Abstract
Foraging animals have several tools for managing the risk of predation, and the foraging games between them and their predators. Among these, time allocation is foremost, followed by vigilance and apprehension. Together, their use influences a forager's time allocation and giving-up density (GUD) in depletable resource patches. We examined Allenby's gerbils (Gerbilus andersoni allenbyi) exploiting seed resource patches in a large vivarium under varying moon phases in the presence of a red fox (Vulpes vulpes). We measured time allocated to foraging patches electronically and GUDs from seeds left behind in resource patches. From these, we estimated handling times, attack rates and quitting harvest rates (QHRs). Gerbils displayed greater vigilance (lower attack rates) at brighter moon phases (full < wane < wax < new). Similarly, they displayed higher GUDs at brighter moon phases (wax > full > new > wane). Finally, gerbils displayed higher QHRs at new and waxing moon phases. Differences across moon phases not only reflect changing time allocation and vigilance, but changes in the state of the foragers and their marginal value of energy. Early in the lunar cycle, gerbils rely on vigilance and sacrifice state to avoid risk; later they defend state at the cost of increased time allocation; finally their state can recover as safe opportunities expand. In the predator–prey foraging game, foxes may contribute to these patterns of behaviours by modulating their own activity in response to the opportunities presented in each moon phase.
Keywords: foraging theory, risk management, time allocation and vigilance, state-dependent foraging, moonlight avoidance, gerbils
1. Introduction
While seeking food, an animal exposes itself to predators and faces tradeoffs between food and safety (Brown & Kotler 2004, 2007) that may select for sophisticated responses (Brown 1999). Through time allocation, the animal can bias its foraging towards resources patches with more favorable combinations of food availability and risk. Through vigilance and other apprehensive behaviours, the animal gains safety while sacrificing aptitude at harvesting food. Both of these risk management tools influence the animal's overall exposure to predators and its energetic state. At the same time, the animal's state feeds back on the appropriate levels of time allocation and vigilance—a hungry or emaciated animal has less to lose from predation and more to gain from food than a sated or fat animal (Brown 1992; McNamara & Houston 1992; Clark 1994; Olsson et al. 2002; van Gils et al. 2009).
When is it necessary to understand this complex interplay between time allocation, vigilance and state-dependent foraging? Here we show that a predator–prey foraging game typified by the response of gerbils to moonlight (Brown et al. 2001) requires just such an understanding. Moonlight avoidance occurs frequently. Among rodents, examples include kangaroo rats and pocket mice (Heteromyidae), Old World porcupines (Hytrix sp.), gerbils (Gerbillus, Gerbillurus), wood rats (Neotoma), spiny mice (Acomys), degus (Phyllotis) and deer mice (Peromyscus maniculatus; see Brown & Kotler 2004). Examples among other taxa include prairie rattlesnakes (Clarke et al. 1996), nocturnal sea birds (Mougoet & Bretagnolle 2000), Galapagos fur seals (Archocephalus galapagoensis; see Trillmich & Mohren 1980) and lake-inhabiting zooplankton (Zaret & Suffern 1976). Moonlight probably makes organisms more conspicuous to their predators (Kotler et al. 1991). All may represent examples of the interplay of foraging, risk management and state.
Increased illumination appears to aid predators such as owls and foxes more than it aids their rodent prey (Kotler et al. 1988, 1991; Longland & Price 1991). From the rodent's point of view, it is better that the predator does not encounter the prey, but this is more difficult on bright nights. The increased risk of predation with moonlight increases the animal's cost of foraging, requiring a higher harvest rate to compensate. Of particular importance is how long a forager devotes to each resource patch, i.e. its time allocation. In food patches with diminishing returns, this manifests as higher giving-up densities (GUD, the amount of food a forager leaves behind in a resource patch) under full moon than new moon (Kotler et al. 1993a), or with lights on versus lights off in aviary experiments (Kotler et al. 1988, 1991).
In studies where animals manage risk through time allocation, researchers often assume that animals adopt the same feeding tactics under safe and risky conditions. But, animals probably vary their apprehension and tailor their tactics to the particular risk circumstances. Apprehension refers to the attention that a forager directs towards predator detection at the expense of attention paid towards foraging related tasks (Dall et al. 2001). An extreme form of apprehension is vigilance where all of a forager's attention is directed towards predator detection at the expense of all other activities. More generally, an apprehensive animal can look for and harvest food while at the same time being wary of predators.
Apprehension brings greater safety, but at the expense of harvest rate. Consequently, there is an optimal level (Brown 1999; Brown et al. 2001) that should increase with the encounter rate with predators and their lethality. The effectiveness of apprehension in lowering risk also alters optimal behaviours, with foragers needing little apprehension when it is highly effective, wanting little when it is largely useless, and using higher amounts when its effectiveness is intermediate.
The state of the forager should influence time allocation and apprehension through the foraging cost of predation. Foragers in a high state have much to lose should they be killed by a predator. They should assess a higher predation cost (increased GUD), forage less, and be more apprehensive (McNamara & Houston 1992; Brown 1999; Olsson et al. 2002; van Gils et al. 2009). But foraging decisions based on state may in turn affect state. For many circumstances, the state of the animal may remain relatively constant between changing risk environments, for instance, across safe and risky microhabitats within the same foraging bout. However, the same animal may experience large, predictable seasonal changes in state, and its responses should reflect this.
Moonlight avoidance may provide an ideal setting for testing for the combined use of time allocation and apprehension. Changes in moonlight probably change the optimal levels of apprehension. For instance, moonlight should increase the prey's encounter rate with predators, increase the predator's lethality, and perhaps make apprehension more effective. Like time allocation, apprehension should cycle with moon phase.
Moonlight avoidance is generally viewed without regard to state dependencies, but the regular progression from a low foraging cost, low GUD new moon, through the waxing moon, to a high cost, high GUD full moon and back means that the animal's state may also cycle. State may be increasing during the new moon, declining through the full moon, and then increasing again over the lunar cycle. Integrating state and predation risk with moon phase, we predict that state will be greatest going into the waxing moon and lowest entering the waning moon: waxing > new > full > waning. In terms of predation risk per unit time spent foraging, we predict: full > waning ≈ waxing > new.
Here, we quantify over the course of a lunar cycle the manner in which gerbils under threat of fox predation use time allocation and apprehension while exploiting depletable resource patches. In a large outdoor vivarium, we subjected a population of gerbils to the threat of fox predation and measured the gerbils' use of experimental food patches, their apprehension, and their activity levels over an entire lunar cycle. The results show that in their foraging game with foxes the gerbils manage risk in a sophisticated manner consistent with a feedback of state and moon phase on optimal behavioural decisions.
2. Material and Methods
Gerbils are small, granivorous rodents from Old World deserts. Particularly well studied are the gerbils from sandy habitats in the Negev Desert of Israel. There, gerbils visit fewer resource patches in response to risk factors such as open microhabitat, moonlight, and the presence of predators (e.g. Kotler et al. 1991, 1993b; Brown et al. 1994) and exploit those resource patches less thoroughly (e.g. Kotler et al. 1991). Furthermore, they switch their activity more towards the safer bush microhabitat in response to both natural and artificial moon light (Kotler et al. 1991, 1993a; Brown et al. 1994) and in response to owls (Kotler et al. 1991, 1992; Kotler & Blaustein 1995) and foxes (Mukherjee et al. 2009). In experiments in a large vivarium, moonlight levels of illumination interacted with the presence of predators to determine patch use by gerbils. Gerbils also display greater apprehension at full moon, in the open microhabitat, and at times of the night when predators are most active (Kotler et al. 2002, 2004a,b). Experimental food augmentations intensified the gerbils' response to predators and increased their apprehension (Kotler 1997; Kotler et al. 2004a,b). Moonlight avoidance in gerbils involves time allocation and apprehension in a manner that changes with risk and forager state.
We examined foraging behaviour of Allenby's gerbil (Gerbillus andersoni allenbyi) under the threat of predation from a red fox (Vulpes vulpes) in a large (34 × 17 m) outdoor enclosure situated in the Negev Desert, Israel. This vivarium is divided into halves, and a gate in the dividing partition allowed the fox (but not the gerbils) to move freely between sides.
We provided cover by arranging 36 wooden trellises in an evenly spaced 6 × 6 array on each side of the vivarium. Each trellis measured 76 × 60 cm and stood 16 cm off the ground. To mimic bushes, we topped each trellis with a pile of cut brush.
We used plastic trays (28 × 38 × 8 cm) to make seed patches for the gerbils. Each tray contained 3 g of millet seeds mixed into 3 l of sifted sand. We placed pairs of trays at 18 of the 36 stations on each side of the vivarium, one pair located at every alternate trellis of a row (one tray of a pair placed under the trellis (bush microhabitat) and the other just beyond the canopy (open microhabitat)), for a total of 36 trays per side, and 72 overall. Gerbils foraging in these trays experience diminishing returns (Kotler & Brown 1990). The amount of seeds the gerbils leave behind, the GUD, provides a measure of foraging efficiency and foraging costs (Brown 1988). In particular, animals exploiting these food patches should use each patch so long as the benefit of patch use, i.e. the harvest rate, is greater than the sum of the energetic, predation and missed opportunity costs of foraging (Brown 1988). Also, GUDs provide an estimate of quitting harvest rates (QHRs).
We quantified the amount of time that gerbils spent in seed trays by marking each gerbil with a uniquely numbered passive integrated transponder (PIT) tag and placing specially designed PIT tag reader/loggers (Model SQID, Vantro Systems, Burnsville, MN, USA) under 7–8 seed trays per side. These readers recorded the identity of each gerbil to visit the seed tray, the time of the visit and the duration of each visit. We used this information to derive the cumulative amount of time that gerbils spent at each resource patch each night. We then used this information along with the amount of seeds harvested from each patch to calculate QHRs and to plot harvest rate curves, i.e. the relationship between the seeds remaining in a tray and the expected instantaneous harvest rate of the gerbil exploiting that tray (see below; Kotler & Brown 1990).
To begin the experiment, we added 32 wild-caught individuals (16 males and 16 females) of G. a. allenbyi to the vivarium, with 16 gerbils to a side. Prior to experiments, the gerbils had five nights to acclimate to the vivarium and food patches. On each experimental night, we provisioned the food patches with millet before dusk, and released a muzzled fox into the vivarium. The following morning, we removed the fox, sifted seeds from each tray, downloaded data from the loggers, and provisioned trays with another 3 g of millet for the following night. We cleaned the seeds of debris and weighed them to obtain the GUDs. We collected data for three consecutive nights centred on each of four moon phases: new, waxing half (moonset around midnight), full and waning half moon (moonrise around midnight; all nights offered clear skies).
3. Results
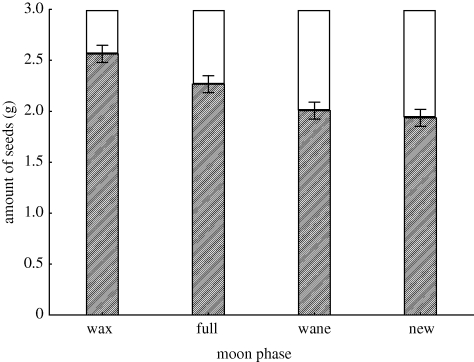
Gerbils exhibited moonlight avoidance (figure 1) by using seed patches less intensively during brighter moon phases and leaving significantly higher GUDs (M.S. = 6.8478, F3,269 = 12.869, p ≪0.001). Highest GUDs occurred during the waxing half moon, with a rank ordering of waxing half moon > full moon > new moon = waning half moon. These results reflect changing foraging costs as reflected in the GUD. They also reflect changes in the gerbils' state since the GUD is the mirror image of the amount of seeds harvested by gerbils from the trays, and the trays ultimately provide all of the gerbils' food within the vivarium, even if the gerbils might cache food for later consumption. See the appendix in the electronic supplementary material showing where gerbils here help manage risk through higher QHRs in the open microhabitat.
Figure 1.
Giving-up density (filled bars) and amount of food (seeds; white bars) taken by foraging gerbils during four moon phases. Vertical bars indicate 95% CIs.
At new moon, gerbils visited and foraged an average of 64.25 trays per night, at waxing moon 61.75 trays, at full moon 60 trays, and at waning moon 63 trays. Taking into consideration the number of trays foraged as well as amount of seeds taken, gerbils took the most seeds at new moon (2.39 g of seed per individual gerbil per night), followed by the waning half moon (2.28 g per gerbil), followed by full moon (1.65 g), followed by the waxing half moon (0.98 g; all tray types), compared with an energetic requirement of approximately 2 g of seed per night (Degen et al. 1998) Therefore, gerbils were in a high and increasing state during the new moon. Comparatively, the gerbils' state dropped severely during the waxing moon and continued to drop at full moon although not as severely. The gerbils' state probably rebounded during the waning moon as dark hours became more frequent and gerbils exploited food patches more thoroughly.
Gerbils responded to moon phase by altering the amount of time they spent in resource patches (M.S. = 9.308 × 10−6, F3,269 = 5.746, p < 0.001). They spent the most time in each exploited resource patch during the waning moon (24.8 min per foraged tray), approximately equal amounts of time during full (21.9 min) and new moon (21.5 min) and the least time during the waxing moon phase (10.2 min). These times can be multiplied by the number of food patches foraged (see above) and divided by 32 gerbils to calculate the time spent foraging per gerbil during each night (on average 1.94).
The amount of time spent in resource patches and the subsequent GUD for a particular set of conditions can be used to calculate QHRs in resource patches based on Holling's (1959) disc equation. Following Kotler & Brown (1990), we obtained estimates of attack rate or instantaneous area of discovery, a (per second), and handling time, h (s g−1). Once these coefficients are estimated, they can be used in Holling's disc equation to convert GUDs into QHRs. Over all, we estimated handling time to be 22.9 s g−1 of millet. The low value gives the curves in figure 2 an appearance of linearity, although they are not. Following Olsson et al. (2002), we used this value and then estimated attack rate for each moon phase (see the electronic supplementary material, appendix). Gerbils showed their highest attack rates at new moon (5.42 × 10−4 s−1), followed by the waning moon (4.90 × 10−4), and the waxing moon (4.34 × 10−4), with lowest attack rates at the full moon (4.04 × 10−4).
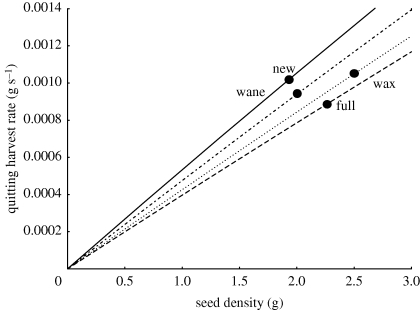
Figure 2.
Harvest rate curves for gerbils foraging during four moon phases. Estimates of quitting harvest rates appear as functions of seed density in the resource patch. Curves are arrived at by estimating attack rates from data, using previously estimated estimates of handling time, and fitting them to a Holling disc equation. Then we plot for each moon phase, the estimated quitting harvest rate derived from the mean giving-up density and the disc equation. Shallower slopes correspond to higher levels of vigilance; giving-up densities lying closer to the origin correspond to greater time allocation. See the appendix in the electronic supplementary material for more details.
QHRs varied significantly with moon phase (M.S. = 2.278 × 10−2, F3,269 = 17.9705, p ≪ 0.001). QHRs were highest during the waxing moon phase (1.02 × 10−3 g seeds s−1), followed by new moon (9.35 × 10−4 g seeds s−1), then waning moon (7.92 × 10 −4 g seeds s−1), and close behind, the full moon (7.76 × 10 −4 g seeds s−1; figure 2).
4. Discussion
Moonlight avoidance in gerbils is probably a sophisticated strategy of time allocation and apprehension. The gerbils' response to moonlight included the direct effect of moonlight on predation risk. More moonlight is riskier. And, the gerbils' response reflected the indirect feedback caused by fluctuations in the gerbils' energy states. The gerbils ate well during the new moon and the waning moon, and ate poorly during the waxing moon and full moon phases.
In terms of predation risk, prior work tells us that full moon > waxing moon = waning moon > new moon. Furthermore, moon phases cycle regularly from new to waxing to full to waning and back to new. We had four related metrics for determining the gerbils' responses: GUDs, average harvest of seeds per gerbil per night, average foraging time per patch and QHR.
Gerbil GUDs varied with moon phase: waxing > full > new = waning. In the absence of other responses, full should be the highest and new the lowest. But, gerbil state also changed with moon phase as indicated by the gerbils' average harvest of seeds per night. When a gerbil gathers greater than 2 g of seeds per night, we can expect it to maintain or increase its energy state (Degen et al. 1998). Well below this, and a gerbil's state declines. Hence, the gerbils' states at the beginning of each moon phase should vary, with waxing > new = full > waning. As gerbils enter the waxing moon they have enjoyed two phases of increasing state. Conversely, entering the waning moon, gerbils have had two consecutive phases of declining state.
The foraging cost of predation increases with risk and with the forager's state. Hence, we unambiguously expect the foraging cost of predation to be higher at full than new moon for reasons of risk. And, we expect the foraging cost of predation to be higher on a waxing than a waning moon for reasons of state. Differences in GUDs support these two comparisons.
Time spent in the food patches provides an estimate of overall activity. By this measure, activity with moon phases ranked as: waning > new = full > waxing. We see the waning moon as a period of playing catch-up in terms of foraging, and waxing moon as a period of letting one's state slide in the name of safety. The fairly equal activity time during the new and full moon suggest shifts in foraging tactics via apprehension and time allocation. Since the gerbils harvested more seeds on new than full moon phases, the gerbils of a new moon forage quickly and inattentively vis-a-vis predators, while on a full moon they forage slowly and attentively. This can be seen in the attack rates (the slopes of the cumulative harvest curves of figure 2) that show: new > waning > waxing > full.
It may be that in the dark, apprehension is less valuable and less needed than on a bright night. This slow and apprehensive foraging strategy of brighter nights coupled with faster and less apprehensive foraging on dark nights yields a pattern of QHRs of: waxing > new > waning = full. QHRs, more so than GUDs, directly estimate foraging costs. That QHRs are lower under full than new moon is at first paradoxical. But, QHRs reflect costs after adjusting for apprehension and state. The gerbils' low state and high use of apprehension at the full moon appears to leave a lower foraging cost of predation at full than new moon! Figure 2 tells the story.
Figure 2 plots QHR versus GUD for each moon phase (see the electronic supplementary material, appendix). The resulting graph is uniquely informative. The slopes of the harvest rate curves reflect gerbil foraging while exploiting the resource patch, and the GUDs and the QHRs reflect when foragers decided to leave resource patches. Different slopes correspond to different levels of apprehension. Steeper slopes represent faster harvest for the same density of seeds, and indicate foragers paying more attention to their harvesting task and less to predator detection. Thus steeper slopes for the harvest rate curves correspond to less apprehensive animals. The locations of the GUD points reflect time allocation. The curve plots the forager's harvest rate in the patch, staring from an unexploited resource patch at the upper right and tracing patch depletion towards the origin. Points located on the curve closer to the origin represent greater time allocation to resource patches.
Animals that use time allocation to manage risk would devote more time to a safer resource patch than a riskier one. Thus they would have both higher QHRs and higher GUDs in the riskier patch. When looking at the harvest rate curve, the point representing the QHR for the safer situation would be located lower down the curve towards the origin, reflecting the greater time allocation to that patch. In this manner, the risk management behaviour of the foragers can be deconstructed into components of time allocation and vigilance.
Figure 2 suggests the following scenario. Starting at new moon, gerbils forage with low apprehension as indicated by the steep slope of the harvest rate curve, and with moderate time allocation, as indicated by the GUD. During this time period, gerbils should be in a high and increasing state.
Soon, the safety of darkness begins to give way to more and more moonlit hours as the moon waxes towards half. In response, gerbils respond by increasing solely their level of apprehensiveness. QHRs do not change, but it now takes a higher level of resources in a patch to return the same harvest rate to the more apprehensive gerbils. The correspondingly higher GUDs mean that gerbils are now harvesting fewer resources than before, and their state must drop unless they can make up the difference from seed caches.
Next, the moon reaches its full phase, and dark, starlit hours are no longer available. Gerbils can only forage in bright moonlight, and they increase their apprehension further. Interestingly, their GUDs drop dramatically. Despite the greater risk, gerbils allocate more time to resource patches, apparently to help defend state and protect against starvation risk.
At last, the moon starts to wane, and dark, moonless hours begin to appear. As the waning half moon appears, gerbils reduce their apprehension, directing more attention to foraging tasks. They continue to allocate much time towards resource patches in order to rebuild state. Finally, dark hours become abundant as the moon wanes to new, gerbils reduce their apprehension yet more, and perhaps reduce their time allocation as they finish rebuilding their state.
The data and the scenario they suggest tell of a feedback of risk and state over the lunar cycle. This feedback manifests in both time allocation and apprehension. The gerbils appear to rely mostly on apprehension to manage risk, especially when they are in good state. They alter their apprehensiveness across all four moon phases, and the transitions from new to waxing half moon and from full to waning half moon reflect this. Changes in apprehension then lead to changes in state, requiring gerbils to alter their behaviour yet more. Lowered state reduces the cost of predation for a given level of risk (Brown 1988, 1992, 1999), and perhaps encourages the gerbils at full moon to use time allocation to preserve their state.
How well does the vivarium match what free-ranging gerbils experience? The gerbils used here inhabit sand dunes. On the dunes, the mobile characteristic of the sandy substrate along with the daily afternoon winds cause sand and seed to accumulate in depressions and wind shadows. This leads to daily renewal of seed resource patches (Ben-Natan et al. 2004). Gerbils then deplete these resource patches at night (e.g. Kotler et al. 1993c). Thus early evening hours are the most profitable hours for foraging, and gerbils are most active then (Kotler et al. 2002, 2004a,b). They are also the most dangerous because of the activity of owls and foxes responding to gerbil activity (Kotler et al. 2002; Magen 2007), and gerbils are correspondingly more apprehensive early in the night, adjusted for moon phase (Kotler et al. 2002, 2004a,b). In our experiments, we mimicked the pulse and depletion of the natural system by giving gerbils fresh seed trays each evening and allowing them to deplete the trays during the night. We also exposed gerbils each night to the presence of a fox. The responses of gerbils to moon phase may therefore in part be a response to foxes that are managing the fear and catchability of their prey by altering their activity times in a larger foraging game (Brown et al. 2001; Kotler et al. 2002). We used densities of gerbils and foxes that far exceed natural densities. Still, the resources available nightly per gerbil are on the order required for maintaining body mass, and in past experiments, field data when available agree at least qualitatively with vivarium results (Brown & Kotler 2004).
Studies that focus on vigilance or patch use alone may fall short of fully characterizing risk management strategies, the factors that affect and tailor risk management, and their consequences. In gerbils, examining GUDs alone (figure 1) or apprehension alone does not reveal the importance of state or how time allocation, apprehension and state combine to form a monthly cycle of moonlight avoidance in the tradeoff of food and safety. The gerbils' cycling of state, apprehension and time allocation should factor into their predator–prey foraging game with owls and foxes. Perhaps under the cover of darkness, the fox became stealthier, further reducing the value of vigilance and encouraging the gerbils to forage swiftly. Under full moon, the fox may have chosen to be less stealthy, thus amplifying the slow, apprehensive foraging tactics of the gerbils.
Many animals face repeatable, cyclical changes in state on time scales ranging from annual (e.g. bears) to seasonal (e.g. rhinoceros) to monthly (e.g. crested porcupines; Brown & Alkon 1990) to daily (e.g. granivorous birds, Caraco 1981), and often in the context of a predator–prey foraging game. Perhaps all of these cases manifest cycles of foraging behaviour based on food, safety and state.
Acknowledgements
We carried out this research under permit no. 2006/25 159 from the Israel Nature and Parks Authority.
This is publication no. 663 of the Mitrani Department of Desert Ecology. This work was supported by the United States-Israel Binational Science Foundation (Grant 2004-105).
References
- Ben-Natan G., Abramsky Z., Kotler B. P.2004Daily renewal of seed resource patches provide the necessary environmental variability for two temporally partitioning gerbils. Oikos 105, 325–335 (doi:10.1111/j.0030-1299.2004.12948.x) [Google Scholar]
- Brown J. S.1988Patch use as an indicator of habitat preference, predation risk, and competition. Behav. Ecol. Sociobiol. 22, 37–47 (doi:10.1007/BF00395696) [Google Scholar]
- Brown J. S.1992Patch use under predation risk. I. Models and predictions. Ann. Zool. Fenn. 29, 301–309 [Google Scholar]
- Brown J. S.1999Vigilance, patch use, and habitat selection: foraging under predation risk. Evol. Ecol. Res. 1, 49–71 [Google Scholar]
- Brown J. S., Alkon P.1990Patch use by the Indian crested porcupine (Hystris indica). Oecologia 83, 512–518 [DOI] [PubMed] [Google Scholar]
- Brown J. S., Kotler B. P.2004Hazardous duty pay: studying the foraging cost of predation. Ecol. Lett. 7, 999–1014 (doi:10.1111/j.1461-0248.2004.00661.x) [Google Scholar]
- Brown J. S., Kotler B. P.2007The ecology of fear. In Foraging (eds Stephens D., Ydenberg R., Brown J. S.), pp. 437–480 Chicago, IL: University of Chicago Press [Google Scholar]
- Brown J. S., Kotler B. P., Mitchell W. A.1994Foraging theory, patch use, and the structure of a Negev Desert rodent community. Ecology 75, 2286–2300 (doi:10.2307/1940884) [Google Scholar]
- Brown J. S., Kotler B. P., Bouskila A.2001The ecology of fear and the foraging game between owls and gerbils. Ann. Zool. Fenn. 38, 71–87 [Google Scholar]
- Clark C. W.1994Antipredator behavior and the asset-protection principle. Behav. Ecol. 5, 159–170 (doi:10.1093/beheco/5.2.159) [Google Scholar]
- Clarke J. A., Chopko J. T., Mackessy S. P.1996The effect of moonlight on activity patterns of adult and juvenile prairie rattlesnakes (Crotalus viridus viridus). J. Herpetol. 30, 192–197 (doi:10.2307/1565509) [Google Scholar]
- Caraco T.1981Energy budgets, risk and foraging preferences in dark-eyed juncos (Junco hyemalis). Behav. Ecol. Sociobiol. 8, 213–217 (doi:10.1007/BF00299833) [Google Scholar]
- Dall S. R. X., Kotler B. P., Bouskila A.2001Attention, apprehension, and gerbils searching in patches. Ann. Zool. Fenn. 38, 15–23 [Google Scholar]
- Degen A. A., Kamm M., Khokhlava I. S., Krasnov B. R., Barraclough T. G.1998Average daily metabolic rate of rodents: habitat and dietary comparisons. Funct. Ecol. 12, 63–73 (doi:10.1046/j.1365-2435.1998.00162.x) [Google Scholar]
- Holling C. S.1959Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398 [Google Scholar]
- Kotler B. P.1997Patch use by gerbils in a risky environment: manipulating food and safety to test four models. Oikos 78, 274–282 (doi:10.2307/3546294) [Google Scholar]
- Kotler B. P., Blaustein L.1995Titrating food and safety in a heterogeneous environment: when is the risky patch of equal value? Oikos 74, 251–258 (doi:10.2307/3545654) [Google Scholar]
- Kotler B. P., Brown J. S.1990Rates of seed harvest by two species of gerbilline rodents. J. Mammal. 71, 591–596 (doi:10.2307/1381798) [Google Scholar]
- Kotler B. P., Brown J. S.1999Mechanisms of coexistence of optimal foragers as determinants of local abundances and distributions of desert granivores. J. Mammal. 80, 361–374 (doi:10.2307/1383285) [Google Scholar]
- Kotler B. P., Brown J. S., Smith R. J., Wirtz W. O., II1988The effects of morphology and body size on rates of owl predation on desert rodents. Oikos 53, 145–152 (doi:10.2307/3566056) [Google Scholar]
- Kotler B. P., Brown J. S., Hasson O.1991Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72, 2249–2260 (doi:10.2307/1941575) [Google Scholar]
- Kotler B. P., Blaustein L., Brown J. S.1992Predator facilitation: the combined effect of snakes and owls on the foraging behaviour of gerbils. Ann. Zool. Fenn. 29, 199–206 [Google Scholar]
- Kotler B. P., Brown J. S., Mitchell W. A.1993aEnvironmental factors affecting patch use in two species of gerbilline rodents. J. Mammal 74, 614–620 (doi:10.2307/1382281) [Google Scholar]
- Kotler B. P., Brown J. S., Slotow R., Goodfriend W., Strauss M.1993bThe influence of snakes on the foraging behavior of gerbils. Oikos 67, 309–318 (doi:10.2307/3545476) [Google Scholar]
- Kotler B. P., Brown J. S., Subach A.1993cMechanisms of species coexistence of optimal foragers: temporal partitioning by two species of sand dune gerbils. Oikos 67, 548–556 (doi:10.2307/3545367) [Google Scholar]
- Kotler B. P., Brown J. S., Dall S. R. X., Gresser S., Ganey D., Bouskila A.2002Foraging games between owls and gerbils: temporal dynamics of resource depletion and apprehension in gerbils. Evol. Ecol. Res. 4, 495–518 [Google Scholar]
- Kotler B. P., Brown J. S., Bouskila A.2004aApprehension and time allocation in gerbils: the effects of predatory risk and energetic state. Ecology 85, 917–922 (doi:10.1890/03-3002) [Google Scholar]
- Kotler B. P., Brown J. S., Bouskila A., Mukherjee S., Goldberg T.2004bForaging games between gerbils and their predators: seasonal changes in schedules of activity and apprehension. Isr. J. Zool. 50, 256–271 (doi:10.1560/K8D7-8KCX-BLAW-Y2K5) [Google Scholar]
- Longland W. S., Price M. V.1991Direct observations of owls and heteromyid rodents—can predation risk explain microhabitat use? Ecology 72, 2261–2273 (doi:10.2307/1941576) [Google Scholar]
- Magen T.2007A game theoretic approach to studying foraging related decisions made under predation risk. MSc. Thesis, Ben-Gurion University of the Negev, Be'er Sheva, Israel. [Google Scholar]
- McNamara J. M., Houston A. I.1992Evolutionarily stable levels of vigilance as a function of group size. Anim. Behav. 43, 641–658 [Google Scholar]
- Mougoet F., Bretagnolle V.2000Predation risk and moonlight avoidance in nocturnal seabirds. J. Avian Biol. 31, 376–386 [Google Scholar]
- Mukherjee S., Zelcer M., Kotler B. P.2009Patch use in time and space for a meso-predator in a risky world. Oecologia 159, 661–668 (doi:10.1007/s00442-008-1243-3) [DOI] [PubMed] [Google Scholar]
- Olsson O., Brown J. S., Smith H. G.2002Long-term and short-term state-dependent foraging under predation risk: an indication of habitat quality. Anim. Behav. 63, 981–989 [Google Scholar]
- Trillmich F., Mohren W.1980Effects of lunar cycle on the Galapagos fur seal, Archocephalus galapagoensis). Oecologia 48, 85–92 (doi:10.1007/BF00346992) [DOI] [PubMed] [Google Scholar]
- van Gils J. A., Kraan C., Dekinga A., Koolhaas A., Drent J., de Goeij P., Piersma T.2009Reversed optimality and predictive ecology: burrowing depth forecasts population change in a bivalve. Biol. Lett. 5, 5–8 (doi:10.1098/rsbl.2008.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret T. M., Suffern J. S.1976Vertical migration as a predator avoidance mechanism. Limnol. Oceanogr. 21, 804–813 [Google Scholar]