Abstract
This is the first direct physiological evidence in support of the ionoregulatory hypothesis, challenging the long-held assumption that teleost gills develop initially for gas exchange. Resting unidirectional sodium (Na+) uptake and oxygen (O2) uptake across the skin and gills were measured simultaneously in larval rainbow trout, Oncorhynchus mykiss, during development. In soft and hard water, Na+ uptake shifted to the gills by 15 and 16 days post-hatch (dph) while O2 uptake took 50–80% longer and shifted by 23 and 28 dph, respectively. This suggests that gills are required for ionoregulation prior to gas exchange in developing rainbow trout. The age of transition for Na+ uptake, gill Na+, K+-ATPase (NKA) α-subunit protein expression and gill NKA enzyme activity were not significantly different between soft and hard water-reared groups, which suggests a lack of plasticity in gill ionoregulatory development.
In rainbow trout, the gills assume a dominant role in ionoregulation before gas exchange, suggesting that ionoregulation may be the initial driving force for gill development. Further investigation is required to determine whether this pattern is consistent with other teleosts and more basal fishes during early development to gain insight into the role of ionoregulation in vertebrate gill evolution.
Keywords: ionoregulation, gas exchange, development, rainbow trout, gill, metabolic rate
1. Introduction
The gills of adult fish are a multifunctional organ involved in, gas exchange, ionoregulation, acid–base regulation and ammonia excretion (reviewed in Evans et al. 2005). Of these functions, gas exchange, in particular O2 uptake, is generally considered most critical. Blocking O2 uptake at the gill of adult fish rapidly leads to death by asphyxiation. Oxygen uptake is also generally considered the primary function driving gill development (Krogh 1941).
Gills form relatively late in larval development and until they appear, O2 uptake is restricted to the skin (Rombough 1988). Since skin surface area (SA) expands at a rate proportional to M2/3 (M = mass), while the demand for O2 expands at a rate proportional to M1.0, skin SA eventually becomes limiting (Rombough 2007). According to Krogh (1941), the solution to this problem is to develop gills which because of their morphology are not subject to the same geometric constraints as the skin. To date, the view that gills develop for gas exchange (often termed the oxygen hypothesis) is the accepted explanation for why fish develop gills.
However, recent evidence suggests that ionoregulation may be more important than O2 uptake as a driving force during the initial stages of gill development (reviewed in Rombough 2007). Like O2, ion uptake in freshwater is initially entirely cutaneous and is subject to similar, if not more stringent geometric constraints (Rombough 2007). The major site of ion uptake in developing fish, as in adult fish, is the mitochondrion-rich cells (MRCs; reviewed in Hwang 2009; Hwang & Lee 2007). MRCs are initially restricted to cutaneous surfaces but begin to appear on the gills soon after they start to develop (Li et al. 1995; Rombough 1999). In all freshwater and marine fish species examined to date, MRCs appear on the gills in advance of gill lamellae (reviewed in Rombough 2007). Since gill lamellae are considered the definitive site of O2 uptake, this suggests that the gills initially may be more important for ion exchange than for gas exchange. Functional ablation experiments with zebrafish (Danio rerio) appear to confirm this: the ionoregulatory functions normally provided by the developing gills become critical for survival at about 7 days post-fertilization (dpf) in zebrafish larvae while respiratory services do not become critical until about 14 dpf (Rombough 2002). Based on the balance of evidence, there would appear to be a strong case for ion exchange being the more important factor driving gill development. This view has been termed the ionoregulatory hypothesis (Rombough 2007).
To date, no direct physiological evidence exists to differentiate between the O2 and ionoregulatory hypotheses. For the most part, the evidence is either theoretical (e.g. skin SA : M ratios) or circumstantial (e.g. relative timing of the appearance of branchial MRCs and lamellae) and, as such, is open to interpretation. To our knowledge, no one has directly measured ion and gas uptake across the skin and gills at the same stage of development in the same species under the same conditions. The primary goal of our study was to determine how the partitioning of Na+ and O2 uptake between the skin and gills changes during early gill development. Comparison of the partitioning patterns would then allow us to directly test whether the ionoregulatory or respiratory hypothesis applies for that species under our experimental conditions. We picked rainbow trout, Oncorhynchus mykiss, as our animal model because (i) the methodologies for measuring skin and gill O2 uptake (Rombough 1998) and whole body Na+ uptake (Brauner & Wood 2002a,b) have already been developed and proven reliable and (ii) the respiratory and ionoregulatory physiology of juvenile and adult rainbow trout is well understood. This allowed us to put the results of the current study into perspective. Our study had two main objectives:
—To determine whether ion or gas uptake is first to make the transition from the skin to the gills in larval rainbow trout. This was assessed by comparing the relative contributions of the skin and gills to total O2 and total Na+ uptake at various stages throughout the course of larval development.
—To determine whether the rate at which ionoregulatory activity shifts from the skin to the gills is affected by water hardness. Shen & Leatherland (1978) reported that the ionic strength of the rearing medium had no impact on the number or appearance of gill MRCs in developing rainbow trout. This suggests that water hardness is unlikely to affect the timing of the shift of ionoregulatory activity to the gills, yet the possibility remains since gill ion uptake rates were not measured directly in that study. Hence, a subsidiary goal of this study was to determine whether water hardness indeed has no significant effect on the ionoregulatory activity of the developing gill. This was evaluated by comparing Na+ uptake rates, gill Na+, K+-ATPase (NKA) concentrations and NKA activities in soft and hard water.
2. Material and methods
(a). Animals and rearing
Rainbow trout, O. mykiss, were obtained as eyed embryos from Rainbow Springs Trout Hatchery in Thamesford, Ontario, Canada, and transported to the University of British Columbia, Vancouver, Canada. The soft water-acclimated group was reared in March 2008 in soft dechlorinated tap water from the City of Greater Vancouver (in mM: Na+, 0.06; Cl−, 0.05; Ca2+, 0.03; Mg2+, 0.007; K+, 0.004; alkalinity, 3.3 mg as CaCO3 l−1; hardness 3.55 mg as CaCO3 l−1; pH 6.2–6.7; Metro Vancouver 2007), and the hard water-acclimated group was reared in April 2008 in artificial hard water made by adding salts to distilled deionized water (nominal concentrations in mM: Na+, 2.3; Cl−, 0.1; Ca2+, 0.9; Mg2+, 1.0; K+, 0.1; HCO3−, 2.3; alkalinity, 110–120 mg as CaCO3 l−1; hardness 160–180 mg as CaCO3 l−1; pH 7.6–8.0). Both groups were reared in the dark at 10°C (actual temperature 9.5 ± 0.1°C) in an egg incubation tray supplied by a 40 l recirculating system.
(b). Flux chamber design and use
Flux chambers similar to those of Wells & Pinder (1996) were designed to partition larvae into two compartments, enabling in vivo measurement of O2 and Na+ uptake by the anterior compartment (consisting of the head and opercula epithelia and gills) separately from the posterior compartment (consisting of the remaining body and yolk sac epithelia). Each flux chamber consisted of two identical rectangular chambers made of acrylic plastic, each approximately 5 ml in volume and had a micro-stir bar, an air inlet and an O2 probe/radioisotope injection port.
Larvae were randomly selected for either oxygen (O2) or sodium (Na+) uptake measurements at 0, 6, 9, 12 and 18 days post-hatch (dph), with the exception that no measurements were performed on 12 dph in soft water larvae due to technical difficulties.
Uptake measurements required positioning an individual larva into a hole in a stretched rubber membrane of dental dam latex. Prior to positioning, larvae were calmed with 100 ppm clove oil (Rougier Pharma, Quebec, Canada) for approximately 5 min to attain stage 5 anaesthesia (total loss of equilibrium and swimming motion with weak opercular motion and total loss of reactivity; Keene et al. 1998). Water in flux chambers contained 10 ppm clove oil to reduce struggling without impairing opercular motion. During a 1 h recovery period prior to the start of measurements, flux chambers were aerated and submerged in a 10°C water bath (actual temperature 9.6 ± 0.1°C).
(c). Measurement of unidirectional Na+ uptake rate
After the recovery period, either the anterior or the posterior chamber (chosen randomly) was injected with 0.5 µCi of the radioisotope 22Na (PerkinElmer, MA, USA). Both chambers were gently aerated and stirred for the duration of the 1.5 h flux period. Water samples of 25 µl were removed in duplicate 10 min post-injection and again at 1.5 h for measurement of water radioactivity. At the end of the flux period, larvae were immediately removed from the dam and flux chambers, rinsed three times with 5 mM NaCl to displace surface-bound 22Na and then rinsed once with deionized water. Water samples and larvae were measured for radioactivity in counts per minute using a gamma counter (PerkinElmer, Turku, Finland). Na+ uptake, expressed in µmol Na+ h−1 larvae−1, was calculated as in Brauner & Wood (2002b). Water concentration of Na+ was determined by flame atomic absorption spectrometry (Varian Australia Pty Ltd, Australia).
(d). Measurement of O2 uptake rate
Following the recovery period, air bubbles were removed from the flux chambers and the chambers were completely filled. Needle-type housing fibre-optic O2 microoptodes (Loligo Systems ApS, Denmark) were positioned in both chambers. A data acquisition system (Precision Sensing GMbH, Germany) recorded the decline of O2 in the water in the anterior and posterior chambers as mg O2 l−1 min−1 at 10°C for 30 min. After the completion of measurements, larvae were placed in aerated, dechlorinated water to recover for 1 h. Larvae were euthanized with 1000 ppm tricaine methane sulphonate (MS-222, Finquel, Argent Chemical Laboratories), and body wet mass (with and without yolk) was determined to the nearest 0.1 mg. Data were discarded for individuals that failed to recover within 1 h or had visible physical damage. Data are expressed as mg O2 h−1 larvae−1.
(e). Measurement of skin SA
Animals were fixed in paraformaldehyde solution in phosphate-buffered saline (pH 7.4, as described in Presnell & Schreibman 1997) for 24 h at 4°C, then stored in 70 per cent ethanol at 4°C until analysis. To measure total skin SA, the body was divided into the head, pectoral fins, trunk and associated fins, tail and yolk sac regions and skinned. All skin components were immersed briefly in Wright–Giemsa stain (SureStain, Fisher Scientific, Pittsburgh, USA) and rinsed with deionized water prior to mounting on coverslips to improve visibility for imaging. Slides were photographed with a Leica MZ16A dissecting microscope with a JVC KY-F75U digital camera using the software Auto-Montage Pro 5.01.0005 (Synoptics Ltd). SAs were then measured using the area measurement function in trace mode using the software SigmaScan Pro Image Analysis 5.0.0 (SPSS). The average percentages of total skin SA in the anterior and posterior chambers were calculated for each age group (n = 3–6 per group), with the exception of hard water larvae at 0 dph because they were too small to handle.
(f). Calculation of gill and skin Na+ and O2 uptake rates
Rates of O2 and Na+ uptake by the gills and skin (consisting of head, opercula, body and yolk sac epithelia) were calculated from the uptake rates measured in the anterior and posterior chambers as described in Wells & Pinder (1996). Gill and skin absolute value uptake rates were also expressed as percentages of total uptake rates, where total O2 uptake rate = anterior O2 uptake rate + posterior O2 uptake rate (obtained simultaneously on the same larvae) and total Na+ uptake rate = mean anterior Na+ uptake rate + mean posterior Na+ uptake rate (measurements were on different larvae, and consequently, in some cases, addition of the two deviates slightly from 100%).
(g). Measurement of NKA protein concentration
Five to seven larvae from each age group in soft and hard water were euthanized with 1000 ppm MS-222, immediately frozen intact in liquid nitrogen and stored at −80°C until analysis. Larvae were thawed on ice and the gills, yolk sac membrane and skin were dissected and placed separately in 100 µl SEI buffer (150 mM sucrose, 10 mM ethylenediaminetetraacetic acid, 50 mM imidazole, pH 7.3). Tissues were homogenized by sonication (Sonics & Materials Ltd, Newtown, CT, USA), and then centrifuged. The supernatant was serially diluted 10-fold in a four-step series in 50 mM imidazole buffer (IB, pH 7.5) and dot blotted using a 96-well vacuum manifold (Convertible, Life Technology) onto PVDF membranes (Hybond P, GE HealthCare Inc., Carnaxide, Portugal). Wells were rinsed three times with IB and dried at 37°C for storage.
Membranes were rehydrated and blocked with 5 per cent blotto in TTBS (0.05% Tween 20, 20 mM Tris–HCl, 500 mM NaCl, pH 7.5) and probed with a rabbit polyclonal anti-NKA α-subunit antibody (Wilson et al. 2007) diluted 1 : 10 000 in blocking buffer overnight at room temperature on an orbital shaker. Membranes were washed three times in TTBS and incubated with goat anti-rabbit horse radish peroxidase-conjugated secondary antibody diluted 1 : 100 000 in TTBS for 1 h at room temperature on an orbital shaker. Membranes were rinsed three times in TTBS and incubated with ECL solution (Immobilon, Millipore) for 5 min. The signal was detected with a FujiFilm LAS 4000 mini imager (FujiFilm, Porto). Membranes were stripped with 25 mM glycine–HCl, 1 per cent SDS, pH 2 for 30 min at room temperature and reprobed with a mouse monoclonal anti-actin antibody (clone AC-40, Sigma-Aldrich Chemical Co., St Louis, MO, USA) diluted 1 : 1000 in blocking buffer as described above with the exception of the use of a goat anti-mouse horse radish peroxidase-conjugated secondary antibody diluted 1 : 50 000 in TTBS. Spot intensity from both NKA and actin dotblots were measured using MultiGauge Image Analysis software (FujiFilm). Results are presented as a ratio of intensities of luminescence of NKA α-subunit to actin.
(h). Measurement of NKA enzyme activity
Four to six larvae from each age group of the soft and hard water groups were euthanized with 1000 ppm MS-222, immediately frozen intact in liquid nitrogen and stored at −80°C until analysis. Samples of the gills (on larvae 6 dph and older), body epithelium and yolk sac epithelium were homogenized by sonication (Kontes Micro Ultrasonic Cell Disrupter, Mandel Scientific Co., Guelph, Canada) in 300 µl SEI buffer on ice and then centrifuged at 5000 g for 5 min. Activity was measured in the supernatant according to McCormick (1993) at 25°C using a plate reader (SpectraMAX 190, Molecular Devices, Sunnyvale, USA) and is expressed in µmol ADP mg−1 protein h−1. Protein content of the homogenate supernatant was measured using Bradford reagent (Sigma-Aldrich) and bovine serum albumin as a standard.
(i). Statistical analyses
O2 and Na+ uptake rates, NKA α-subunit protein expression and NKA enzyme activity were plotted against age. In general, the F-test showed that linear regressions were a better fit to the data than second-order polynomial regressions (Prism 5.02, GraphPad Software, Inc., CA, USA). The F-test was used to test for slopes significantly different from zero and significant differences between pairs of slopes (Prism 5.02, GraphPad Software, Inc.).
Gill and skin O2 and Na+ uptake rates as percentages of total uptake rates were plotted against age. The age at which each pair of gill and skin lines intersected (corresponding to 50% gill uptake and 50% skin uptake) was determined. The mean and standard error for points of intersection were calculated by fitting data to the equation Y = Ycross + (X−Xcross) slope, where Xcross and Ycross were defined as shared parameters to find a best-fit value that applies to both the gill and skin lines. Pairs of crossing points were tested for significant differences using Student's t-test (Prism 5.02, GraphPad Software, Inc.). Statistical significance was assumed at p < 0.05. All values are reported as mean ± s.e.m.
3. Results and discussion
During early development, the gills take on a primary role in Na+ uptake in just over half the time relative to that observed for O2 uptake in rainbow trout (15–16 versus 23–28 dph) highlighting the importance of the developing fish gill to ionoregulation. Furthermore, the timing of the transition to the gills for Na+ uptake, gill NKA α-subunit protein expression and gill NKA activity levels were similar between soft and hard water groups, suggesting that gill ionoregulatory development is unaffected by water hardness. These findings support the ionoregulatory hypothesis and have important implications for our current understanding of developmental physiology and the function of larval gills.
(a). Concerns with measurement under calmed, resting conditions in normoxia
Measurements of resting O2 uptake rate in normoxia usually underestimate metabolic rate in the natural environment (Wieser 1985), but this discrepancy is probably minimal in developing fish larvae in general because of their relatively low factorial aerobic scope (ratio of maximum to standard metabolic rate) due to the high O2 requirements of development (Killen et al. 2007). The factorial aerobic scope of rainbow trout larvae is only 2.7, considerably lower than 5.2 for fry (Wieser 1985) and 6 to 20 for adults (Wilson et al. 1994; Shingles et al. 2001). Consequently, resting measurements in larval rainbow trout may more closely represent levels in their natural habitats relative to juvenile and adult stages.
Larvae must be calmed to prevent struggling and injury while in the rubber dam. Preliminary studies revealed that calming with 10 ppm clove oil only reduced resting Na+ uptake by 12 per cent (not statistically significant, data not shown) and O2 uptake by 24 per cent (p = 0.003), while MS 222 had much greater effects on Na+ uptake precluding its use. Given that the effects of clove oil were relatively small, it is unlikely that its use significantly influenced the timing of the skin-to-gill transition for Na+ and O2 uptake, but we have assumed that the effect of clove oil was similar on gills and skin and that they were consistent throughout development.
(b). Na+ uptake rate partitioning between the gills and the skin
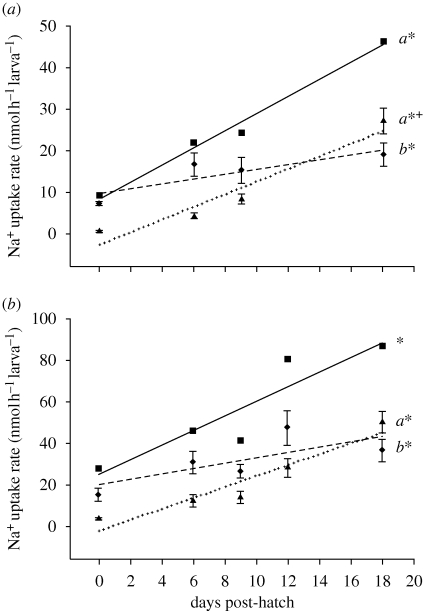
Total Na+ uptake rate increased with development (figure 1). Total Na+ uptake rate was lower overall and increased at a slower rate in soft water than in hard water, most probably because Na+ uptake rate is dependent on water Na+ content (figure 1 and table S2 in the electronic supplementary material; Kirschner 1970). Mass-specific total Na+ uptake rates are comparable to those reported in Brauner & Wood (2002a) at equivalent developmental stages.
Figure 1.
Total (filled squares, solid line), gill (filled triangles, dotted line) and skin (filled diamonds, dashed line) Na+ uptake rates in larval rainbow trout reared in (a) soft water and (b) hard water from 0 to 18 dph. Linear regressions are based on raw data. Regression statistics are presented in table S2 in the electronic supplementary material. Asterisks denote slopes significantly different from zero. Letters denote significant differences in slopes within soft and hard water groups. Plus denotes the significant differences in slope between soft and hard water gill or skin regressions (p < 0.05). Note vertical scale in hard water is two-fold of soft water. Data shown are mean ± s.e.m.
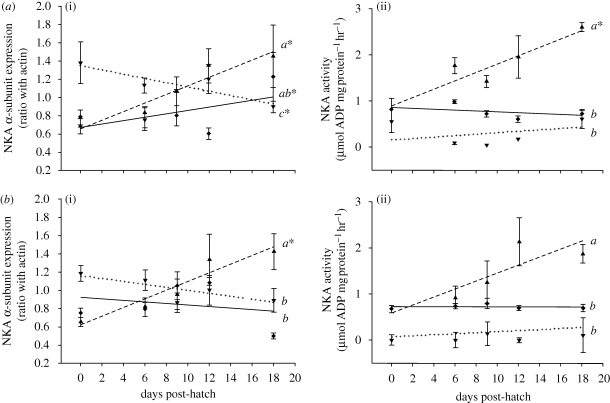
Gill Na+ uptake increased linearly with age (figure 1). This is consistent with the increases in gill NKA α-subunit expression and NKA activity (figure 2) and the number of MRCs per filament which increased approximately threefold from 0 to 18 dph (Rombough 1999). Na+ uptake rate increased at a slower rate at the skin than the gills indicating that the ionoregulatory capacity of the gills increased more rapidly than the skin (figure 1). Ion uptake efficiency at the skin probably declines with age due to thickening of the skin, increasing distance of MRCs from blood vessels, decreasing MRC density after hatch and reabsorption of the yolk sac epithelia (Rombough 1999, 2004).
Figure 2.
NKA α-subunit expression and NKA activity at the gills (filled triangles, dashed line), body epithelium (filled diamonds, solid line) and yolk sac epithelium (filled inverted triangles, dotted line) in rainbow trout reared in (a) soft water and (b) hard water from 0 to 18 dph. No NKA activity data are available for the gills at 0 dph. Refer to the caption of figure 1 for all other details.
(c). Demands for increased total Na+ uptake rate with age
The observed increase in total Na+ uptake rate with development in both soft and hard water (figure 1) may be attributed to two major demands for Na+. Firstly, uptake rate must be elevated to supply ions to growing tissues. This is evident in the near doubling in whole body Na+ content in rainbow trout during the first 14 dph (Brauner & Wood 2002a). Secondly, expansion of respiratory SA exacerbates diffusive ion loss at the lamellae, requiring greater ion uptake rates to balance increased efflux (Evans et al. 1999).
Based on changes in whole body Na+ content reported in Brauner & Wood (2002a), we estimate that approximately 90 per cent (hard water) and 75 per cent (soft water) of Na+ uptake balances diffusive Na+ loss, while only 10 and 25 per cent of Na+ uptake, respectively, is incorporated into the body. Assuming that there were no differences in Na+ incorporated into larvae reared in soft and hard water of the present study (which were not measured) Na+ efflux must have been lower in soft water. The specific mechanisms that may reduce ion loss in a low ion content environment are not known, but may consist of tightening of paracellular junctions or reduction in gill perfusion. It is not known why diffusive efflux in developing trout is so high and represents such a large proportion of unidirectional influx, but it may be associated with acid–base regulation and the need to modulate net Na+/H+ exchange; however, this remains to be investigated.
Given that the majority of Na+ uptake appears to balance efflux, Na+ uptake is expected to scale proportionately with total gill SA, assuming that unidirectional Na+ uptake is not environmentally limited (i.e. uptake rates are near Vmax) and total gill SA is an indication of potential Na+ efflux. Indeed, gill Na+ uptake rate in larvae reared in hard water (where Na+ uptake rates are near maximal in adult rainbow trout; Kirschner et al. 1973) scaled to unity with gill SA (estimated from Rombough 1999; see figure S1 in the electronic supplementary material).
(d). O2 uptake rate and partitioning between the gills and skin
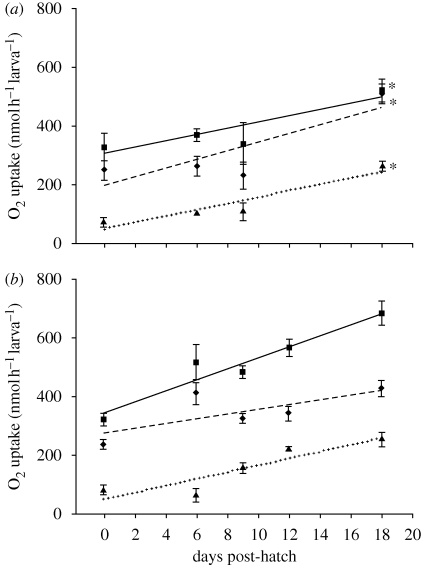
Total O2 uptake rate increased with development at the same rate in soft and hard water (figure 3). Total O2 uptake rates in mass-matched larvae were similar to those reported by Rombough (1998). The trends of increasing total, gill and skin O2 uptake rate per larva with tissue mass were consistent with those reported for larval chinook and Atlantic salmon in earlier partitioning studies (data not shown; Rombough & Ure 1991; Wells & Pinder 1996).
Figure 3.
Total (filled squares, solid line), gill (filled triangle, dotted line) and skin (filled diamonds, dashed line) O2 uptake rates in larval rainbow trout reared in (a) soft water and (b) hard water from 0 to 18 dph. Refer to the caption of figure 1 for all other details.
(e). Contribution of the gills to Na+ and O2 uptake rate
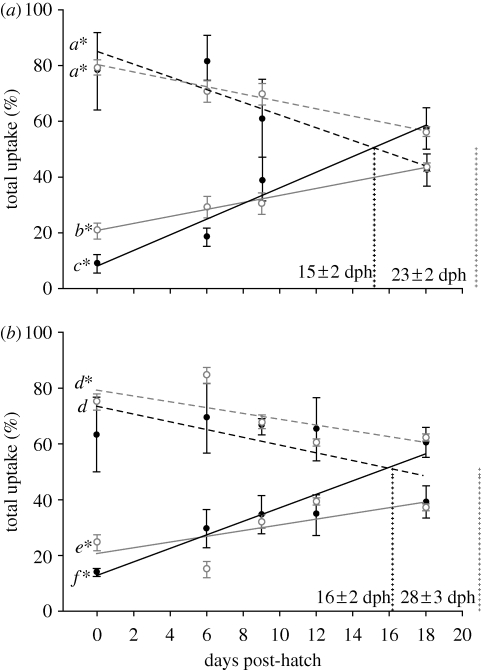
Studies on the function of larval gills have traditionally focused on their role as a gas exchange organ, while their importance in ionoregulation has been seen as secondary. Direct comparison of the contribution of the gills to total Na+ and O2 uptake suggests that the gills develop more rapidly for ionoregulation than gas exchange in rainbow trout. At hatch, the gills account for 21 per cent (soft water) and 25 per cent (hard water) of total O2 uptake (figure 4). These values are in agreement with published values of 27 per cent in rainbow trout (Rombough 1998), 26 per cent in chinook salmon (Rombough & Ure 1991) and 28 per cent in Atlantic salmon (Wells & Pinder 1996). By contrast, the gills at hatch accounted for only 9 per cent (soft water) and 14 per cent (hard water) of Na+ uptake rate (figure 4). By 18 dph, the gills account for 57 per cent (soft water) and 61 per cent (hard water) of total Na+ uptake while at this stage they only accounted for 37 per cent (hard water) to 43 per cent (soft water) of total O2 uptake (figure 4). Thus, the gills increase their involvement in Na+ uptake faster than for O2 uptake.
Figure 4.
Per cent contributions of the gill (solid line) and skin (dashed line) to total Na+ (filled circles) and O2 (open circles) uptake rates in larval rainbow trout reared in (a) soft water and (b) hard water from 0 to 18 dph. Calculated skin-to-gill transitions are indicated by vertical dotted lines. Times of Na+ or O2 transition are not significantly different between soft and hard water; times of Na+ and O2 transition are significantly different within soft and hard water (p = 0.003). Refer to the caption of figure 1 for all other details.
(f). Timing of skin-to-gill transitions for Na+ and O2 uptake
The time at which Na+ and O2 uptake transitions from skin to gills was defined as the age at which the gills account for 50 per cent of total uptake rate. This was estimated as the intersection between the gills' and skin's per cent contribution to total uptake (figure 4). As predicted by the ionoregulatory hypothesis, the skin-to-gill transition occurred significantly earlier for Na+ uptake than for O2 uptake in both soft and hard water environments (p < 0.003). The Na+ transition occurred at 15 ± 2 dph in soft water and at 16 ± 2 dph in hard water (not statistically different; figure 4). Intersection points for O2 uptake were extrapolated to occur at 23 ± 2 dph in soft water and 28 ± 3 dph in hard water (not statistically different; figure 4). This is consistent with predictions based on morphological data: MRCs appeared on the gills as early as 15 days prior to the appearance of lamellae (Gonzalez et al. 1996), and the gills accounted for 50 per cent of all MRCs by 18 dph while it took until day 35 dph for the gills to account for 50 per cent of total respiratory SA (Rombough 1999). In gill function ablation experiments on larval zebrafish, ionoregulation by the gills were essential to survival by 7 dpf but O2 uptake by the gills were only essential to survival by 14 dpf (Rombough 2002). It appears that the gills play a major role in ionoregulation before doing so for gas exchange in both rainbow trout and zebrafish.
(g). The lack of plasticity of gill ionoregulatory development: timing of skin-to-gill transition for Na+ uptake, NKA α-subunit protein expression and NKA activity
The timing of skin-to-gill transition for Na+ uptake was not affected by water hardness. Hardness also had little effect on NKA α-subunit expression and NKA activity. This is in agreement with an early study on rainbow trout embryos and larvae which reported that gill MRCs did not differ in number or appearance in larvae reared at different salinities (deionized water, 11 ppm and 13 ppm sea water; Shen & Leatherland 1978). Taken together, it appears that variations in the ion content of water have little impact on the ionoregulatory development of the rainbow trout gill, at least within the relatively broad range in ion content employed in this study.
(h). Conclusion and future directions
Until the ionoregulatory hypothesis was proposed (Li et al. 1995), it was assumed that the gills of all fish developed initially for the purpose of gas exchange. Ionoregulation was assumed to be a secondary function acquired at a later stage. Our findings suggest the reverse is true in at least some species. In rainbow trout, the gills become the primary site of Na+ uptake 8–12 days sooner than for O2 uptake. In environments that differ markedly in ion content (hard versus soft water), there were no differences in the timing of skin-to-gill transition for Na+ uptake during development in rainbow trout. Furthermore, no differences in gill NKA protein and activity during development were observed between soft and hard water rearing environments, suggesting that variations in water ion content may have little impact on the rate of ionoregulatory development of the gills.
The findings of our study may have important implications on our current understanding of gill evolution. It is commonly assumed that gills evolved from the branchial baskets of protochordates to complement O2 uptake across the skin as O2 demands increased due to the evolution of more active, predatory lifestyles and increased body size (reviewed in Coolidge et al. 2007). Such pressures are similar to those encountered by larvae during gill development (Rombough 2004). It has been suggested that exchange at the skin became limiting for ionoregulation before gas exchange during the course of gill evolution, as is believed to be the case for the gills during development (Rombough 2004). This hypothesis is supported by studies on the gill function of primitive fishes. Studies on gill function in osmoconforming marine hagfishes, extant members of the earliest fish lineage, suggest that the first function of the vertebrate gills may be acid–base regulation (Mallat et al. 1987; reviewed in Evans et al. 2005). It has been proposed that the mechanism of acid–base relevant NaCl exchange at the gills were an exaptation for ionoregulatory functions that became important when fish invaded freshwater habitats (Evans 1984; reviewed in Wright 2007). In subsequently evolving species including lampreys (reviewed in Wright 2007), the gills are also believed to play an important, if not a primary, role in acid–base regulation and/or ionoregulation. In summary, we emphasize the need to investigate the functions of gills in phylogenetically varied species. The role of larval fish gills in ionoregulation is a good starting point for future research. We can then make a well-informed hypothesis on the relative strength of the forces that drive ontogenic gill development and, on a larger scale, the selective pressures that may have influenced the evolution of the vertebrate gill.
Acknowledgements
The University of British Columbia Animal Care Committee approved all procedures performed on animals.
This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant to C.J.B. and C.F. was supported by an NSERC CGSM.
References
- Brauner C. J., Wood C. M.2002aEffect of long-term silver exposure on survival and ionoregulatory development in rainbow trout (Oncorhynchus mykiss) embryos and larvae, in the presence and absence of added dissolved organic matter. Comp. Biochem. Physiol. C 133, 161–173 [DOI] [PubMed] [Google Scholar]
- Brauner C. J., Wood C. M.2002bIonoregulatory development and the effect of chronic silver exposure on growth, survival and sublethal indicators of toxicity in early life stages of rainbow trout (Oncorhynchus mykiss). J. Comp. Physiol. B 172, 153–162 (doi:10.1007/s00360-001-0238-8) [DOI] [PubMed] [Google Scholar]
- Coolidge E., Hedrick M. S., Milsom W. K.2007Ventilatory systems. In Fish physiology XXVI (eds McKenzie D. J., Brauner C. J., Anthony F. P.), pp. 181–211 San Diego, CA: Academic Press, Elsevier [Google Scholar]
- Evans D. H.1984Gill Na+/H+ and Cl−/HCO3− exchange systems evolved before the vertebrates entered fresh water. J. Exp. Biol. 113, 465–469 [DOI] [PubMed] [Google Scholar]
- Evans D. H., Piermarini P. M., Potts W. T. W.1999Ionic transport in the gill epithelium. J. Exp. Zool. 283, 641–652 (doi:10.1002/(SICI)1097-010X(19990601)283:7<641::AID-JEZ3>3.0.CO;2-W) [Google Scholar]
- Evans D. H., Piermarini P. M., Choe K. P.2005The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol. Rev. 85, 97–177 (doi:10.1152/physrev.00050.2003) [DOI] [PubMed] [Google Scholar]
- Gonzalez M. E., Blanquez M. J., Rojo C.1996Early gill development in the rainbow trout, Oncorhynchus mykiss. J. Morphol. 229, 201–217 (doi:10.1002/(SICI)1097-4687(199608)229:2<201::AID-JMOR5>3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- Hwang P.2009Ion uptake and acid secretion in zebrafish (Danio rerio). J. Exp. Biol. 212, 1745–1752 (doi:10.1242/jeb.026054) [DOI] [PubMed] [Google Scholar]
- Hwang P.-P., Lee H.2007New insights into fish ion regulation and mitochondrionfich cells. Comp. Biochem. Physiol. A 148, 479–497 (doi:10.1016/j.cbpa.2007.06.416) [DOI] [PubMed] [Google Scholar]
- Keene J. L., Noakes D. L. G., Moccia R. D., Soto C. G.1998The efficacy of clove oil as an anaesthetic for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 29, 89–101 [Google Scholar]
- Killen S. S., Costa I., Brown J. A., Gamperl K. A.2007Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc. R. Soc. B 274, 431–438 (doi:10.1098/rspb.2006.3741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner L. B.1970The study of NaCl transport in aquatic animals. Am. Zool. 10, 365–376 [DOI] [PubMed] [Google Scholar]
- Kirschner L. B., Greenwald L., Kerstetter T. H.1973Effect of amiloride on sodium transport across body surfaces of freshwater animals. Am. J. Physiol. 224, 832–837 [DOI] [PubMed] [Google Scholar]
- Krogh A.1941The comparative physiology of respiratory mechanisms Philadelphia, PA: University of Pennsylvania Press [Google Scholar]
- Li J., Eygensteyn J., Lock R., Verbost P., Heijden A., Bonga S., Flik G.1995Branchial chloride cells in larvae and juveniles of freshwater tilapia Oreochromis mossambicus. J. Exp. Biol. 198, 2177–2184 [DOI] [PubMed] [Google Scholar]
- Mallat J., Conley D. M., Ridgway R. L.1987Why do hagfish have gill ‘chloride cells’ when they need not regulate plasma NaCl concentration? Can. J. Zool. 65, 1956–1965 (doi:10.1139/z87-298) [Google Scholar]
- McCormick S. D.1993Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can. J. Fish. Aquat. Sci. 50, 656–658 (doi:10.1139/f93-075) [Google Scholar]
- Metro Vancouver 2007. Water: The Greater Vancouver Water District Quality Control Annual Report 2007. Volume I See http://www.metrovancouver.org/about/publications/Publications/QualityControlAnnualWaterReport2007-Volume1.pdf [Google Scholar]
- Presnell J. K., Schreibman M. P.1997Humason's animal tissue techniques, 5th edn Baltimore, MD: The John Hopkins University Press [Google Scholar]
- Rombough P. J.1988Respiratory gas exchange, aerobic metabolism and effects of hypoxia during early life. In Fish physiology XIA (eds Hoar W. S., Randall D. J.), pp. 59–161 New York, NY: Academic Press [Google Scholar]
- Rombough P. J.1998Partitioning of oxygen uptake between the gills and skin in fish larvae: a novel method for estimating cutaneous oxygen uptake. J. Exp. Biol. 201, 1763–1769 [DOI] [PubMed] [Google Scholar]
- Rombough P. J.1999The gill of fish larvae. Is it a primarily a respiratory or an ionoregulatory structure? J. Fish Biol. 55, 186–204 (doi:10.1111/j.1095-8649.1999.tb01055.x) [Google Scholar]
- Rombough P. J.2002Gills are needed for ionoregulation before they are needed for O2 uptake in developing zebrafish, Danio rerio. J. Exp. Biol. 205, 1787–1794 [DOI] [PubMed] [Google Scholar]
- Rombough P. J.2004Gas exchange, ionoregulation, and the functional development of the teleost gill. Am. Fish. Soc. Symp. 40, 47–83 [Google Scholar]
- Rombough P. J.2007The functional ontogeny of the teleost gill: which comes first, gas or ion exchange? Comp. Biochem. Physiol. A 148, 732–742 (doi:10.1016/j.cbpa.2007.03.007) [DOI] [PubMed] [Google Scholar]
- Rombough P. J., Ure D.1991Partitioning of oxygen uptake between cutaneous and branchial surfaces in larval and young juvenile Chinook salmon Onchorhynchus tshawytscha. Physiol. Zool. 64, 717–727 [Google Scholar]
- Shen A., Leatherland J.1978Structure of the yolksac epithelium and gills in the early developmental stages of rainbow trout (Salmo gairdneri) maintained in different ambient salinities. Environ. Biol. Fish. 4, 345–354 (doi:10.1007/BF00000526) [Google Scholar]
- Shingles A., McKenzie D. J., Taylor E. W., Moretti A., Butler P. J., Ceradini S.2001Effects of sublethal ammonia exposure on swimming performance in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 204, 2691–2698 [DOI] [PubMed] [Google Scholar]
- Wells P. R., Pinder A. W.1996The respiratory development of Atlantic salmon II. Partitioning of oxygen uptake among gills, yolk sac and body surfaces. J. Exp. Biol. 199, 2737–2744 [DOI] [PubMed] [Google Scholar]
- Wieser W.1985Developmental and metabolic constraints of the scope for activity in young rainbow trout (Salmo gairdneri). J. Exp. Biol. 118, 133–142 [Google Scholar]
- Wilson R. W., Bergman H. L., Harold L., Wood C. M.1994Metabolic costs and physiological consequences of acclimation to aluminum in juvenile rainbow trout (Oncorhynchus mykiss). 2: gill morphology, swimming performance, and aerobic scope. Can. J. Fish. Aquat. Sci. 51, 536–544 (doi:10.1139/f94-056) [Google Scholar]
- Wilson J. M., et al. 2007Modulation of branchial ion transport protein expression by salinity in glass eels (Anguilla anguilla L.). Mar. Biol. 151, 1633–1645 (doi:10.1007/s00227-006-0579-7) [Google Scholar]
- Wright P. A.2007Ionic, osmotic and nitrogenous waste regulation. In Fish physiology XXVI (eds McKenzie D. J., Brauner C. J., Farrell A. P.), pp. 284–318 San Diego, CA: Academic Press, Elsevier [Google Scholar]