Abstract
Theory predicts that mutualisms should be vulnerable to invasion by cheaters, yet mutualistic interactions are both ancient and diverse. What prevents one partner from reaping the benefits of the interaction without paying the costs? Using field experiments and observations, we examined factors affecting mutualism stability in six fig tree–fig wasp species pairs. We experimentally compared the fitness of wasps that did or did not perform their most basic mutualistic service, pollination. We found host sanctions that reduced the fitness of non-pollinating wasps in all derived, actively pollinated fig species (where wasps expend time and energy pollinating), but not in the basal, passively pollinated fig species (where wasps do not). We further screened natural populations of pollinators for wasp individuals that did not carry pollen (‘cheaters’). Pollen-free wasps occurred only in actively pollinating wasp species, and their prevalence was negatively correlated with the sanction strength of their host species. Combined with previous studies, our findings suggest that (i) mutualisms can show coevolutionary dynamics analogous to those of ‘arms races’ in overtly antagonistic interactions; (ii) sanctions are critical for long-term mutualism stability when providing benefits to a host is costly, and (iii) there are general principles that help maintain cooperation both within and among species.
Keywords: mutualism, cooperation, exploitation, cheating, sanctions, fig
1. Introduction
From the mycorrhizal fungi that are essential for the growth of most forest trees to the intestinal bacteria that provide nutrients we otherwise could not access, mutualisms are of fundamental ecological and evolutionary importance (Herre et al. 1999). Despite this, the evolution and maintenance of mutualisms remains a largely unsolved puzzle (Trivers 1971; Axelrod & Hamilton 1981; Bull & Rice 1991; Sachs et al. 2004; West et al. 2007). In a mutualistic system where the partners trade costly services, individuals that reap the benefits of the interactions without paying the cost of providing service to the mutualist (i.e. ‘cheaters’) are expected to realize higher fitness than non-cheaters, and increase in their relative frequency. Therefore, the long-term stability of the mutualism likely requires mechanisms that limit or prevent cheating. Some studies have documented the existence of host ‘sanctions’ that lower the fitness of uncooperative symbionts (Nefdt 1989; Pellmyr & Huth 1994; Richter & Weis 1995; Wilson & Addicott 1998; Huth & Pellmyr 2000; Jousselin & Kjellberg 2001; Kiers et al. 2003, 2006; Edwards et al. 2006; Simms et al. 2006; Tarachai et al. 2008; Bever et al. 2009; Heath & Tiffin 2009). For example, both cultivated and wild legumes can selectively suppress rhizobial strains that feed off plants but are inefficient in fixing nitrogen (Kiers et al. 2003, 2006; Simms et al. 2006), and yuccas differentially abort inflorescences that are relatively underpollinated or overexploited by their pollinating moth (Pellmyr & Huth 1994; Richter & Weis 1995; Wilson & Addicott 1998; Huth & Pellmyr 2000). However, no study has examined variation in sanction strength across related host species. Further, although distinct cheating/parasitic species are known to exploit many mutualisms (Sachs & Simms 2006), examples of cheating individuals within a mutualistic species are rare (Bronstein 2001). Here we use the fig tree–fig wasp system to test whether cheating levels in symbionts are related to sanction strength.
The fig tree–fig wasp system is well suited for studying the evolution and stability of mutualisms. This mutualism is both ancient (80 Mya) and diverse (more than 700 fig species) (Machado et al. 2001; Rønsted et al. 2005), and each fig species is generally pollinated by one or a few highly host-specific wasp species (Molbo et al. 2003; Haine et al. 2006). Each partner is totally dependent on the other for reproductive success, and lifetime reproductive success of the wasp is easily measured (Herre 1989). Fig flowers are located on the inside of the distinctive, enclosed inflorescences that define the genus Ficus (formally syconia; hereafter figs). Pollination is completely dependent on having one or more pollen-bearing female fig wasp (foundress) enter each fig and pollinate the flowers. Fig wasps, in turn, can only oviposit and reproduce in fig flowers.
Wasps generally pollinate both the flowers in which they oviposit, and those that do not receive eggs (Jousselin & Kjellberg 2001; Jandér 2003), then die inside the fig. In monoecious fig species (the roughly 50% of fig species that produce seeds and wasps in the same fig, as opposed to on different trees (dioecious)), each flower produces either a seed or is transformed into a gall that hosts a single wasp larva, causing a direct trade-off between producing fig seeds or wasp offspring (Verkerke 1989; Herre & West 1997). When mature, the wasp offspring mate and females gather pollen within their natal fig before they fly off in search for a new receptive fig tree. Thus, monoecious fig trees depend on the foundress generation to pollinate their own flowers and initiate seed production (thereby realizing ‘female function’) and on the females of the offspring generation to use their pollen to initiate seed production in another tree (thereby realizing ‘male function’).
There are two distinct pollination syndromes in figs that demand different levels of effort from the pollinating wasps: (i) passive pollination, the ancestral condition, and (ii) active pollination, the more derived condition (Jousselin et al. 2003b). Passively pollinated fig species produce numerous, large male flowers that release abundant pollen onto the wasps as they leave the fig to disperse. Typically, male-to-female flower ratios range from 0.25 to 1, and pollen to ovule ratios can be as high as 44 000 to 1 (Cruden 1997; Kjellberg et al. 2001). Therefore, in these fig species, trees invest considerable resources in producing abundant pollen, and no aspect of pollen transfer relies on specialized wasp behaviour.
In contrast, actively pollinated fig species produce relatively few, small male flowers. Male-to-female flower ratios range from 0.01 to 0.15, and pollen to ovule ratios are generally 5 to 10 times lower than that in passively pollinated species (Cruden 1997; Kjellberg et al. 2001). In these fig species, pollen transfer is completely dependent on specialized wasp morphology and pollination behaviour. Female wasps search for the male flowers inside their natal fig, gather pollen using their front legs, and store it in specialized thoracic pollen pockets (Galil & Snitzer-Pasternak 1970; Frank 1984). When wasps reach a receptive fig, they oviposit, then use their front legs to transfer pollen grains to the stigmas (Galil & Eisikowitch 1969; Frank 1984). Overall, 2–5% of the wasps' total time within a receptive fig is spent actively depositing pollen (Jandér 2003; K. C. Jandér 2009, unpublished data). Active pollination thus appears to be beneficial for trees as less pollen production is required, but it requires time and energy from the short-lived wasps. Previous studies suggest that wasps ovipositing in pollinated figs produce more offspring than wasps ovipositing in unpollinated figs (reviewed in Herre et al. 2008).
Here, we use field experiments and observations to examine factors that affect the host–pollinator relationships in six monoecious fig species—four actively pollinated (where wasps actively expend time and energy pollinating) and two passively pollinated (where pollination is a byproduct of the wasps' activities). First, we experimentally measured sanction strength in the respective fig species by relating total lifetime reproductive success for a single foundress wasp to whether or not the wasp was carrying pollen. Second, for the different wasp species we estimated the likelihood that a wasp would be a single foundress—the situation in which a cheating wasp would be most fully exposed to any host sanctions. Third, we screened natural populations of pollinator wasps for wasp individuals that did not carry pollen (‘cheaters’). We thus were able to examine: (i) whether host sanctions were present in these fig species, and if that was related to the pollination syndrome (passive or active), and (ii) whether pollinator cheating levels were related to the strength of sanctions or the likelihood of being a single foundress.
2. Material and methods
(a). Study system
We studied natural populations of trees and wasps near the Panama Canal, Republic of Panama. The passively pollinated fig species represent the most basal of all fig lineages, subgenus Pharmacosycea, section Pharmacosycea: Ficus maxima and F. insipida (Herre et al. 1996; Machado et al. 2001; Jousselin et al. 2003b; Rønsted et al. 2005). All the actively pollinated fig species belong to the more derived subgenus Urostigma, section Americana: F. citrifolia, F. nymphaefolia, F. obtusifolia and F. popenoei. The respective pollinator wasp species and mean number of female flowers are specified in electronic supplementary material, table S1. For simplicity, here we will use the fig species name as a proxy also for its associated wasp species.
(b). Pollen exclusion experiment
For each fig tree–pollinator species-pair, we experimentally produced pollen-carrying (P + ) and artificially pollen-free (AP−) wasps, and introduced one wasp into each fig to produce pollinated (P+) and unpollinated (P−) figs (Jousselin et al. 2003a). We quantified two components of fig sanctions that strongly influence wasp fitness: (i) the proportion of P− and P+ figs that the tree aborted prior to maturation (fig abortion leads to 100% mortality of the enclosed wasp larvae), and (ii) the reproductive success of AP− and P+wasps in the unaborted figs.
We first surveyed several hundred fig trees to match pairs of nearly ripe trees (producing wasps) with nearly receptive conspecific experimental trees. We prevented uncontrolled pollination by enclosing multiple twigs on each pre-receptive tree in mesh bags. To obtain artificially pollen-free (AP−) wasps of the pollinator species, we gathered nearly ripe figs from different, conspecific trees, and opened the figs when male wasps were mating with the females, but when females were still within their galls. We removed all male flowers to prevent female wasps from accessing pollen when they emerged. Control wasps with pollen loads (P+) emerged normally from ripe figs into mesh-covered vials (Jousselin et al. 2003a).
When figs on the experimental tree were receptive, a single AP− or P+ female wasp was introduced into each randomly assigned fig. To determine the effects of no foundress (F−, i.e. no oviposition and no pollination), some figs were left without any wasp entering. All experimental figs on each tree were approximately the same size, and when possible paired figs were used for the P+ and P− treatments. We then re-bagged the twigs to prevent attacks by parasites. During the weeks following the experimental introductions we collected any aborted figs and checked them—figs in which a foundress had been introduced but had not successfully entered the internal cavity of the fig were excluded from the study. The majority of aborted but entered figs showed macroscopic signs of gall development, indicating wasp oviposition. At the end of the experiment, we collected the non-aborted figs just before wasps emerged, so that wasps could emerge in vials and be counted. In a few cases where wasps had already emerged, we counted empty wasp galls to quantify the number of offspring. Experimental figs on F. maxima tree no. 2 were lost because of a neighbouring tree fall a few days before maturation (well after any abortions); hence abortions could be assessed but wasp offspring could not be counted. We counted seeds in each fig to confirm a successful treatment. In some cases there were a few seeds in the P− treatment (usually less than 1% of seeds in the P+ treatment). These figs were included in the P− treatment in the analyses; the results did not change if only figs with zero seeds were included.
To enable direct comparisons across species, we calculated the following values for each tree:
— MP−, the proportion of figs in the P− treatment that matured (did not abort).
— MP+, the proportion of figs in the P+ treatment that matured (did not abort).
— OP−, the mean number of wasp offspring in P− figs that matured.
— OP+, the mean number of wasp offspring in P+ figs that matured.
— MR, MP−/MP+, the relative proportion of P− figs that matured.
— OR, OP−/OP−, the relative number of offspring in unaborted P− figs.
— WR, MR × OR, the relative fitness of a single foundress P− wasp. Assuming P− and P+ foundresses laid similar numbers of eggs (electronic supplementary material), this is equivalent to the relative survival of P− eggs to P+ eggs (Wenseleers & Ratnieks 2006).
Thus, each tree produced a single value of MR, OR and WR, and we compared these variables across species using ANOVAs. The parameter WR was square root-transformed to meet the assumption of homogeneity of variances for the ANOVA, but we used untransformed data for graphs and magnitude comparisons. Performing the ANOVA on untransformed data did not change the results.
(c). Proportion of single foundress wasps in each species
To estimate the proportion of wasps associated with each species that are likely to be single foundresses, we collected figs within a week after pollinator arrival (with few exceptions more than 100 figs per crop; number of crops: F. pop. 12, F. nym. 5, F. cit. 6, F. obt. 8), and counted the number of dead foundress wasps in each fig. We then calculated the proportion of wasps that were single foundresses (e.g. if 50% of figs had one foundress and 50% had two, then one-third of wasps were single foundresses), and tested whether this proportion differed across species using a generalized linear model with binomial errors, a logit link and an overdispersion parameter, using single foundress wasps (out of total number of wasps) for each crop as the response variable, and species as the explanatory variable; contrasts were pairwise and sequential Bonferroni corrected. Results did not change if we instead used a Kruskal–Wallis test to examine whether the proportion of single foundress wasps differed across species.
(d). Prevalence of pollen-free wasps in natural populations
Unmanipulated, naturally occurring wasps of the pollinator species were collected on sticky traps or by using an aspirator as they were arriving at receptive trees. In two cases we collected wasps emerging from ripe figs by placing mesh bags around individual figs; only a single wasp per fig fruit was examined to assure independence. In all fig species except one we sampled from several independent flowering or fruiting events (crops); number of examined wasps per event: F. max. 723; F. ins. 311, 1117; F. pop. 564, 699, 620, 988, 715, 979, 459; F. obt. 107, 396, 206, 160, 241; F. nym. 709, 302, 411; F. cit. 621, 479, 1017, 687, 919. Wasps were examined under a light microscope, with the examiner blind to species when possible, to detect presence or absence of pollen grains in their pollen pockets (active pollinators) or on their body (passive pollinators). We fitted a generalized linear model with binomial errors, a logit link and an overdispersion parameter, using natural pollen-free (NP−) wasps (out of total number of wasps) for each crop as the response variable, and species as the explanatory variable. Results did not change if we instead used a Kruskal–Wallis test to examine whether the proportion of NP− wasps differed across species.
(e). Phylogenetically independent contrasts
We used the PDAP module of Mesquite to calculate phylogenetically independent contrasts (Midford et al. 2008). Because the dependent variable is a wasp characteristic, we based calculations on the best known wasp phylogeny (Machado et al. 2005; C. A. Machado 2009, personal communication). We used molecular branch lengths; results did not change if we used equal branch lengths. We treated each host as associated with only one (the most common) wasp species. The results did not change if we instead based calculations on an alternative wasp phylogeny (Jackson et al. 2008) or the fig phylogeny (Jackson et al. 2008), using either equal or molecular branch lengths.
3. Results
(a). Experimental investigation of fitness cost for wasps that do not pollinate
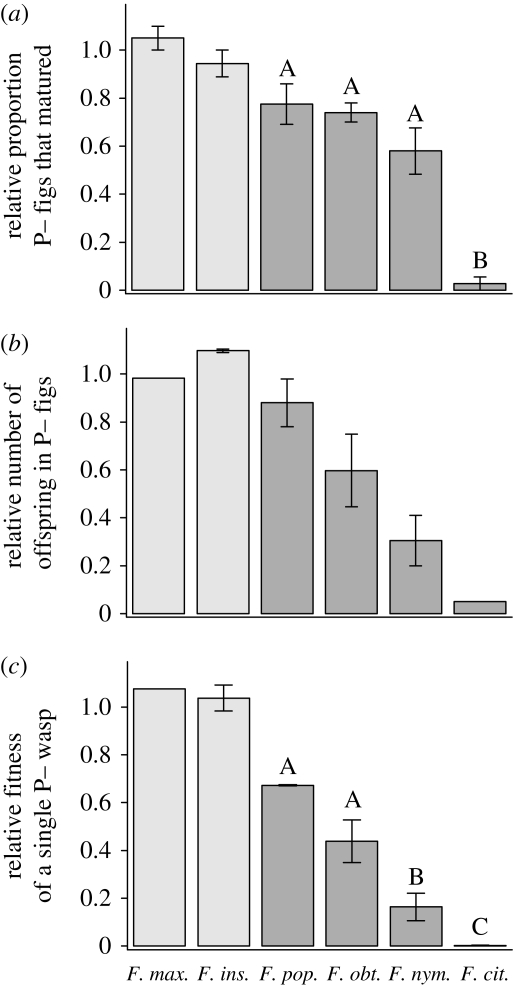
We found host sanctions against cheating (AP−) wasps in all actively pollinated fig species. These sanctions were a combination of (i) increased abortion of P− figs (MR), and (ii) reduced number of wasp offspring in P− figs that were not aborted (OR) (electronic supplementary material, table S2). In contrast, the passively pollinated fig species showed no evidence of sanctions: there was no difference between P− and P+ figs in either the likelihood of abortion, or the number of wasp offspring that developed from a fig (electronic supplementary material, table S2). Specifically, the relative proportion of P− figs that matured (MR) was significantly lower in the actively pollinated species than the passive (ANOVA: F5,9 = 39.5, p < 0.0001; planned contrast: t9 = −7.7, p < 0.0001; figure 1a). Further, the relative number of offspring in unaborted P− figs (OR) was significantly lower in the actively pollinated species than the passive (ANOVA: F5,5 = 7.7, p < 0.05; planned contrast: t5 = −4.1, p < 0.01; figure 1b). The resulting relative fitness for a single foundress P− wasp (WR) was significantly lower in actively pollinated species than in passive (ANOVA: F5,8 = 70.7, p < 0.0001; planned contrast: t8 = −10.6, p < 0.0001; figure 1c). There was no evidence that the experimental AP− treatment reduced the number of eggs a wasp carried or could lay (electronic supplementary material). Across all species, all figs aborted if neither a wasp nor pollen entered (electronic supplementary material, table S2).
Figure 1.
The two components of sanctions and the resulting relative fitness of a cheating wasp compared across species. (a) Relative proportion of figs that matured (did not abort) after experimental introductions of wasps without pollen (AP−) compared to wasps with pollen (P+) (MR). (b) Relative number of wasp offspring emerging from unaborted P− figs compared with P+ figs (OR). (c) The resulting experimentally determined relative fitness of a single foundress AP− wasp compared with a P+ wasp (WR). Letters represent significantly different subsets within the actively pollinated species. Light grey bar, passive pollination; dark grey bar, active pollination. Error bars represent 1 s.e.m.
Sanction strength (defined as 1 − WR (see Wenseleers & Ratnieks 2006)) varied greatly across the actively pollinated species. The relative proportion of P− figs that matured (MR) ranged from 2.8 per cent (F. citrifolia) to 78 per cent (F. popenoei) (ANOVA: F3,7 = 32.3, p<0.001; Tukey HSD: p < 0.05) (figure 1a). The relative number of wasp offspring produced in unaborted P− figs (OR) ranged from 4.9 per cent (F. citrifolia) to 88 per cent (F. popenoei) of the number produced in P+ figs (ANOVA: F3,4 = 6.3, p = 0.053) (figure 1b). Therefore, across the actively pollinated species, the combined effects of abortion and offspring reduction produced large differences in estimated relative fitness for P− wasps (WR), ranging from 0.14 per cent (F. citrifolia) to 67 per cent (F. popenoei) (ANOVA: F3,7 = 55.6, p < 0.0001; Tukey HSD: p < 0.05) (figure 1c).
(b). Foundress distributions
The proportion of wasps that were single foundresses varied considerably across the actively pollinated fig species: in F. obtusifolia (A), 71 ± 8.2 (s.e.m.) per cent, in F. citrifolia (A) 52 ± 5.3 per cent, in F. nymphaefolia (B) 24 ± 10.6 per cent, and in F. popenoei (B) 6.9 ± 2.2 per cent (GLM: binomial errors, χ23 = 76.1, p < 0.0001; letters represent significantly different subsets). Therefore, in addition to the difference in sanction strength described above, an average P− wasp in F. popenoei would be seven times less likely to experience full sanctions than an average P− wasp in F. citrifolia.
(c). Field survey of natural pollen-free wasps
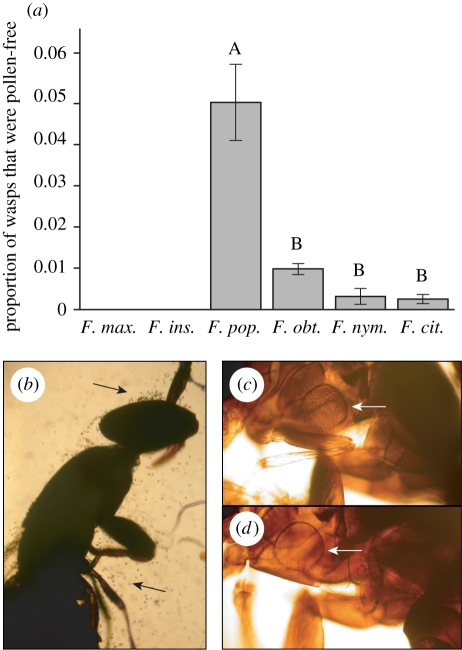
The proportion of natural pollen-free (NP−) wasps varied significantly across species (GLM: binomial errors, χ25 = 93.0, p < 0.0001; figure 2). No passively pollinating wasp (out of 2151 sampled) was caught without pollen, whereas all actively pollinating wasp species exhibited some individuals that did not carry any pollen (planned contrasts, p < 0.0001). Within the actively pollinated species, pollen-free wasps were an order of magnitude more common in wasps associated with F. popenoei (5%) than in the other species (0.5%) (GLM: binomial errors, χ23 = 65.9, p < 0.0001; pair-wise sequential Bonferroni corrected contrasts, p < 0.0001) (figure 2).
Figure 2.
Wasps of the pollinator species do not always carry pollen. (a) The proportion of naturally occurring pollen-free wasps (NP−) varied across the studied fig species. NP− wasps were only found in association with actively pollinated fig species. Letters represent significantly different subsets within the actively pollinated species; error bars indicate 1 s.e.m. Light grey bar, passive pollination; dark grey bar, active pollination. (b) Passive pollinator of F. insipida with pollen grains scattered all over her body. (c,d) Two active pollinator wasps of F. nymphaefolia, one with her pollen pocket full of pollen grains (arrow, c), the other with an empty pollen pocket (arrow, d). In both (c,d), the wasp's head is just outside the lower right corner.
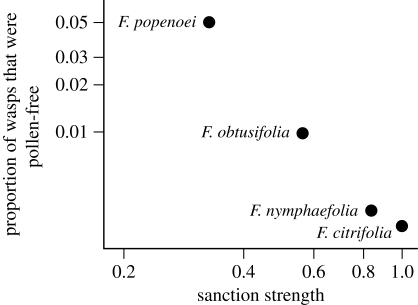
Further, across the actively pollinated species the proportion of pollen-free wasps negatively correlated with sanction strength (Pearson correlation on log-transformed data, r = −0.996, p < 0.01; figure 3). This relationship persisted when we controlled for phylogenetic dependencies (PIC: r = −0.995, p < 0.01). Across the actively pollinated species studied, there was no significant relationship between the proportion of pollen-free wasps and the proportion of wasps that were single foundresses (Pearson correlation: r = −0.46, p = 0.54).
Figure 3.
The proportion of naturally occurring pollen-free wasps was negatively correlated with sanction strength (1 − WR) across actively pollinated fig species.
Ficus popenoei and F. obtusifolia each have two cryptic pollinator species (Molbo et al. 2003). We found pollen-free wasps in each of these pollinator species; there was no support for pollen-free wasps belonging exclusively to one of the cryptic wasp species (electronic supplementary material, table S3). Ficus citrifolia and F. nymphaefolia have only one known pollinator species each (Molbo et al. 2003; Machado et al. 2005); all tested NP− and P+ wasps in F. citrifolia belonged to the known species.
4. Discussion
This study provides three novel findings relevant to mutualism stability. First, we show that host sanctions against non-cooperative symbionts vary dramatically in form and intensity across fig species. Second, we document the existence of pollen-free individuals (‘cheaters’) within the otherwise mutualistic pollinator wasp species. Third, across the actively pollinated fig species, we show that the proportion of pollen-free wasps is negatively correlated with sanction strength. Finally, we combine the results from our study with previous fig studies to give a phylogenetic overview of our current knowledge of host sanctions and wasp cheating in the fig tree–fig wasp mutualism. Together, these studies demonstrate that the form and strength of sanctions in the host, and the corresponding characteristics of the pollinators vary greatly across the fig tree–fig wasp mutualism.
(a). Host sanctions in figs
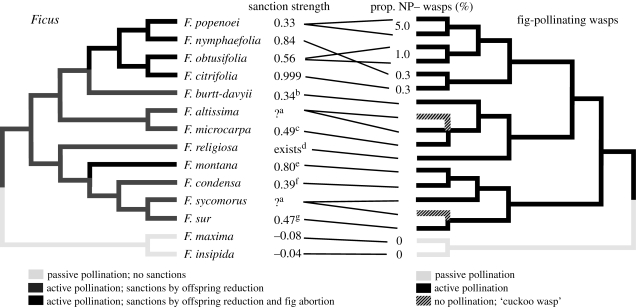
Results from previous pollen exclusion experiments in figs show or suggest lower offspring numbers for wasps that did not pollinate, and/or increased abortion of figs that received wasp eggs but no pollen (Galil & Eisikowitch 1971; Nefdt 1989; Jousselin & Kjellberg 2001; Jousselin et al. 2003a; Tarachai et al. 2008). Host sanctions have been detected in fig species representing all major subgenera of Ficus (see the phylogenetic overview in figure 4). However, the previous studies only examined actively pollinated species, with little or no replication either of species, or of trees within species (figure 4; summary in Herre et al. 2008). Our study design allows us to directly compare sanction strength both across several closely related actively pollinated fig species, and across distantly related groups of figs that represent different pollination syndromes. In the actively pollinated species, the sanction strengths we measured here (0.33–0.999 in F. popenoei and F. citrifolia, respectively) bracket those reported previously (0.34–0.80 in F. burtt-davyii and F. montana, respectively; figure 4).
Figure 4.
Phylogenetic relationship between the fig species that have been studied for sanctions and their pollinating wasps. Because the sanction strength value does not take into account the likelihood of being a single foundress in the respective species, effective sanction strength is likely to be lower in species with multiple foundresses. The proportion of naturally occurring pollen-free wasps (NP−) in the pollinating species is indicated where known. Phylogenetic relationships based on Machado et al. 2001, 2005; Rønsted et al. 2005; Jackson et al. 2008; C. A. Machado 2009, personal communication. Placement of wasps associated with F. altissima and F. religiosa are inferred from the wasp taxonomy. aF. altissima and F. sycomorus are associated with ‘cuckoo’ wasp species that have lost their active pollination behaviour; we hypothesize that they have weak, if any, sanctions. In earlier pollen-free experiments, figs are monoecious and the fitness reduction significant unless otherwise noted; sample sizes (P+, P−) and relevant notes: b(6,12) (Nefdt 1989); c(12,11), p = 0.07 (Jousselin et al. 2003a); dno data or statistical tests presented (Galil & Eisikowitch 1971); e(60,60), dioecious (Tarachai et al. 2008); f(16,16), dioecious (Jousselin & Kjellberg 2001); g(4,3), experimental figs had two foundresses (Nefdt 1989).
The passively pollinated species in our study represent the most basal lineage of Ficus, and in contrast with the previously studied fig species (Herre et al. 2008; figure 4), there was no indication of host sanctions in these species. Further, we found no evidence of pollen-free individuals in the associated wasp species. Passively pollinating wasps do not actively expend energy pollinating, and they cannot easily avoid carrying pollen. We suggest that although passively pollinated fig species invest more in pollen production, they benefit from a low incidence of pollen-free wasps, which makes sanction mechanisms unnecessary. In contrast, actively pollinating wasps actively expend time and energy on pollination, and omitting even one of a chain of required pollination behaviours would prevent a fig from being pollinated. Therefore, this behaviour could easily be lost, and there may be wasp incentives to do so. Although actively pollinated fig species benefit from considerably lower costs of pollen production (Kjellberg et al. 2001), all existing studies of actively pollinated fig species suggest that they need effective sanction mechanisms in order to maintain highly cooperative pollinators (figure 4; Herre et al. 2008; this study).
(b). The existence of ‘cheaters’ within the mutualistic wasp species
In the fig tree–fig wasp system there are two well-known groups of non-mutualistic wasp species: (i) numerous taxa of parasitic wasps that mostly oviposit from the outside of figs and do not pollinate (West et al. 1996; Rasplus et al. 1998; Kerdelhue et al. 2000; but see Jousselin et al. 2001), and (ii) two species of wasps from lineages of active pollinators that have lost their ability to pollinate, and have become parasites or ‘cuckoos’ (Galil & Eisikowitch 1968; Compton et al. 1991; Peng et al. 2008; figure 4). In contrast, we here report for the first time the existence of pollen-free, ‘cheating’, wasp individuals that belong to otherwise mutualistic pollinating wasp species, and not to separate, parasitic species. Since these wasps will be unable to pollinate but still will be able to lay eggs, they are effective cheaters with respect to the tree's seed production (female function). If the tendency not to collect pollen is heritable, then such wasps are also detrimental to the tree's male function because their daughters are not likely to disperse pollen.
We found pollen-free individuals in natural populations of all actively pollinating wasp species, including all known cryptic wasp species of F. popenoei and F. obtusifolia. Interestingly, although the cryptic species in F. obtusifolia appear to be sister species, those in F. popenoei are not (Molbo et al. 2003; Jackson et al. 2008), suggesting that the high levels of pollen-free wasps found in the two cryptic species associated with F. popenoei may represent two independent evolutionary events. It is currently unclear if the pollen-free wasps derive any benefit, and if so how large, from not carrying pollen. Potential benefits include energy savings from not carrying the pollen weight, and time savings from not collecting and depositing pollen.
(c). The association between sanction strength and the prevalence of pollen-free wasps
In systems with repeated interactions between individuals, direct punishment of uncooperative individuals is known to induce future cooperative behaviour. For example, in the reef fish–cleaner fish mutualism, host punishment of cheating cleaners increased cooperation levels in future interactions with the same host individual (Bshary & Grutter 2002; Bshary & Grutter 2005). Many relationships, however, are not characterized by repeated interactions between the punisher and the punished. Recent studies of intraspecific systems lacking repeated interactions suggest that the level of cheating in a population will negatively correlate with the expected level of punishment. In social insects, reproductive workers (cheaters) are rarer in species where the probability of nestmates killing worker-laid eggs is higher (Wenseleers & Ratnieks 2006), and across human societies, the tendency for cooperation in economic games is positively correlated with the tendency to punish uncooperative individuals (Henrich et al. 2006).
Here we have shown a similar pattern in a mutualism, where the interacting individuals belong to different species and do not interact repeatedly. Across the actively pollinated fig species in our study, the prevalence of naturally pollen-free (NP−) wasps was negatively correlated with host sanction strength, and this relationship persisted when we controlled for phylogenetic dependencies. Although data for the prevalence of NP− wasps is currently unavailable for the previously studied fig–wasp pairs (figure 4), we expect NP− wasps to be relatively more common in fig species where sanctions are weak. We would similarly expect the fig species associated with the ‘cuckoo’ wasps (F. sycomorus and F. altissima) to have relatively weak sanctions (Herre et al. 2008; figure 4).
The fig sanctions described in this study are likely to be a modification of the universal plant trait of aborting unpollinated flowers. Fig trees, too, abort figs that are both unpollinated and unoviposited (this study and (Bronstein 1988), also see (Herre 1989)). However, as shown in this study and others (Galil & Eisikowitch 1971; Nefdt 1989; Jousselin & Kjellberg 2001; Jousselin et al. 2003a; Tarachai et al. 2008), fig trees often retain unpollinated figs in which wasps have oviposited. We note that in monoecious species, a seedless fig can still contribute to a fig tree's fitness if at least some of the offspring wasps disperse pollen from their natal fig. While we suspect that the immediate reason for trees to apply ‘sanctions’ is likely to direct resources to those figs that are the most profitable (most seeds and wasps per tree investment), such sanctions would also restrain the spread of the pollen-free trait in the wasp populations if the pollen-free trait is heritable. In contrast, wasps should be selected to increase the likelihood that oviposited flowers will be provisioned, and reduce the likelihood of fig abortion, regardless of pollination status. We suspect that whether individual flowers are provisioned or entire figs are aborted will be determined from the chemical/physical interaction between the fig inflorescences and some combination of pollination and the liquid deposited by the wasps during oviposition (Verkerke 1989).
The relationship between the spatial precision of sanctions and the spatial distribution of symbionts will be important in determining the effectiveness of sanctions in any mutualism where multiple symbionts interact simultaneously with a host (see also Denison 2000; Bever et al. 2009). For example, if sanctions operate on the fig level, pollen-free wasp might largely evade sanctions in fig species that routinely have multiple foundresses (such as F. popenoei and F. sycomorus; Herre 1989; Compton et al. 1991) by free-riding on the pollination efforts of other foundresses. Alternatively, if sanctions operate on the level of individual flowers within figs, pollen-free wasps would be relatively more exposed to sanctions even in fig species with many foundresses. We found a negative but non-significant relationship between the likelihood of wasps being single foundresses and the proportion of pollen-free wasps, the direction being consistent with sanctions acting on the fig level. Further studies of the figs are needed to identify the level of precision and mechanism of sanctions, and to attempt to quantify the relative costs of sanctions across species. Further studies of the wasps are needed to determine if naturally occurring pollen-free wasps inherit this trait from their mothers, and whether any fitness benefits of the pollen-free trait are large enough to explain its persistence despite the sanctions.
In conclusion, we found host sanctions in all actively pollinated fig species, but not in passively pollinated fig species. Further, we found pollen-free individuals in all species where wasps can easily cheat by omitting any of the time-consuming behaviours associated with active pollination. Within these actively pollinated fig species, pollen-free wasps were most common in the species with the weakest sanctions. Combined with previous studies, our results indicate that the mechanisms that maintain mutualism stability are not fixed in form or intensity, and that within the fig tree–fig wasp mutualism they have changed dramatically over the course of 80 Mya of co-adaptation. Such change in host sanction and symbiont response is likely to be a more general property across other mutualisms, analogous to ‘arms races’ in overtly antagonistic interactions.
Acknowledgements
We thank L. Berg, D. Castle, J. Coenen, J. Ek-Jandér, A. Gomez, M. Lopez and Z. Maynard for help with fieldwork and laboratory work, and S. A. Mangan for extensive advice. M. Björklund, A. Dafoe, L. D. Harder, R. Lande, E. G. Leigh Jr, C. A. Machado, D. Molbo, T. D. Seeley, P. W. Sherman, E. L. Simms and the Simms laboratory, A. R. Smith, E. O. Suurmeyer, H. K. Reeve, S. A. Van Bael, M. J. West-Eberhard, and several anonymous reviewers provided helpful comments and suggestions. A. A. Agrawal, J. Booth, C. A. Machado, D. R. Nash and R. J. Rowe provided advice on the statistical and phylogenetic analyses. Special thanks to C. A. Machado for allowing us to use unpublished wasp phylogeny branch lengths. This study was part of K.C.J.'s PhD thesis at Cornell University. We thank Cornell University Graduate School for supporting K.C.J., and the Smithsonian Tropical Research Institution for providing funding (E.A.H. and K.C.J.) and maintaining the research facilities and intellectual environment that made this study possible.
References
- Axelrod R., Hamilton W. D.1981The evolution of cooperation. Science 211, 1390–1396 (doi:10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- Bever J. D., Richardson S. C., Lawrence B. M., Holmes J., Watson M.2009Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol. Lett. 12, 13–21 (doi:10.1111/j.1461-0248.2008.01254.x) [DOI] [PubMed] [Google Scholar]
- Bronstein J. L.1988Limits to fruit production in a monoecious fig: consequences of an obligate mutualism. Ecology 69, 207–214 (doi:10.2307/1943176) [Google Scholar]
- Bronstein J. L.2001The exploitation of mutualisms. Ecol. Lett. 4, 277–287 (doi:10.1046/j.1461-0248.2001.00218.x) [Google Scholar]
- Bshary R., Grutter A. S.2002Asymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim. Behav. 63, 547–555 (doi:10.1006/anbe.2001.1937) [Google Scholar]
- Bshary R., Grutter A. S.2005Punishment and partner switching cause cooperative behaviour in a cleaning mutualism. Biol. Lett. 1, 396–399 (doi:10.1098/rsbl.2005.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J. J., Rice W. R.1991Distinguishing mechanisms for the evolution of co-operation. J. Theor. Biol. 149, 63–74 (doi:10.1016/S0022-5193(05)80072-4) [DOI] [PubMed] [Google Scholar]
- Compton S. G., Holton K. C., Rashbrook S., van Noort S. L., Vincent S. L., Ware A. B.1991Studies of Ceratosolen galili, a non-pollinating agaonid fig wasp. Biotropica 23, 188–194 (doi:10.2307/2388305) [Google Scholar]
- Cruden R. W.1997Implications of evolutionary theory to applied pollination ecology. In Acta Horticulturae 437: VII International Symposium on Pollination (ed. Richards K. W.), pp. 27–51 Leuven, Belgium: International Society for Horticultural Science [Google Scholar]
- Denison R. F.2000Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am. Nat. 156, 567–576 [DOI] [PubMed] [Google Scholar]
- Edwards D. P., Hassall M., Sutherland W. J., Yu D. W.2006Selection for protection in an ant-plant mutualism: host sanctions, host modularity and the principal-agent game. Proc. R. Soc. B 273, 595–602 (doi:10.1098/rspb.2005.3273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. A.1984The behaviour and morphology of the fig wasps Pegoscapus assuetus and P. jimenezi: descriptions and suggested behavioural characters for phylogenetic studies. Psyche 91, 289–308 (doi:10.1155/1984/35653) [Google Scholar]
- Galil J., Eisikowitch D.1968On the pollination ecology of Ficus sycomorus in East Africa. Ecology 49, 259–269 (doi:10.2307/1934454) [Google Scholar]
- Galil J., Eisikowitch D.1969Further studies on the pollination ecology of Ficus sycomorus L. (Hymenoptera, Chalcidoidea, Agaonidae). Tijdschrift voor Entomologie 112, 1–13 [Google Scholar]
- Galil J., Eisikowitch D.1971Studies on mutualistic symbiosis between syconia and sycophilous wasps in monoecious figs. New Phytol. 70, 773–787 (doi:10.1111/j.1469-8137.1971.tb02578.x) [Google Scholar]
- Galil J., Snitzer-Pasternak Y.1970Pollination in Ficus religiosa L. as connected with the structure and mode of action of the pollen pockets of Blastophaga quadraticeps Mayr. New Phytol. 69, 775–784 (doi:10.1111/j.1469-8137.1970.tb02462.x) [Google Scholar]
- Haine E. R., Martin J., Cook J. M.2006Deep mtDNA divergences indicate cryptic species in a fig-pollinating wasp. BMC Evol. Biol. 6, 83 (doi:10.1186/1471-2148-6-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath K. D., Tiffin P.2009Stabilizing mechanisms in a legume–rhizobium mutualism. Evolution 63, 652–662 (doi:10.1111/j.1558-5646.2008.00582.x) [DOI] [PubMed] [Google Scholar]
- Henrich J., et al. 2006Costly punishments across human societies. Science 312, 1767–1770 (doi:10.1126/science.1127333) [DOI] [PubMed] [Google Scholar]
- Herre E. A.1989Coevolution of reproductive characteristics in 12 species of New World figs and their pollinator wasps. Experientia 45, 637–647 (doi:10.1007/BF01975680) [Google Scholar]
- Herre E. A., West S. A.1997Conflict of interest in a mutualism: documenting the elusive fig wasp–seed trade-off. Proc. R. Soc. Lond. B 264, 1501–1507 (doi:10.1098/rspb.1997.0208) [Google Scholar]
- Herre E. A., Machado C. A., Bermingham E., Nason J. D., Windsor D. M., McCafferty S. S., van Houten W., Bachmann K.1996Molecular phylogenies of figs and their pollinator wasps. J. Biogeogr. 23, 521–530 (doi:10.1111/j.1365-2699.1996.tb00014.x) [Google Scholar]
- Herre E. A., Knowlton N., Mueller U. G., Rehner S. A.1999The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol. Evol. 14, 49–53 (doi:10.1016/S0169-5347(98)01529-8) [DOI] [PubMed] [Google Scholar]
- Herre E. A., Jandér K. C., Machado C. A.2008Evolutionary ecology of figs and their associates: ongoing progress and outstanding puzzles. Ann. Rev. Ecol. Syst. 39, 439–458 [Google Scholar]
- Huth C. J., Pellmyr O.2000Pollen-mediated selective abortion in yuccas and its consequences for the plant-pollinator mutualism. Ecology 81, 1100–1107 [Google Scholar]
- Jackson A. P., Machado C. A., Robbins N., Herre E. A.2008Multi-locus phylogenetic analysis of neotropical figs does not support co-speciation with the pollinators: the importance of systematic scale in fig/wasp cophylogenetic studies. Symbiosis 45, 57–72 [Google Scholar]
- Jandér K. C.2003Fig wasp behaviour and stability of the fig–fig wasp mutualism. Undergraduate thesis, Department of Animal Ecology, Uppsala University, Uppsala, Sweden, pp. 1–41 [Google Scholar]
- Jousselin E., Kjellberg F.2001The functional implications of active and passive pollination in dioecious figs. Ecol. Lett. 4, 151–158 (doi:10.1046/j.1461-0248.2001.00209.x) [Google Scholar]
- Jousselin E., Rasplus J.-Y., Kjellberg F.2001Shift to mutualism in parasitic lineages of the fig/fig wasp interaction. Oikos 94, 287–294 (doi:10.1034/j.1600-0706.2001.940209.x) [Google Scholar]
- Jousselin E., Hossaert-McKey M., Herre E. A., Kjellberg F.2003aWhy do fig wasps actively pollinate monoecious figs? Oecologia 134, 381–387 [DOI] [PubMed] [Google Scholar]
- Jousselin E., Rasplus J.-Y., Kjellberg F.2003bConvergence and coevolution in a mutualism: evidence from a molecular phylogeny of Ficus. Evolution 57, 1255–1269 [DOI] [PubMed] [Google Scholar]
- Kerdelhue C., Rossi J.-P., Rasplus J.-Y.2000Comparative community ecology studies on old world figs and fig wasps. Ecology 81, 2832–2849 [Google Scholar]
- Kiers E. T., Rousseau R. A., West S. A., Denison R. F.2003Host sanctions and the legume–rhizobium mutualism. Nature 425, 78–81 (doi:10.1038/nature01931) [DOI] [PubMed] [Google Scholar]
- Kiers E. T., Rousseau R. A., Denison R. F.2006Measured sanctions: legume hosts detect quantitative variation in rhizobium cooperation and punish accordingly. Evol. Ecol. Res. 8, 1077–1086 [Google Scholar]
- Kjellberg F., Jousselin E., Bronstein J. L., Patel A., Yokoyama J., Rasplus J.-Y.2001Pollination mode in fig wasps: the predictive power of correlated traits. Proc. R. Soc. Lond. B 268, 1113–1121 (doi:10.1098/rspb.2001.1633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C. A., Jousselin E., Kjellberg F., Compton S. G., Herre E. A.2001Phylogenetic relationships, historical biogeography and character evolution of fig-pollinating wasps. Proc. R. Soc. Lond. B 268, 685–694 (doi:10.1098/rspb.2000.1418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C. A., Robbins N., Gilbert M. T. P., Herre E. A.2005Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism. Proc. Natl Acad. Sci. USA 102, 6558–6565 (doi:10.1073/pnas.0501840102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midford P. E., Garland T., Jr, Maddison W. P.2008PDAP Package of Mesquite. Version 1.12. See http://mesquiteproject.org. [Google Scholar]
- Molbo D., Machado C. A., Sevenster J. G., Keller L., Herre E. A.2003Cryptic species of fig pollinating wasps: implications for sex allocation, precision of adaptation, and the evolution of the fig–wasp mutualism. Proc. Natl Acad. Sci. USA 100, 5867–5872 (doi:10.1073/pnas.0930903100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefdt R. J. C.1989. Interactions between fig wasps and their host figs. PhD thesis, Rhodes University, Grahamstown, South Africa, pp. 1–170 [Google Scholar]
- Pellmyr O., Huth C. J.1994Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372, 257–260 (doi:10.1038/372257a0) [Google Scholar]
- Peng Y. Q., Duan Z. B., Yang D. R., Rasplus J. Y.2008Co-occurrence of two Eupristina species on Ficus altissima in Xishuangbanna, SW China. Symbiosis 45, 9–14 [Google Scholar]
- Rasplus J.-Y., Kerdelhue C., La Clainche I., Mondor G.1998Molecular phylogeny of fig wasps. Agaonidae are not monophyletic. C.R. Acad. Sci. Paris, Sciences de la vie 321, 517–527 [DOI] [PubMed] [Google Scholar]
- Richter K. S., Weis A. E.1995Differential abortion in the yucca. Nature 376, 557–558 (doi:10.1038/376557b0)7637801 [Google Scholar]
- Rønsted N., Weiblen G. D., Cook J. M., Salamin N., Machado C. A., Savolainen V.200560 million years of co-divergence in the fig–wasp symbosis. Proc. R. Soc. B 272, 2593–2599 (doi:10.1098/rspb.2005.3249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. L., Simms E. L.2006Pathways to mutualism breakdown. Trends Ecol. Evol. 21, 585–592 (doi:10.1016/j.tree.2006.06.018) [DOI] [PubMed] [Google Scholar]
- Sachs J. L., Mueller U. G., Wilcox T. P., Bull J. J.2004The evolution of cooperation. Q. Rev. Biol. 79, 135–160 (doi:10.1086/383541) [DOI] [PubMed] [Google Scholar]
- Simms E. L., Taylor D. L., Povich J., Shefferson R. P., Sachs J. L., Urbina M., Tausczik Y.2006An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proc. R. Soc. B 273, 77–81 (doi:10.1098/rspb.2005.3292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarachai Y., Compton S. G., Trisonthi C.2008The benefits of pollination for a fig wasp. Symbiosis 45, 29–32 [Google Scholar]
- Trivers R. L.1971The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57 (doi:10.1086/406755) [Google Scholar]
- Verkerke W.1989Structure and function of the fig. Experientia 45, 612–622 (doi:10.1007/BF01975678) [Google Scholar]
- Wenseleers T., Ratnieks L. W.2006Enforced altruism in insect societies. Nature 444, 50 (doi:10.1038/444050a) [DOI] [PubMed] [Google Scholar]
- West S. A., Griffin A. S., Gardner A.2007Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672 (doi:10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- West S. A., Herre E. A., Windsor D. M., Green P. R. S.1996The ecology and evolution of the New World non-pollinating fig wasp communities. J. Biogeogr. 23, 447–458 (doi:10.1111/j.1365-2699.1996.tb00006.x) [Google Scholar]
- Wilson R. D., Addicott J. F.1998Regulation of mutualism between yuccas and yucca moths: is oviposition behaviour responsive to selective abscission of flowers? Oikos 81, 109–118 (doi:10.2307/3546473) [Google Scholar]