Abstract
A fundamental feature of vertebrate muscle is that maximal force can be generated only over a limited range of lengths. It has been proposed that locomotor muscles operate over this range of lengths in order to maximize force production during movement. However, locomotor behaviours like jumping may require muscles to shorten substantially in order to generate the mechanical work necessary to propel the body. Thus, the muscles that power jumping may need to shorten to lengths where force production is submaximal. Here we use direct measurements of muscle length in vivo and muscle force–length relationships in vitro to determine the operating lengths of the plantaris muscle in bullfrogs (Rana catesbeiana) during jumping. We find that the plantaris muscle operates primarily on the descending limb of the force–length curve, resting at long initial lengths (1.3 ± 0.06 Lo) before shortening to muscle's optimal length (1.03 ± 0.05 Lo). We also compare passive force–length curves from frogs with literature values for mammalian muscle, and demonstrate that frog muscles must be stretched to much longer lengths before generating passive force. The relatively compliant passive properties of frog muscles may be a critical feature of the system, because it allows muscles to operate at long lengths and improves muscles' capacity for force production during a jump.
Keywords: length-tension, stiffness, passive tension, locomotion, biomechanics
1. Introduction
The anuran body plan is highly specialized for jumping. Features such as long hindlimbs, a stout vertebral column and a relatively small body size are considered specializations associated with enhanced jumping performance (Zug 1972; Emerson 1978; Shubin & Jenkins 1995). Although these morphological features are undoubtedly important, the frog jump is ultimately reliant on the mechanical force and power supplied by the hindlimb musculature.
The muscles that power frog jumping, like all vertebrate skeletal muscles, are governed in their mechanical function by well-known contractile properties. Gordon and coworkers described one such property more than 40 years ago when they showed that force output in a contracting muscle varied with muscle length (Gordon et al. 1966). The familiar force–length relationship shows that muscle force reaches a plateau at intermediate lengths, the midpoint of which defines the muscle's optimal length for force production (Lo). The plateau of the force–length relationship is relatively narrow and as a result vertebrate skeletal muscles can generate maximal force only within about ±5 per cent of Lo. At lengths longer and shorter than this range, the force output of the muscle declines. Because high force output is probably desirable in muscle contractions, this region is generally considered physiologically optimal and previous investigators have suggested that muscles' operating lengths in vivo should be limited to this region (e.g. Lieber et al. 1992; Rome 1998).
Do frog muscles function optimally during jumping by operating only at lengths where force is maximized? A consideration of the mechanics of jumping suggests that a restricted range of muscle operating lengths may not maximize performance. Jump distance is ultimately determined by the muscle work done during takeoff (Marsh 1994), and work is the product of muscle force and shortening distance. Thus, to maximize work output and power in a single contraction, muscles should shorten substantially during the takeoff phase of a jump.
Recent measurements of muscle fascicle length changes in jumping frogs support this prediction. Some muscles have been shown to shorten by as much as 30 per cent of their initial length during a jump (Olson & Marsh 1998; Roberts & Marsh 2003). Based on a typical force–length relationship, a muscle shortening by 30 per cent cannot avoid the significant influence of length on force output. A muscle starting at Lo and shortening by 30 per cent would end the contraction at a length where force output approaches only about 50 per cent of the muscles' maximum force. Thus, the predicted optimal function for frog muscles during jumping faces the problem that the substantial shortening associated with increased muscle work would seemingly require a tradeoff in force.
Here we used direct measurements of muscle length in vivo and muscle force–length relationship in vitro to determine the operating lengths of the plantaris muscle in bullfrogs (Rana catesbeiana) during jumping. We tested the hypothesis that, owing to large shortening strains associated with the high work requirements of a jump, the plantaris muscle would shorten to lengths well below the plateau of the force–length curve, where force output is submaximal.
2. Material and methods
(a). Animals
Four adult bullfrogs (R. catesbeiana) ranging in body mass from 135–163 g were purchased from a herpetological vendor. Animals were housed in the Brown University Animal Care facility in large aquaria, which included both aquatic and terrestrial regions. Frogs were kept on a diet of large crickets provided ad libitum.
(b). Surgical procedures
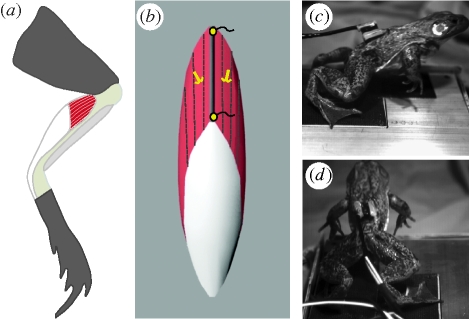
Frogs were anaesthetized using buffered tricain methanosulphonate (MS222, 0.25 g l−1). Once anaesthetized, a small incision along the dorsal midline was used to feed sonomicrometry and electromyography (EMG) transducers under the skin along the leg, past the hip and knee. A second incision was made along the skin covering the plantaris muscle. Sonomicrometry transducers (1 mm, Sonometrics Inc., London, Ontario, CA, USA) were implanted along a proximal fascicle of the plantaris muscle and secured using 6-0 silk and a small drop of VetBond adhesive (figure 1b). Two fine-wire bipolar EMG (Medwire Corp., Mt. Vernon, NY, USA) electrodes were inserted into the muscle just medial and lateral to the fascicle implanted with sonomicrometers (figure 1b). EMG electrodes were implanted using a 26G hypodermic needle and were sutured in place. Once transducers were in place, all incisions were closed and the frogs were given 24 h for recovery.
Figure 1.
Methods for measuring plantaris lengths and activity during jumping. (a) The plantaris is highlighted in ventral view showing the substantial aponeurosis and tendon (white) associated with the muscle. The plantaris acts as the primary ankle extensor. (b) Sonomicrometry transducers were placed along superficial, proximal fascicles to measure length. Two bipolar EMG electrodes were also implanted in the same region to measure muscle activation patterns. (c,d) Jumps were recorded in two views with high-speed video at 250 frames s−1.
(c). In vivo data collection
Measurements of fascicle length (sonomicrometry) and muscle electrical activity (EMG) were taken during jumps. Sonomicrometry data were collected using a Sonometrics TRX8 system (Sonometrics Inc.). The raw sonomicrometry signals were monitored using an oscilloscope in order to ensure that the transducers were consistently triggering off the first analogue peak. EMG signals were amplified (1000×) with a DAM50 differential amplifier (World Precision Instruments, Sarasota, FL, USA). All data were collected at 4000 Hz using a 16-bit A/D converter (National Instruments, TX, USA). Jumps were imaged at 250 frames s−1 (figure 1c) using two Fastcam 1280 high-speed video cameras (Photron Inc., CA, USA). All data were synchronized using a common external trigger. The seven longest jumps from each individual were analysed. The length of jumps used in this study ranged from 37 to 55 cm.
(d). In vitro data collection
Once all necessary jumps were collected from an individual, the force–length relationship of the same plantaris muscle was quantified using an in vitro preparation. The frogs were euthanized with a double pithing protocol. The leg previously implanted with sonomicrometry crystals for jumping trials was isolated. The distal tendon of the plantaris was severed and the muscle freed from the ankle and the tibiafibula. The proximal attachment of the muscle at the knee was kept intact. Along the femur, the sciatic nerve was freed from the surrounding tissue and a nerve cuff, constructed from silver wire was attached to stimulate the muscle. The sonomicrometry transducers implanted prior to jumping trials were left in place and used for fascicle length measurements. Care was taken to limit any disruption of the sonomicrometry transducers during the isolation process. The femur and the tibiafibula were attached to a rigid plate and the distal end of the plantaris tendon was placed in a custom-made clamp. The preparation was placed in an amphibian Ringer's solution (100 mM NaCl, 2.5 mM KCl, 2.5 mM NaHCO3, 1.6 mM CaCl, 10.5 mM Dextrose), which was continuously aerated with oxygen and kept at 22°C. The tendon clamp was attached to a servomotor (310 B-LR, Aurora Scientific Inc., Ontario, CA, USA) with stiff aircraft cable. The implanted sonomicrometry transducers were used to measure muscle fascicle length, while the servomotor was used to measure muscle force.
To determine the optimum stimulation voltage, a muscle's twitch force was monitored as the voltage was increased by 1 V increments. The voltage that resulted in maximum twitch force was increased by 1 V and used to supramaximally stimulate the muscle. The muscle was then stimulated tetanically at varying lengths in order to characterize its active force–length properties. Each tetanic stimulation was performed using 0.2 ms pulses, at 100 pulses s−1 for a duration of 300 ms. The passive force–length properties were also quantified using the sonomicrometer-measured length and force prior to stimulation. All data were collected at 1000 Hz using a 16-bit A/D converter (National Instruments, TX, USA). Following data collection, the morphological properties of the muscle, including fascicle length, muscle-tendon length, muscle mass and tendon length were measured. The muscle's physiological cross-sectional area was calculated according to Sacks & Roy (1982).
(e). Data analysis
All sonomicrometry, EMG and force data were processed and analysed using Igor Pro software (Wavemetrics, OR, USA). Sonomicrometry data required little processing. Occasional level-shifts were removed from the data using a custom algorithm. EMG data were processed with a band-pass filter with a 100 Hz low cut-off and a 1000 Hz high cut-off.
Data from high-speed video were analysed using Matlab (Mathworks, Inc. MA, USA). The location on the back of the frogs where transducer leads exited was used to approximate the frogs' centre of mass. This location has been previously used as a reliable indicator of the centre of mass (Marsh & John-Alder 1994; Roberts & Marsh 2003). This location was digitized in both camera views. Direct linear transformation of XY coordinates were used to convert data to XYZ positions. The displacements of this digitized point were smoothed with a quintic spline interpolation (s.d. = 0.05) and differentiated to calculate the velocity of the centre of mass during jumps.
Data from isolated muscle experiments were analysed using Igor Pro software. Given the pennate architecture of the plantaris, the aponeurosis of the muscle could not be removed. Therefore, all contractions included some degree of fascicle shortening against the stretch of series elastic structures. As a result, active force and length data were taken, where peak force reached a constant plateau for each contraction. To calculate only the active contribution to force, the passive force at the length corresponding to peak force was subtracted from the total force. This protocol allowed us to account for the fascicle shortening that occurs during the contraction when series elastic elements are present (Macintosh & Macnaughton 2005). Active force–length data were fit with the following function:
| 2.1 |
where F is force, L is length, and a, b and s represent the roundness, skewness and width of the force–length curve, respectively (Otten 1987). Based on the fit applied to the active force–length data, the peak isometric force (Po) and the fascicle length at peak force (Lo) were determined for each muscle. The passive force–length data were fit with a standard exponential function (Fung 1967).
| 2.2 |
Each muscle's optimal length (Lo) was used to convert in vivo length changes during jumps to strains ((L − Lo)/Lo). Total fascicle strain as well as the fascicle strain that occurred prior to centre of mass movement was calculated. The duration of EMG activity for the entire jump as well as the period prior to centre of mass movement was calculated.
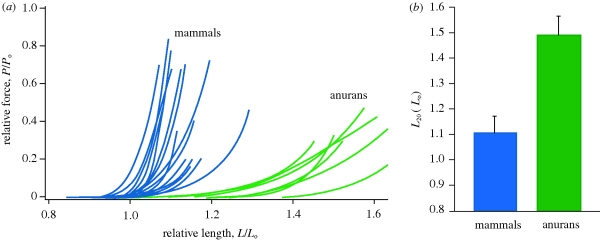
In addition to the experimental data collected, a meta-analysis of data from the literature was conducted to compare the passive force–length properties of frog hindlimb muscles to mammalian hindlimb muscles. Studies used for this analysis included a wide range of methodologies from whole muscle in situ studies to single fibre preparations. Only data from studies that measured both active and passive force–length properties were included. This allowed normalization relative to peak isometric force (Po) and optimal length (Lo). Measurements from chemically or mechanically skinned fibres were not included in the analysis because these methods eliminate connective tissue elements surrounding the fibre that have been shown to contribute to passive tension (Prado et al. 2005). All passive curves were used to determine the relative length at which the passive force reached 20 per cent of Po. This variable was termed L20 and was used to statistically compare frog and mammalian hindlimb muscles with a one-way analysis of variance.
3. Results
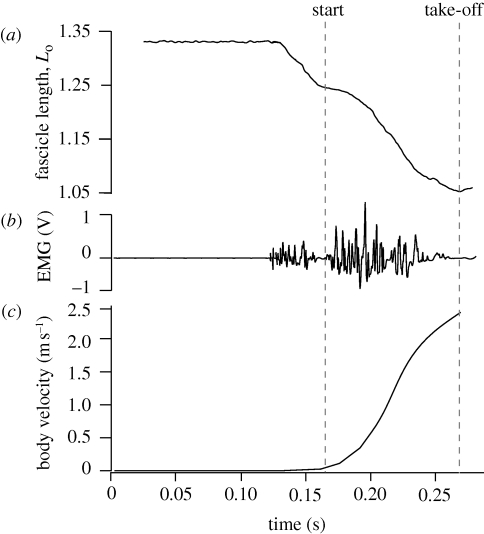
The plantaris muscle was active and began shortening in advance of any perceptible movement (figure 2). The plantaris muscle was active for about 42 ms and shortened by about 10 per cent before any jump motion was detectable. The beginning of body movement often corresponded to a short period of reduced muscle activity such that the EMG pattern appeared as two distinct bursts (figure 2b). In addition, initial movements of the body often corresponded to a brief slowing of fascicle shortening (figure 2a). Muscle fascicles continued to shorten throughout the jump, reaching a total shortening strain of 25–30% Lo before take-off.
Figure 2.
Muscle length, EMG and body velocity for a representative jump. (a) Fascicle lengths measured by sonomicrometry normalized to the muscle's optimal length (Lo). (b) Muscle activity during jumps. The two separate bursts of EMG activity shown occurred in many but not all jumps. (c) Plot of the body velocity during the jump measured from high-speed video. This plot shows that little external movement occurred during the period of initial muscle activation and shortening. The beginning of the jump as measured from external video often coincided with a slowing of fascicle shortening and reduced EMG activity.
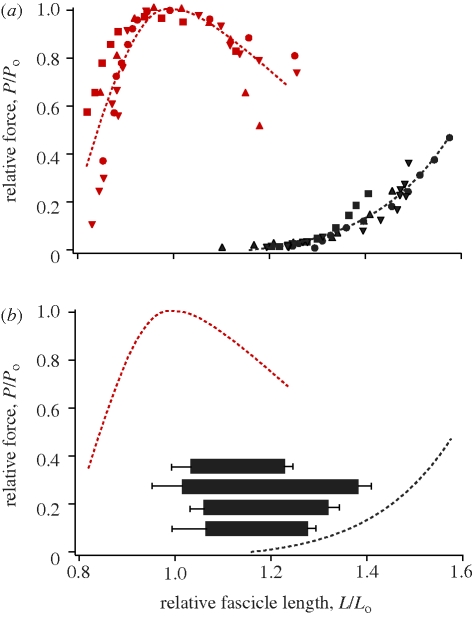
The active force–length curve of the plantaris had the commonly described parabolic relationship, consisting of an ascending, plateau and descending portion (figure 3a). Maximum isometric force normalized for cross-sectional area ranged from 25–29 N cm−2 in the four muscle preparations (table 1). The muscles did not develop passive force until stretched to relatively long lengths (approx. 1.2Lo), beyond which passive force increased exponentially with increasing length (figure 3a).
Figure 3.
The force–length properties and operating lengths of the plantaris muscle. (a) Normalized force–length curves from plantaris muscles of four individuals. Active properties are shown in red and passive properties are shown in black. Each individual is represented by a different symbol. (b) Muscle operating lengths for the plantaris muscle during jumps. Fascicle length was measured with the same sonomicrometry transducers during both in vivo jumps and in vitro characterization of the force–length curves, allowing for the accurate determination of operating length during jumping. Each horizontal bar represents the mean (±s.d.) operating length for one individual. All muscles started at lengths corresponding to the descending limb of the active force–length curve and shortened onto or near the plateau. Note that the horizontal bars correspond only to values on the x-axis.
Table 1.
Plantaris muscle properties. Lo, length at max isometric force; Po, max isometric force; L20, length at which passive force reaches 20 per cent.
| individual no. | mass (g) | Lo (mm) | Po (N cm−2) | L20 (Lo) |
|---|---|---|---|---|
| 1 | 2.31 | 11.32 | 26.59 | 1.46 |
| 2 | 2.29 | 10.26 | 25.91 | 1.39 |
| 3 | 2.41 | 9.35 | 24.96 | 1.45 |
| 4 | 2.56 | 10.95 | 28.76 | 1.43 |
The range of fascicle lengths observed during jumping corresponded to the descending limb and plateau of the force–length curve (figure 3b). We found that while frogs were at rest in the crouched pre-jump position, fascicles were on average 30 per cent longer than the muscle's optimum length (1.3Lo). During the take-off phase of the jump, fascicles shortened by an average of 27 per cent, resulting in final lengths corresponding to the plateau of the force–length curve (figure 3b). On average, fascicle length at take-off did not differ significantly from muscle optimal length (p < 0.001).
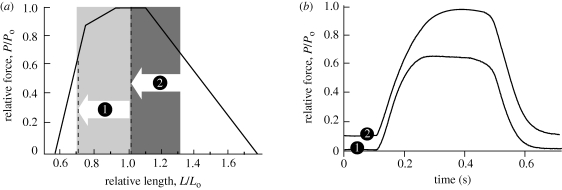
A comparison of fixed-end contractions in vitro demonstrates the influence of the initial and final length on maximum force output in a contraction (figure 4). In contraction (1), the muscle begins on the plateau but shortens down to a final length corresponding to the ascending limb of the force–length curve (figure 4). In contraction (2), the muscle begins on the descending limb but shortens down to a final length within the plateau region (figure 4). As a result of differences in the final length, peak force developed in the second contraction is much higher than the force of the first contraction. This example shows that peak force production may be more influenced by muscle lengths at the end of a contraction than by the muscle's initial length.
Figure 4.
The effect of operating length on the peak force production in a muscle. Using an in vitro protocol we show the same muscle undergoing two contractions (1 and 2) where the fascicles shorten by about 30 per cent. (a) Operating lengths for two ‘fixed-end’ contractions in the same muscle shortening against elastic structures in vitro. In both contractions the muscle shortens by about 30 per cent, but contraction (1) starts on the plateau, while contraction (2) starts on the descending limb. The classic Gordon Huxley & Julian (1966) force–length curve illustrates the hypothetical effect of length on force for maximally activated, isometric muscle. (b) Developed force versus time for two sample contractions consisting of length changes depicted in panel (a). Higher peak force is developed when the muscle starts at a longer length. This example shows that peak force production may be influenced more by a muscle's length at the end of a contraction than by its initial length.
The relatively long fascicle lengths in the frog plantaris before the jump would, in many muscles, be associated with high passive tension. However, we found that the passive tension at these lengths (approx. 1.3Lo) corresponded to less than 10 per cent of Po (figure 3). This result prompted a comparison of the passive properties of various hindlimb muscle in order to understand whether this pattern was unique to our study. Our meta-analysis confirmed that the passive compliance found in our study was broadly observed in other studies of frog hindlimb muscles (figure 5). A comparison of the passive force–length properties of frog and mammalian hindlimb muscles showed that frog muscles do not develop passive tension until much longer lengths (figure 5). To provide a statistical comparison of the passive properties of frog and mammalian hindlimb muscles, we quantified the length (L20) at which each muscle develops passive tensions corresponding to 20 per cent of Po (table 1). We found that frog muscles have a significantly higher L20 than mammalian muscles (p < 0.0001; figure 5b). Based on this analysis, we conclude that increased passive compliance is an important functional feature of frog hindlimb muscles.
Figure 5.
Meta-analysis of passive muscle properties. (a) Passive force–length curves are shown for 15 mammalian and 7 anuran hindlimb muscles. The data show that frog muscles can be stretched to longer muscle lengths relative to their optimal length (Lo) for a given force. (b) A comparison of the length (L20) at which muscles reach passive forces that correspond to 20 per cent of maximum active isometric force. Anuran muscles can be stretched to significantly (p < 0.0001) longer lengths for the same level of force. Data included in this analysis were limited to published studies of hindlimb muscles that measured both active and passive force–length properties and could therefore be normalized to Lo. Mammalian data are from: Gareis et al. (1992), Woittiez et al. (1983), Hawkins & Bey (1997), Davis et al. (2003), Witzmann et al. (1982), Rack & Westbury (1969) and Askew & Marsh (1998). Anuran data are from: Bagni et al. (1988), Julian & Moss (1980), this study, Talbot & Morgan (1998), Edman (1979), Altringham & Bottinelli (1985) and Ter Keurs et al. (1978). See electronic supplementary material, figure for more detail.
4. Discussion
(a). Operating lengths of the frog plantaris
The plantaris muscle shortens significantly during a jump, with fascicles shortening by about 30 per cent of their initial length during the take-off phase. This relatively large magnitude of shortening is consistent with some previous studies (Olson & Marsh 1998; Roberts & Marsh 2003). However, other studies have shown strains to be lower in the plantaris of Bufo marinus (Gillis & Biewener 2000) and the semimembranosus of Rana pipiens (Lutz & Rome 1994, 1996). These differences may, in part, be due to variation in the jump distance in different experiments, as shorter jumps will require less mechanical work, which may be produced with less muscle shortening. Nonetheless, the large strains observed in the plantaris suggest that excursions are not likely to be restricted to the plateau of the force–length curve.
Despite the large strains observed in the plantaris during a jump, our hypothesis that the muscle would shorten to lengths well below the plateau of the force–length curve was not supported. Rather than starting at the plateau and shortening to the ascending limb of the curve, the plantaris started at long lengths, on the descending portion of the curve, and shortened to final lengths on the plateau. Operating on either the ascending or descending limb of the curve comes at a cost of reduced average force. A consideration of the dynamics of force production in a single contraction, however, suggests that the pattern observed in the bullfrog plantaris may be advantageous for generating higher peak forces.
The consequences for force production for two different starting lengths are illustrated in figure 4. The figure depicts two contractions, where a muscle starts at an initial length corresponding to either (i) the plateau, or (ii) the descending limb and then actively shortens by 30 per cent of Lo (figure 4). In both contractions, the muscle-tendon length is kept constant and fascicle shortening is done against the stretch of elastic structures. Although in both cases the muscle operates over lengths where force production on average would be submaximal, the peak force developed is much higher in the second contraction, when the muscle begins on the descending limb (figure 4b). The reason for this difference is that when the muscle begins on the plateau, it shortens beyond the optimal lengths for force production during the early period of force development, when the muscle is not yet fully active. In contrast, when the contraction begins on the descending limb, the muscle operates at the optimal lengths for force production when the force is fully developed. As a result, the peak force ultimately developed for a contraction that begun on the descending limb is nearly twice as high when compared with the contraction that begun on the plateau (figure 4). Thus, under the contractile conditions approximating those expected during a jump, peak forces are more likely to be determined by fascicle lengths near the end of force production as opposed to lengths during the initial period of force development. Developing high peak forces may be particularly important for muscles like the plantaris that operate with an in-series tendon, as energy storage and recovery in elastic elements will be proportional to peak force.
One challenge in relating the in vivo behaviour of muscles to their in vitro properties is that recruitment patterns of a muscle are difficult to recreate in vitro. As a result, an important caveat to our results is that the force–length curve of the muscle is characterized under maximal stimulation. One advantage to frog jumping as a model system is that an anti-predator behaviour like a frog jump likely involves maximal recruitment (Lutz & Rome 1994). However, even under conditions of submaximal recruitment, a muscle's force–length curve should accurately describe the effect of length on force in the subset of fibres that are active. One other concern in translating force–length behaviour measured in vitro to in vivo function is a potential effect of stimulation frequency. Several studies have shown that a muscle's force–length curve shifts toward longer lengths at lower stimulation frequencies (Rack & Westbury 1969; Roszek et al. 1994; Zuurbier et al. 1998; Brown et al. 1999). Brown and colleagues (1999) found that a threefold drop in stimulation frequency (from 120 to 40 pps) resulted in a shift of approximately 10 per cent Lo. Such a shift, if it exists in vivo, would affect the values of operating range that we measured for the plantaris. However, even with a 10 per cent shift in the value of Lo, the plantaris would operate largely on the descending limb.
(b). Dangers of the descending limb
Few muscles have been shown to operate primarily on the descending limb of the force–length curve during locomotion. It is generally accepted that the descending limb of the force–length curve is avoided because of the increased susceptibility of muscle fibres to damage when actively stretched at long lengths (Lieber & Friden 1993; Proske & Morgan 2001). Many locomotor activities (i.e. downhill running, braking, jump landing) require active muscle lengthening in order to absorb energy and decelerate the body (Lindstedt et al. 2001). These eccentric contractions can disrupt the cytoskeletal components of myofibrils and result in decreased capacity for force generation (Lieber et al. 1996; Proske & Morgan 2001). The likelihood of eccentric muscle damage has been shown to increase with muscle length such that muscles operating on the descending limb of the force–length curve suffer greater disruption at the level of the sarcomere (Morgan 1990; Gosselin & Burton 2002). Therefore, in muscles that may be required to absorb energy, avoiding the descending limb of the force–length curve may be considered a protective mechanism against the potential of eccentric muscle damage.
Unlike most terrestrial vertebrates, the hindlimb muscles of frogs are rarely loaded eccentrically. During swimming, hindlimb muscles shorten and produce positive work to generate the necessary hydrodynamic forces (Richards & Biewener 2007). Similarly, during the take-off phase of jumping and hopping, hindlimb muscles shorten and produce positive work in order to accelerate the mass of the frog. Although the landing phase of a jump or a hop requires the absorption of energy, most frogs use their forelimbs and bodies rather than their hindlimbs for this function (Nauwelaerts & Aerts 2006). In fact, no EMG activity is observed in the hindlimb muscles of anurans during the landing phase of a jump or a hop (Olson & Marsh 1998; Ahn et al. 2003). It is possible that the lack of eccentric loading allows frog hindlimb muscles to safely operate on the descending limb of the force–length curve with little threat of eccentric muscle damage.
(c). Passive muscle forces and anuran muscle function
In many muscles, the feasibility of operating on the descending limb of the force–length curve may also be limited by high passive tensions at long muscle lengths. Stretching muscles to initial lengths that correspond to the descending limb can require high forces, which must ultimately come from either antagonistic muscles, or gravitational or inertial forces on the body. Since passive forces in muscles increase exponentially with length, getting to such long lengths may simply require too much force in many muscles.
A comparison of anuran and mammalian passive muscle forces reveal a striking difference in passive muscle properties that explains why frog muscles are capable of operating at such long lengths (figure 3). Compared with mammalian muscles, frog hindlimb muscles can be stretched to significantly longer lengths before developing high passive tensions. Literature values for passive force–length relationships indicate that, if frog muscles had the same passive properties as those of mammals, the passive forces developed at the pre-jump resting length of 1.3Lo would be extremely high. Most literature values for passive muscle stiffness indicate that mammalian muscles would develop forces as much as or exceeding the muscle's peak active force at 1.3Lo (figure 5). In fact, such high passive tensions may limit the ability to assume the highly crouched pre-jump limb posture common in most frogs.
It has previously been suggested that increased passive muscle stiffness limits a limb's range of motion (Brown et al. 1996). Therefore, the observed compliance of frog hindlimb muscles may allow for the large joint excursions associated with a jump and may imply a broader relationship between changes in passive muscle properties and diversity in limb posture.
Variation in a muscle's passive stiffness can arise from changes in either intra-sarcomeric proteins or extra-sarcomeric connective tissues. Studies have shown that the spring-like sarcomeric protein titin is responsible for some of the passive elastic properties of muscle (Wang et al. 1991). Titin isoforms vary in size and an increase in size has been shown to be inversely proportional to passive stiffness (Granzier & Labeit 2006). A muscle's passive elastic properties can also be attributed to extra-sarcomeric collagenous structures surrounding myofibers (Williams & Goldspink 1984; Purslow 1989). An increase in the collagen content of the perimyosium and endomyosium can result in a significant increase in a muscle's passive stiffness (Williams & Goldspink 1984). Although it is well established that both titin and extra-sarcomertic connective tissues contribute to passive tension, the relative contribution of each component can vary substantially between different muscles (Prado et al. 2005). It remains unclear whether the differences in the passive properties of frog and mammalian hindlimb muscles observed in this study arise from differences in myofibrillar proteins like titin or ultrastructural differences in extra-sarcomeric connective tissues. Future comparative studies may shed light on the passive structures responsible for changes in stiffness and how such changes may ultimately accompany shifts in locomotor function.
5. Conclusions
During a jump the plantaris muscle begins on the descending limb of the force–length curve and shortens onto the plateau. The observed operating lengths suggest little decrement in force production despite significant fascicle shortening. Our findings suggest that the passive compliance of frog hindlimb muscles is a critical and unique feature of the system, which facilitates the use of the descending limb of the force–length curve and improves force production during jumping.
Acknowledgements
All husbandry and experimental protocols were approved by the Brown University Institutional Animal Care and Use Committee. This work was funded by NSF grant 0642428 to T.J.R. and NIH grant F32AR054246 to E.A.
The authors would like to thank Emily Abbott and Kevin Matzurik for assistance in data collection and Dr Rich Marsh for valuable discussions.
References
- Ahn A. N., Monti R. J., Biewener A. A.2003In vivo and in vitro heterogeneity of segment length changes in the semimembranosus muscle of the toad. J. Physiol-London 549, 877–888 (doi:10.1113/jphysiol.2002.038018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altringham J. D., Bottinelli R.1985The descending limb of the sarcomere length-force relation in single muscle fibers of the frog. J. Muscle Res. Cell Motil. 6, 585–600 (doi:10.1007/BF00711916) [DOI] [PubMed] [Google Scholar]
- Askew G., Marsh R.1998Optimal shortening velocity (V/Vmax) of skeletal muscle during cyclical contractions: length–force effects and velocity-dependent activation and deactivation. J. Exp. Biol. 201, 1527–1540 [DOI] [PubMed] [Google Scholar]
- Bagni M. A., Cecchi G., Colomo F., Tesi C.1988Plateau and descending-limb of the sarcomere length-tension relation in short length-clamped segments of frog-muscle fibers. J. Physiol. 401, 581–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. E., Liinamaa T. L., Loeb G. E.1996Relationships between range of motion, Lo, and passive force in five strap-like muscles of the feline hind limb. J. Morphol. 230, 69–77 (doi:10.1002/(SICI)1097-4687(199610)230:1<69::AID-JMOR6>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- Brown I. E., Cheng E. J., Loeb G. E.1999Measured and modeled properties of mammalian skeletal muscle. II. The effects of stimulus frequency on force–length and force–velocity relationships. J. Muscle Res. Cell. Motil. 20, 627–643 (doi:10.1023/A:1005585030764) [DOI] [PubMed] [Google Scholar]
- Davis J., Kaufman K. R., Lieber R. L.2003Correlation between active and passive isometric force and intramuscular pressure in the isolated rabbit tibialis anterior muscle. J. Biomech. 36, 505–512 (doi:10.1016/S0021-9290(02)00430-X) [DOI] [PubMed] [Google Scholar]
- Edman K. A. P.1979Velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle-fibers. J. Physiol. 291, 143–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. B.1978Allometry and jumping in frogs: helping twain to meet. Evolution 32, 551–564 (doi:10.2307/2407721) [DOI] [PubMed] [Google Scholar]
- Fung Y. C. B.1967Elasticity of soft tissue in simple elongation. Am. J. Physiol. 213, 219–249 [DOI] [PubMed] [Google Scholar]
- Gareis H., Solomonow M., Baratta R., Best R., Dambrosia R.1992The isometric length force models of 9 different skeletal muscles. J. Biomech. 25, 903–916 (doi:10.1016/0021-9290(92)90230-X) [DOI] [PubMed] [Google Scholar]
- Gillis G. B., Biewener A. A.2000Hindlimb extensor muscle function during jumping and swimming in the toad (Bufo marinus). J. Exp. Biol. 203, 3547–3563 [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J.1966Variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol-London 184, 170–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin L. E., Burton H.2002Impact of initial muscle length on force deficit following lengthening contractions in mammalian skeletal muscle. Muscle Nerve 25, 822–827 (doi:10.1002/mus.10112) [DOI] [PubMed] [Google Scholar]
- Granzier H. L., Labeit S.2006The giant muscle protein titin is an adjustable molecular spring. Exerc. Sport. Sci. Rev. 34, 50–53 (doi:10.1249/00003677-200604000-00002) [DOI] [PubMed] [Google Scholar]
- Hawkins D., Bey M.1997Muscle and tendon force-length properties and their interactions in vivo. J. Biomech. 30, 63–70 (doi:10.1016/S0021-9290(96)00094-2) [DOI] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L.1980Sarcomere length-tension relations of frog skinned muscle-fibers at lengths above the optimum. J. Physiol-London 304, 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber R. L., Friden J.1993Muscle damage is not a function of muscle force but active muscle strain. J. Appl. Physiol. 74, 520–526 [DOI] [PubMed] [Google Scholar]
- Lieber R. L., Raab R., Kashin S., Edgerton V. R.1992Sarcomere-length changes during fish swimming. J. Exp. Biol. 169, 251–254 [DOI] [PubMed] [Google Scholar]
- Lieber R. L., Thornell L. E., Friden J.1996Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J. Appl. Physiol. 80, 278–284 [DOI] [PubMed] [Google Scholar]
- Lindstedt S. L., LaStayo P. C., Reich T. E.2001When active muscles lengthen: properties and consequences of eccentric contractions. News Physiol. Sci. 16, 256–261 [DOI] [PubMed] [Google Scholar]
- Lutz G. J., Rome L. C.1994Built for jumping: the design of the frog muscular system. Science 263, 370–372 (doi:10.1126/science.8278808) [DOI] [PubMed] [Google Scholar]
- Lutz G. J., Rome L. C.1996Muscle function during jumping in frogs. I. Sarcomere length change, EMG pattern, and jumping performance. Am. J. Physiol. Cell. Physiol. 40, C563–C570 [DOI] [PubMed] [Google Scholar]
- MacIntosh B. R., MacNaughton M. B.2005The length dependence of muscle active force: considerations for parallel elastic properties. J. Appl. Physiol. 98, 1666–1673 (doi:10.1152/japplphysiol.01045.2004) [DOI] [PubMed] [Google Scholar]
- Marsh R. L.1994Jumping ability of anuran amphibians. Adv. Vet. Sci. Comp. Med. 38B, 51–111 [PubMed] [Google Scholar]
- Marsh R. L., John-Alder H. B.1994Jumping performance of hylid frogs measured with high-Speed cine film. J. Exp. Biol. 188, 131–141 [DOI] [PubMed] [Google Scholar]
- Morgan D. L.1990New Insights into the behavior of muscle during active lengthening. Biophys. J. 57, 209–221 (doi:10.1016/S0006-3495(90)82524-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauwelaerts S., Aerts P.2006Take-off and landing forces in jumping frogs. J. Exp. Biol. 209, 66–77 (doi:10.1242/jeb.01969) [DOI] [PubMed] [Google Scholar]
- Olson J. M., Marsh R. L.1998Activation patterns and length changes in hindlimb muscles of the bullfrog Rana catesbeiana during jumping. J. Exp. Biol. 201, 2763–2777 [DOI] [PubMed] [Google Scholar]
- Otten E.1987A Myocybernetic Model of the jaw system of the rat. J. Neurosci. Methods 21, 287–302 (doi:10.1016/0165-0270(87)90123-3) [DOI] [PubMed] [Google Scholar]
- Prado L. G., Makarenko I., Andresen C., Kruger M., Opitz C. A., Linke W. A.2005Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J. Gen. Physiol. 126, 461–480 (doi:10.1085/jgp.200509364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U., Morgan D. L.2001Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J. Physiol-London 537, 333–345 (doi:10.1111/j.1469-7793.2001.00333.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purslow P. P.1989Strain-induced reorientation of an intramuscular connective tissue network: implications for passive muscle elasticity. J. Biomech. 22, 21–31 (doi:10.1016/0021-9290(89)90181-4) [DOI] [PubMed] [Google Scholar]
- Rack P. M. H., Westbury D. R.1969The effects of length and stimulus rate on tension in the isometric cat soleus. J. Physiol-London 204, 443–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. T., Biewener A. A.2007Modulation of in vivo muscle power output during swimming in the African clawed frog (Xenopus laevis). J. Exp. Biol. 219, 3147–3159 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Marsh R. L.2003Probing the limits to muscle-powered accelerations: lessons from jumping bullfrogs. J. Exp. Biol. 206, 2567–2580 (doi:10.1242/jeb.00452) [DOI] [PubMed] [Google Scholar]
- Rome L. C.1998Some advances in inte grative muscle physiology. Comp. Biochem. Phys. B 120, 51–72 (doi:10.1016/S0305-0491(98)00023-6) [DOI] [PubMed] [Google Scholar]
- Roszek B., Baan G. C., Huijing P. A.1994Decreasing stimulation frequency-dependent length-force characteristics of rat muscle. J. Appl. Physiol. 77, 2115–2124 [DOI] [PubMed] [Google Scholar]
- Sacks R. D., Roy R. R.1982Architecture of the hindlimb muscles of cats: functional significance. J. Morphol. 173, 185–195 (doi:10.1002/jmor.1051730206) [DOI] [PubMed] [Google Scholar]
- Shubin N. H., Jenkins F. A.1995An early Jurassic jumping frog. Nature 377, 49–52 (doi:10.1038/377049a0) [Google Scholar]
- Talbot J. A., Morgan D. L.1998The effects of stretch parameters on eccentric exercise-induced damage to toad skeletal muscle. J. Muscle Res. Cell. Motil. 19, 237–245 [DOI] [PubMed] [Google Scholar]
- Ter Keurs H. E. D. J., Iwazumi T., Pollack G. H.1978Sarcomere length-tension relation in skeletal-muscle. J. Gen. Physiol. 72, 565–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., McCarter R., Wright J., Beverly J., Ramirez-Mitchell R.1991Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc. Natl Acad. Sci. USA 88, 7101–7105 (doi:10.1073/pnas.88.16.7101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G.1984Connective tissue changes in immobilized muscle. J. Anat. 138, 343–350 [PMC free article] [PubMed] [Google Scholar]
- Witzmann F. A., Kim D. H., Fitts R. H.1982Hindlimb immobilization: length-tension and contractile properties of skeletal muscle. J. Appl. Physiol. 53, 335–345 [DOI] [PubMed] [Google Scholar]
- Woittiez R. D., Huijing P. A., Rozendal R. H.1983Influence of muscle architecture on the length-force diagram of mammalian muscle. Pflugers Arch. 399, 275–279 (doi:10.1007/BF00652752) [DOI] [PubMed] [Google Scholar]
- Zug G. R.1972Anuran locomotion: structure and function. I. Preliminary observations on relation between jumping and osteometrics of appendicular and postaxial skeleton. Copeia 1972, 613–624 (doi:10.2307/1442720) [Google Scholar]
- Zuurbier C. J., Lee-de Groot M. B., Van der Laarse W. J., Huijing P. A.1998Effects of in vivo-like activation frequency on the length-dependent force generation of skeletal muscle fibre bundles. Eur. J. Appl. Physiol. Occup. Physiol. 77, 503–510 (doi:10.1007/s004210050367) [DOI] [PubMed] [Google Scholar]