confidence interval |
|||||
| Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Lower | Upper | ||
| Lateral | |||||||||
| AG distance (μm) | 735 ± 151 | 14 | 557 ± 131 | 16 | 0.002** | 640 ± 165 | 30 | 593 | 699 |
| Angle (degrees) | 150 ± 9 | 14 | 146 ± 6 | 16 | 0.093 | 148 ± 8 | 30 | 145 | 151 |
| Area (μm2) | 9031 ± 3167 | 14 | 10 307 ± 3282 | 16 | 0.290 | 9712 ± 3238 | 30 | 8459 | 10 879 |
| Medial | |||||||||
| AG distance (μm) | 1412 ± 214 | 14 | 1178 ± 264 | 16 | 0.013* | 1287 ± 266 | 30 | 1204 | 1386 |
| Angle (degrees) | 203 ± 4 | 14 | 206 ± 4 | 16 | 0.139 | 205 ± 4 | 30 | 203 | 206 |
| Area (μm2) | 12 160 ± 3484 | 14 | 10 113 ± 2435 | 16 | 0.070 | 11 068 ± 3097 | 30 | 10 024 | 12 249 |
| AP distance (μm) | 1961 ± 120 | 14 | 1809 ± 128 | 16 | 0.002** | 1880 ± 144 | 30 | 1838 | 1932 |
Data are from 5-week-old mice, N = 30 bulbs. Only the largest glomerulus (GFmax) in each of the 2 bulb domains (lateral and medial) was used in calculating coordinates for this table. SD, standard deviation.
*p < .05; **p < .01.
Age-related changes—AP bulb dimensions and AG distance
To determine if there were age-related changes in the odorant receptor maps, we measured and compared the coordinate positions (AG distance and angle) of GF glomeruli in mice at 2, 5, 13, 26, 52, 78, 104, and 130 weeks of age. A total of 393 glomeruli from 120 olfactory bulbs were identified and analyzed. We first noticed an increase in AG distance, especially between the ages of 2, 5, and 13 weeks. This shift in AG distance in the caudal (posterior) direction appears to be related to a corresponding increase in bulb length (AP distance) and is most likely associated with normal bulb growth. Figure 3A shows changes in bulb length (AP distance) with age and body weight. Significant increases in bulb length occur between 2 and 5 weeks (364 μm, 24% increase) and 5 and 13 weeks (267 μm, 14% increase). The corresponding increase (caudal shift) in AG distance for GF glomeruli in the medial domain is illustrated in Figure 3B. It is likely that the absolute position (AG distance) will shift as the bulb grows and elongates in size; therefore, we also calculated the relative GF position (AG/AP ratio) to adjust for changes in overall bulb length. To determine if there are age-related changes in the relative position of GF glomeruli, we first plotted the GF coordinates for each age group using the AG/AP distance ratio and angle measurements (see Figure 4). In this figure, GF glomeruli in the lateral domain are located between 131 and 152 degrees and those in the medial domain 203 and 210 degrees. Variability in the angle measurement is greater in the lateral domain compared with the medial domain. AG/AP distance, relative position of glomeruli, in younger mice appears to be more caudal (toward the back of the bulb) when compared with older mice. In the medial domain, GF position appears to be more evenly distributed among age groups with the exception of the 2-week mice. A statistical comparison of GF mapping between young (2 weeks to 6 months) and old (1.0–2.5 years) mice is given in Table 6. This table shows that in the lateral domain, there is a significant difference in GF mapping between young and old mice both for AG/AP distance and angle. No significant difference was found in the medial domain. We also found that there was an age-related increase in the size of GF glomeruli between 2 and 5 weeks in both the lateral and the medial domains of the olfactory bulb (Table 7). However, after 5 weeks of age, there were no significant differences in the size of GF glomeruli between any of the older age groups.
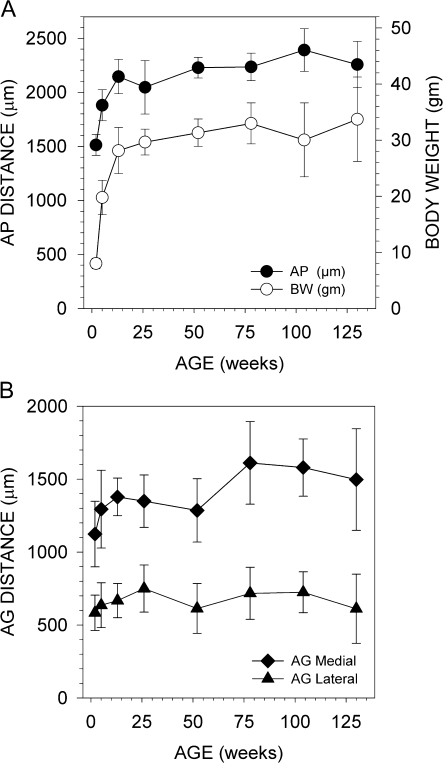
Figure 3.
Graphs showing age-related changes in the olfactory bulbs. (A) Olfactory bulb length (AP distance) and body weight both increase with age. (B) The AG distance of P2 glomeruli shifts as the bulb increases in length. This shift is most noticeable during a period of rapid growth (2–13 weeks).
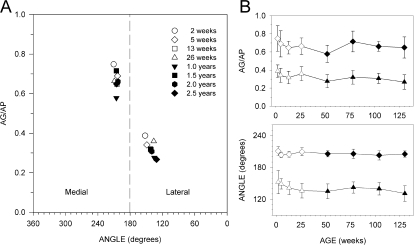
Figure 4.
Graphs illustrating the relative position of GF glomeruli for different age groups. (A) Graph shows P2 glomeruli mapped in bulb space using AG/AP (relative position in bulb) and angle (medial lateral position) measurements. P2 glomeruli in younger mice tend to be in a more caudal (posterior) location than that for older mice. (B) Graphs showing changes in relative position (AG/AP) and angle with age. Data (means ± standard deviations) for GFs in the medial (diamonds) and lateral (triangles) domains are plotted separately.
Table 6.
Comparison of relative position of GF glomeruli in young versus old mice
| Domain | Young |
Old |
P | |||
| Mean ± SD | N | Mean ± SD | N | |||
| Lateral | AG/AP (ratio) | 0.35 ± 0.08 | 118 | 0.29 ± 0.07 | 77 | 0.000** |
| Angle | 146 ± 15 | 118 | 138 ± 13 | 77 | 0.000** | |
| Medial | AG/AP (ratio) | 0.69 ± 0.13 | 117 | 0.65 ± 0.11 | 81 | 0.051 NS |
| Angle | 206 ± 7 | 117 | 205 ± 8 | 81 | 0.228 NS | |
Data represent means for all GF glomeruli (GF1, GF2, and GF3). Young mice (2, 5, 13, and 25 weeks); old mice (1.0, 1.5, 2.0, and 2.5 years); SD, standard deviation; NS, not significant.
**p < .01.
Table 7.
Glomerular size measurements (GF area square micron) for different age groups
| Age group | Lateral domain |
Medial domain |
||||
| Weeks | N | Meana ± SEM | 95% CI | N | Meana ± SEM | 95% CI |
| 2b | 23 | 5250 ± 669 | 3931–6569 | 21 | 5372 ± 643 | 4105–6640 |
| 5 | 52 | 7993 ± 445 | 7116–8871 | 54 | 8683 ± 401 | 7892–9473 |
| 13 | 22 | 7613 ± 684 | 6264–8962 | 21 | 7155 ± 643 | 5887–8422 |
| 26 | 21 | 7082 ± 700 | 5702–8463 | 21 | 6766 ± 643 | 5498–8033 |
| 52 | 22 | 7732 ± 684 | 6383–9081 | 19 | 8130 ± 675 | 6798–9462 |
| 78 | 22 | 7954 ± 684 | 6605–9303 | 22 | 7347 ± 628 | 6109–8585 |
| 104 | 20 | 8090 ± 717 | 6676–9505 | 23 | 8042 ± 614 | 6831–9253 |
| 130 | 13 | 7675 ± 889 | 5920–9430 | 17 | 7583 ± 714 | 6174–8992 |
aMean cross-sectional areas (square micron) for GF glomeruli in the lateral and medial bulb domains. SEM, standard error of the mean; CI, confidence interval.
bComparison between groups (analysis of variance and Tukey post hoc test) found that glomeruli in the 2-week age group were significantly smaller (P < 0.001) than other groups.
Age-related changes in P2 axon convergence and targeting
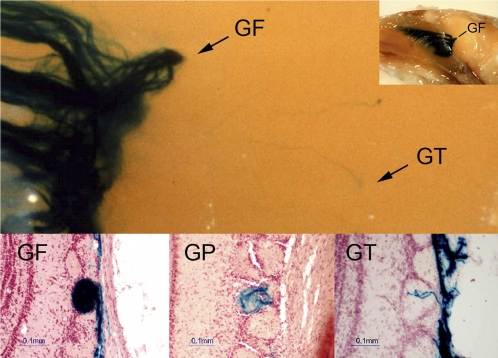
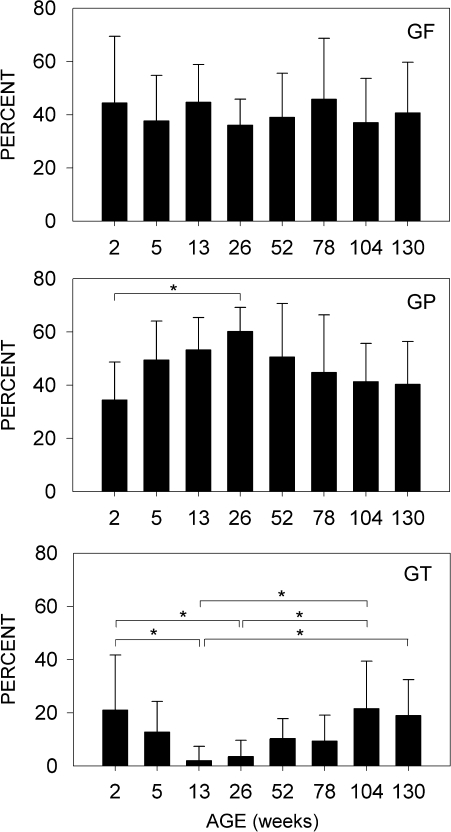
In this study, we analyzed data from 60 mice ranging in age from 2 weeks to 2.5 years and found over 1000 glomeruli that received input from axons expressing the P2 odorant receptor. For most glomeruli, there was a high degree of convergence and targeting. These glomeruli were either completely filled (GF) or partially filled (GP) with P2 axons. Most olfactory bulbs had 2, and sometimes 3, GF glomeruli in each of their 2 domains (lateral and medial). GF glomeruli averaged 4 per bulb and were always present along with GP glomeruli that typically numbered 4–5 per bulb. GT glomeruli, having only trace amounts of P2 axons, were relatively rare and varied in number depending on the age group. Examples of GF, GP, and GT glomeruli are shown in Figure 5. There was no change in the relative number of GF glomeruli present among the different age groups from 2 weeks to 2.5 years (Figure 6). GP glomeruli gradually increased between the ages of 2 and 26 weeks and then decreased as mice began to age. Occasionally, GT glomeruli were found in adult mice (13–26 weeks), but they represented only 2–4% of all the P2 glomeruli. There was, however, a significant (P < 0.05) increase in the number of GT glomeruli observed in older mice, reaching levels of 19–22% in the 2.0- and 2.5-year-old age groups. GTs were relatively common in the 2-week age group, as would be expected during early development where there is diffuse targeting (Royal and Key 1999).
Figure 5.
Images of glomeruli showing differences in P2 target specificity and amounts of innervation. Photo above shows the convergence of P2 axons (dark blue stain) onto a single glomerulus (GF) and a stray axon targeting additional glomeruli (GT). Inset (top right) shows low-power view of olfactory bulb and nasal cavity whole mount preparation. Histological sections in lower panels give examples of glomeruli filled (GF), partially filled (GP), and those with only trace (GT) amounts of P2 axons.
Figure 6.
Graphs showing the percentages of GF, GP, and GT glomeruli present in the olfactory bulb for different age groups. The percentage of GF glomeruli was similar across all age groups. GP glomeruli increased during growth and maturation (2–26 weeks) and then declined with increasing age. GT glomeruli were relatively common (20%) at 2 weeks, decrease in adults (13 and 26 weeks), and then gradually increase in old age (104–130 weeks, 2–2.5 years). Error bars, ±standard deviation; * denotes P < 0.05. Multiple comparisons of the means for the different age groups were tested with an analysis of variance and the Tukey honestly significant difference post hoc test.
Discussion
The results from this study provide a quantitative description of age-related changes in the mapping of P2 odorant receptors onto glomeruli in the olfactory bulb. Research investigating mechanisms underlying the formation of odorant maps often depend upon the measurement and analysis of shifts in the position of targeted glomeruli. In this study, we report position differences that are due to elongation of the olfactory bulb and occur during periods of rapid growth (2–13 weeks). We also found disrupted targeting increases in frequency with aging and that there are male versus female differences in the position of P2 glomeruli. These findings could prove important for the design, analysis, and interpretation of future studies investigating mechanisms of odorant receptor guidance, targeting, and mapping.
Position of P2 glomeruli in 5-week-old mice
The method we used to map the position of glomeruli was first described and validated in 5-week-old mice (Schaefer et al. 2001). In that study, researchers mapped the position of P2 glomeruli in both the lateral and the medial domains of 12 olfactory bulbs from 6 female mice. This 5-week time point is commonly used in studies because mice become sexually mature around 30 days. We selected the 5-week age group to make detailed measurements for P2 glomeruli in both male (N = 7) and female (N = 8) mice. This not only allowed us to test for sex differences but also provided sufficient numbers of glomeruli to make detailed comparisons. For example, we were able to show that when there are multiple glomeruli present in a single domain (G1, G2, and G3), the first 2 glomeruli targeted are in close proximity and similar in size. The third glomerulus, G3, is typically smaller in size, located farther away from G1 and G2, and its position is more variable (Table 3). We also confirmed that there were no significant differences in the coordinate positions, or size, of P2 glomeruli when comparing the left and right olfactory bulbs. Odor maps in the 2 bulbs are essentially mirror images of each other.
Schaefer and his colleagues were careful to point out that the AP and AG distance measurements obtained when mapping glomerular positions depend upon the plane of sectioning (Schaefer et al. 2001). In the appendix of their paper, they provide an equation for converting data when bulb sections are cut at different angles. In this study, we did not remove the olfactory bulbs from the skull, and coronal sections were cut through the nasal cavity and olfactory bulbs in a plane perpendicular to the dorsal surface of the brain (e.g., from tip of the nose to frontal cortex). Most of the data reported in the study of Schaefer et al. (2001) were obtained from sections cut perpendicular to the lateral olfactory tract at a 10.6 degree angle to the ventral surface of the brain. However, they did report data from 2 olfactory bulbs cut perpendicular to the ventral surface. The coordinates for these P2 glomeruli located in both the lateral (AP distance 837 μm, angle 128 degrees) and the medial (1539 μm, 210 degrees) domains are in close agreement with those for the 5-week-old females in our study (Table 5, lateral, 735 ± 151 μm, 150 degrees; medial, 1412 ± 214, 203 degrees). We found that the location of P2 glomeruli for male mice was significantly different (more anterior) than for female mice. This was true for both the lateral and the medial domains of the bulb. These findings may reflect sex differences in growth rates and corresponding increases in bulb size and elongation for this age group (Table 5, AP distance).
Growth and age-related shifts in position of P2 glomeruli
In a study of early postnatal development, it was shown that dorsoventral compression of the olfactory bulb alters the topographic positioning of P2 glomeruli (Chehrehasa et al. 2007). We found that between the ages of 2 weeks and 3 months, there is a rapid and significant increase (42%, 631 μm) in bulb length (Figure 3A). Corresponding to this elongation of the bulb is a shift in the position (AG distance) of P2 glomeruli in both the medial and the lateral domains (Figure 3B). This shift appears to be greater in the medial domain and may be due to the relatively linear arrangement of the glomerular layer compared with the lateral side. To determine if this position shift was due to the elongation of the olfactory bulbs, we also calculated the position of each glomerulus relative to the AP length of each bulb (AG/AP ratio). Figure 4 shows age-related changes for the relative positions of P2 glomeruli. Whereas the AG position moves caudally, in a posterior direction, as the bulb elongates, the relative position of glomeruli remains stationary or in the case of young mice shifts in a rostral anterior direction (Figure 4). Statistical analysis of the data comparing young (2–15 weeks) with old (1–2.5 years) mice reveals a significant (P < 0.001) shift of P2 glomeruli in the lateral domain and an almost significant (P < 0.051) shift in the medial domain (Table 6). These results suggest that the position of P2 glomeruli in young mice is different than the position in older mice. In addition, position changes are more likely to be detected among younger mice due to development, growth, and maturation of the olfactory bulb.
The guidance and targeting of axons to glomeruli localized in fixed regions of the olfactory bulb is an essential factor in the formation of odorant receptor maps. Studies investigating guidance molecules and other factors contributing to the formation of olfactory sensory maps depend on the accurate measurement and comparison of the coordinate positions used to localize glomeruli. Data from this study suggest that the use of ratio, or relative position measurements, is the method of choice, especially when comparing data obtained from young mice. Bulb size can change rapidly within just a few days or weeks (Figure 3A). In addition, the olfactory bulb size of mice that have been genetically altered may differ from the wild-type controls. Some studies have been careful to use ratio measurements when testing for changes in mapping locations among genetically altered strains of mice (Cutforth et al. 2003).
Olfactory impairment in elderly adult mice
A major finding in this study was the significant increase in disrupted targeting, GTs, that is present in old mice. Diffuse targeting of P2 axons is commonly observed during the early development of the olfactory bulb (Royal and Key 1999). The precision of axon targeting improves as mice mature and become adults. Our data comparing GF, GP, and GT glomeruli are consistent with studies of early development and demonstrate that there is a significant decrease in GT glomeruli (diffuse targeting) as mice become mature adults (13–26 weeks). However, we also found that diffuse targeting increases again with age and reaches high levels at 104 and 130 months (2.0 and 2.5 years old). These new findings suggest that the odor map is disrupted in old age. This age-dependent increase in GT glomeruli (Figure 6) and a corresponding change in the precision of the odor map could provide a possible explanation for why so many elderly adults have impaired odor discrimination and odor identification function. A possible reason for this change may be associated with the loss of guidance cues as the olfactory bulb ages (Schwarting et al. 2004). One of the candidate guidance molecule, ephrin-A, is present on the axons of olfactory neurons. When these molecules are modulated, there is a corresponding shift in the position to which P2 axons target glomeruli within the olfactory bulb (Cutforth et al. 2003). The loss of important guidance molecules in old age could contribute to an increase in poorly targeted axons and provide a plausible explanation for the increase in GT glomeruli observed in this study.
Although age-related changes in the olfactory epithelium could contribute to targeting changes observed in the bulb, several studies have shown that cells in the epithelium live longer in adults than younger animals (Weiler and Farbman 1998) and that olfactory neurons retain their sensitivity while only a few subtypes, not the P2s, actually decrease in density (Lee et al. 2009). The number of glomeruli in the olfactory bulb are also known to decrease with age (Meisami et al. 1998). This may contribute further to the problem of maintaining precise targeting to glomeruli and result in increases in the frequency of GT glomeruli.
In this study, we confirmed findings from previous reports and provided new data demonstrating that P2 odorant receptors project onto multiple glomeruli located in lateral and medial domains within the olfactory bulb. In addition, we showed that when there are more than 2 targeted glomeruli, additional glomeruli are smaller in size and more distant from the others. We also found that significant changes in bulb length occur between 2 and 13 weeks of age and corresponding shifts in the position of P2 glomeruli. These findings suggest that mapping studies performed on young, or old, mice should adjust for differences in bulb size or use relative position (ratio) measurements when comparing data between experimental age groups. Our findings also show that there are age-related changes in target specificity and that there is a gradual and significant increase in disrupted glomerular targeting as mice begin to age. Further studies are needed to determine if this disrupted targeting may contribute in some way to impaired odor discrimination function.
Funding
National Institute on Deafness and Other Communication Disorders (DC000165 to R.M.C.).
References
- Cain WS, Stevens JC. Uniformity of olfactory loss in aging. Ann N Y Acad Sci. 1989;561:29–38. doi: 10.1111/j.1749-6632.1989.tb20967.x. [DOI] [PubMed] [Google Scholar]
- Chehrehasa F, Key B, St John JA. The shape of the olfactory bulb influences axon targeting. Brain Res. 2007;1169:17–23. doi: 10.1016/j.brainres.2007.06.073. [DOI] [PubMed] [Google Scholar]
- Conley DB, Robinson AM, Shinners MJ, Kern RC. Age-related olfactory dysfunction: cellular and molecular characterization in the rat. Am J Rhinol. 2003;17:169–175. [PubMed] [Google Scholar]
- Cutforth T, Moring L, Mendelsohn M, Nemes A, Shah NM, Kim MM, Frisen J, Axel R. Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell. 2003;114:311–322. doi: 10.1016/s0092-8674(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Elsner RJ. Environment and medication use influence olfactory abilities of older adults. J Nutr Health Aging. 2001a;5:5–10. [PubMed] [Google Scholar]
- Elsner RJ. Odor threshold, recognition, discrimination and identification in centenarians. Arch Gerontol Geriatr. 2001b;33:81–94. doi: 10.1016/s0167-4943(01)00175-3. [DOI] [PubMed] [Google Scholar]
- Hinds JW, McNelly NA. Aging in the rat olfactory system: correlation of changes in the olfactory epithelium and olfactory bulb. J Comp Neurol. 1981;203:441–453. doi: 10.1002/cne.902030308. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lee AC, Tian H, Grosmaitre X, Ma M. Expression patterns of odorant receptors and response properties of olfactory sensory neurons in aged mice. Chem Senses. 2009;34:695–703. doi: 10.1093/chemse/bjp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisami E, Mikhail L, Baim D, Bhatnagar KP. Human olfactory bulb: aging of glomeruli and mitral cells and a search for the accessory olfactory bulb. Ann N Y Acad Sci. 1998;855:708–715. doi: 10.1111/j.1749-6632.1998.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Rawson NE. Olfactory loss in aging. Sci Aging Knowledge Environ. 2006;2006:e6. doi: 10.1126/sageke.2006.5.pe6. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Royal SJ, Key B. Development of P2 olfactory glomeruli in P2-internal ribosome entry site-tau-LacZ transgenic mice. J Neurosci. 1999;19:9856–9864. doi: 10.1523/JNEUROSCI.19-22-09856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo E, Zhang C, Kronberg E, Restrepo D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chem Senses. 2005;30:615–626. doi: 10.1093/chemse/bji055. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Finger TE, Restrepo D. Variability of position of the P2 glomerulus within a map of the mouse olfactory bulb. J Comp Neurol. 2001;436:351–362. [PubMed] [Google Scholar]
- Schwarting GA, Raitcheva D, Crandall JE, Burkhardt C, Puschel AW. Semaphorin 3A-mediated axon guidance regulates convergence and targeting of P2 odorant receptor axons. Eur J Neurosci. 2004;19:1800–1810. doi: 10.1111/j.1460-9568.2004.03304.x. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Cain WS. Old-age deficits in the sense of smell as gauged by thresholds, magnitude matching, and odor identification. Psychol Aging. 1987;2:36–42. doi: 10.1037//0882-7974.2.1.36. [DOI] [PubMed] [Google Scholar]
- Sullivan SL, Ressler KJ, Buck LB. Spatial patterning and information coding in the olfactory system. Curr Opin Genet Dev. 1995;5:516–523. doi: 10.1016/0959-437x(95)90057-n. [DOI] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Proliferation decrease in the olfactory epithelium during postnatal development. Ann N Y Acad Sci. 1998;855:230–234. doi: 10.1111/j.1749-6632.1998.tb10572.x. [DOI] [PubMed] [Google Scholar]