Abstract
Ecological invasions, where non-native species spread to new areas, grow to high densities and have large, negative impacts on ecological communities, are a major worldwide problem. Recent studies suggest that one of the key mechanisms influencing invasion dynamics is personality-dependent dispersal: the tendency for dispersers to have a different personality type than the average from a source population. We examined this possibility in the invasive mosquitofish (Gambusia affinis). We measured individual tendencies to disperse in experimental streams and several personality traits: sociability, boldness, activity and exploration tendency before and three weeks after dispersal. We found that mosquitofish display consistent behavioural tendencies over time, and significant positive correlations between all personality traits. Most notably, sociability was an important indicator of dispersal distance, with more asocial individuals dispersing further, suggesting personality-biased dispersal on an invasion front. These results could have important ecological implications, as invasion by a biased subset of individuals is likely to have different ecological impacts than invasion by a random group of colonists.
Keywords: personality traits, social tendency, invasion dynamic, dispersal, colonization success
1. Introduction
Ecological invasions are gaining attention as a major threat to biodiversity and an important element of global change (Dukes & Mooney 1999). Ecological invasions occur when a species introduced to areas beyond its native range (i.e. non-indigenous species) spreads from the point of introduction and becomes abundant. At high densities, invasive species often have substantial negative impacts on native species (Mack et al. 2000; Salo et al. 2007). Identifying conditions that allow a successful invasion therefore represents a crucial research area. One approach has been to identify characteristics that predispose a species to becoming a successful invader (Kolar & Lodge 2001; Marchetti et al. 2004; Rehage et al. 2005a). Because invasion is a multi-stage process (introduction, spread, establishment, growth to high density and high impact on an invaded community; Lodge 1993), different characteristics probably affect a species's ability to complete each transition successfully. For dispersal and spread, high dispersal rate and long-distance dispersal are likely to be key traits for successful invasion (Rehage & Sih 2004; Bubb et al. 2006). In addition, the ability to establish, grow rapidly to high density and have high impacts appears to be associated with aggressiveness and a ‘fast lifestyle’ (e.g. high activity, boldness, r-type life history; Lodge 1993). Invasive pests might be those species that exhibit both high dispersal tendencies and the traits that facilitate attaining high densities and exerting large impacts (Rehage & Sih 2004).
While many previous studies have focused on species characteristics that might explain between-species differences in invasiveness, a new exciting approach looks at how individual variation in traits within species might influence invasion dynamics (Duckworth & Badyaev 2007; Pintor et al. 2009). In particular, an aspect of within-species variation that has garnered substantial recent interest involves individual variation in animal personalities; for example, where some individuals are consistently more bold, aggressive or sociable than others (Dall et al. 2004; Sih et al. 2004; Bell 2007; Reale et al. 2007; Sih & Bell 2008).
For invasions, a key issue is personality-dependent dispersal (e.g. where boldness, sociability or aggressiveness are associated with dispersal tendency; Fraser et al. 2001; Dingemanse et al. 2003; Cote & Clobert 2007; Duckworth & Badyaev 2007). If the disperser's personality type enhances its colonization success (Cote & Clobert 2007; Duckworth & Badyaev 2007; Clobert et al. 2009; Duckworth & Kruuk 2009), then personality-dependent dispersal might play an important role in invasions. For instance, Duckworth & Badyaev (2007) showed a biased dispersal of highly aggressive western bluebirds to the invasion front, allowing this species to outcompete and displace less aggressive mountain bluebirds. The general hypothesis is that with personality-dependent dispersal, individuals leading an invasion front might often display behavioural characteristics that facilitate the colonization of new habitats and hasten the spread of an invasive species. Although this idea seems compelling, few studies to date have actually tested for personality-dependent dispersal, and in particular this issue has only rarely been examined in a known invasive species (Duckworth & Badyaev 2007). No previous studies have quantified personality-dependent dispersal in an invasive species under controlled environmental conditions in order to address mechanistic underpinnings.
Sociability-dependent dispersal behaviour has received minimal attention (Cote & Clobert 2007; Jokela et al. 2008), despite theoretical and empirical work suggesting its importance (Ims 1990; Sinervo & Clobert 2003; Le Galliard et al. 2005). In particular, studies on common lizards (Cote & Clobert 2007) and on humans (Jokela et al. 2008) suggest that asocial individuals are more likely to disperse, especially when population density is high. Sociability-dependent dispersal behaviour might thus be an important behavioural mechanism in invasion processes where invaders leave high-density populations to colonize empty habitats.
Here, we quantify the existence of personality types in the invasive mosquitofish (Gambusia affinis) and examine whether personality types are correlated with dispersal behaviour. Gambusia includes approximately 45 species of small, live-bearing fishes (Poeciliidae). Most of what we know about this genus comes from extensive study of the two most temperate, widely distributed and highly invasive species, G. holbrooki and G. affinis. These sister species (both known as mosquitofish) have been introduced for mosquito control worldwide and have spread successfully to over 40 countries (Welcomme 1992). Their invasion success and negative impacts on native communities (Lloyd et al. 1986; Courtenay & Meffe 1989; Gamradt & Kats 1996; Webb & Joss 1997; Goodsell & Kats 1999) have led them to be considered among the 100 worst invasive species worldwide (Lowe et al. 2000). Understanding Gambusia invasions is thus an issue of immediate importance. In particular, information on dispersal behaviour is needed to better understand mosquitofish spread after introduction (Alemadi & Jenkins 2008).
Previous studies of Gambusia demonstrated behavioural differences (e.g. in boldness) between invasive and non-invasive species (Rehage & Sih 2004; Rehage et al. 2005a,b). Both personality types and dispersal are easily measured in this genus, allowing us to study mechanisms at the individual level. We measured individual tendencies to disperse (dispersal distance) in experimental streams, sociability (tendency to shoal), boldness (latency to emerge from refuge), exploration tendency and activity (movement in a novel environment). Three weeks later, individuals were run again through the behavioural and dispersal assays to explore individual consistency. We tested the hypotheses that: (i) individual mosquitofish exhibit consistent behaviours (i.e. significant correlations between each behaviour or dispersal tendency over a three week period); (ii) mosquitofish exhibit behavioural syndromes (i.e. significant correlations between sociability, boldness, exploratory tendency and activity); and (iii) personality type is correlated with dispersal distance.
2. Material and methods
To characterize personality types, we ran two behavioural assays separated by 1 h. First, we characterized sociability as a tendency to shoal. Second, we characterized boldness as the latency to exit from a refuge into a novel environment, and exploratory behaviour and activity as movement in a novel environment. These two assays represent an individual's reaction to a social context and to a novel environment, respectively. This allows us to test for two major factors affecting dispersal and colonization. After behavioural assays, all fish were placed in experimental streams where we measured their dispersal tendency. Behavioural and dispersal assays were repeated three weeks later to test for individual consistency in behaviour.
To match the fact that mosquitofish are commonly introduced by mosquito control agencies, our experimental mosquitofish (G. affinis) were supplied by the Sacramento-Yolo Mosquito and Vector Control District. These fish represent a mix of hatchery-reared and field-collected fish. Three hundred fish were transported to the Center for Aquatic Biology and Aquaculture (CABA), University of California, Davis, on 18 March 2008, held in groups of 60 in 80 l flow-through fibreglass tanks on a natural photoperiod (for early May, L∶D = 14∶10) at 22°C and fed Tetramin flakes ad libitum. Mosquitofish were acclimated to these conditions for more than one month prior to the first behavioural observations, which were carried out between 29 April and 2 May (four replicates) and between 14 May and 18 May 2008 (four replicates). Twelve hours before behavioural observations began individual mosquitofish were placed in 37.9 l aquaria, with 30 l of well water, a 12 cm piece of 5 cm diameter PVC pipe that served as refuge and an airstone. Each day for eight days, 15 females and 15 males were randomly caught and run through behavioural assays, for a total of 240 fish. The aquaria that held our fish before experiments were small enough that it was not difficult for us to catch any fish that we tried to catch and we also used all the fish from a tank over the experiment. Capture biases towards a specific personality are thus unlikely.
(a). Tendency to shoal (sociability)
Here, we recorded the amount of time spent near a shoal of conspecifics (Ward et al. 2004). The experimental arena was an aquarium (30 cm high × 25 cm wide × 50 cm long) filled to a depth of 13.6 cm with 17 l of well water and divided lengthwise into three compartments (two small and one large centre compartment) using two transparent glass partitions 12.5 cm away from each side wall. The partitions allowed visual, but not physical or olfactory, interaction between the shoal and the focal individual. One of six designated stimulus shoals was introduced to one of the smaller compartments 1 h before the experiments began, while the other small compartment was left empty as a control. Stimulus shoals comprised 14 mosquitofishes, none of which had previous experience with the focal individual. After 1 h, the focal fish was introduced into the centre of the larger (central) compartment and allowed to acclimate for 10 min. The aquarium was surrounded by black curtains with a small slit that allowed us to observe fish without disturbing them. The position of the focal fish was continuously recorded for 10 min using Observer 2.01. The large compartment was divided with vertical marks every 2 cm; time spent shoaling was defined as time spent by the focal fish within the 2 cm closest to the stimulus shoal (Ward et al. 2004) and is a metric of individual social attraction to a group of strangers in a novel environment. This is likely to be an ecologically relevant measure as it simulates what mosquitofish experience when they are introduced in a novel environment or disperse and join a new population. When the assay was complete, individuals were returned to their individual home aquaria.
(b). Measuring boldness and exploration in a novel environment
One hour after the sociability assay, boldness, exploration and activity levels were assessed by recording behaviour in a novel environment (Yoshida et al. 2005; Brown et al. 2007). The experimental arena was a well-lit, opaque, white plastic tank (80 cm long × 80 cm wide × 20 cm high), filled with 10 cm of well water, and furnished with half flower pots that served as additional refuges in two corners. Individual fish were added gently to an upright, cylindrical (9 cm diameter), black, opaque, covered refuge chamber placed on the opposite end from the flower pots. After 10 min, we remotely opened a 4 cm wide door on the refuge chamber, allowing fish access to the experimental arena. Black curtains surrounded the arena while cameras recorded behaviour. Trials ended either 5 min after fish left the refuge, or after 45 min (2700 s).
Boldness was measured as the maximum time allowed for fish to exit the refuge (2700 s) minus the latency (s) to exit from refuge, and to stay for greater than 10 consecutive seconds out of refuge; shorter latency to exit indicates higher boldness. Exploratory tendency was quantified by area covered (see below), and activity was measured as percentage of time spent moving during the 5 min after the entire fish left the refuge.
While some have suggested that latency to emerge in a novel environment should be termed exploratory behaviour and not boldness (Reale et al. 2007), we follow several earlier papers (e.g. Yoshida et al. 2005; Brown et al. 2007) in our assessment that for small, schooling fish, a short latency to emerge alone from a dark refuge into an open, novel environment represents boldness, while exploratory tendency is well measured by space use after emergence from refuge. While we would like independent assessments of exploration and activity, in fact, the two might not be functionally separable. To explore, animals must be active. To distinguish the two somewhat, we define activity as movement per se, and exploratory tendency as area covered (explored) while moving. Because the water was shallow (10 cm deep), area (as opposed to volume) covered provided a useful measure of space use. In principle, an animal can be highly active and yet explore little area. In fact (§3), activity and exploratory tendency were strongly positively correlated.
Videos were collected on a dedicated Micros Digital-Sprite2 DVR system and downloaded as avi files before being exported as image stacks (one frame per second) using VirtualDub. These image stacks were imported into ImageJ where the fish's position (x–y coordinates) was tracked over the 5 min assay. The percentage of time that the fish spent moving (activity) was the percentage of frames in which the fish moved greater than one body length in the previous second. Area explored incorporates both the distance an individual moved and the spatial pattern of those movements. Given x–y coordinates from each frame, we tracked each individual's continuous path (assuming that movements between frames were straight). Explored area was calculated (in Matlab R2007) as the percentage of the arena that fell within 5 cm of the fish's path.
(c). Marking and morphological measurements
At the end of each observation day, mosquitofish were marked with an elastomer tag (northwest Marine Technologies, Shaw Island, WA, USA) under a low dose (5 mg l−1) of anaesthetic (MS-222). Each fish received a randomly assigned unique identifier by injecting one of four colours (yellow, orange, blue or red) subcutaneously into four locations on the caudal peduncle (two on each side). Fish were weighed to the nearest 0.001 g and photographed to estimate body size using ImageJ. Fish were allowed to recover from anaesthesia in an opaque bucket before being transferred back to their individual aquaria. No differences in mortality rate were observed between marked and unmarked fish (marked fish, 2.5% over 2 days; unmarked fish, 2.7–6.3% over the same 2 days), and we checked for normal behaviour after marking by checking that behaviour was similar between sets of unmarked and marked fish. After 30 fish were observed and marked, they were transferred to an 80 l fibreglass tank and maintained for 2 days before being transferred to the experimental stream for the dispersal assay.
(d). Dispersal in an experimental stream
We conducted our dispersal assay in an artificial stream at CABA, consisting of five plastic pools positioned in line (each 1.5 m diameter, filled with 40 cm of well water) connected by riffles (1.3 m long, 30 cm wide). Our artificial stream simulates the natural situation where Gambusia often reside in relatively small stream pools except when undergoing dispersal (Pyke 2005). The stream is located outdoors under a roof with open sides (about 5 m high) that screened out rain and direct sunlight. A 34 HP pump at the downstream end pumped water (370.7 ml s−1) to the top pool where it then flowed downstream through the system. Pools simulated the slow-moving backwaters typically inhabited by Gambusia, whereas flow in the riffles was too fast for mosquitofish to maintain position or to swim upstream against the current. Each pool had three half flower pots and three PVC pipes that served as refuges. Algae on the tank walls and mud in the bottom of each pool made conditions in the artificial streams reasonably similar to conditions experienced in the field. Water temperature was noted prior to fish introduction and the continual input of well water ensured consistency in water temperature between trials (19°C).
Two days after behavioural assays, 30 fish were introduced into the top, upstream pool. This density is within the natural range; indeed, it is relatively low compared with the high local densities often seen in natural populations (e.g. Martin 1975). A removable barrier at the downstream end kept the fish from dispersing during the acclimation period, while still allowing water to flow out into the riffle. Fish were allowed 2 h acclimation in the pool after which the barrier was removed and fish were free to disperse or stay in the pool. The flow of water downstream precluded movement upstream, so fish that dispersed out of an upstream pool were unable to return. After 24 h, we removed fish and recorded the pool in which each individual was found (a measure we refer to as dispersal distance). All 30 individuals were then returned to group housing in an 80 l tank. To emphasize, current velocity in our artificial stream pools was low enough that fish that entered a pool on the upstream end were never rapidly swept by the current through the pool and out the downstream end. That is, getting to the 5th (most downstream) pool required a fish to undergo four distinct dispersal events. Although our maximum dispersal distance is certainly less than the occasional long-distance dispersal seen in nature, because mosquitofish usually move locally over relatively small areas (Pyke 2005), our dispersal assay probably simulates natural, daily dispersal distances.
Eight blocks of 30 individuals were run through behavioural and dispersal assays over two 4-day periods (29 April–2 May, 14–17 May). Three weeks after the last assay, the four sets of 30 individuals were randomly divided into new groups of 30. These new groups were then run through the behavioural and dispersal assays again to test for individual consistency (19–22 May, 3–6 June).
(e). Statistics
Six individuals were excluded from analyses because they exhibited behaviours indicating poor health (swimming on their side or lying immobile on the floor of the tank); 234 fish remained for analyses on the first set of behavioural observations. Mortality and irregular behaviour (e.g. owing to apparent illness or unknown causes) reduced sample sizes to 157–160 for the second set of assays three weeks later. Individuals that died or exhibited irregular behaviour during this three week period did not display irregular behaviour during the first set of assays, and did not appear morbid or die within the first few days after the first dispersal assay. ANOVAs comparing fish that were included in the second set of observations with those that were included in the first observations but excluded from the second set showed no significant differences in personality types (on principal component analysis axis (§3); PC1: F1,217 = 2, p = 0.16; PC2: F1,217 = 0.30, p = 0.58) or personality-dependent dispersal (interaction between death status and PC2 (§3): F1,202 = 0.95, p = 0.33). Therefore, in behavioural correlation analyses shown below, we used all fish that survived to the end of the first dispersal assay.
(i). Behavioural correlations and consistencies
We analysed correlations among the four personality parameters (sociability, boldness, exploratory behaviour and activity) as well as their consistency. Because one of these metrics (boldness) was not normally distributed, correlations between these behaviours were calculated using Spearman's rank correlation, which tests for rank-order consistency in multiple behaviours (i.e. behavioural syndrome). Behavioural consistency across time (i.e. repeatability) was assessed by Spearman's rank correlations between the earlier and later measurements. We also computed intraclass correlation coefficients (ICC; Lessells & Boag 1987). One-way ANOVA on standardized behavioural values was carried out in order to obtain variance components. Repeatability is a measure of change in trait expression of individuals across time (within-individual variance), relative to the change of the study population (Lessells & Boag 1987; Bell et al. 2009). The time taken to emerge from the shelter was log transformed (log maximum time minus log time to emerge from the shelter) to approximate normal distributions. No other transformations were necessary.
The consistency of dispersal distance over three weeks was analysed using a likelihood-ratio χ2-test on the contingency table of dispersal distances for the two dispersal assays. Dispersal distance can be consistent either because the same individuals tend to stay in the original pool while others disperse, or because the distance dispersed was consistent for those that left the first pool. We tested, in particular, for the latter by running a Spearman's rank correlation test for distance dispersed using only the relatively few fish that left the first pool in both dispersal trials.
(ii). Principal component analysis
Because our behavioural metrics were correlated, we performed a principal component analysis (PCA) with varimax rotation (Quinn & Keough 2002) in JMP v.7 to define possible personality trait dimensions. Eleven individuals that never emerged from shelter could not be assessed for exploration/activity and were thus excluded from the PCA (n = 223). Including these 11 animals in an analysis that directly used behavioural metrics instead of PCA axes did not alter the qualitative conclusions on effects of sociability and boldness on dispersal. Based on the scree plot and a bootstrapped Kaiser–Guttman, we identified two key PCA factors for further analyses (Jackson 1993). Behaviours with a loading of at least 0.32 were considered to contribute to the meaning of a component (Tabachnick & Fidell 1996). The relation between principal components and sex, body size and body condition was analysed using a generalized linear model (Proc GLM). Body condition could be measured by residuals from the regression of body mass on body length cubed. Residuals can, however, be problematic in some situations (Darlington & Smulders 2001; Garcia-Berthou 2001), but adding body size and body mass in the same model would lead to collinearity problems. We decided to perform a PCA on body mass and body size data (Quinn & Keough 2002). From the factor loadings of this PCA, the first axis clearly measures overall body size (factor loadings, body size: 0.99 and body mass: 0.99) while the second axis is the part of body mass not explained by body size (i.e. body condition; factor loadings: body size = −0.15 and body mass = 0.15). Thereafter, we named these two axes body size and body condition, respectively. We also checked that we find the same results using residuals as a metric of body condition.
(iii). Personality-dependent dispersal
We used dispersal distance—the pool where the fish was found (pools 1–5) at the termination of the first dispersal trial—rather than dispersal decision (the probability of leaving pool 1) when analysing if/how dispersal depends on personality traits, because it reduces the effect of individuals that left the first pool accidentally. Because seven individuals died before dispersal assays, n = 216 for the dispersal analysis over the eight observation days (population size ranged from 25 to 28). We analysed the relationship between PCA scores and dispersal distance using a mixed generalized linear model with a cumulative logit link and a multinomial error distribution in SAS (Littell et al. 1996; Quinn & Keough 2002). The fixed effects were the PCA scores, body size, body condition, sex and the interactions, and the random effect was the experimental population for dispersal (observation day), which allows to control for differences between populations. We can also show that there were no differences between populations for the two PCA axes (p > 0.05). We used type III F-tests for fixed effects. The model was simplified by using backward elimination of the non-significant terms.
3. Results
Individuals displayed significant rank-order consistency over three weeks in all four behaviours assayed (table 1). Although the behaviours were significantly repeatable over this period, repeatability values were relatively low, revealing a time effect (table 1).
Table 1.
Behavioural consistency (Spearman's rank correlation) and the repeatability (intraclass correlation coefficients) of the four behaviours measured (before dispersal assay and three weeks after dispersal).
| rank consistency | repeatability | |
|---|---|---|
| sociability | 0.28, p = 0.0004 | F159,160 = 1.48, p = 0.006, ICC = 0.20 |
| boldness | 0.29, p = 0.0002 | F159,160 = 1.69, p = 0.0005, ICC = 0.25 |
| exploratory behaviour | 0.21, p = 0.01 | F156,157 = 1.37, p = 0.024, ICC = 0.16 |
| activity | 0.20, p = 0.01 | F156,157 = 1.42, p = 0.015, ICC = 0.17 |
The four behaviours were also significantly correlated to each other, indicating an overall behavioural syndrome (table 2). Boldness, exploration and activity were highly significantly positively correlated. In contrast, while sociability was significantly positively correlated to the three other behaviours, the correlations were only barely significant. Scree plot and Kaiser–Guttman analysis of the PCA revealed two factors that explained 75.7 per cent of the variance (table 3). PC1 had strong component loadings for boldness, exploration and activity, while PC2 represented sociability (table 3). Thus, fish that had higher PC1 scores took less time to emerge from the shelter, explored a larger area and spent more time moving. Fish that had higher scores on PC2 spent more time close to the shoal. These two PC scores were unrelated to sex, body size and body condition (p > 0.10).
Table 2.
Spearman's rank correlations between the four behaviours measured before dispersal assay.
| boldness | exploratory behaviour | activity | |
|---|---|---|---|
| sociability | 0.14, p = 0.027 | 0.13, p = 0.045 | 0.13, p = 0.045 |
| boldness | 0.27, p < 0.0001 | 0.30, p < 0.0001 | |
| exploratory behaviour | 0.84, p < 0.0001 |
Table 3.
Component loadings of behaviours observed on two orthogonally rotated principal components. Boldface indicates the highest component loadings for each behaviour.
| principal components |
||
|---|---|---|
| behaviour | boldness–exploration–activity | sociability |
| sociability | 0.08 | 0.99 |
| boldness | 0.52 | 0.11 |
| exploratory behaviour | 0.93 | 0.01 |
| activity | 0.94 | 0.06 |
| variance explained (%) | 50.6 | 25.1 |
| total variance (%) | 75.7 | |
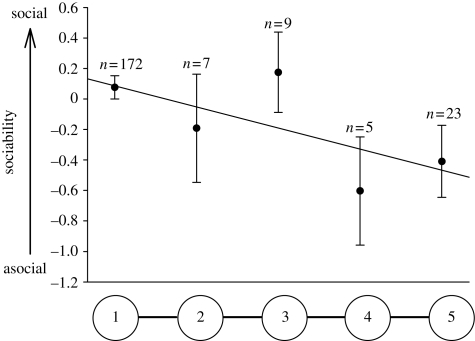
Dispersal distance was related to the sociability axis but not to the boldness–exploration–activity axis (table 4). Asocial individuals moved further downstream (figure 1; analysis of covariance with sociability axis as dependent variable and dispersal distance (pool) as the explicative covariable: F1,214 = 5.54, p = 0.02). In particular, dispersers reaching the last two pools were more asocial than fish staying in pool 1 (multiple contrasts for differences between pools in mean sociability: pools 4–5 lumped versus pool 1, F = 5.09, d.f. = 1, 211, p = 0.03). Dispersal distance was not related to body size (table 4), but individuals in better condition dispersed further (table 4). Males moved further than females (table 4), but independently of personality types (sociability axis × sex: F1,202 = 2.16, p = 0.14; boldness–exploration–activity axis × sex: F1,202 = 0.69, p = 0.41). Finally, the distance moved during dispersal was repeatable across the two dispersal assays three weeks apart (likelihood-ratio χ2 = 25.84, n = 159, p = 0.01, r = 0.28), even when we use only the individuals that left pool 1 in both assays (n = 11; Spearman = 0.63, p = 0.04).
Table 4.
Dispersal distance (the pool where the fish was found (pools 1–5) at the termination of the first dispersal trial) in relation to personality traits, sex, body size and body mass. Mixed generalized linear model with a cumulative logit link and a multinomial error with the experimental population as a random effect. Simplification of the model was made using backward elimination of the non-significant terms.
| statistical test | |
|---|---|
| sociability | F1,205 = 5.49, p = 0.02 |
| boldness–exploration–activity | F1,204 = 0.25, p = 0.62 |
| sex | F1,205 = 9.49, p = 0.02 |
| body size | F1,203 = 0.69, p = 0.41 |
| body condition | F1,205 = 4.76, p = 0.03 |
Figure 1.
Mean sociability value in relation to dispersal distance. Mean values (±s.e.) of the sociability score as a function of the pool where the fish was found at the termination of the dispersal trial (pools 1, 2, 3, 4 or 5) are shown. n, the number of fish found in each of these pools.
4. Discussion
(a). Individual consistency and behavioural syndromes
Mosquitofish exhibited two key components of a behavioural syndrome: (i) individual consistency over time (here, three weeks) for focal behaviours and (ii) significant correlations among behaviours expressed in different contexts (behavioural syndrome). With regard to behavioural consistency, rank-order correlations were significantly positive for all four behavioural metrics over several weeks; individual differences in behaviours were therefore consistent. The fact that repeatability was low (but significant) is consistent with a recent review that found that behavioural repeatabilities are often low, but significant (Bell et al. 2009). In our case, although we cannot rule out the possibility that these behaviours intrinsically have a low repeatability, we suspect that part of this relatively low repeatability represents uncontrolled variability in individual motivational state that arises even in controlled, standardized laboratory conditions. Interestingly, dispersal distance was also repeatable over the three-week period. This result matches recent studies showing a repeatability of dispersal (Bonte et al. 2009; Doligez et al. 2009). The fact that individuals vary systematically in their dispersal distances suggests that they either disperse actively (voluntarily) or, if they disperse accidentally (passively), something consistent and non-random about their behaviour makes some more susceptible than others to passive dispersal.
We also found that mosquitofish display positive correlations between measures of boldness, exploration and activity. Although the existence of a bold/exploratory/active syndrome seems intuitively reasonable, few previous studies have actually quantified all three aspects of personality in one study, especially in an invasive species. Here, the strong correlations between these three behaviours were due, in part, to the fact that all three were assessed in the same trials. Still, PC1, with high loadings for boldness, activity and exploratory behaviour, represents a general axis of response to a novel environment, ranging from ‘emerge early and explore actively’ to ‘emerge only after a long delay, and move around rather little’. A previous study examined parallel differences between species in the Gambusia genus (some invasive, others not) in boldness and exploration (Rehage & Sih 2004). However, our study is the first to examine behavioural syndromes within the invasive species, and one of few to quantify a behavioural syndrome in any invasive species (see also Duckworth & Badyaev 2007; Pintor et al. 2009).
Boldness, activity and exploratory behaviour were also positively correlated to sociability—individual variation in attraction to a social group—a personality trait that has been relatively rarely studied (but see Cote & Clobert 2007; Reale et al. 2007). Our results suggest that in mosquitofish, fish that are bolder (active and exploratory) tend to school more. It is worth noting, however, that sociability was only weakly correlated to the other personality traits. While PC1 (explaining 50% of the variance in behaviour) encompasses the bold/exploratory/active syndrome, PC2 describes only individual sociability differences (table 3). Social attraction thus emerged here as a behaviour that is partly independent of other behaviours. In particular, while we did not test individuals for aggressiveness, we observed that individuals display a low (if any) level of aggressive behaviour, and thus sociability differences are partly independent of aggressiveness differences. However, more studies in this and other systems are needed to establish the patterns of correlation between boldness and sociability or aggressiveness.
One might wonder why individuals vary in sociability, especially in a highly social species. One explanation might be that differences in social attraction are related to differences in competitive abilities, in which asocial individuals avoid groups (i.e. disperse) because they are poor competitors. Alternatively, sociability might relate to producer/scrounger competitive strategies, with asocial individuals representing producers seeking to avoid scroungers. Competitive ability and strategy tend to be associated with dominance or size; larger, dominant individuals tend to be scroungers and better competitors (Pilastro et al. 2003). Here, however, because sociability was not related to body size or body mass, it seems unlikely that variation in sociability is due primarily to differences in competitive abilities or strategies. An alternative explanation is that asocial and social individuals differ in their strategies for coping with predation risk. Shoaling can decrease predation risk through the dilution effect, earlier predator detection owing to increased overall vigilance and predator confusion (Krause & Ruxton 2002). On the other hand, predators may attack groups more frequently because they are more easily detected (Botham et al. 2005). Individuals that have a high likelihood of escaping a predator (e.g. through swimming speed or efficient use of shelter) might avoid social groups as the benefit provided is lower than the costs (i.e. competition or predators attraction), whereas individuals that have poorer escape ability might rely on shoaling for safety. Further experiments are needed to assess the link between sociability, foraging abilities and predator avoidance.
(b). Sociability and dispersal behaviour
As noted above, individual variation in sociability has the potential to strongly affect population dynamics through effects on competition and ability to cope with predators (Cote & Clobert 2007; Reale et al. 2007). The impact of a personality type on population dynamics becomes even more interesting and complex if that personality type is also related to dispersal, and thus to spatial ecological dynamics. In this context, it is particularly notable that we found that sociability is related to dispersal distance. The distance an individual moved during the dispersal assay was negatively related to its sociability (i.e. asocial individuals dispersed farther downstream). In the common lizard (Lacerta vivipara), dispersal behaviour depended on the relationship between individual sociability and local population density, explaining the existence of variable density-dependent habitat preferences within a given species (Cote & Clobert 2007). Our results support the idea that individual variation in social tolerance partly modulates dispersal decisions and may thus affect spatial population dynamics.
We did not find the positive relationship between boldness–exploration and dispersal distance that has been observed in other species (Fraser et al. 2001; Dingemanse et al. 2003). One possible explanation for this discrepancy might be that the tendency for bold individuals, but not shy ones, to disperse only arises if dispersal requires overcoming challenging barriers (e.g. Alemadi & Jenkins 2008) or if dispersal per se is viewed as a particularly dangerous activity. Our artificial streams did not have physical barriers or obvious risks associated with dispersal. Alternatively, predation risk may be necessary to induce boldness-dependent dispersal.
While our dispersal assay in an experimental stream was conducted over a shorter period and a smaller spatial scale than dispersal in natural invasions, we believe that our experiment realistically assessed individual variation in dispersal behaviour and how it relates to behavioural types in the wild. We introduced fish into the upstream release pool in a way that resembles how they are often released by mosquito control agencies. Most fish then stayed in the release pool; that is, we saw no sign of high dispersal rates that might be associated with stress owing to handling or to being in a new, unfamiliar habitat. A few fish that preferred not to disperse might have accidentally dispersed from the release pool; these would presumably stay in the second pool. Importantly, very few of those that dispersed stayed in the second pool. Instead, many individuals that left the original release pool dispersed all the way down to the fifth, most downstream pool (i.e. they apparently made four distinct dispersal decisions that took them as far downstream as they could go in our experimental system). As noted, these individuals were more asocial individuals. Given that dispersal distance was repeatable (i.e. the same individuals that dispersed as far as they could go in the first assay tended to again disperse as far as they could go in a second assay three weeks later), we believe that our experiment realistically detects individual variation in tendency to disperse from the area of introduction. If individuals leaving their pond of introduction have different personality traits than individuals staying, it would have important implications for the invasion process. Obviously, our measurement of dispersal might not precisely match dispersal distance in the wild. However, our aim is not to provide quantitative prediction of dispersal distance in the wild but to show that dispersal behaviour might depend on personality traits in this invasive species.
(c). Behavioural syndromes and the spread and impact of invasion
If dispersal is personality-dependent, then personality traits can play an important role in generating individual variability in dispersal decisions that can affect metapopulation dynamics (Cote & Clobert 2007; Clobert et al. 2009) and invasion processes (Duckworth & Badyaev 2007). Indeed, the ability of an invasive species to spread is likely to depend on individual dispersal distances and rates (Swingland 1983; Bradford & Taylor 1997; Parker & Reichard 1998). While previous studies have compared the dispersal ability of invasive and non-invasive species, individual variation within a given species in dispersal tendency has only rarely been integrated into the way we think about the invasion process (Duckworth & Badyaev 2007). Our study confirms that this idea is both applicable and potentially important to invasion processes.
Note that the different traits necessary to successfully complete different stages of the invasion process might be in conflict. For instance, a tendency for frequent dispersal might be incompatible with reaching high enough densities to overcome poor population growth rates associated with low density (i.e. Allee effects). This conflict can be alleviated if dispersers that colonize new habitats tend to remain in their new habitat rather than disperse again. In our system, the least socially tolerant individuals (i.e. asocial individuals) might fit these criteria. They move farther/faster and are therefore likely to be the first to colonize new habitats. Crucially, there is evidence that asocial individuals tend to stay in low-density patches, and leave only when populations become dense (Cote & Clobert 2007; J. Cote, S. Fogarty, T. Brodin & A. Sih 2009, unpublished data on Gambusia). These early ‘colonizers’ can then allow the population in a patch to build numbers and overcome any Allee effect.
One can ask what the benefits are for individual asocial fish to stay in a newly colonized habitat. A complementary study on common lizards showed that asocial individuals have increased fitness at low density and that it might explain why asocial individuals prefer to stay away from conspecifics at high densities (Cote et al. 2008). In addition, the fact that females can store sperm and bear live young further enhances the ability of low numbers of fish to establish in new habitats (Chesser et al. 1984). The gradual build-up of asocial individuals may then facilitate the settlement of other, more social individuals (i.e. ‘joiners’). Over time, the more socially tolerant individuals may eventually disperse and join patches previously colonized by asocial individuals. Importantly, the increase in population size should then drive asocial individuals out, leading to colonization of additional empty patches. In theory, this multi-stage dynamic, which requires a mix of asocial colonizers and social joiners, results in a more rapid invasive spread to higher densities than an invasion featuring only one personality type (J. Cote, S. Fogarty, T. Brodin & A. Sih 2009, unpublished data).
Because personality types differ in their ability to cope with various ecological factors (e.g. with high density, competition or predation; Smith & Blumstein 2008), a second ecological implication of personality-dependent dispersal arises when invasion by a biased subset of individuals has different ecological impacts than invasion by a group of random colonists. Recent evidence that bold or aggressive animals tend to be dispersers to new habitats (Fraser et al. 2001; Rehage & Sih 2004; Duckworth & Badyaev 2007; Duckworth 2008) can explain that these species have larger impact on invaded communities than on their home communities. The direct link between sociability and performance in dealing with such challenges as interspecific competition and predation could be important, but has not been well studied. How ecological factors (density, competition and predation) influence both sociability and more generally personality-dependent dispersal and performance within patches is thus a crucial future direction in understanding invasions and, in general, in understanding the link between behaviour and ecology. Finally, it has recently been shown that the expression of personality might depend on group composition and even that the personality of others matters in the formation of a group (Harcourt et al. 2009; Magnhagen & Bunnefeld 2009) and the behaviours of conspecifics also matter in dispersal decisions. More experiments are needed to test how individual social behaviour interacts with average social behaviour in the population.
Acknowledgements
We thank Demetri Dokos and the Sacramento-Yolo Mosquito and Vector Control District for providing us with mosquitofish, and David Harris and two anonymous referees for comments. This research programme was supported by a Fyssen Foundation fellowship to J.C. and by a postdoctoral fellowship grant from the Swedish Research Council to T.B.
References
- Alemadi S., Jenkins D.2008Behavioral constraints for the spread of the eastern mosquitofish, Gambusia holbrooki (Poeciliidae). Biol. Invas. 10, 59–66 (doi:10.1007/s10530-007-9109-x) [Google Scholar]
- Bell A.2007Future directions in behavioural syndromes research. Proc. R. Soc. B 274, 755–761 (doi:10.1098/rspb.2006.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. M., Hankison S. J., Laskowski K. L.2009The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783 (doi:10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte D., Clercq N. d., Zwertvaegher I., Lens L.2009Repeatability of dispersal behaviour in a common dwarf spider: evidence for different mechanisms behind short- and long-distance dispersal. Ecol. Entomol. 34, 271–276 (doi:10.1111/j.1365-2311.2008.01070.x) [Google Scholar]
- Botham M., Kerfoot C., Louca V., Krause J.2005Predator choice in the field: grouping guppies, Poecilia reticulata, receive more attacks. Behav. Ecol. Sociobiol. 59, 181–184 (doi:10.1007/s00265-005-0018-7) [Google Scholar]
- Bradford M. J., Taylor G. C.1997Individual variation in dispersal behaviour of newly emerged chinook salmon (Oncorhynchus tshawytscha) from the Upper Fraser River, British Columbia. Can. J. Fish. Aquat. Sci. 54, 1585–1592 (doi:10.1139/cjfas-54-7-1585) [Google Scholar]
- Brown C., Burgess F., Braithwaite V. A.2007Heritable and experiential effects on boldness in a tropical poeciliid. Behav. Ecol. Sociobiol. 62, 237–243 (doi:10.1007/s00265-007-0458-3) [Google Scholar]
- Bubb D. H., Thom T. J., Lucas M. C.2006Movement, dispersal and refuge use of co-occurring introduced and native crayfish. Freshwater Biol. 51, 1359–1368 (doi:10.1111/j.1365-2427.2006.01578.x) [Google Scholar]
- Chesser R. K., Smith M. W., Smith M. H.1984Biochemical genetics of mosquitofish: incidence and significance of multiple insemination. Genetica 64, 77–81 (doi:10.1007/BF00120257) [Google Scholar]
- Clobert J., Le Galliard J. F., Cote J., Meylan S., Massot M.2009Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209 (doi:10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- Cote J., Clobert J.2007Social personalities influence natal dispersal in a lizard. Proc. R. Soc. B 274, 383–390 (doi:10.1098/rspb.2006.3734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J., Dreiss A., Clobert J.2008Social personality trait and fitness. Proc. R. Soc. B 275, 2851–2858 (doi:10.1098/rspb.2008.0783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay W. R., Meffe G. K.1989Small fish in strange places: a review of introduced poeciliids. In Ecology and evolution of livebearing fishes (ed. Meffe G. K.), pp. 319–332 New York, NY: Prentice-Hall [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 (doi:10.1111/j.1461-0248.2004.00618.x) [Google Scholar]
- Darlington R. B., Smulders T. V.2001Problems with residual analysis. Anim. Behav. 62, 599–602 (doi:10.1006/anbe.2001.1806) [Google Scholar]
- Dingemanse N. J., Both C., van Noordwijk A. J., Rutten A. L., Drent P. J.2003Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747 (doi:10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doligez B., Gustafsson L., Part T.2009‘Heritability’ of dispersal propensity in a patchy population. Proc. R. Soc. B 276, 2829–2836 (doi:10.1098/rspb.2009.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R. A.2008Adaptive dispersal strategies and the dynamics of a range expansion. Am. Nat. 172, S4–S17 (doi:10.1086/588289) [DOI] [PubMed] [Google Scholar]
- Duckworth R. A., Badyaev A. V.2007Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R. A., Kruuk L. E. B.2009Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution 63, 968–977 [DOI] [PubMed] [Google Scholar]
- Dukes J. S., Mooney H. A.1999Does global change increase the success of biological invaders? Trends Ecol. Evol. 14, 135–139 (doi:10.1016/S0169-5347(98)01554-7) [DOI] [PubMed] [Google Scholar]
- Fraser D. F., Gilliam J. F., Daley M. J., Le A. N., Skalski G. T.2001Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 158, 124–135 (doi:10.1086/321307) [DOI] [PubMed] [Google Scholar]
- Gamradt S. C., Kats L. B.1996Effect of introduced crayfish and mosquitofish on California newts. Conserv. Biol. 10, 1155–1162 (doi:10.1046/j.1523-1739.1996.10041155.x) [Google Scholar]
- Garcia-Berthou E.2001On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. J. Anim. Ecol. 70, 708–711 (doi:10.1046/j.1365-2656.2001.00524.x) [Google Scholar]
- Goodsell J. A., Kats L. B.1999Effect of introduced mosquitofish on pacific treefrogs and the role of alternative prey. Conserv. Biol. 13, 921–924 (doi:10.1046/j.1523-1739.1999.98237.x) [Google Scholar]
- Harcourt J. L., Sweetman G., Johnstone R. A., Manica A.2009Personality counts: the effect of boldness on shoal choice in three-spined sticklebacks. Anim. Behav. 77, 1501–1505 (doi:10.1016/j.anbehav.2009.03.004) [Google Scholar]
- Ims R. A.1990Determinants of natal dispersal and space use in gray-sided voles, Clethrionomys rufocanus—a combined field and laboratory experiment. Oikos 75, 106–113 (doi:10.2307/3565743) [Google Scholar]
- Jackson D. A.1993Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74, 2204–2214 (doi:10.2307/1939574) [Google Scholar]
- Jokela M., Elovainio M., Kivimäki M., Keltikangas-Järvinen L.2008Temperament and migration patterns in Finland. Psychol. Sci. 19, 831–837 (doi:10.1111/j.1467-9280.2008.02164.x) [DOI] [PubMed] [Google Scholar]
- Kolar C. S., Lodge D. M.2001Progress in invasion biology: predicting invaders. Trends Ecol. Evol. 16, 199–204 (doi:10.1016/S0169-5347(01)02101-2) [DOI] [PubMed] [Google Scholar]
- Krause J., Ruxton G. D.2002Living in groups Oxford, UK: Oxford University Press [Google Scholar]
- Le Galliard J. F., Ferrière R., Dieckmann U.2005Adaptive evolution of social traits: origin, trajectories, and correlations of altruism and mobility. Am. Nat. 165, 206–224 (doi:10.1086/427090) [DOI] [PubMed] [Google Scholar]
- Lessells C. M., Boag P. T.1987Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 [Google Scholar]
- Littell R. C., Miliken G. A., Stroup W. W., Wolfinger R. D.1996SAS system for mixed models Cary, NC: SAS Institute, Inc [Google Scholar]
- Lloyd L. N., Arthington A. H., Milton D. A.1986The mosquitofish—a valuable mosquito-control agent or a pest? In The ecology of exotic animals and plants: some Australian case histories (ed. Kitching R. L.), pp. 6–25 Brisbane, Queensland: John Wiley & Sons [Google Scholar]
- Lodge D. M.1993Biological invasions: lessons for ecology. Trends Ecol. Evol. 8, 133–137 (doi:10.1016/0169-5347(93)90025-K) [DOI] [PubMed] [Google Scholar]
- Lowe S., Browne M., Boudjelas S., De Poorter M.2000100 of the world's worst invasive alien species: a selection from the Global Invasive Species database Auckland, New Zealand: Invasive Species Specialist Group [Google Scholar]
- Mack R. N., Simberloff D., Mark Lonsdale W., Evans H., Clout M., Bazzaz F. A.2000Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710 (doi:10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2) [Google Scholar]
- Magnhagen C., Bunnefeld N.2009Express your personality or go along with the group: what determines the behaviour of shoaling perch? Proc. R. Soc. B 276, 3369–3375 (doi:10.1098/rspb.2009.0851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M. P., Moyle P. B., Levine R.2004Invasive species profiling? Exploring the characteristics of non-native fishes across invasion stages in California. Freshwater Biol. 49, 646–661 (doi:10.1111/j.1365-2427.2004.01202.x) [Google Scholar]
- Martin R. G.1975Sexual and aggressive behavior, density and social structure in a natural population of mosquitofish, Gambusia affinis holbrooki. Copeia 1975, 445–454 (doi:10.2307/1443641) [Google Scholar]
- Parker I. M., Reichard S. H.1998Critical issues in invasion biology for conservation science. In Conservation biology for the coming decade (eds Fiedler P. L., Kareiva P. M.). New York, NY: Chapman and Hall [Google Scholar]
- Pilastro A., Benetton S., Bisazza A.2003Female aggregation and male competition reduce costs of sexual harassment in the mosquitofish Gambusia holbrooki. Anim. Behav. 65, 1161–1167 (doi:10.1006/anbe.2003.2118) [Google Scholar]
- Pintor L. M., Sih A., Kerby J. L.2009Behavioral correlations provide a mechanism for explaining high invader densities and increased impacts on native prey. Ecology 90, 581–587 (doi:10.1890/08-0552.1) [DOI] [PubMed] [Google Scholar]
- Pyke G. H.2005A review of the biology of Gambusia affinis and G. holbrooki. Rev. Fish Biol. Fisher. 15, 339–365 (doi:10.1007/s11160-006-6394-x) [Google Scholar]
- Quinn G. P., Keough M. J.2002Experimental design and data analysis for biologists Cambridge, UK: Cambridge University Press [Google Scholar]
- Reale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- Rehage J. S., Sih A.2004Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol. Invas. 6, 379–391 (doi:10.1023/B:BINV.0000034618.93140.a5) [Google Scholar]
- Rehage J. S., Barnett B. K., Sih A.2005aForaging behaviour and invasiveness: do invasive Gambusia exhibit higher feeding rates and broader diets than their noninvasive relatives? Ecol. Freshw. Fish 14, 352–360 (doi:10.1111/j.1600-0633.2005.00109.x) [Google Scholar]
- Rehage J. S., Barnett B. K., Sih A.2005bBehavioral responses to a novel predator and competitor of invasive mosquitofish and their non-invasive relatives (Gambusia sp.). Behav. Ecol. Sociobiol. V57, 256–266 [Google Scholar]
- Salo P., Korpimaki E., Banks P. B., Nordstrom M., Dickman C. R.2007Alien predators are more dangerous than native predators to prey populations. Proc. R. Soc. B 274, 1237–1243 (doi:10.1098/rspb.2006.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Bell A. M.2008Insights for behavioral ecology from behavioral syndromes. Advances in the Study of Behavior 38, 227–281 New York: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004Behavioral syndromes: an integrative overview. Quart. Rev. Biol. 79, 241–277 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- Sinervo B., Clobert J.2003Morphs, dispersal behavior, genetic similarity, and the evolution of cooperation. Science 300, 1949–1951 (doi:10.1126/science.1083109) [DOI] [PubMed] [Google Scholar]
- Smith B. R., Blumstein D. T.2008Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 (doi:10.1093/beheco/arm144) [Google Scholar]
- Swingland I. R.1983Intraspecific differences in movement. In The ecology of animal movement (eds Swingland I. R., Greenwood P. J.). Oxford, UK: Clarendon Press [Google Scholar]
- Tabachnick B. G., Fidell L. S.1996Using multivariate statistics New York, NY: HarperCollins [Google Scholar]
- Ward A., Thomas P., Hart P. J. B., Krause J.2004Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. V55, 561–568 (doi:10.1007/s00265-003-0751-8) [Google Scholar]
- Webb C., Joss J.1997Does predation by the fish Gambusia holbrooki (Atheriniformes: Poeciliidae) contribute to declining frog populations? Aust. Zool. 30, 316–323 [Google Scholar]
- Welcomme R.1992A history of international introductions of inland aquatic species. ICES Mar. Sci. Symp. 194, 3–14 [Google Scholar]
- Yoshida M., Nagamine M., Uematsu K.2005Comparison of behavioral responses to a novel environment between three teleosts, bluegill Lepomis macrochirus, crucian carp Carassius langsdorfii, and goldfish Carassius auratus. Fish. Sci. 71, 314–319 (doi:10.1111/j.1444-2906.2005.00966.x) [Google Scholar]