Abstract
The differential allocation hypothesis assumes that animals should weigh costs and benefits of investing into reproduction with a current mate against the expected quality of future mates, and predicts that they should invest more into reproduction when pairing with a high-quality mate. In the broad-nosed pipefish (Syngnathus typhle), males care for the embryos in a brood pouch and females compete for access to male mating partners. Both sexes prefer mating with large partners. In the present study, we show that the same female provides both large and small mating partners with eggs of similar size, weight and lipid content when mated to two males in succession. Importantly, however, eggs provided to small males (less preferred) had higher egg protein content (11% more) than those provided to large males (preferred). Thus, contrary to the differential allocation hypothesis, eggs did not contain more resources when females mated with a larger male. Instead, the pattern observed in our results is consistent with a compensatory reproductive strategy.
Keywords: Syngnathidae, sex-role reversal, paternal care, female choice, maternal effects, reproductive compensation
1. Introduction
Life-history theory predicts that parents should assess the value of their current brood in relation to other possible investments (e.g. their own growth or survival), and increase their investment in the current brood only when marginal benefits are higher than those of the alternatives (Williams 1966; Roff 1992; Stearns 1992). Generally, reproduction is costly for females and a large share of a female's fitness value may depend on the quality of the male partner (Trivers 1972; Vincent et al. 1994). Thus, female mate choice aims to discriminate in favour of good-quality partners in order to maximize fitness returns (Kokko & Johnstone 2002). Mate choice benefits are central to the evolution of mating preferences. However, experimental studies of mate choice often suffer from confounding effects arising from the differential allocation hypothesis (Burley 1988; Sheldon 2000) because individual flexibility in reproductive allocation in response to mate quality is not taken into account.
According to the differential allocation hypothesis, individuals that mate more than once should weigh the costs and benefits of investing in reproduction with their current partner against the potential reproductive investment for mates in the future. Specifically, differential allocation can generate a parental effect through which individuals regulate their reproductive investment by weighing the attractiveness of their current mate (Loyau et al. 2007) against the probability of obtaining a better quality partner in the future (Burley 1988; Sheldon 2000). Individuals should therefore allocate more resources to reproduction when breeding with a mate whose quality is higher, and allocate less resources, or simply skip reproduction altogether, when their mate is of lower quality. The extra resources can be allocated to increased egg size (Kolm 2001), egg number (Locatello & Neat 2005), more carotenoids (Surai et al. 2001), antibodies (Saino et al. 2003) or hormones (Schwabl et al. 1997; McCormick 1999; Lovern & Wade 2001).
By contrast, rather than positive differential allocation strategies, reproductive compensatory strategies have been described in some systems, encompassing a wide range of species (house mouse: Drickamer et al. 2000; Gowaty et al. 2003; mallard: Bluhm & Gowaty 2004a; Drosophila pseudoobscura: Anderson et al. 2007). The reproductive compensation hypothesis (Gowaty et al. 2007; Gowaty 2008), states that when individuals are constrained in their mate choice and mate with less-preferred partners, they should attempt to make up for lowered offspring viability (Bluhm & Gowaty 2004b) via induced parental physiology or behaviour or via increased fecundity (Bluhm & Gowaty 2004a; Michl et al. 2005; Navara et al. 2006). Thus, an adaptive flexibility is predicted that allows constrained individuals to increase their reproductive allocation into the offspring in order to make their current reproductive bout more successful than if they had not compensated (Gowaty et al. 2007; Gowaty 2008). Possible constraints to mate choice may be environmental, such as risk of predation (Booksmythe et al. 2008) and limitations to dispersal (Byers et al. 2005) or mate availability, or they may be social, such as in intra-sexual competition (Villanelli & Gherardi 1998) and sexual conflict (Shine et al. 2004). Although both theories have been in the literature for at least a decade, only recently has the first attempt to model under which conditions each allocation strategy should be optimal (Harris & Uller 2009), and careful consideration of the prerequisites necessary for reproductive compensation to occur (Gowaty 2008; Bolund et al. 2009) been published.
Despite the huge diversity of mating systems and reproductive strategies among fishes, few studies have investigated parental effects resulting from mate choice in this animal group. Nevertheless, in the blenny Aidablennius sphinx, females spawn more eggs and faster when paired with large partners compared with females mating with small males (Locatello & Neat 2005). Also, manipulation of egg size and clutch weight has been observed in the Banggai cardinalfish Pterapogon kauderni in response to large partner size (Kolm 2001). Both cases are in line with the differential allocation hypothesis.
Syngnathus typhle, the broad-nosed pipefish, is a polygynandrous marine fish (Berglund et al. 1988, 1989; Jones et al. 1999). During copulation, a female transfers her eggs into the pouch of a male by inserting her ovipositor in the opening on the top part of the pouch. There is a great variation in the number of eggs that a female transfers into a male's pouch, owing to the female's own decision to transfer (or the male's decision to accept) more or less eggs. Also, the movements and alignment between the couple when mating may influence the transfer of eggs. As a result, it is common to take several attempts before any eggs are transferred, and even when successful, only a limited number of eggs are transferred in each attempt. Parental care is provided solely by the males during embryonic development in vascularized brood pouches, providing the embryos with oxygen, osmoregulation and nutrients (Quast & Howe 1980; Haresign & Shumway 1981; Berglund et al. 1986b; Ripley & Foran 2009; C. Kvarnemo, K. B. Mobley, C. Patridge, A. G. Jones & I. Ahnesjö 2003–2004, unpublished data), for about a month. Thus, there are ample opportunities for phenotypic differences in quality of care to arise, which in turn may provide females with an incentive to adjust their reproductive allocations in relation to a mate's predicted paternal quality.
To date, it is not clear whether larger males actually contribute more than smaller males to the developing embryos in pipefish. It is known that large males produce larger offspring (Ahnesjö 1992a), but it is still unknown whether: (i) this is owing to large males attracting larger females, which produce larger eggs (Berglund et al. 1986a); (ii) this is owing to large males providing better care compared with smaller males; (iii) it is generated or reinforced by females investing more into their eggs when mating with large males; or (iv) this is owing to a combination of any or all of these processes. Alternatively, quality of paternal care may be unrelated to male size and females may choose partners on characteristics other than parental quality (Watanabe & Watanabe 2002).
Given the possibilities explained above, three predictions can be made: (A) in accordance with the differential allocation hypothesis, females should invest more in each offspring when mating with larger males if by mating with such males they gain direct or indirect fitness benefits; (B) in accordance with the reproductive compensation hypothesis, females should increase their investment per offspring when mating with smaller partners compared with when mating with larger ones, if small males provide substantially poorer quality care, incurring high costs on female fitness; and (C) no differences in female investment should be predicted if females are selecting partners based on characteristics unrelated to quality of parental care.
Here, we investigate whether females distribute their reproductive resources, in response to partner size, following a pattern consistent with either the differential allocation hypothesis or the reproductive compensation hypothesis or neither. To our knowledge, no previous studies have focused on how nutrients (lipids and proteins) of fish eggs may be distributed by females in response to mate quality. We measured egg diameter and dry weight, lipid and protein content of the eggs that females transferred into the brood pouches of small and large males. Since there is substantial variance in egg size between females, we used a paired design, allowing each individual female to mate both with a large and a small male in succession.
2. Material and methods
The study was carried out at Kristineberg Marine Research Station (58°15′ N, 11°28′ E), in Sweden, between May and July 2006, during S. typhle's breeding season. Specimens were caught with a beam trawl (4 mm mesh size), pulled behind a boat, in eelgrass (Zostera marina) meadows in waters (1–6 m) near the research station.
All fish were initially held in large storage barrels (225 l), and separated by sex and size. Individuals were kept at densities of around 60 individuals per barrel. Both barrels and experimental aquaria were provided with artificial eelgrass and a flow-through system of continuously renewed natural sea water. Light regimes approximated natural conditions (L : D 16 : 8), and both air and water temperatures were maintained at 14°C. Fish were fed twice daily with cultured newly hatched and adult Artemia, and their diet was supplemented with wild-caught mysid shrimp (Mysidae), common shrimp (Crangon crangon) and copepod spp. collected from nearby waters.
Relatively large females were chosen for this experiment (standard length: mean ± s.e.: 214.8 ± 3.6 mm, n = 29). Each female mated with one smaller male (S male; range 135–164 mm, mean ± s.e.: 151.3 ± 1.4 mm, n = 29) and one larger male (L male; range 169–236 mm, mean ± s.e.: 194.8 ± 3.4 mm, n = 29). Females were only allowed to mate with one male at a time and the order in which the two males were provided to the females was alternately assigned (treatment: mate order).
At the beginning of each trial, the female was placed in a net bag (8 l) in the same aquarium as the male, thus enabling contact while preventing copulation, for a period of about 12 h. Females were kept in the net overnight and released into the aquarium the following morning. Pairs were allowed to mate until the male carried a minimum of 20 eggs. The first male was then removed while the female remained in the experimental tank until the evening. She was then placed in the net again and the second male, of a different size, was introduced to the tank, following the same procedure as in the first mating.
After a male was successfully mated and removed from the experimental tank, he was anaesthetized with 2-phenoxyethanol (60 μl l−1 sea water). The eggs were gently removed from the pouch using a stainless steel spatula. This was always done within 4 h of mating. The removed eggs were randomly divided into three subsets for analysis; egg diameter and dry weight, egg soluble protein content and egg lipid content. After eggs were removed, males were moved to a container with fresh sea water to recover from the anaesthetic. All males and females were returned to the sea upon completion of trials.
(a). Data collection
(i). Mating time
Time, measured in days, was recorded between the time a female was released from the net bag and the time when the male carried enough eggs (more than 20) for the analysis. The minimum time registered was 1 day, referring to the cases when males were introduced early in the morning and had mated by late afternoon.
(ii). Egg diameter
Three eggs were gently separated from each other with a spatula and their diameters were measured using a graticule in a magnifying lens (×60) and standardized using a micrometer, and the average diameter (mm) was calculated for each male.
(iii). Dry weight
Between seven and 29 eggs from each male were carefully counted and dried in a heating cupboard at 60°C (minimum time more than one week). All egg samples were weighed two to three times on a Sartorius microbalance (LE26P) to the nearest 0.002 mg, and the average weight per egg was calculated for each male.
(iv). Protein extraction
Samples of three eggs from each male brood were preserved in ethanol and later dissolved in NaOH (0.5 M) to extract and quantify the amount of soluble protein per egg using the Bradford technique (Bradford 1976). Assays were performed following the instructions of Protein Assay kit II from Bio-Rad (Sundbyberg, Sweden). Subsamples were diluted (1/20) and transferred into 96-well tissue culture plates. Soluble protein content (nanograms per egg) was measured using a Labsystems iEMS reader spectrophotometer and Transmit Software Revision 4.2 (595 nm) and standardized to known concentrations of bovine serum albumin.
(v). Lipid extraction
Using the same samples as for the dry weight measurements, lipid extractions were performed using petroleum ether. The samples were placed in individual 20 ml glass containers and immersed in 15 ml of petroleum ether for 12 h. Samples were subsequently removed and left to dry for 2 h in a fume cabinet to allow for complete evaporation of the petroleum ether. Eggs were then returned to the drying oven at 60°C for another 12 h (Lissåker et al. 2003). All samples were reweighed and the weight difference divided by the number of eggs in the sample was used as an estimate of lipid content per egg (μg).
(vi). Length and body depth measurements
The standard lengths of all adult fish were measured to the nearest millimetre on a measuring board. Body depth was measured at the thickest part of the female's trunk with the use of callipers to the nearest 0.1 mm.
(b). Statistical analysis
SPSS 15.0 (SPSS Inc., Chicago, IL, USA) for Windows was used to perform the statistical analyses. Wilcoxon signed-rank tests were performed on the time dataset. Egg protein content was log-transformed to conform to linearity assumptions (Sokal & Rohlf 1981).
We used a repeated-measures ANOVA model to analyse the effect of male size (within-subjects variable) and mate order (between-subjects treatment) on egg diameter, weight, protein and lipid content. Neither female length nor body depth significantly affected the results when introduced as covariates, so they were removed from these models. Effect sizes with confidence limits were calculated (according to Nakagawa & Cuthill 2007) to assess the strength of the effect of male size on all measured egg variables.
3. Results
Twenty-nine females mated both with a large and a small male. Of these, 15 females were mated with a smaller male followed by a large male (treatment A), and 14 females were mated with a larger male followed by a small male (treatment B).
Females took similar numbers of days to mate with each male, irrespective of male size (Wilcoxon signed-rank test: Z = −0.91, p = 0.36, S males: 2.9 ± 0.3 days, L males: 3.3 ± 0.3 days) and of mate order (Z = −1.27, p = 0.20, first mates: 2.9 ± 0.3 days, second mates: 3.4 ± 0.3 days).
Egg dry weight was significantly and positively correlated to both egg diameter and egg protein content. Egg lipid content was not correlated to any of the other egg characteristics measured (table 1).
Table 1.
Correlation matrix (n = 29) and partial correlation coefficients (keeping the remaining two variables constant, d.f. = 25) for egg diameter (mm), egg dry weight (mg), egg protein content (ng) and egg lipid content (μg). Significance indicated by *p < 0.05, ***p < 0.001.
| partial correlations |
||||
|---|---|---|---|---|
| diameter (r) | dry weight (r) | protein (r) | lipids (r) | |
| Pearson correlations | ||||
| diameter | — | 0.444* | 0.016 | 0.091 |
| dry weight | 0.630*** | — | 0.653*** | 0.240 |
| protein | 0.464* | 0.734*** | — | −0.152 |
| lipids | 0.257 | 0.303 | 0.125 | — |
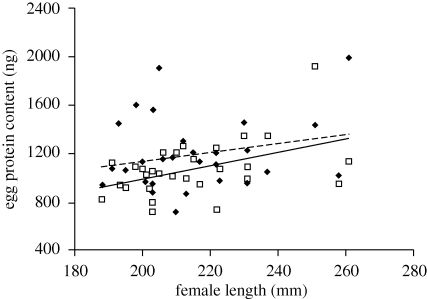
When assessing the average values from both male sizes for each female, female length was significantly and positively correlated to mean egg diameter (Pearson's correlation: r = 0.46, n = 29, p = 0.01), mean egg dry weight (r = 0.42, n = 29, p = 0.02) and to mean egg protein content (r = 0.43, n = 29, p = 0.02; figure 1), but not to mean lipid content of eggs (r = 0.10, n = 29, p = 0.61). By contrast, female body depth was significantly correlated to mean egg lipid content (Pearson's correlation: r = 0.45, n = 29, p = 0.01), but to none of the other egg characteristics (Pearson's correlation: egg diameter: r = −0.21, n = 29, p = 0.28; egg dry weight: r = 0.12, n = 29, p = 0.54; egg protein content: r = −0.01, n = 29, p = 0.96).
Figure 1.
Average protein content (nanograms per egg) of eggs provided to smaller (filled diamonds, semidotted line) and larger males (open squares, continuous line). Protein content of eggs is significantly and positively correlated to female length. n = 58.
Mate order had no effect on any of the egg characteristics measured (table 2). Similarly, there were no significant second-order interactions between mate order and male size, i.e. female investment when mating with small or large males did not differ depending on whether these were the female's first or second partners (table 2).
Table 2.
Effects of male size (small or large) and treatment (mate order) on egg diameter, egg weight, egg protein content and on egg lipid content, analysed using repeated-measures ANOVA. d.f. = 1, 25. p-values in bold indicate significance.
| variable | source of variation | F | p |
|---|---|---|---|
| egg diameter | male size | <0.01 | 0.98 |
| treatment | 0.22 | 0.65 | |
| male size * treatment | 0.54 | 0.47 | |
| egg dry weight | male size | 0.04 | 0.56 |
| treatment | 0.14 | 0.71 | |
| male size * treatment | 1.91 | 0.18 | |
| egg protein content | male size | 4.22 | 0.050 |
| treatment | 0.94 | 0.34 | |
| male size * treatment | 0.94 | 0.27 | |
| egg lipid content | male size | 0.08 | 0.78 |
| treatment | 1.36 | 0.26 | |
| male size * treatment | 2.4 | 0.13 |
Male body size had no effect on egg diameter (mean ± s.e., S males: 1.88 ± 0.02 mm, L males: 1.88 ± 0.02 mm, effect size (ES): 0, lower and upper confidence interval (CI): −0.40; 0.40) or on egg dry weight (mean ± s.e., S males: 1.337 ± 0.031 mg, L males: 1.324 ± 0.028 mg, ES: 0.08, CI: −0.19; 0.35; table 2). However, in terms of nutritional investment, the eggs transferred to small males had significantly greater amounts of proteins per egg (mean ± s.e., S males: 1193.38 ± 55.14 ng, L males: 1076.07 ± 42.36 ng, ES: 0.45, CI: 0.01; 0.88), but not lipids (mean ± s.e., S males: 0.050 ± 0.003 μg, L males: 0.054 ± 0.005 μg, ES: 0.21, CI: −0.18; 0.60), than eggs transferred to large males (table 2). Egg protein content was, on average, 11 per cent higher in eggs given to small males than in eggs given to larger males (figure 1).
4. Discussion
This study clearly shows that broad-nosed pipefish (S. typhle) females do not increase their investment into reproduction with larger males over smaller ones, in terms of resources provided to the eggs. Thus, we find no evidence that females exhibit allocation patterns with respect to male size that are consistent with the differential allocation hypothesis (Burley 1988; Sheldon 2000), thereby ruling out prediction A (§1; which states that females should invest more per offspring when mating with large males if by doing so they gain substantial fitness benefits). Instead, we found evidence that when females were mated to small males, the eggs transferred to those males contained more proteins than the eggs transferred when females mated with large males. This lends support to prediction B, which states that females should increase their allocation to each offspring when mating with smaller partners compared with larger ones, in order to compensate for possible lower quality care. This result excludes prediction C, which states that no differences in egg quality should be observed in response to male size if size is not an indicator of paternal quality.
Mating order affected neither female willingness to mate nor any of the egg parameters investigated. Similarly, latency to mate, egg diameter and dry weight were not affected by male size. The small variation in egg diameter and egg dry weight, both within and between treatments, suggests that females may lack flexibility in short-term control of egg size and weight.
Proteins and lipids are the most important constituents of fish eggs (Brooks et al. 1997). Although exact amounts of proteins and lipids vary both within and between species, in general, around 60 per cent of egg dry weight is made up of proteins, while lipids usually account for about 20–50%. Egg lipids are used mostly in the production of cell membranes and as sources of energy for developing embryos. Since fat has the potential to affect body depth more than length, the positive correlation that we found between lipid allocation and body depth suggests that lipid content in the eggs may depend on the female's current fatness. Interestingly, broad-nosed pipefish females have an ornament that forms stripes across the body that accentuate body depth (Berglund 2000), possibly signalling, when displayed towards males, an ability to produce eggs with a high lipid content. Expanded female trunks and stripy patterns across the trunk are also common in other species of pipefishes such as Kaupus costatus, Leptonotus blainvilleanus, Leptonotus norae and Mitotichthys semistriatus (Dawson 1985).
Pipefishes of the genus Syngnathus are known to produce eggs continuously and asynchronously (Begovac & Wallace 1987, 1988), and are able to spawn multiple times and with multiple partners within short periods of time (e.g. 48 h; Berglund et al. 1988). In relation to the physiology of developing eggs, as far as we are aware, very little is known, and therefore we cannot explain why the observed difference in the current study was on egg protein content rather than on egg size or lipid content. However, a recent study on Syngnathus floridae and Syngnathus fuscus (Ripley & Foran 2009) shows that males transfer proteins, but not lipids, to the embryos during development, suggesting that all lipids have to be supplied by the female.
Our results show that the protein content of the eggs was significantly higher when females were mated to small males, which is consistent with a compensatory pattern. Mean protein content was significantly and positively correlated to female length, suggesting that protein allocation may be partially dependent on the intrinsic quality of the female.
Egg proteins are used in two main contexts: as a source of amino acids for tissue growth, and energy through catabolism (Heming & Buddington 1988). Therefore, egg proteins can have important effects upon offspring survival and development (Brooks et al. 1997), and increased protein concentrations are likely to improve embryonic development (Satia et al. 1974; Brooks et al. 1997). Also, in pipefishes, despite being provided in small amounts, proteins are very important to the development of embryos (Haresign & Shumway 1981). As mentioned, in this genus males have the potential to supply proteins to developing offspring (S. fuscus and S. floridae: Ripley & Foran 2009; S. typhle: C. Kvarnemo, K. B. Mobley, C. Partridge, A. G. Jones & I. Ahnesjö 2003–2004, unpublished data). Thus, more proteins in the eggs may have the potential to improve offspring viability in a compensatory way, in particular if smaller males have a lower capacity to supply proteins to developing offspring compared with larger males. In the present study, egg protein content was, on average, 11 per cent higher in eggs given to smaller males than in eggs given to larger males. This result strongly suggests that broad-nosed pipefish females are able to adjust their reproductive investment within a very short time frame (24 h) and allocate proteins to eggs in response to male size in a pattern expected by the reproductive compensation hypothesis.
There is at least one biological reason that may explain why broad-nosed pipefish females would allocate more proteins into the eggs provided to smaller males. Females have a higher potential reproductive rate than males, that is, on average, they produce eggs faster than males can brood them, a situation which becomes more pronounced with increasing female size (Berglund et al. 1989). Consequently, female reproductive rate is limited by that of males (Berglund et al. 1989; Vincent et al. 1994). Limited male availability leads to competition among females for access to males, resulting in sex-role reversal in this population (Berglund & Rosenqvist 1993; Vincent et al. 1994). Also, sexual selection acts on body length, with both sexes showing a strong preference for large partners over smaller ones (Berglund et al. 1986a; Berglund & Rosenqvist 2003). Furthermore, since large males mature earlier in the season than small males (Berglund & Rosenqvist 1990), the males available to mate with will be of smaller size as the breeding season progresses. Thus, owing to this and to the female-biased operational sex ratio that results from the higher potential reproductive rates of females than males (Berglund & Rosenqvist 1990; Vincent et al. 1994), individual females are often likely to face a constraint in their mate choice. Moreover, females may not be able to forego reproduction if they do not find a suitable and available partner because they do not seem to be able to resorb their eggs once matured (I. Braga Goncalves, K. B. Mobley, I. Ahnesjö, G. Sagebakken, A. G. Jones & C. Kvarnemo 2006, unpublished data). Indeed, a field study has found that both large and small females mate with both large and small males in nature (K. B. Mobley 2005, unpublished data) and, nevertheless, in the field, small males give birth to smaller offspring compared with large males (Ahnesjö 1992b). Furthermore, an experimental study, also conducted on the same population, has shown that mating females with non-preferred partners (character under selection not known by the researchers) results in offspring viability deficits (Sandvik et al. 2000). Consequently, females mate with preferred (large) males when available, but since males are in short supply, females should invest in any available males, regardless of quality. Taken together, this may explain the pattern observed in egg protein content in the current study, as females might be compensating for poor male quality by allocating more proteins into the eggs when mating with non-preferred (smaller) males compared with when they mate with higher quality (large) ones. By doing so, these females would be expected to increase their offspring viability to be closer to that of offspring from matings with preferred partners (Gowaty et al. 2007). A study with a similar setup as the one presented here has shown that, after a brooding period of 18 days, brood reduction and embryo length were similar in broods of small and large males (I. Braga Goncalves, K. B. Mobley, I. Ahnesjö, G. Sagebakken, A. G. Jones & C. Kvarnemo 2006, unpublished data). In other words, the higher protein content of eggs brooded by smaller males did not result in larger or more viable offspring. This result, coupled with the observations that large males give birth to larger offspring than small males even though both small and large females mate with both small and large males in the wild, leads us to suggest that reproductive compensation being carried out in this population is more likely than differential allocation, albeit it might be incomplete compensation as has been observed in several other species (reviewed in Gowaty et al. 2007).
It can be argued for broad-nosed pipefish males that smaller individuals may be less willing to invest into the current reproductive situation if future reproductive opportunities have higher fitness pay-offs (Magnhagen 1990). A similar scenario has been described for collared flycatcher males (Michl et al. 2005), where females laid higher yolk testosterone concentrations in eggs when mated with young rather than older males. In the broad-nosed pipefish, if females compensate for small males' lower parental quality, selection may act on small males to reduce parental investment and instead canalize resources into their own growth. In males, the number of newborns is positively related to body size as larger males have larger and longer brood pouches, which can accommodate more eggs (Berglund et al. 1986a; Berglund & Rosenqvist 1990).
According to Harris & Uller (2009), differential allocation is the most common optimal strategy for females when compared with reproductive compensation. Reproductive compensation was predicted by their model to be optimal only under restricted conditions, which include: when the relative impact of parental investment on the offspring is low, i.e. offspring of high-quality males benefit less from female differential allocation compared with the costs incurred to the females from the added investment; when the expected future partner quality is low; and when females cannot forego reproduction. In addition, Bolund et al. (2009) argued that a low reproductive skew is further necessary for reproductive compensation to become stable and optimal. Female broad-nosed pipefish seem to fit most requirements necessary for reproductive compensation to be the optimal reproductive strategy. As mentioned above, females are not able to forego reproduction without incurring energetic costs since they do not seem to be able to resorb their eggs if unable to mate (Berglund & Rosenqvist 1990; I. Braga Goncalves, K. B. Mobley, I. Ahnesjö, G. Sagebakken, A. G. Jones & C. Kvarnemo 2006, unpublished data). Also, egg size is relatively large in this species so that increasing reproductive investment when mating with high-quality partners may bear little benefit to the offspring compared with the costs incurred by the females, and given the strong competition for males among females, combined with smaller males maturing later, expected future partner quality should be relative low in our study population (Berglund & Rosenqvist 1993; Vincent et al. 1994). However, reproductive skew in the population is thought to be high given the intense female–female competition and unequal potential reproductive rates of males and females, and thus, is not in accordance with the argument posed by Bolund et al. (2009). Instead, we argue again that the lack of long-term pair bonds in this species (Vincent et al. 1995) and the intensity of competition for mates, together with the already large female reproductive investment (large nutritious eggs), make it more likely for reproductive compensation rather than differential allocation to occur.
In conclusion, in this experimental study of the sex-role reversed pipefish S. typhle, we found that eggs which females transfer to small males contain more proteins than eggs the same females transfer to large males, contrary to what might be expected based on the differential allocation hypothesis. Instead, the pattern observed in our data is consistent with the reproductive compensation hypothesis (Gowaty et al. 2007; Gowaty 2008), as a response to male partner size. Whether smaller males provide lower quality care to the offspring or whether they provide fewer indirect fitness benefits to the females remains to be explored.
Acknowledgements
Ethical approval was given by the Swedish Animal Welfare Agency (diarie nr. 196-2005).
We thank L. Mendoza for her assistance in the field and A. Berglund and G. Rosenqvist for support and equipment. We are also grateful to J. Wright, M. Andersson, A. F. Russell, N. Kolm and J. Pienaar for valuable discussions. We are very thankful for the facilities and support provided at Kristineberg Marine Research Station, Gothenburg University. This study was supported by grants from Fundação para a Ciência e Tecnologia - Portugal (to I.B.G.), the Inez Johansson's foundation and the Royal Swedish Academy of Sciences (to I.A.), the National Science Foundation (to K.B.M. and A.G.J.), Gothenburg Marine Research Center (to I.B.G., G.S. and C.K.) and the Swedish Research Council (to C.K.).
References
- Ahnesjö I.1992aConsequences of male brood care: weight and number of newborn in a sex-role reversed pipefish. Funct. Ecol. 6, 274–281 (doi:10.2307/2389517) [Google Scholar]
- Ahnesjö I.1992bFewer newborn result in superior juveniles in the paternally brooding pipefish Syngnathus typhle L. J. Fish Biol. 41, 53–63 (doi:10.1111/j.1095-8649.1992.tb03868.x) [Google Scholar]
- Anderson W., Kim Y.-K., Gowaty P.2007Experimental constraints on female and male mate preferences in Drosophila pseudoobscura decrease offspring viability and reproductive success of breeding pairs. Proc. Natl Acad. Sci. USA 104, 4484–4488 (doi:10.1073/pnas.0611152104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begovac P. C., Wallace R. A.1987Ovary of the pipefish Syngnathus scovelli. J. Morphol. 193, 117–133 (doi:10.1002/jmor.1051930202) [DOI] [PubMed] [Google Scholar]
- Begovac P. C., Wallace R. A.1988Stages of oocyte development in the pipefish, Syngnathus scovelli. J. Morphol. 197, 353–369 (doi:10.1002/jmor.1051970309) [DOI] [PubMed] [Google Scholar]
- Berglund A.2000Sex role reversal in a pipefish: female ornaments as amplifying handicaps. Ann. Zool. Fennici 37, 1–13 [Google Scholar]
- Berglund A., Rosenqvist G.1990Male limitation of female reproductive success in a pipefish: effects of body-size differences. Behav. Ecol. Sociobiol. 27, 129–133 (doi:10.1007/BF00168456) [Google Scholar]
- Berglund A., Rosenqvist G.1993Selective males and ardent females in pipefishes. Behav. Ecol. Sociobiol. 32, 331–336 [Google Scholar]
- Berglund A., Rosenqvist G.2003Sex role reversal in pipefish. Adv. Study Behav. 32, 131–167 (doi:10.1016/S0065-3454(03)01003-9) [Google Scholar]
- Berglund A., Rosenqvist G., Svensson I.1986aMate choice, fecundity and sexual dimorphism in two pipefish species (Syngnathidae). Behav. Ecol. Sociobiol. 19, 301–307 (doi:10.1007/BF00300646) [Google Scholar]
- Berglund A., Rosenqvist G., Svensson I.1986bReversed sex-roles and parental energy investment in zygotes of two pipefish (Syngnathidae) species. Mar. Ecol. Prog. Ser. 29, 209–215 (doi:10.3354/meps029209) [Google Scholar]
- Berglund A., Rosenqvist G., Svensson I.1988Multiple matings and paternal brood care in the pipefish Syngnathus typhle. Oikos 51, 184–188 (doi:10.2307/3565641) [Google Scholar]
- Berglund A., Rosenqvist G., Svensson I.1989Reproductive success of females limited by males in two pipefish species. Am. Nat. 133, 506–516 (doi:10.1086/284932) [Google Scholar]
- Bluhm C. K., Gowaty P. A.2004aReproductive compensation for offspring viability deficits by female mallards, Anas platyrhynchos. Anim. Behav. 68, 985–992 (doi:10.1016/j.anbehav.2004.01.012) [Google Scholar]
- Bluhm C. K., Gowaty P. A.2004bSocial constraints on female mate preferences in mallards, Anas platyrhynchos, decrease offspring viability and mother productivity. Anim. Behav. 68, 977–983 (doi:10.1016/j.anbehav.2004.01.013) [Google Scholar]
- Bolund E., Schielzeth H., Forstmeier W.2009Compensatory investment in zebra finches: females lay larger eggs when paired to sexually unattractive males. Proc. R. Soc. B 276, 707–715 (doi:10.1098/rspb.2008.1251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booksmythe I., Detto T., Backwell P. R. Y.2008Female fiddler crabs settle for less: the travel costs of mate choice. Anim. Behav. 76, 1775–1781 (doi:10.1016/j.anbehav.2008.07.022) [Google Scholar]
- Bradford M. M.1976A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 72, 248–254 (doi:10.1016/0003-2697(76)90527-3) [DOI] [PubMed] [Google Scholar]
- Brooks S., Tyler C. R., Sumpter J. P.1997Quality in fish: what makes a good egg? Rev. Fish. Biol. Fish. 7, 387–416 (doi:10.1023/A:1018400130692) [Google Scholar]
- Burley N.1988The differential-allocation hypothesis: an experimental test. Am. Nat. 132, 611–628 (doi:10.1086/284877) [Google Scholar]
- Byers J. A., Wiseman P. A., Jones L., Roffe T. J.2005A large cost of female mate sampling in Pronghorn. Am. Nat. 166, 661–668 (doi:10.1086/497401) [DOI] [PubMed] [Google Scholar]
- Dawson C. E.1985Indo-Pacific pipefishes (Red Sea to the Americas) Ocean Springs, MS: The Gulf Coast Research Laboratory [Google Scholar]
- Drickamer L. C., Gowaty P. A., Holmes C. M.2000Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim. Behav. 59, 371–378 (doi:10.1006/anbe.1999.1316) [DOI] [PubMed] [Google Scholar]
- Gowaty P.2008Reproductive compensation. J. Evol. Biol. 21, 1189–1200 (doi:10.1111/j.1420-9101.2008.01559.x) [DOI] [PubMed] [Google Scholar]
- Gowaty P. A., Drickamer L. C., Schmid-Holmes S.2003Male house mice produce fewer offspring with lower viability and poorer performance when mated with females they do not prefer. Anim. Behav. 65, 95–103 (doi:10.1006/anbe.2002.2026) [Google Scholar]
- Gowaty P. A., Anderson W. W., Bluhm C. K., Drickamer L. C., Kim Y. K., Moore A. J.2007The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc. Natl Acad. Sci. USA 104, 15 023–15 027 (doi:10.1073/pnas.0706622104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haresign T., Shumway S.1981Permeability of the marsupium of the pipefish, Syngnathus fuscus to (14C)-alpha amino isobutyris acid. Comp. Biochem. Phys. 69(A), 603–604 [Google Scholar]
- Harris W. E., Uller T.2009Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Phil. Trans. R. Soc. B 364, 1039–1048 (doi:10.1098/rstb.2008.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heming T. A., Buddington R. K.1988Yolk absorption in embryonic and larval fishes. In Fish physiology. The physiology of developing fish (eds Hoar W., Randall D.), pp. 407–446 San Diego, CA: Academic Press [Google Scholar]
- Jones A. G., Rosenqvist G., Berglund A., Avise J. C.1999The genetic mating system of a sex-role-reversed pipefish (Syngnathus typhle): a molecular inquiry. Behav. Ecol. Sociobiol. 46, 357–365 (doi:10.1007/s002650050630) [Google Scholar]
- Kokko H., Johnstone R. A.2002Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Phil. Trans. R. Soc. Lond. B 357, 319–330 (doi:10.1098/rstb.2001.0926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolm N.2001Females produce larger eggs for large males in a paternal mouthbrooding fish. Proc. R. Soc. Lond. B 268, 2229–2234 (doi:10.1098/rspb.2001.1792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissåker M., Kvarnemo C., Svensson O.2003Effects of a low oxygen environment on parental effort and filial cannibalism in the male sand goby, Pomatoschistus minutus. Behav. Ecol. 14, 374–381 (doi:10.1093/beheco/14.3.374) [Google Scholar]
- Locatello L., Neat F. C.2005Reproductive allocation in Aidablennius sphynx (Teleostei, Blenniidae): females lay more eggs faster when paired with larger males. J. Exp. Zool. A Comp. Exp. Biol. 303A, 922–926 (doi:10.1002/jez.a.204) [DOI] [PubMed] [Google Scholar]
- Lovern M. B., Wade J.2001Maternal plasma and egg yolk testosterone concentrations during embryonic development in green anoles (Anolis carolinensis). Gen. Comp. Endocrinol. 124, 226–235 (doi:10.1006/gcen.2001.7704) [DOI] [PubMed] [Google Scholar]
- Loyau A., Saint Jalme M., Mauget R., Sorci G.2007Male sexual attractiveness affects the investment of maternal resources into the eggs in peafowl (Pavo cristatus). Behav. Ecol. Sociobiol. 61, 1043–1052 (doi:10.1007/s00265-006-0337-3) [Google Scholar]
- Magnhagen C.1990Reproduction under predation risk in the sand goby, Pomatoschistus minutus, and the black goby, Gobius niger: the effect of age and longevity. Behav. Ecol. Sociobiol. 26, 331–335 [Google Scholar]
- McCormick M. I.1999Experimental test of the effect of maternal hormones on larval quality of a coral reef fish. Oecologia 118, 412–422 (doi:10.1007/s004420050743) [DOI] [PubMed] [Google Scholar]
- Michl G., Török J., Péczely P., Garamszegi L. Z., Schwabl H.2005Female collared flycatchers adjust yolk testosterone to male age, but not to attractiveness. Behav. Ecol. 16, 383–388 (doi:10.1093/beheco/ari002) [Google Scholar]
- Nakagawa S., Cuthill I. C.2007Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605 (doi:10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- Navara K. J., Hill G. E., Mendonca M. T.2006Yolk androgen deposition as a compensatory strategy. Behav. Ecol. Sociobiol. 60, 392–398 (doi:10.1007/s00265-006-0177-1) [Google Scholar]
- Quast W. D., Howe N. R.1980The osmotic role of the brood pouch in the pipefish Syngnathus scovelli. Comp. Biochem. Physiol. 67, 675–678 (doi:10.1016/0300-9629(80)90259-5) [Google Scholar]
- Ripley J. L., Foran C. M.2009Direct evidence for embryonic uptake of paternally-derived nutrients in two pipefishes (Syngnathidae: Syngnathus spp.). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 179, 325–333 (doi:10.1007/s00360-008-0316-2) [DOI] [PubMed] [Google Scholar]
- Roff D.1992The evolution of life histories: theory and analysis New York, NY: Chapman & Hall [Google Scholar]
- Saino N., Romano M., Ferrari R. P., Martinelli R., Moller A. P.2003Maternal antibodies but not carotenoids in barn swallow eggs covary with embryo sex. J. Evol. Biol. 16, 516–522 (doi:10.1046/j.1420-9101.2003.00534.x) [DOI] [PubMed] [Google Scholar]
- Sandvik M., Rosenqvist G., Berglund A.2000Male and female mate choice affects offspring quality in a sex-role-reversed pipefish. Proc. R. Soc. Lond. B 267, 2151–2155 (doi:10.1098/rspb.2000.1262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satia B. P., Donaldson L. R., Smith L. S., Nightingale J. N.1974Composition of ovarian fluid and eggs of the University of Washington strain of rainbow trout (Salmo gairdneri). J. Fish. Res. Board Can. 31, 1796–1799 [Google Scholar]
- Schwabl H., Mock D. W., Gieg J. A.1997A hormonal mechanism for parental favouritism. Nature 386, 231 (doi:10.1038/386231a0)9069278 [Google Scholar]
- Sheldon B. C.2000Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15, 397–402 (doi:10.1016/S0169-5347(00)01953-4) [DOI] [PubMed] [Google Scholar]
- Shine R., Phillips B., Langkilde T., Lutterschmidt D. I., Waye H., Mason R. T.2004Mechanisms and consequences of sexual conflict in garter snakes (Thamnophis sirtalis, Colubridae). Behav. Ecol. 15, 654–660 (doi:10.1093/beheco/arh058) [Google Scholar]
- Sokal R. R., Rohlf F. J.1981Biometry New York, NY: W. H. Freeman and Company [Google Scholar]
- Stearns S.1992The evolution of life histories Oxford, UK: Oxford University Press [Google Scholar]
- Surai P. F., Speake B. K., Wood N. A. R., Blount J. D., Bortolotti G. R., Sparks N. H. C.2001Carotenoid discrimination by the avian embryo: a lesson from wild birds. Comp. Biochem. Physiol. B 128, 743–750 (doi:10.1016/S1096-4959(00)00369-9) [DOI] [PubMed] [Google Scholar]
- Trivers R.1972Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- Villanelli F., Gherardi F.1998Breeding in the crayfish, Austropotamobius pallipes: mating patterns, mate choice and intermale competition. Freshw. Biol. 40, 305–315 (doi:10.1046/j.1365-2427.1998.00355.x) [Google Scholar]
- Vincent A., Ahnesjö I., Berglund A.1994Operational sex-ratios and behavioral sex-differences in a pipefish population. Behav. Ecol. Sociobiol. 34, 435–442 (doi:10.1007/BF00167335) [Google Scholar]
- Vincent A. C. J., Berglund A., Ahnesjö I.1995Reproductive ecology of five pipefish species in one eelgrass meadow. Environ. Biol. Fishes 44, 347–361 (doi:10.1007/BF00008250) [Google Scholar]
- Watanabe S., Watanabe Y.2002Relationship between male size and newborn size in the seaweed pipefish, Syngnathus schlegeli. Environ. Biol. Fishes 65, 319–325 (doi:10.1023/A:1020510422509) [Google Scholar]
- Williams G.1966Adaptation and natural selection Princeton, NJ: Princeton University Press [Google Scholar]