Abstract
The influence of male territorial and foraging behaviours on female choice has received little attention in studies of resource-defence mating systems even though such male behaviours are thought to affect variation in their territory quality and mating success. Here we show that female purple-throated carib hummingbirds Eulampis jugularis preferred to mate with males that had high standing crops of nectar on their flower territories. A male's ability to maintain high nectar standing crops on his territory not only depended on the number of flowers in his territory, but also on his ability to enhance his territory through the prevention of nectar losses to intruders. We observed that males defended nectar supplies that were two to five times greater than their daily energy needs and consistently partitioned their territories in order to provide some resources to attract intruding females as potential mates. Such territorial behaviour resulted in males defending some flowers for their own food and other flowers as food for intruding females. Collectively, our results suggest that variation in mating success among males is driven primarily by variation in territory quality, which ultimately depends on a male's fighting ability and size.
Keywords: hummingbird, Eulampis jugularis, mate choice, mate competition, territoriality, foraging
1. Introduction
Males of many animal species defend food supplies in order to acquire mates (Andersson 1994). A major question in studies of such resource-based mating systems is the relative importance of male competition versus mate choice in sexual selection on male traits (Alatalo et al. 1986; Hews 1990; Andersson 1994; Kelly 2008; Jones & Ratterman 2009). Less studied is how male territorial defence and foraging behaviour contribute to variation in food resources and territory quality, which may occur in two ways. First, males will vary in their ability to evict intruders and thereby prevent resource losses. Such variation in behaviour would be expected to contribute to variation in territory quality and mating success, i.e. better defenders would have higher quality territories containing more food resources not just because they are able to claim better territories, but because they are able to prevent resource losses to intruders. Second, if the defended food resource is used by both males and potential female mates, then the extent and manner by which the food resource is shared should affect territory quality and mating success.
Investigations linking mate choice and territory quality to male defence and foraging behaviours require a system in which food resources can be easily quantified. Hummingbirds have been model organisms for studies of resource defence and foraging behaviour owing to the relative ease by which their food resource, nectar, can be measured (e.g. Wolf et al. 1972; Carpenter et al. 1983; Powers & McKee 1994; Bateson et al. 2003; Temeles et al. 2005, 2009). Despite studies of male courtship and display behaviours in a number of hummingbird species (e.g. Powers 1987; Hurly et al. 2001; Ficken et al. 2002; Camfield 2003; Clark 2009; Clark & Dudley 2009), the link between male defence of nectar resources, foraging behaviour and sexual selection has not been established. A major problem has been the difficulty of observing hummingbird mating in the wild.
Here we examine how variation in male territorial and foraging behaviours affect mating success in the purple-throated carib Eulampis jugularis, a hummingbird native to the mountainous islands of the Eastern Caribbean. Although sexes are monomorphic in plumage, males are considerably larger and more massive in body size than females, with wings 8.6 per cent longer and body masses 25 per cent greater than those of females (Wolf 1975; Temeles et al. 2000). With regard to feeding traits, females have bills that are 20 per cent longer and 30 per cent more curved than bills of males (Temeles et al. 2009). These differences between the sexes are strongly associated with sexual resource partitioning of two species of Heliconia, a primarily neotropical genus of plants (Temeles et al. 2000; Temeles & Kress 2003). Males are associated with Heliconia caribaea, which they defend against conspecifics and heterospecifics. Females are associated primarily with Heliconia bihai, which they visit in a trap-lining fashion, although they also feed at H. caribaea. A close correspondence between floral traits and energy rewards of these two heliconias to the bill morphology and energetic requirements of each sex of purple-throated carib provides strong evidence for a role of ecology in the evolution of sexual dimorphisms in this hummingbird species. Nonetheless, because males of E. jugularis defend the same plants in both the breeding and non-breeding seasons (Wolf & Hainsworth 1971; Temeles et al. 2005), we have argued that sexual selection may also play a role in the evolution of their larger size (Temeles et al. 2000; Temeles & Kress 2003).
In the investigations reported here, we focused on two aspects of male territoriality at H. caribaea. First, we examined the male traits and territory characteristics associated with male–male competition and female choice. Second, we examined how male territoriality and foraging behaviour affected nectar supplies within territories. We show that males defended nectar supplies in excess of their energy needs and specifically maintained flowers for their own needs and flowers for the use of females as potential mates. We also demonstrate that a female's choice of a male depended on the nectar supplies within his territory, which in turn depended on his prevention of nectar losses to intruders.
2. Material and methods
Our study was conducted at a continuous tract of H. caribaea approximately 1.6 km in length on Morne Joy along Warner Road, Dominica, West Indies (15°23′33″ N, 61°22′16″ W), from 20 March to 31 May 2007. These dates encompassed the breeding cycle of purple-throated caribs at this site, although dates of breeding for this species vary from year to year and from site to site (Wolf 1975; E. J. Temeles & W. J. Kress 2007–2009, personal observations).
(a). The birds
Prior to the start of observations and experiments, we captured hummingbirds with mist nets and marked them with unique combinations of coloured leg bands. For each bird, we measured (in mm) the length of the exposed culmen, total bill length, wing chord, tarsus length and length of the first retrix (Temeles et al. 2009). We banded 12 territorial males, two non-territorial males and 28 females. With the exception of one female, all birds were adults in after hatching-year plumage (Schuchmann 1999).
We identified the boundary of each territory by observing the point at which territorial males evicted intruders, which in our study included conspecific males and females, green-throated caribs (Eulampis holosericeus), female Antillean crested hummingbirds (Orthorhynchus cristatus), bananaquits (Coereba flaveola) and Lesser Antillean bullfinches (Loxigilla noctis). The latter two species either pierced flowers to rob nectar (C. flaveola) or physically removed flowers and consumed them (L. noctis; Temeles et al. 2005). We then measured the number of plants, inflorescences per plant and bracts per inflorescence within each male's territory and marked each plant with a unique number using liquid paper. Within our study area, we identified 12 contiguous territories.
We observed territorial males from the hours of 0630 to 1430 Atlantic standard time from 19 April to 25 May 2007. To ensure that each male was observed under similar meteorological and temporal conditions, we staggered observations in 2 h blocks from 06.30 to 08.30, 08.30 to 10.30, 10.30 to 12.30 and 12.30 to 14.30 over a 9 day period so that each male was observed for a total of 8 h from 06.30 to 14.30 over 9 days. We repeated this 9 day observation cycle twice. For each male in each observation session, we recorded seconds spent sitting, foraging, flycatching, chasing, perch shifting and gone (see Temeles et al. (2005) for descriptions of these behaviours) and the identity and the number of flowers of the plant visited when feeding. We also recorded whether heterospecific intruders successfully fed, and if so, the number of flowers visited during each intrusion. We used the average across all time-observation periods for each male in our data analyses.
Wolf (1975) and Schuchmann & Schuchmann-Wegert (1984) described the mating sequence of the purple-throated carib as a multi-stage process in which one or more of the following stages may occur, in or out of sequence. In stage 1, a female intrudes onto a male territory and is chased repeatedly by the male until she is allowed to remain. In stage 2, the female is allowed to feed at flowers by the male, who hops near her, often with his wings outspread. In stage 3, both sexes display to each other with outstretched wings and usually the hovering pair circles in the air. Stage 4 of the mating sequence is copulation, in which the male mounts the female from the rear or both sexes attempt cloacal contact with appressed abdomens. We noted these four stages and referred to stage 1 as ‘time spent chasing females’ (s) and stages 2–4 as ‘time spent courting’ (s) in calculations of time and energy budgets. During each female intrusion, we also recorded whether the female fed, the number of flowers and identity of the plant at which she fed, whether the male allowed her to feed or whether she fed without interacting with the male and whether the sequence resulted in copulation.
We used the time budgets to calculate 24 h energy budgets for 12 territorial males. Calculations of energy budgets were based only on the proportions of time activities during which birds were under observation; i.e. we excluded the time that a male was gone from his territory. We converted time budgets to energy budgets using measurements of flying, standard and resting metabolism of purple-throated caribs (see Temeles et al. (2005) and the electronic supplementary material for details).
(b). The plants
Individual plants of Heliconia may live up to 20 years and flower for up to four months (Berry & Kress 1991); thus, during our study, territory boundaries remained fixed. Flowers produce nectar during the late hours of the night as well as during the day, and we measured 24 h nectar production by covering inflorescences with mesh bags to exclude animal visitors and then extracting nectar from picked flowers using 25 µl capillary pipets. We sampled three flowers from each of three different plants for each of the 12 male territories, and used the average nectar production per plant in our statistical analyses. Similarly, we measured standing crops of nectar per flower by sampling the amount of nectar in one flower for each of three plants during each of the four observation time intervals for a total of 12 flowers sampled for each territory and five undefended sites. On Dominica, H. caribaea contains up to 20 flowers per bract, and within bracts, each flower opens sequentially and lasts for a single day. At peak flowering, all of the bracts of an inflorescence may each bear a single flower on the same day, but during our study, percentages of flower-bearing bracts per inflorescence ranged from approximately 25–50% at peak flowering. We therefore multiplied the number of bracts on a male's territory by 0.25 and 0.50 to estimate the number of flowers on his territory. Based on our measurements of 24 h nectar production, we used 160 µl and 22 per cent sucrose (weight : weight) as the volume and concentration of nectar per flower, which is equivalent to 0.946 cal µl−1.
(c). Statistical methods
We estimated relative fitness by dividing the number of copulations obtained by individual males by the mean number of copulations of all 12 males. Although we cannot exclude the possibility that females may store sperm from multiple males to use in the production of a clutch, our observations suggest that the number of copulations is a reliable correlate of reproductive success. We never observed a marked female mate with two different males throughout our observations. We did, however, observe two females mating twice with the same male within the same 2 h observation interval. We assume that mating success within one breeding cycle is correlated with lifetime reproductive success.
Male traits (total culmen, wing, tail and tarsus lengths) and measures of territory quality (territory area, bract numbers, nectar standing crop) were log(e)-transformed and standardized to have zero mean and unit variance prior to analyses. We estimated directional selection differentials by conducting separate regressions of these variables against relative mating success. We then estimated the standardized selection gradients (β′) as the partial regression coefficients from a ‘best-model’ stepwise multiple regression of relative mating success on these variables (e.g. Hews 1990). Owing to small sample sizes (n = 12 males), we did not examine quadratic (stabilizing and disruptive) selection. We employed a similar procedure to examine factors affecting territory quality. All regressions were performed using the PROC REG procedure in SAS v. 8.2 (SAS Institute, Cary, NC).
We used χ2-tests of independence and exact tests to compare the number of feeding visits to plants between territorial males and intruding females for each of the 12 territories. We used χ2 goodness-of-fit tests to examine whether their visits to plants were non-random compared with expectations based on relative availabilities of bracts per plant within territories. Because expected frequencies were small for many of these comparisons, we used Monte Carlo estimations with 106 draws to compute p-values for these tests. We excluded plants that were not visited by either sex and combined adjacent plants that received fewer than five visits from either the territorial male or intruding females for the analyses. All tests of independence and goodness-of-fit tests were performed using the PROC FREQ procedure in SAS v. 8.2. For regression analyses of factors affecting territory quality, we used Morista's overlap measure as a summary statistic of the degree of overlap in plant use by territorial males and intruding females (Smith & Zaret 1982).
We conducted a multivariate repeated measures analysis of variance (MANOVA) from the GLM procedure in SAS v. 8.2 to examine patterns of nectar standing crops and female intrusions, with time interval (i.e. 06.30–08.30 to 12.30–14.30) as the repeated measure and male mating status (mated or unmated, undefended) as the subject variable. We used t-tests and paired t-tests with Bonferroni sequential adjustments of p-values (Rice 1989) to compare means within and between subject variables following these analyses. Statistics are presented as means ± s.e., and percentages were arcsine-transformed for statistical tests.
3. Results
(a). Territory characteristics and female intrusions
Territories of the 12 males studied averaged 108 ± 11 m2 in area and contained an average of 676 ± 44 H. caribaea bracts. These territories would hold 169 ± 11 flowers at 25 per cent of population flowering and 338 ± 22 flowers at 50 per cent (peak) of population flowering. At 25 per cent flowering, the average territory offered 25 ± 2 kcal per day, and at peak flowering, 50 ± 4 kcal per day. The energy rewards from territories greatly exceeded the average 24 h energy expenditure of males, which was 10.7 ± 0.1 kcal.
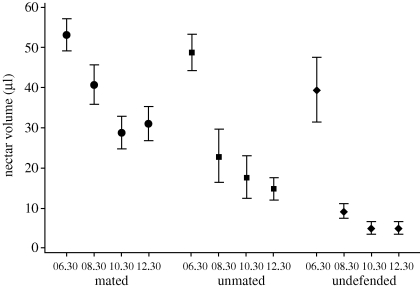
Standing crops of nectar per flower were highest in early morning (06.30–08.30), and we observed no significant differences in standing crops of nectar between undefended sites and territories of seven mated or five unmated males at this time (figure 1; t-tests with Bonferroni adjustments, p>0.05). Standing crops per flower, however, declined rapidly at undefended plants as the morning progressed and were significantly lower relative to standing crops on territories of mated males from 08.30–10.30, 10.30–12.30 and 12.30–14.30, and to standing crops on territories of unmated males from 12.30–14.30 (figure 1; repeated measures MANOVA: F3,12 = 36.6, p < 0.0001; t-tests with Bonferroni corrections, p < 0.05 overall). In addition, standing crops per flower of unmated males were significantly lower than standing crops per flower of mated males from 08.30–10.30, 10.30–12.30 and 12.30–14.30 (figure 1; t-tests with Bonferroni corrections, p < 0.05). Differences in standing crops between males were not a consequence of differences in the quality of plants on territories: 24 h nectar production per flower on territories of mated males (160.7 ± 3.1 µl per flower) did not differ significantly from 24 h nectar production on territories of unmated males (158.3 ± 4.0 µl per flower; t8 = 0.47, p=0.65).
Figure 1.
Mean standing crops of nectar ± s.e. (µl per flower) of Heliconia caribaea on territories of seven mated and five unmated male purple-throated caribs and five undefended sites at four different time intervals (06.30–08.30, 08.30–10.30, 10.30–12.30 and 12.30–14.30, abbreviated 06.30, 08.30, 10.30 and 12.30). Standing crops on undefended sites were not significantly different from those on male territories for 06.30–08.30, but were significantly lower than on territories of mated males for the latter three time categories and on territories of unmated males for 12.30–14.30. Standing crops also were significantly lower for unmated as opposed to mated males for 08.30–10.30, 10.30–12.30 and 12.30–14.30 (t-tests and paired t-tests with Bonferroni adjustments, p < 0.05 overall).
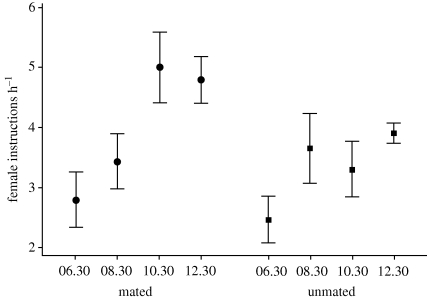
The pattern of female intrusions onto male territories was exactly opposite the pattern of nectar standing crops per flower on undefended areas. The intrusion rate of females onto territories was lowest in the early morning, when nectar standing crop was highest on undefended areas, and then increased progressively over the course of the day as nectar standing crop on undefended areas declined (figures 1 and 2; repeated measures MANOVA: F3,8 = 8.25, p = 0.008). Female intrusion rate was significantly higher on territories of mated males relative to unmated males from 10.30–14.30 (figure 2; t9 = 2.3, p = 0.045); however, female intrusion rate did not differ significantly between mated and unmated males across the entire 06.30–14.30 observation period (repeated measures MANOVA: F1,10 = 1.87, p = 0.201).
Figure 2.
Mean rate of intrusion ± s.e. (intrusions per hour) of female purple-throated caribs on territories of seven mated and five unmated males at four different time intervals (see figure 1). For each time interval, differences between unmated and mated males were not significant, but female intrusion rates were significantly lower on territories of unmated males than on territories of mated males for the time intervals 10.30–12.30 and 12.30–14.30 combined (t-tests with Bonferroni adjustments, p < 0.05 overall).
(b). Mating success
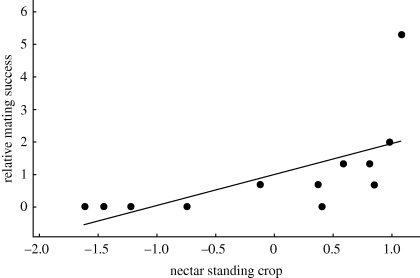
Of the variables wing, tail and tarsus lengths and territory area, bract numbers and nectar standing crop per flower, only nectar standing crop was significantly related to male mating success (figure 3), although both wing length and bract numbers had p-values < 0.1. Of these six variables, only nectar standing crop exhibited a statistically significant directional selection gradient (figure 3), accounting for 35 per cent of the variation in male mating success. The average standing crop of nectar on territories of mated males (41 ± 2 µl per flower; n = 7 males) was significantly greater than the average standing crop of nectar on territories of unmated males (25 ± 3 µl per flower; n = 5 males; t6 = 4.14; p < 0.006). Differences between mated and unmated males for the five male traits and other measures of territory quality were not significant.
Figure 3.
Relationship between relative mating success (copulation rate per male/mean copulation rate) and nectar standing crop (µl per flower, log(e) transformed and standardized) for 12 territorial male purple-throated caribs. (β′ ± s.e. = 0.97 ± 0.41, F1,11 = 5.37, p = 0.043, r2 = 0.35). The regression remains significant excluding the outlier in the upper right (p = 0.026).
(c). Territory defence
We observed no significant difference in the rate of intrusions by heterospecifics onto territories of mated (3.2 ± 0.5 intrusions per hour) versus unmated (2.6 ± 0.5 intrusions per hour) males (t9 = 0.89, p = 0.39). Mated males, however, chased a greater percentage of heterospecific intruders (98 ± 1%) than unmated males (67 ± 11%; t4 = 2.7, p = 0.05). As a result, heterospecific intruders fed at a significantly greater number of flowers on territories of unmated males (2.9 ± 0.8 flowers per hour) than on territories of mated males (0.6 ± 0.3 flowers per hour; t5 = 2.6, p = 0.04). Unmated males also sustained a significantly greater rate of intrusions onto their territories by other conspecific males (0.40 ± 0.11 intrusions per hour) than mated males (0.02 ± 0.02 intrusions per hour; t4 = 3.40, p = 0.03). Consequently, male intruders fed at a greater number of flowers on territories of unmated males (0.6 ± 0.3 flowers per hour) than on territories of mated males (0.0 ± 0.0 flowers per hour; t4 = 2.2, p = 0.09).
(d). Plant use by males and females
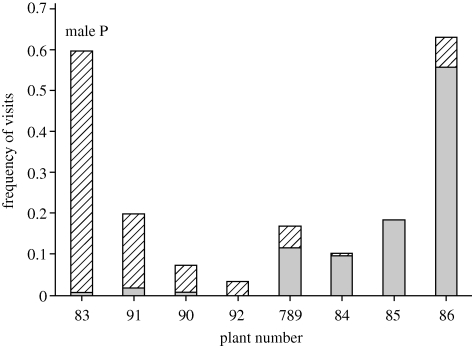
As noted above, males defended nectar-containing plants in excess of their daily needs. Our observations revealed that these males specifically maintained plants for their own use and other plants for use by intruding females, and with the exception of three unmated males having the fewest plants and bracts in their territories, territorial males and intruding females differed significantly in their feeding visits to specific plants and flowers within each territory (χ2 and exact tests, p's < 0.01; figure 4 and the electronic supplementary material). These sexual differences in selective feeding at specific floral resources resulted from non-random plant use by males and females. Visits by males and females differed from expectations based on the availability of bracts, and hence flowers (χ2-tests, p's < 0.01, and the electronic supplementary material). Consistent with these analyses, Morista's overlap in plant use between territorial males and intruding females was significantly lower for mated males (0.48 ± 0.09) than for unmated males (0.86 ± 0.09; t9 = 3.21, p = 0.011).
Figure 4.
Example of plant partitioning by a territorial male and intruding females. This graph shows the relative frequency of feeding visits by territorial male P (= M, hatched) and intruding females (= F, solid) to H. caribaea plants on his territory. On nine of the 12 male territories studied, males and females differed significantly in their visits to plants (χ2 and exact tests of independence, p's < 0.05; striped bar, M, n = 167; filled grey bar, F, n = 102).
(e). Determinants of territory quality
To assess the relative importance of factors affecting nectar standing crop per flower on the territories of the 12 males, we examined its relationship to six variables (male wing, tail and tarsus lengths; numbers of bracts on male territories; nectar losses to heterospecifics (heterospecific thefts per hour); and plant-partitioning with females (Morista's overlap)). Of these variables, numbers of bracts, tarsus length, the number of heterospecific thefts per hour, and degree of plant-partitioning (Morista's overlap) with females were significantly related to nectar standing crops (table 1). In stepwise regressions, only the number of heterospecific thefts per hour and tarsus length entered the regression as significant predictors of nectar standing crop, explaining 52 and 20 per cent of the variation among males in this measure of territory quality (table 2).
Table 1.
Standardized regression coefficients (β) from simple linear regressions of six male traits and territory characteristics against nectar standing crop for 12 territorial male purple-throated caribs.
| male character | β ± s.e. | statistic | p |
|---|---|---|---|
| number of bracts | 0.54 ± 0.24 | t1 = 2.21 | 0.052 |
| wing length | 0.37 ± 0.21 | t1 = 1.67 | 0.126 |
| tail length | 0.17 ± 0.29 | t1 = 0.59 | 0.566 |
| tarsus length | 0.56 ± 0.24 | t1 =2.33 | 0.042 |
| Morista's overlap | −0.62 ± 0.22 | t1 =− 2.79 | 0.019 |
| heterospecific theft per hour | −0.68 ± 0.21 | t1 = −3.29 | 0.008 |
Table 2.
Standardized selection gradients (β′) from stepwise multiple regression of six male traits and territory characteristics against nectar standing crop for 12 territorial male purple-throated caribs. Only variables with p < 0.05 are shown.
| male character | β′± s.e. | statistic | p |
|---|---|---|---|
| heterospecific thefts per hour | −0.58 ± 0.17 | t1 = −3.35 | 0.008 |
| tarsus length | 0.42 ± 0.17 | t1 = 2.45 | 0.037 |
4. Discussion
On the island of Dominica female purple-throated carib hummingbirds prefer to mate with males that have high standing crops of nectar in the flowers of H. caribaea in their territories (figure 3). Females clearly responded to changes in nectar standing crops on and off territories, because rates of female intrusions were highest on territories in late morning and early afternoon (figure 2), at times when nectar standing crops on undefended areas were lowest (figure 1). These high nectar standing crops depended not only on the number of bracts and flowers on a male's territory, but also upon the male's ability to partition his territory through the differential use of flowering plants for his own energy needs and for those of the females (figure 4), as well as through the prevention of nectar losses to intruders. We suggest that females initially assess the quality of males on the basis of the number of bracts in their territories and then select mates on the basis of nectar standing crops as accumulated and maintained by male defence and foraging. Such choices result from comparative evaluation of standing crops of males in a population at a given point in time (Bateson & Healy 2005).
(a). Male resource holding potential
A male's ability to maintain a high-quality territory in the form of high nectar standing crops was best explained by his prevention of nectar losses to heterospecific intruders, and to a lesser extent, large size as indexed by tarsus length (table 2). Males that were most successful at preventing nectar losses to heterospecific intruders also were more successful at preventing nectar losses to male conspecific intruders, at dominating neighbouring males, and at feeding on neighbours' territories. Nectar losses to heterospecifics is a proxy for resource holding potential (RHP; including fighting ability) and territory defence, and we cannot rule out other factors such as condition, plumage (which we did not examine), prior residency (Davies 1978; Shutler & Weatherhead 1991), or individual personality (Drent et al. 2002), all of which could affect RHP in addition to large size. Individual Heliconia plants may live for 20 years and we have observed banded male purple-throated caribs defending the same plants for as many as five years. In some cases, males continued to inspect patches in previously defended territories even during periods when the heliconias were not in flower. Strong site fidelity and possible residence effects may explain why some males defended poor-quality patches, and also why such males were more frequently absent from territories. It is possible that these males were younger individuals and simply prospecting for new territories (Amrhein et al. 2004).
Our study indicates that RHP is crucial for the quality of a male's territory and hence his mating success. Successful defence is based in part on large size, but further experimental manipulations are needed, not just of territory quality but also of male traits and behaviours (e.g. residency), to completely understand the determinants of aggressive outcomes.
(b). Nectar partitioning
Studies of nectar-feeding birds on feeding territories demonstrate that the benefit of floral defence is that it allows territorial birds to accumulate a large supply of nectar on their territories (Gill & Wolf 1975; Temeles et al. 2005). In the case of male purple-throated caribs on breeding territories, territoriality serves not only as a defence of a valuable and rich nectar resource by males for their own energy needs, but more importantly as an attractant for potential female mates. Males defended flower territories that contained two to five times the energy required to meet their daily needs and our observations indicate that the defence of excess plants was specifically for mate attraction. In nine of 12 territories, the floral resources were divided into sections that included bracts and flowers visited primarily by the territorial male or bracts and flowers that potential female mates were allowed to visit (see figure 4 and the electronic supplementary material). Such defence and partitioning of resources, which we term ‘nectar farming,’ is analogous to the activities of farmers who actively manage their crop resources to maximize productivity and success.
Territorial rufous hummingbirds Selasphorus rufus (Paton & Carpenter 1984) and other avian species (Davies & Houston 1983) use food resources on their territories non-randomly as a defence against intruders. We suggest that for some hummingbirds, such as the purple-throated carib, the same non-random foraging behaviour may be incorporated into mating behaviour and territory-partitioning in which some flowers are defended and maintained not for the use of the territorial male but rather for the use of intruding females. Wolf & Stiles (1970) also found that male fiery-throated hummingbirds (Panterpe insignis) partitioned or shared clumps of flowers on their territories with one to two females, depending on flower availability. Male hummingbirds typically do not participate in parental care (Schuchmann 1999) and these researchers viewed the nectar provided to nesting females from male territories as indirect parental assistance by males. In P. insignis, female choice presumably evolved because the higher-quality resources conferred by sharing nectar increased reproductive success (Andersson 1994).
In Dominica we did not observe the same females repeatedly visiting male territories over the breeding season, although we observed marked females regularly visiting undefended patches of H. caribaea (presumably while they were nesting and rearing young). Differences in indirect male parental assistance between purple-throated caribs and P. insignis may depend upon the quantity of floral resources. Wolf & Stiles (1970) concluded that in the case of P. insignis, female reproductive success might be limited by flower availability, and indirect assistance might allow those females, and hence their mates, to rear more young. By contrast, the greater availability of food to female purple-throated caribs may emancipate males from any indirect parental assistance, and the benefits received by females through mate choice on the basis of territory quality and male behaviour seem to be entirely genetic (Andersson 1994).
(c). Concluding remarks
A number of studies of resource defence mating systems have found that females choose males on the basis of territory quality, rather than male physical attributes. As shown here, variation in territory quality may arise through variation in male defence, foraging behaviour, and food- resource partitioning. We suggest that consideration of the breadth of male behaviours—specifically the prevention of food-resource losses to intruders and non-random foraging on the territory—may provide a better understanding of sexual selection in resource-defence mating systems by linking variation in territory quality to measurable aspects of male behaviour.
Acknowledgements
This research was reviewed and approved by the Forestry Department of the Commonwealth of Dominica, West Indies.
We thank D. Williams, A. James, V. Gowda and E. Hypolite for field assistance, S. and A. Peyer Loerner and A. Jno Baptiste for accommodation on Dominica, and R. H. Wagner and two anonymous reviewers for comments. Our research was supported by Amherst College, the Smithsonian Institution, and NSF grant DEB 0614218.
References
- Alatalo R. V., Lundberg A., Glynn C.1986Female pied flycatchers choose territory quality and not male characteristics. Nature 323, 152–153 (doi:10.1038/323152a0) [Google Scholar]
- Amrhein V., Kunc H. P., Naguib M.2004Non-territorial nightingales prospect territories during the dawn chorus. Proc. R. Soc. Lond. B 271, S167–S169 (doi:10.1098/rsbl.2003.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M.1994Sexual selection Princeton, NJ: Princeton University Press [Google Scholar]
- Bateson M., Healy S. D.2005Comparative evaluation and its implications for mate choice. Trends Ecol. Evol. 20, 559–664 [DOI] [PubMed] [Google Scholar]
- Bateson M., Healy S. D., Hurly T. A.2003Context-dependent foraging decisions in rufous hummingbirds. Proc. R. Soc. Lond. B 270, 1271–1276 (doi:10.1098/rspb.2003.2365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry F., Kress W. J.1991Heliconia: an identification guide Washington, DC: Smithsonian Institution Press [Google Scholar]
- Camfield A. F.2003Quality of food source affects female visitation and display rates of male broad-tailed hummingbirds. Condor 105, 603–606 (doi:10.1650/7118) [Google Scholar]
- Carpenter F. L., Paton D. C., Hixon M. A.1983Weight gain and adjustment of feeding territory size in migrant hummingbirds. Proc. Natl Acad. Sci. USA 80, 7259–7263 (doi:10.1073/pnas.80.23.7259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. J.2009Courtship dives of Anna's hummingbird offer insights into flight performance limits. Proc. R. Soc. B 276, 3047–3052 (doi:10.1098/rspb.2009.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. J., Dudley R.2009Flight costs of long, sexually-selected tails in hummingbirds. Proc. R. Soc. B 276, 2109–2115 (doi:10.1098/rspb.2009.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. B.1978Territory defence in the speckled wood butterfly (Parage aegeria): the resident always wins. Anim. Behav. 26, 138–147 (doi:10.1016/0003-3472(78)90013-1) [Google Scholar]
- Davies N. B., Houston A. I.1983Owners and satellites: the economics of territory defence in the pied wagtail, Motacilla alba. J. Anim. Ecol. 50, 157–180 [Google Scholar]
- Drent P. J., Van Oers K., Van Noordwijk A. J.2002Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. Lond. B 270, 45–51 (doi:10.1098/rspb.2002.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficken M. S., Rusch K. M., Taylor S. J., Powers D. R.2002Reproductive behavior and communication in blue-throated hummingbirds. Wilson Bull. 114, 197–209 (doi:10.1676/0043-5643(2002)114[0197:RBACIB]2.0.CO;2) [Google Scholar]
- Gill F. B., Wolf L. L.1975Economics of feeding territoriality in the golden-winged sunbird. Ecology 56, 333–345 (doi:10.2307/1934964) [Google Scholar]
- Hews D. K.1990Examining hypotheses generated by field measures of sexual selection on male lizards, Uta palmieri. Evolution 44, 1956–1966 (doi:10.2307/2409606) [DOI] [PubMed] [Google Scholar]
- Hurly T. A., Scott R. D., Healy S. D.2001The function of displays of male rufous hummingbirds. Condor 103, 647–651 (doi:10.1650/0010-5422(2001)103[0647:TFODOM]2.0.CO;2) [Google Scholar]
- Jones A. D., Ratterman N. L.2009Mate choice and sexual selection: what have we learned since Darwin? Proc. Natl Acad. Sci. USA 106, 10 001–10 008 (doi:10.1073/pnas.0901129106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C. D.2008The interrelationships between resource-holding potential, resource-value and reproductive success in territorial males: how much variation can we explain? Behav. Ecol. Sociobiol. 62, 855–871 (doi:10.1007/s00265-007-0518-8) [Google Scholar]
- Paton D. C., Carpenter F. L.1984Peripheral foraging by territorial rufous hummingbirds: defense by exploitation. Ecology 65, 1808–1819 (doi:10.2307/1937777) [Google Scholar]
- Powers D. R.1987Effects of variation in food quality on the breeding territoriality of the male Anna's hummingbird. Condor 89, 103–111 (doi:10.2307/1368763) [Google Scholar]
- Powers D. R., McKee T.1994The effect of food availability on time and energy expenditures of territorial and non-territorial hummingbirds. Condor 96, 1064–1075 (doi:10.2307/1369115) [Google Scholar]
- Rice W. R.1989Analyzing tables of statistical tests. Evolution 43, 223–225 (doi:10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- Schuchmann L.1999Family Trochilidae (hummingbirds). In Handbook of birds of the world, vol. 5 (eds del Hoyo J., Elliott A., Sargatal J.), pp. 468–680 Barcelona, Spain: Lynx Edicions [Google Scholar]
- Schuchmann K. -L., Schuchmann-Wegert G.1984Notes on the displays and mounting behaviour in the purple-throated carib hummingbird. Bonn. Zool. Beitr. 35, 327–334 [Google Scholar]
- Shutler D., Weatherhead P. J.1991Owner and floater red-winged blackbirds: determinants of status. Behav. Ecol. Sociobiol. 28, 235–241 [Google Scholar]
- Smith E. P., Zaret T. M.1982Bias in estimating niche overlap. Ecology 63, 1248–1253 (doi:10.2307/1938851) [Google Scholar]
- Temeles E. J., Kress W. J.2003Adaptation in a plant-hummingbird association. Science 300, 630–633 (doi:10.1126/science.1080003) [DOI] [PubMed] [Google Scholar]
- Temeles E. J., Pan I. L., Brennan J. L., Horwitt J. N.2000Evidence for ecological causation of sexual dimorphism in a hummingbird. Science 289, 441–443 (doi:10.1126/science.289.5478.441) [DOI] [PubMed] [Google Scholar]
- Temeles E. J., Goldman R. S., Kudla A. U.2005Foraging and territory economics of sexually-dimorphic Purple-throated caribs, Eulampis jugularis, at three heliconias. Auk 122, 187–204 (doi:10.1642/0004-8038(2005)122[0187:FATEOS]2.0.CO;2) [Google Scholar]
- Temeles E. J., Koulouris C. R., Sander S. E., Kress W. J.2009Effect of flower shape and size on foraging performance and trade-offs in a tropical hummingbird. Ecology 90, 1147–1161 (doi:10.1890/08-0695.1) [DOI] [PubMed] [Google Scholar]
- Wolf L. L.1975‘Prostitution’ behavior in a tropical hummingbird. Condor 77, 140–144 (doi:10.2307/1365783) [Google Scholar]
- Wolf L. L., Hainsworth F. R.1971Time and energy budgets of territorial hummingbirds. Ecology 52, 980–988 (doi:10.2307/1933803) [Google Scholar]
- Wolf L. L., Stiles F. G.1970Evolution of pair cooperation in a tropical hummingbird. Evolution 24, 759–773 (doi:10.2307/2406556) [DOI] [PubMed] [Google Scholar]
- Wolf L. L., Hainsworth F. R., Stiles F. G.1972Energetics of foraging: rate and efficiency of nectar extraction by hummingbirds. Science 176, 1351–1352 (doi:10.1126/science.176.4041.1351) [DOI] [PubMed] [Google Scholar]