Abstract
The aims of this study were (i) to quantify chondrocyte mechanics in fully intact articular cartilage attached to its native bone and (ii) to compare the chondrocyte mechanics for cells in healthy and early osteoarthritis (OA) tissue. We hypothesized that cells in the healthy tissue would deform less for given articular surface pressures than cells in the early OA tissue because of a loss of matrix integrity in early OA and the associated loss of structural integrity that is thought to protect chondrocytes. Chondrocyte dynamics were quantified by measuring the deformation response of the cells to controlled loading of fully intact cartilage using a custom-designed confocal indentation system. Early OA was achieved nine weeks following transection of the anterior cruciate ligament (ACL) in rabbit knees. Experiments were performed on the retropatellar cartilage of early OA rabbit knees (four joints and 48 cells), the corresponding intact contralateral control knees (four joints and 48 cells) and knees from normal control rabbits (four joints and 48 cells). Nine weeks following ACL transection, articular cartilage of the experimental joints showed substantial increases in thickness, and progression towards OA as assessed using histological grading. Local matrix strains in the superficial zone were greater for the experimental (38 ± 4%) compared with the contralateral (27 ± 5%) and normal (28 ± 4%) joints (p = 0.04). Chondrocyte deformations in the axial and depth directions were similar during indentation loading for all experimental groups. However, cell width increased more for the experimental cartilage chondrocytes (12 ± 1%) than the contralateral (6 ± 1%) and normal control chondrocytes (6 ± 1%; p < 0.001). On average, chondrocyte volume increased with indentation loading in the early OA cartilage (8 ± 3%, p = 0.001), while it decreased for the two control groups (−8 ± 2%, p = 0.002 for contralateral and −8 ± 1%, p = 0.004 for normal controls). We conclude from these results that our hypothesis of cell deformations in the early OA tissue was only partially supported: specifically, changes in chondrocyte mechanics in early OA were direction-specific with the primary axial deformations remaining unaffected despite vastly increased average axial matrix deformations. Surprisingly, chondrocyte deformations increased in early OA in specific transverse directions which have received little attention to date but might be crucial to chondrocyte signalling in early OA.
Keywords: articular cartilage, chondrocytes, osteoarthritis, in situ cell mechanics, anterior cruciate ligament transection
1. Introduction
Osteoarthritis (OA) is the most common joint disease and is defined by degeneration of the cartilage surface, swelling and pain in the joint, osteophyte formation and changes to the subchondral bone architecture. About 10 per cent of all Canadians suffer from OA, and the corresponding costs are estimated to be approximately $5 billion per year in Canada alone.
Animal models of OA often include transection of ligaments or the removal of menisci to produce joint instabilities. Such instabilities are associated with increased cartilage thickness and water content and a decrease in proteoglycan content in the surface zone in early OA (Young et al. 2006). In the later stages of OA, there is fibrillation of the cartilage and eventually cartilage erodes away from the articular surfaces (Bullough 1992). In early OA, cartilage becomes softer, peak contact pressures are reduced and contact areas are generally increased (Seedholm et al. 1979; Hasler & Herzog 1998), indicating that the mechanics of force transmission across joints are altered prior to any symptomatic signs of OA. Chondrocyte metabolic activity is increased in early OA (Wang et al. 2003), chondrocytes proliferate (Poole et al. 1991) and extracellular matrix (ECM) components are synthesized at an increased rate (Lippiello et al. 1977; Aigner et al. 1999). This increase in metabolic activity is thought to limit the rate and degree of cartilage degeneration (Fukui et al. 2001; Sandell & Aigner 2001).
Transection of the rabbit anterior cruciate ligament (ACL) is a well-defined model of OA that has been used to characterize the early stages of the disease mechanically (Yoshioka et al. 1996), biochemically (Vignon et al. 1987; Sah et al. 1997) and biologically (Graverand et al. 2002). The rabbit ACL transection model has been said to go through similar stages as the human disease, although in the animal model disease progression is vastly accelerated (Vignon et al. 1987; Sah et al. 1997).
Early OA is associated with defined structural changes in the articular cartilage matrix, as described above, and these changes alter the environment of chondrocytes (Alexopoulos et al. 2003, 2005; Korhonen & Herzog 2008). Chondrocyte activity is thought to be regulated by a multitude of factors including genetic and environmental influences, the composition of the ECM and mechanical stimuli. Although the detailed regulatory mechanisms remain unknown, there is strong evidence suggesting that mechanical stimuli, such as compression (Sah et al. 1992; Kim et al. 1994; Buschmann et al. 1996; Quinn et al. 1998), shear stress (Smith et al. 1995), hydrostatic pressure (Lipiello et al. 1985; Hall et al. 1991) and osmotic stress (Hall et al. 1996), influence the biosynthetic activity of chondrocytes.
Chondrocyte mechanics have been studied extensively in healthy cartilage and explant tissues under mechanical compression (Kim et al. 1994; Guilak et al. 1995; Buschmann et al. 1996; Wong et al. 1997; Clark et al. 2003; Choi et al. 2007). Comparatively little is known about chondrocyte mechanics in the fully intact tissue and the changes in chondrocyte mechanics associated with early OA under mechanical load (Clark et al. 2005, 2006).
Therefore, the objective of this study was to quantify in situ chondrocyte mechanics for groups of cells in loaded and unloaded states using three-dimensionally reconstructed confocal image analysis. Chondrocytes from normal and early OA tissues were tested and compared. The unilateral ACL transection model in the rabbit was used to produce early OA. A custom-designed indentation system based on a confocal microscope was used to measure chondrocyte mechanics in real time in the fully intact cartilage attached to its native bone. Cell deformations were measured before and at steady state after loading for the same group of cells.
2. Material and methods
2.1. Animals and preparation
Patellar cartilage from 12 knees of 15 months old, skeletally mature female New Zealand white rabbits was used for chondrocyte mechanics analysis. Four knees from four rabbits (two left and two right knees) were harvested nine weeks following ACL transection, and the corresponding intact contralateral controls were harvested at the same time. Four additional knees from two rabbits were harvested from normal (ACL intact) rabbits. ACL transections were carried out using the protocol described by Bray et al. (1992).
2.2. Cartilage specimen preparation
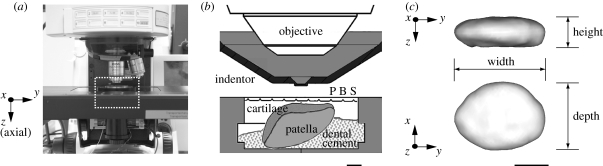
Retropatellar cartilages were grouped according to experimental (ACL-transected) and control (contralateral and normal controls), and were harvested with the intact patellae. Fluorescein conjugated dextran of 3 kDa molecular weight (excitation: 488 nm, emission: 500 nm; Molecular Probes, OR, USA) was suspended in DMEM (Dulbecco's Modified Eagle's Medium, Gibco, OR, USA) at a concentration of 0.8 mg ml−1 (0.26 mM). The patella was incubated in the dextran solution for 4–8 h at 4°C prior to fluorescent confocal imaging. After staining, tissue samples were washed in dye-free phosphate buffered saline (PBS) for 20 min. Patellae were then attached to the specimen holder using dental cement (figure 1). In order to provide a solid binding and rigidity between patellae and the dental cement, a self-tapping bone screw (diameter 1.17 mm and length 4.76 mm, Fine Science Tools, North Vancouver, BC, Canada) was inserted into the patellae. Patellae were then immersed in a PBS solution throughout testing to prevent dehydration of the cartilage surface.
Figure 1.
Custom-designed indentation system: (a) indentation system on confocal microscope; (b) schematic illustration of the rectangular area marked with the dashed line in (a). (c) Definition of cell morphology. Scale bar: (b) 2 mm and (c) 5 µm.
2.3. Cartilage loading and mechanical measurements
A surface pressure of 2 MPa was applied to the mid-region of the medial side of the retropatellar cartilage using a round glass indentor (diameter = 2 mm) at an average speed of 6 µm s−1. Once the desired pressure was reached, the indentor displacement was held constant for 20 min when a steady state was reached (Clark et al. 2003). A 40 × 0.8 n.a. and 0.17 mm cover glass corrected water immersion objective (Zeiss Inc., Germany) was used to capture cell images through the glass indentor with a spatial resolution of 0.41 × 0.41 µm. Optical sections were recorded before and at steady state after loading from the cartilage surface to a depth of 40 µm using a spacing of 0.5 µm in the optical (z) direction. The confocal microscope-based indentation system was developed specifically for the purpose of quantifying chondrocyte mechano-biology in the intact cartilage and has been described in detail previously (Han et al. 2009).
2.4. Histological analysis using Mankin scoring
Retropatellar cartilages in all three groups were assessed by histological analysis and graded according to the Mankin scoring system (Mankin et al. 1971). After indentation testing, patellae were fixed in a 10 per cent neutral buffered formalin solution for 7 days. Following fixation, patellae were decalcified in a mixture of 10 per cent formic acid and formaldehyde (Cal-Ex II solution, Fisher Scientific) for 14 days. Patellae were bisected along the mid-sagittal plane and were dehydrated in graded alcohols, cleared in xylene and embedded in paraffin wax (Paraplast Plus paraffin wax, Fisher Scientific). Sections of the cartilage and subchondral bone were cut in a sagittal plane at 10 µm thickness, adhered to slides (Superfrost Plus slides, Fisher Scientific) and stained with hematoxylin, safranin-O and fast green. Sections were examined every 50 µm and viewed under a light microscope (2.5 × objective, Zeiss Axiostar plus, Zeiss Inc.). Digital images of all sections were obtained (Axiovision imaging system, Zeiss Inc.) and Mankin scores were determined by a blinded examiner for the proximal, middle and distal parts of the patella (table 1, figure 2a).
Table 1.
Mankin histological–histochemical grading scheme used to assess the extent of cartilage damage.
| category | subcategory | score |
|---|---|---|
| cartilage structure | normal surface | 0 |
| irregular surface, no pannus | 1 | |
| pannus and surface irregularities | 2 | |
| superficial cartilage layer absent | 2 | |
| fissures/clefts to transitional zone | 3 | |
| fissures/clefts to radial zone | 4 | |
| fissures/clefts to calcified zone | 5 | |
| cellular abnormality | cellular abnormality | 0 |
| normal in zones 1, 2, 3 | 1 | |
| hypercellularity | 2 | |
| clustering/cloning | 3 | |
| matrix staining (safranin O) | normal | 0 |
| slight reduction | 1 | |
| moderate reduction | 2 | |
| severe reduction | 3 | |
| no stain present | 4 | |
| tidemark integrity | intact tidemark | 0 |
| tidemark crossed by blood vessel | 1 | |
| multiple tidemarks | 2 | |
| total | 14 |
Figure 2.
(a) Definition of the proximal, middle and distal regions of the patella. (b) Histological sample of retropatellar cartilage from an (i) experimental (tissue number 1, Mankin score = 8 in table 2), (ii) contralateral (tissue number 2, Mankin score = 2 in table 2), and (iii) normal knee (tissue number 4, Mankin score = 0 in table 2). Scale bar, 500 µm.
2.5. Confocal image analysis
Twelve cells from each patella (for a total of 48 cells each from experimental, contralateral and normal joints) were used for cell morphology analysis. Cells were located in the superficial zone of the rabbit retropatellar cartilage between the articular surface and 40 µm depth. Three-dimensional reconstructions of chondrocytes were performed using a custom-written code (VTK, the Visualization Toolkit, Kitware Inc., USA). The marching cubes algorithm was used for determining iso-intensity contours of individual cells. Cell volumes were calculated using the divergence theorem applied to the position vector field (Alyassin et al. 1994; Guilak 1994). A best-fit ellipsoid method was used for quantitative analysis of morphological changes of individual cells (Feddema & Little 1997). Cell widths and depths were defined along the major and minor axes of the cross-section taken perpendicular to the cell height, respectively.
The global reference frame for the description of articular cartilage has the axial direction parallel to the tissue depth, and the transverse plane orthogonal to it (and parallel to the articular surface, figure 1). Cells in cartilage are well approximated by ellipsoids with one axis roughly parallel to the axial direction (height). The local axial engineering strain, εaxial = (d0 −d)/d, in the ECM of the superficial zone was calculated by measuring the distance between identified paired cells (n = 9 pairs in each sample) in the unloaded and loaded configuration (d0 and d, respectively), as described previously (Guilak et al. 1995). The ECM engineering strain tensor  in the transverse plane was computed using four ‘marker’ cells. The strain tensor was then diagonalized, so that the higher and lower eigenvalues represent the major and minor principal strain directions, respectively.
in the transverse plane was computed using four ‘marker’ cells. The strain tensor was then diagonalized, so that the higher and lower eigenvalues represent the major and minor principal strain directions, respectively.
2.6. Statistical analysis
Comparisons of chondrocyte morphology were performed using two way repeated ANOVA (SPSS 15.0, SPSS Inc.) with test groupings (experimental, contralateral and normal) as the main between subject factors, and loading conditions (loaded and unloaded) as the main within subject factors. Tissue strain, thickness and Mankin score comparisons between experimental and control (contralateral and normal combined) were performed using non-parametric statistics owing to the small group size. The level of significance for all tests was set at α = 0.05. Results are shown as means ± standard errors of the mean.
3. Results
3.1. Cartilage—histology analysis
Mankin scores for the proximal region of the retropatellar surface were significantly greater for the experimental compared with the contralateral and normal control tissues (table 2, n = 4 per group, *p = 0.005), while they were not different for the middle and distal regions (table 2).
Table 2.
Mankin scores and cartilage thickness for patellae from the three experimental groups. Mankin scores were assessed for the distal, middle and proximal regions of the retropatellar cartilage (*p = 0.005).
| n | experimental |
contralateral |
normal |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ave | 1 | 2 | 3 | 4 | ave | 1 | 2 | 3 | 4 | ave | |
| proximal | 6 | 6 | 6 | 7 | 6.3* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| middle | 2 | 2 | 2 | 0 | 1.5 | 2 | 2 | 0 | 0 | 1.0 | 0 | 0 | 0 | 0 | 0 |
| distal | 8 | 8 | 0 | 0 | 4.0 | 8 | 0 | 0 | 6 | 3.5 | 8 | 5 | 0 | 0 | 3.3 |
| thickness (µm) | 743 | 988 | 819 | 664 | 803* | 641 | 743 | 654 | 658 | 674 | 710 | 713 | 605 | 554 | 646 |
Average cartilage thickness in the experimental joints (803 ± 69 µm) was significantly greater than the contralateral (674 ± 23 µm) and normal joints (646 ± 39 µm; n = 4 per group, table 2, *p = 0.005 and figure 2b).
3.2. Cartilage and chondrocyte deformation with loading
Average compressive tissue strains for 2 MPa surface pressure loading were similar for the experimental (16 ± 3%), contralateral (16 ± 1%) and normal tissue (17 ± 3%). However, average axial local ECM strains were significantly greater for the experimental tissue (38 ± 4%) than the contralateral (27 ± 5%) and normal tissues (28 ± 4%; n = 4 per group, figure 3, **p = 0.04). The average axial local ECM strains were also greater than the average cell strains in all experimental group tissues (figure 3, ***p < 0.005). Average transverse ECM strains were also greater for the major and minor directions (14 ± 3% and 4 ± 1%, respectively) in the experimental compared with the contralateral (8 ± 2% and 0 ± 1%, respectively) and normal tissues (5 ± 2% and 1 ± 1%, respectively; n = 4 per group, table 3, *p = 0.04 for major and = 0.005 for minor directions).
Figure 3.
Compressive nominal tissue strains, ECM strains and cell strains for retropatellar cartilage from experimental, contralateral and normal knees. **Comparisons between the ECM of the experimental (exp) and the combined controls; i.e. the contralateral (con) and normal (nor) controls (n = 4 per group, **p = 0.04, ***p < 0.005). White bar, tissue; grey bar, ECM; black bar, cell.
Table 3.
Average transverse ECM strain in the major and minor directions in the three experimental groups (*p < 0.05).
| experimental | contralateral | normal | |
|---|---|---|---|
| major direction | 14 ± 3* | 8±2 | 5 ± 2 |
| minor direction | 4 ± 1* | 0 ± 1 | 1 ± 1 |
| major : minor ratio | 3.5 | 5 |
There was no significant difference in the average axial cell strains for the 2 MPa load application between the experimental (12 ± 2%), contralateral (18 ± 2%) and normal groups (17 ± 1%; figure 4a; p = 0.055 between the experimental and contralateral groups, p = 0.142 between experimental and normal group). However, average increases in cell width were greater in the experimental (12 ± 1%) than the contralateral (6 ± 1%) and normal joint cartilages (6 ± 1%; n = 48 per group, figure 4b, ***p < 0.001). Cell depth changes were not significantly different between the experimental (11 ± 2%), contralateral (7 ± 2%) and normal groups (6 ± 1%; p = 0.247 between the experimental and contralateral groups, p = 0.068 between experimental and normal group, figure 4c).
Figure 4.
Changes in retropatellar cartilage cell (a) height, (b) width, (c) depth, and (d) volume associated with tissue loading for cartilages from experimental (exp), contralateral (con) and normal (nor) knees (n = 48 per group, ***p < 0.005). White bar, before loading; black bar, after loading.
Before loading, average cell volumes in the experimental (294 ± 12 µm3) and contralateral joints (290 ± 8 µm3) were significantly greater than those observed in the normal joints (250 ± 9 µm3; figure 4d, before loading, ***p = 0.002; appendix A, table 4). Following loading, average cell volumes increased in the experimental joints (8 ± 3%, ***p = 0.001), while they decreased in the contralateral (−8 ± 2%, ***p = 0.002) and normal joints (−8 ± 1%, **p = 0.004; n = 48 per group, figure 4d; appendix A, table 5).
On average, the primary direction of transverse cell strain was aligned with the primary direction of transverse ECM strain (figure 5a–c).
Figure 5.
Comparison of the major direction of transverse ECM strain and the direction of the cell semi-axis that has the largest transverse strain: (a) experimental joints, (b) contralateral joints, and (c) normal joints. Black double arrow head solid line indicates the major direction of transverse ECM strain. Grey double arrow head dashed line (before loading) and solid line (after loading) indicates the direction of greatest transverse cell strain.
4. Discussion
ACL transection in the rabbit produced the standard signs of early OA. Cartilage from the retropatellar surface of the experimental joints was thicker (figure 2), and Mankin scores were higher in the proximal patella compared with contralateral and normal controls (table 2).
When applying a surface pressure of 2 MPa, total tissue strain was similar across all groups but local axial ECM strain in the surface zone cartilage was significantly greater in the experimental than the contralateral and normal control cartilage. This indicates a relative softening of the surface zone and hardening of the middle and deep zone cartilage in the experimental group, which agrees with results reported for the dog model of early OA (Matyas et al. 2001). Local transverse strain in the ECM was also significantly greater in the experimental than the contralateral and normal control cartilage (table 3). The increase in axial compressive and transverse tensile strain in the superficial zone is probably associated with a degradation of matrix integrity caused by a decrease in collagen stiffness and loss of proteoglycans typically found in OA (Bank et al. 1997). Although the direction of collagen fibre orientation relative to the principal transverse ECM strains is not known, we speculate that the observed anisotropy of the transverse strain is linked to the collagen fibre orientation (Woo et al. 1976; Bank et al. 2000).
Chondrocyte volumes in the experimental cartilage were significantly greater than in normal cartilage (figure 4d). This result agrees with those observed for superficial zone chondrocytes in degenerated human tibial plateau cartilage (Bush & Hall 2003). This increase in cell volume has been associated with changes in the osmotic pressure of interstitial cartilage fluid (Bush & Hall 2001). Chondrocyte volume and cartilage thickness were increased in the contralateral joints compared with the normal joints suggesting that the cartilage in the contralateral joints was also undergoing structural changes.
The primary purpose of this study was to analyse cell deformations in intact cartilage during early OA. Compressive axial strains of cells in all three groups were significantly smaller than local ECM strains (figure 3). Isolated chondrocytes have an elastic modulus that is approximately three orders of magnitude smaller (0.7 kPa, chondrocytes from cartilage of human knees, hips, ankles and elbows; Jones et al. 1999) than that typically associated with the ECM of articular cartilage (0.4 MPa, patellofemoral groove of adult bovine; Schinagl et al. 1997). Thus, one would expect cell deformations to be much greater than average matrix deformations. However, axial cell deformations reported in healthy cartilage were often smaller than the corresponding average matrix strains (Guilak et al. 1995; Choi et al. 2007; Han et al. 2009).
Chondrocytes in the intact cartilage are surrounded by the chondron, which consists of a pericellular matrix and capsule (Poole et al. 1987). It has been suggested that the chondron provides protection against excessive deformation of chondrocytes when tissue deformations become substantial. Our novel finding of similar axial strains of cells in early osteoarthritic and normal cartilage suggests that this ‘protective’ mechanism afforded by the chondron remains operative in early OA. In order to understand the possible protective role of the chondron in healthy and OA cartilage tissue, direct measurement of pericellular matrix deformations should be performed in future investigations.
Cells in the early OA cartilage showed a conceptually different deformation pattern than cells from the contralateral and normal control tissues. Specifically, cell volume increased with load application in the experimental cartilage, while it decreased for the control tissues, in agreement with published results obtained in healthy cartilage explant studies (Guilak et al. 1995; Choi et al. 2007). The increase in volume of experimental cartilage cells was primarily caused by the increased cell strain in the primary transverse strain direction (cell width) in the experimental compared with the control tissues. This large increase in chondrocyte width was associated with a corresponding increase in average transverse strain of the experimental compared with the contralateral and normal cartilage ECM. Moreover, the direction of the primary cell semi-axes in the transverse plane tended to correspond to the major direction of transverse ECM strain (figure 5a).
Despite the anisotropy of the transverse ECM strain (table 3, the ratio εmajor/εminor of major to minor average local transverse ECM strain is higher than 5), the average transverse cell strain in the contralateral and normal joints was nearly isotropic in the width and depth directions (figure 4b,c). Therefore, we may infer that the transverse cell strain is weakly affected by the transverse ECM strain, at least in a certain range of ECM strains. For small transverse ECM strains (up to 8%), transverse cell strains were not correlated with the local transverse ECM strains. In this range of ECM strains, the cells deform independently of the ECM by ‘expanding’ into the pericellular matrix (a layer located between the cell and the ECM), which is stiffer than the cell, but softer than the ECM. However, at greater transverse ECM strains (more than 8%), transverse cell strains depend on the transverse ECM strain: the stiff collagen fibres in the ECM surrounding cells and pericelluar matrix might pull the ‘soft’ cells. One interpretation for these experimental findings is that the pericellular matrix serves as a nonlinear mechanical filter that amplifies transverse cell deformations in the low range of ECM strains, and dampens them at large strains (Choi et al. 2007).
Although the detailed mechanisms underlying mechanotransduction in cartilage are not fully understood, local tissue and chondrocyte deformations have been thought to be primary factors affecting cell biosynthesis (Buschmann et al. 1996; Wong et al. 1997). Thus, the conceptual differences in chondrocyte strains observed between early OA and normal tissues might produce differential biosynthetic responses.
5. Conclusions
We conclude from the results of this study (i) that there is an intricate mechanotransduction system that amplifies small tissue strains and dampens large strains in articular cartilage chondrocytes and (ii) that chondrocyte deformations differ between normal and early OA cartilages.
Acknowledgements
The authors acknowledge Dr Tak-Shing Fung, University of Calgary, for his help with the statistical analysis; Dr Salvatore Federico and Dr Fabio Ayres, University of Calgary, for their help with confocal image analysis data processing; Eva Szabo, Doug Bourne, Tim Leonard and Brandon Hisey, University of Calgary, for their help with ACL transection surgery of rabbits; and the financial support of the Canada Research Chair Programme for Molecular and Cellular Biomechanics, the Canadian Institutes of Health Research (CIHR), the Alberta Ingenuity Fund (Canada) and the Alberta Heritage Foundation for Medical Research (AHFMR) Team Grant on Osteoarthritis. This study was carried out according to the guidelines of the Canadian Council on Animal Care and was approved by the committee on Animal Ethics at the University of Calgary.
Appendix A
Table 4.
Outcomes by treatment groups (ANOVA).
| condition |
groups |
effects |
|||||
|---|---|---|---|---|---|---|---|
| outcome measure | before loading /after loading | exp | con | nor | loading | group | interaction |
| mean (SEM) (µm) n = 48 | mean (SEM) (µm) n = 48 | mean (SEM) (µm) n = 48 | F (df) [p] | F (df) [p] | F (df) [p] | ||
| height | before | 5.21 (0.14) | 5.40 (0.17) | 5.10 (0.11) | 162.48 (1,141) [<0.001] | 1.45 (2,141) [0.239] | 1.81 (2,141) [0.168] |
| after | 4.47 (0.11) | 4.33 (0.08) | 4.19 (0.07) | ||||
| width | before | 12.66 (0.34) | 12.47 (0.32) | 11.25 (0.27) | 213.67 (1,141) [<0.001] | 9.44 (2,141) [<0.001] | 16.74 (2,141) [<0.001] |
| after | 14.12 (0.34) | 13.21 (0.31) | 11.86 (0.27) | ||||
| depth | before | 8.90 (0.19) | 8.85 (0.20) | 8.58 (0.15) | 67.23 (1,141) [<0.001] | 2.56 (2,141) [0.081] | 2.97 (2,141) [0.055] |
| after | 9.82 (0.23) | 9.41 (0.17) | 9.06 (0.15) | ||||
| volume | before | 294.25 (11.55) | 290.47 (7.64) | 249.79 (9.20) | 2.38 (1,141) [0.125] | 10.93 (2,141) [<0.001] | 13.76 (2,141) [<0.001] |
| after | 318.69 (17.49) | 268.06 (8.05) | 228.53 (7.75) | ||||
Table 5.
Outcomes by simple effect testing (MANOVA).
|
loading effects broken down by groups | |||
|---|---|---|---|
| groups |
|||
| outcome measure | exp | con | nor |
| F (df) [p] | F (df) [p] | F (df) [p] | |
| height | 36.26 (1,141) [<0.001] | 75.86 (1,141) [<0.001] | 53.98 (1,141) [<0.001] |
| width | 172.13 (1,141) [<0.001] | 44.31 (1,141) [<0.001] | 30.71 (1,141) [<0.001] |
| depth | 44.83 (1,141) [<0.001] | 16.29 (1,141) [<0.001] | 12.04 (1,141) [0.001] |
| volume | 11.51 (1,141) [0.001] | 9.68 (1,141) [0.002] | 8.71 (1,141) [0.004] |
| group effects broken down by loading | |||
| loading | |||
| outcome measure | before | after | |
|
F (df) [p] |
F (df) [p] |
||
| height | 1.15 (2,141) [0.318] | 2.61 (2,141) [0.077] | |
| width | 5.96 (2,141) [0.003] | 13.55 (2,141) [<0.001] | |
| depth | 0.91 (2,141) [0.403] | 7.09 (2,141) [0.016] | |
| volume | 6.60 (2,141) [0.002] | 14.23 (2,141) [<0.001] | |
References
- Aigner T., Zhu Y., Chansky H. H., Matsen F. A., III, Maloney W. J., Sandell L. J. 1999. Reexpression of type IIA procollagen by adult articular chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 42, 1443–1450. ( 10.1002/1529-0131(199907)42:7) [DOI] [PubMed] [Google Scholar]
- Alexopoulos L., Haider M. A., Vail T. P., Guilak F. 2003. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J. Biomech. Eng. 125, 323–333. ( 10.1115/1.1579047) [DOI] [PubMed] [Google Scholar]
- Alexopoulos L., Setton L., Guilak F. 2005. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 1, 317–325. ( 10.1016/j.actbio.2005.02.001) [DOI] [PubMed] [Google Scholar]
- Alyassin A. M., Lancaster J. L., Down J. H., III 1994. Evaluation of new algorithms for the interactive measurement of surface area and volume. Med. Phys. 21, 741–752. [DOI] [PubMed] [Google Scholar]
- Bank R. A., Krikken M., Beekman B., Stoop R., Maroudas A., Lafeber F. P. J. G., TeKoppele J. M. 1997. A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. J. Clin. Invest. 99, 1534–1545. [DOI] [PubMed] [Google Scholar]
- Bank R. A., Soundry M., Maroudas A., Mizrahi J., TeKoppele J. M. 2000. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 43, 2202–2210. ( 10.1002/1529-0131(200010)43:10) [DOI] [PubMed] [Google Scholar]
- Bray R. C., Shrive N. G., Frank C. B., Chimich D. D. 1992. The early effects of joint immobilization on medial collateral ligament healing in an ACL-deficient knee: a gross anatomic and biomechanical investigation in the rabbit model. J. Orthop. Res. 120, 157–166. ( 10.1002/jor.1100100202) [DOI] [PubMed] [Google Scholar]
- Bullough P. G. 1992. The pathology of osteoarthritis. In Osteoarthritis, diagnosis and medical/surgical management (eds Moskowitz R. W., Howell D. S., Goldberg V. M., Mankin H. J.), pp. 39–69, 2nd edn. Philadelphia, PA: W.B. Saunders Company. [Google Scholar]
- Buschmann M. D., Hunziker E. B., Kim Y. J., Grodzinsky A. J. 1996. Altered aggrecan synthesis correlates with cell and nucleus structure in statically compressed cartilage. J. Cell Sci. 109, 499–508. [DOI] [PubMed] [Google Scholar]
- Bush P. G., Hall A. C. 2001. The osmotic sensitivity of isolated and in situ bovine articular chondrocytes. J. Orthop. Res. 19, 768–778. ( 10.1016/S0736-0266(01)00013-4) [DOI] [PubMed] [Google Scholar]
- Bush P. G., Hall A. C. 2003. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthritis Cartilage 11, 242–251. ( 10.1016/S1063-4584(02)00369-2) [DOI] [PubMed] [Google Scholar]
- Choi J. B., Youn I., Cao L., Leddy H. A., Gilchrist C. L., Setton L. A., Guilak F. 2007. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular and extracellular deformation in articular cartilage. J. Biomech. 40, 2596–2603. ( 10.1016/j.jbiomech.2007.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. L., Barclay L. D., Matyas J. R., Herzog W. 2003. In situ chondrocyte deformation with physiological compression of the feline patellofemoral joint. J. Biomech. 36, 553–568. ( 10.1016/S0021-9290(02)00424-4) [DOI] [PubMed] [Google Scholar]
- Clark A. L., Leonard B., Barclay B. A., Matyas J. R., Herzog W. 2005. Opposing cartilages in the patellofemoral joint adapt differently to long-term cruciate deficiency: chondrcyte deformation and reorientation with compression. Osteoarthritis Cartilage 13, 1100–1114. ( 10.1016/j.joca.2005.07.010) [DOI] [PubMed] [Google Scholar]
- Clark A. L., Leonard B., Barclay B. A., Matyas J. R., Herzog W. 2006. Heterogeneity in patellofemoral cartilage adaptation to anterior cruciate ligament transaction; chondrocyte shape and deformation with compression. Osteoarthritis Cartilage 14, 120–130. ( 10.1016/j.joca.2005.08.016) [DOI] [PubMed] [Google Scholar]
- Feddema J. T., Little C. Q. 1997. Rapid world modelling: fitting range data to geometric primitives. In Proc. IEEE Int. Conf. Robototics and Automation, Albuquerque, NM, 20–25 April 1997, vol. 4, pp. 2807–2812. [Google Scholar]
- Fukui N., Purple C. R., Sandell L. J. 2001. Cell biology of osteoarthritis: the chondrocyte's response to injury. Curr. Rheumatol. Rep. 3, 496–505. [DOI] [PubMed] [Google Scholar]
- Graverand M. H. L., Eggerer J., Vignon E., Otterness I. G., Barclay L., Hart D. 2002. Assessment of specific mRNA levels in cartilage regions in a lapine model of osteoarthritis. J. Orthop. Res. 20, 535–544. ( 10.1016/S0736-0266(01)00126-7) [DOI] [PubMed] [Google Scholar]
- Guilak F. 1994. Volume and surface-area measurement of viable chondrocytes in situ using geometric of modelling. J. Microsc. 173, 245–256. [DOI] [PubMed] [Google Scholar]
- Guilak F., Ratcliffe A., Mow V. C. 1995. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J. Orthop. Res. 13, 410–421. ( 10.1002/jor.1100130315) [DOI] [PubMed] [Google Scholar]
- Hall A. C., Urban J. P. G., Gehl K. A. 1991. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J. Orthop. Res. 9, 1–10. ( 10.1002/jor.1100090102) [DOI] [PubMed] [Google Scholar]
- Hall A. C., Starks I., Shoults C. L., Rashidbigi S. 1996. Pathways for K+ transport across the bovine articular chondrocyte membrane and their sensitivity to the cell volume. Am. J. Physiol. 270, C1300–C1310. [DOI] [PubMed] [Google Scholar]
- Han S.-K., Colarusso P., Herzog W. 2009. Confocal microscopy indentation system for studying in situ chondrocyte mechanics. Med. Eng. Phys. 31, 1038–1042. ( 10.1016/j.medengphy.2009.05.013) [DOI] [PubMed] [Google Scholar]
- Hasler E. M., Herzog W. 1998. Quantification of in vivo patellofemoral contact forces before and after ACL transection. J. Biomech. 31, 37–44. ( 10.1016/S0021-9290(97)00105-X) [DOI] [PubMed] [Google Scholar]
- Jones W. R., Ting-Beall H. P., Lee G. M., Kelly S. S., Hochmuth R. M., Guilak F. 1999. Alterations in the Young's modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech. 32, 119–127. ( 10.1016/S0021-9290(98)00166-3) [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Sah R. Y., Grodzinsky A. J., Plaas A. H. K., Sandy J. D. 1994. Mechanical regulation of cartilage biosynthetic behaviour: physical stimuli. Arch. Biochem. Biophys. 311, 1–12. ( 10.1006/abbi.1994.1201) [DOI] [PubMed] [Google Scholar]
- Korhonen R. K., Herzog W. 2008. Depth-dependent analysis of the role of collagen fibrils, fixed changes and fluid in the pericellular matrix of articular cartilage on chondrocyte mechanics. J. Biomech. 41, 480–485. ( 10.1016/j.jbiomech.2007.09.002) [DOI] [PubMed] [Google Scholar]
- Lippiello L., Hall D., Mankin H. J. 1977. Collagen synthesis in normal and osteoarthritic cartilage. J. Clin. Invest. 59, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiello L., Kaye C., Neumata T., Mankin H. J. 1985. In vitro metabolic response of articular cartilage segments to low levels of hydrostatic pressure. Connect. Tissue Res. 13, 99–107. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello J., Zarins A. 1971. Biochemical and metabolic abnormailities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 53, 523–537. [PubMed] [Google Scholar]
- Matyas J., Young D., Hulme P., Duncan N. 2001. Zonal distribution of articular cartilage compressive strain in a model of early experimental osteoarthritis. Trans. Orthop. Res. Soc. 26, 54. [Google Scholar]
- Poole C. A., Flint M. H., Beaumont B. W. 1987. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J. Orthop. Res. 5, 509–522. ( 10.1002/jor.1100050406) [DOI] [PubMed] [Google Scholar]
- Poole C. A., Matsuoka A., Schofield J. R. 1991. Chondrons from articular cartilage. III. Morphologic changes in the cellular microenvironment of chondrons isolated from osteoarthritic cartilage. Arthritis Rheum. 34, 22–35. ( 10.1002/art.1780340105) [DOI] [PubMed] [Google Scholar]
- Quinn T. M., Grodzinsky A. J., Buschmann M. D., Hunziker E. B. 1998. Mechanical compression alters proteoglycan deposition and matrix deformation around individual cells in cartilage explants. J. Cell Sci. 111, 573–583. [DOI] [PubMed] [Google Scholar]
- Sah R. L., Grodzinsky A., Plaas A., Sandy J. 1992. Effect of static and dynamic compression on matrix metabolism in cartilage explants. In Articular cartilage and osteoarthritis (eds Kuettner K., Shleyerbach R., Peyron J., Hascall V.), pp. 373–392. New York, NY: Raven Press. [Google Scholar]
- Sah R. L., Yang A. S., Chen A. C., Hant J. J., Halili R. B., Yoshioka M., Amiel D., Coutts R. D. 1997. Physical properties of rabbit articular cartilage after transection of the anterior cruciate ligament. J. Orthop. Res. 15, 197–203. ( 10.1002/jor.1100150207) [DOI] [PubMed] [Google Scholar]
- Sandell L. J., Aigner T. 2001. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 3, 107–113. ( 10.1186/ar148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinagl R. M., Gurskis D., Chen A. C., Sah R. L. 1997. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J. Orthop. Res. 15, 499–506. ( 10.1002/jor.1100150404) [DOI] [PubMed] [Google Scholar]
- Seedholm B. B., Takeda T., Tsubuku M., Wright V. 1979. Mechanical factors and patellofemoral osteoarthritis. Ann. Rheum. Dis. 38, 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., et al. 1995. Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J. Orthop. Res. 13, 824–831. ( 10.1002/jor.1100130604) [DOI] [PubMed] [Google Scholar]
- Vignon E., Bejui J., Mathieu P., Hartmann J. D., Ville G., Evreux J. C., Descotes J. 1987. Histological cartilage changes in a rabbit model of osteoarthritis. J. Rheumatol. 14, 104–106. [PubMed] [Google Scholar]
- Wang J., Verdonk P., Elewaut D., Veys E. M., Verbruggen G. 2003. Homeostasis of the extracellular matrix of normal and osteoarthritic human articular cartilage chondrocytes in vitro. Osteoarthritis Cartilage 11, 801–809. ( 10.1016/S1063-4584(03)00168-7) [DOI] [PubMed] [Google Scholar]
- Wong M., Wuethrich P., Buschmann M. D., Eggli P., Hunsiker E. 1997. Chondrocyte biosynthesis correlates with local tissue strain in statically compressed adult articular cartilage. J. Orthop. Res. 15, 189–196. ( 10.1002/jor.1100150206) [DOI] [PubMed] [Google Scholar]
- Woo S. L. Y., Akeson W. H., Jemmott G. F. 1976. Measurements of the nonhomogeneous directional properties of articular cartilage in tension. J. Biomech. 9, 785–791. ( 10.1016/0021-9290(76)90186-X) [DOI] [PubMed] [Google Scholar]
- Young A. A., McLennan S., Smith M. M., Cake M. A., Read R. A., Melrose J., Sonnabend D. H., Flannery C. R., Litte C. B. 2006. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res. Therapy 8, R41 ( 10.1186/ar1898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M., Coutts R. D., Amiel D., Hacker S. A. 1996. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage 4, 87–98. ( 10.1016/S1063-4584(05)80318-8) [DOI] [PubMed] [Google Scholar]