Abstract
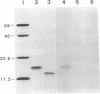
We previously purified an auxin-binding protein (ABP) from the microsomal fraction of maize shoots (Zea mays L. cv. Golden Cross Bantam). In the present study cDNA clones derived from mRNAs encoding the ABP were isolated and sequenced. The nucleotide sequence of the 822-base-pair cDNA includes a 603-base-pair open reading frame. RNA blot hybridization analysis indicated a single mRNA species of approximately 1.0 kilobase. The predicted precursor of ABP is composed of 201 amino acid residues and has a molecular weight of 21,976. The NH2-terminal sequence of 38 residues is hydrophobic and may be a signal peptide for translocation of the ABP across the membrane of the endoplasmic reticulum. The mature ABP, composed of 163 residues with a molecular weight of 18,352, contains a potential N-glycosylation site (Asn-Thr-Thr), and the COOH-terminal tetrapeptide (Lys-Asp-Glu-Leu) may be a signal for retention of the ABP in the lumen of the endoplasmic reticulum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainley W. M., Walker J. C., Nagao R. T., Key J. L. Sequence and characterization of two auxin-regulated genes from soybean. J Biol Chem. 1988 Aug 5;263(22):10658–10666. [PubMed] [Google Scholar]
- Brightman A. O., Barr R., Crane F. L., Morré D. J. Auxin-Stimulated NADH Oxidase Purified from Plasma Membrane of Soybean. Plant Physiol. 1988 Apr;86(4):1264–1269. doi: 10.1104/pp.86.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhout T. J., Young K. A., Low P. S., Morré D. J. In vitro promotion by auxins of divalent ion release from soybean membranes. Plant Physiol. 1981 Aug;68(2):512–515. doi: 10.1104/pp.68.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen R. E., Laties G. G. Ethylene regulation of gene expression in carrots. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4060–4063. doi: 10.1073/pnas.79.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C., Lehle L. Auxin induces rapid changes in phosphatidylinositol metabolites. Nature. 1988 Jan 14;331(6152):176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- Evans M. L. The action of auxin on plant cell elongation. CRC Crit Rev Plant Sci. 1985;2(4):317–365. doi: 10.1080/07352688509382200. [DOI] [PubMed] [Google Scholar]
- Gabathuler R., Cleland R. E. Auxin regulation of a proton translocating ATPase in pea root plasma membrane vesicles. Plant Physiol. 1985 Dec;79(4):1080–1085. doi: 10.1104/pp.79.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983 Jun 17;220(4603):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Jagus R. Hybrid selection of mRNA and hybrid arrest of translation. Methods Enzymol. 1987;152:567–572. doi: 10.1016/0076-6879(87)52063-8. [DOI] [PubMed] [Google Scholar]
- Key J. L., Kroner P., Walker J., Hong J. C., Ulrich T. H., Ainley W. M., Gantt J. S., Nagao R. T. Auxin-regulated gene expression. Philos Trans R Soc Lond B Biol Sci. 1986 Nov 17;314(1166):427–440. doi: 10.1098/rstb.1986.0063. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kutschera U., Briggs W. R. Rapid auxin-induced stimulation of cell wall synthesis in pea internodes. Proc Natl Acad Sci U S A. 1987 May;84(9):2747–2751. doi: 10.1073/pnas.84.9.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Lomax T. L., Mehlhorn R. J., Briggs W. R. Active auxin uptake by zucchini membrane vesicles: quantitation using ESR volume and delta pH determinations. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6541–6545. doi: 10.1073/pnas.82.19.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbler M., Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). II. Localization of a putative auxin receptor. J Biol Chem. 1985 Aug 15;260(17):9854–9859. [PubMed] [Google Scholar]
- Morré D. J., Gripshover B., Monroe A., Morré J. T. Phosphatidylinositol turnover in isolated soybean membranes stimulated by the synthetic growth hormone 2,4-dichlorophenoxyacetic acid. J Biol Chem. 1984 Dec 25;259(24):15364–15368. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988 Apr;7(4):913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Hardwick K. G., Lewis M. J. Sorting of soluble ER proteins in yeast. EMBO J. 1988 Jun;7(6):1757–1762. doi: 10.1002/j.1460-2075.1988.tb03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin and Fusicoccin Enhancement of beta-Glucan Synthase in Peas : An Intracellular Enzyme Activity Apparently Modulated by Proton Extrusion. Plant Physiol. 1985 Jul;78(3):466–472. doi: 10.1104/pp.78.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Specificity of Auxin-binding Sites on Maize Coleoptile Membranes as Possible Receptor Sites for Auxin Action. Plant Physiol. 1977 Oct;60(4):585–591. doi: 10.1104/pp.60.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura S., Sotobayashi T., Futai M., Fukui T. Purification and properties of an auxin-binding protein from maize shoot membranes. J Biochem. 1986 May;99(5):1513–1524. doi: 10.1093/oxfordjournals.jbchem.a135621. [DOI] [PubMed] [Google Scholar]
- Walton J. D., Ray P. M. Evidence for Receptor Function of Auxin Binding Sites in Maize : RED LIGHT INHIBITION OF MESOCOTYL ELONGATION AND AUXIN BINDING. Plant Physiol. 1981 Dec;68(6):1334–1338. doi: 10.1104/pp.68.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]