Abstract
Many animals become active during twilight, a narrow time window where the properties of the visual environment are dramatically different from both day and night. Despite the fact that many animals including mammals, reptiles, birds and insects become active in this specific temporal niche, we do not know what cues trigger this activity. To identify the onset of specific temporal niches, animals could anticipate the timing of regular events or directly measure environmental variables. We show that the Australian bull ant, Myrmecia pyriformis, starts foraging only during evening twilight throughout the year. The onset occurs neither at a specific temperature nor at a specific time relative to sunset, but at a specific ambient light intensity. Foraging onset occurs later when light intensities at sunset are brighter than normal or earlier when light intensities at sunset are darker than normal. By modifying ambient light intensity experimentally, we provide clear evidence that ants indeed measure light levels and do not rely on an internal rhythm to begin foraging. We suggest that the reason for restricting the foraging onset to twilight and measuring light intensity to trigger activity is to optimize the trade-off between predation risk and ease of navigation.
Keywords: temporal niche, twilight, foraging onset, light intensity, navigation, Myrmecia
1. Introduction
Many animals ranging from corals to mammals occupy discrete temporal niches. Being active at different times of the day enables them to avoid predators, follow the temporal distribution of food resources and reduce competition and direct confrontation (Schoener 1974; Kronfeld-Schor & Dayan 2003). Irrespective of why animals occupy temporal niches, it is crucial for them to identify a cue that enables them to determine the appropriate point in time to begin activity. To monitor and identify the onset of specific temporal niches, animals could either anticipate the timing of regular events (e.g. sunrise, sunset) through internal rhythms, or directly measure environmental variables such as temperature, light or the activity states of other animals (Ziv et al. 1993; Fenn & MacDonald 1995). However, the effect of temperature and light on internal rhythms has been studied mostly by alternating warm : cool or light : dark cycles in laboratory conditions (Rosengren 1977; Rosengren & Fortelius 1986). Hence, we know very little about the cues that trigger the onset of activity in natural conditions. This is further surprising, given the large number of animals that begin their activity around sunset (e.g. bats: Welbergen 2008; owls: De Groot 1983; butterflies: Frederiksen & Warrant 2008; dragonflies: Horridge 1978; beetles: Houston & McIntyre 1985; Caveney et al. 1995; glow-worms: Dreisig 1975; ants: Greiner et al. 2007; bees: Kelber et al. 2006; Somanathan et al. 2008; and wasps: Greiner 2006).
Ants are known to exhibit great versatility in their foraging schedules (Hölldobler & Wilson 1990). Activity is thought to depend primarily on temperature, which influences foraging by several mechanisms. In trail-following ants, for instance, high temperature increases the decay rate of the trail, thus altering recruitment strategies (Taylor 1977; Greenaway 1981). High surface temperatures have been suggested to be the selecting force behind solitary foraging behaviour in ants (Ruano et al. 2000). While some ants actively seek extreme thermal niches (e.g. Hölldobler & Taylor 1983; Christian & Morton 1992), most ants prefer moderate temperatures and adapt to the seasonal variation of temperature by switching from bimodal activity in summer to unimodal activity during cooler seasons (e.g. Greenaway 1981; Duncan & Crewe 1994), or by changing from a nocturnal to a diurnal lifestyle (e.g. Hölldobler & Möglich 1980).
By contrast, the effects of light intensity on the timing of foraging activity in ants have been less explored and are generally considered unimportant. This is surprising since the change in illumination is most pronounced between temporal niches, especially around twilight (Johnsen et al. 2006). Evidence that light may play a role in timing ant activity comes from investigations into ant circadian rhythms (McCluskey 1958). Workers of Formica polyctena, entrained to alternating light and dark cycles, exhibited an increase in activity towards the end of the dark period as if anticipating dawn (Rosengren 1977; Rosengren & Fortelius 1986). These rhythmic activities are essential for winged ants (alates) to synchronize the timing of mating flights between nests to mate at species-specific times during twilight (McCluskey 1965, 1992).
The only indication that light may determine the timing of foraging comes from a single observation at one nest in a diurnal leaf-cutter ant, Atta cephalotes (Hodgson 1955). Shining light onto the nest entrance using a gasoline lantern triggered activity ahead of time. Shining the same light on to the nest several hours after the last forager had returned did not trigger any activity. Thus, these diurnal ants responded to the earlier availability of light only when it occurred close to their usual foraging time.
We recently discovered a unique foraging schedule in the primitive ant, Myrmecia pyriformis (Greiner et al. 2007). In the summer, these ants became active just after sunset with nearly 65 per cent of foragers leaving the nest in a period of 40–60 min during evening twilight. During morning twilight just before sunrise, in another burst of activity, 60 per cent of foragers returned to the nest in a period of 40–60 min. Here, we aim to identify whether the timing of the foraging onset is determined by (i) surface temperature, (ii) sunset time (internal rhythm), or (iii) light intensity. If temperature were used as a cue, we would expect temperature at the foraging onset to be relatively constant. As a consequence, foraging onset would have to follow the temperature gradient over the year and would have to switch from the night in summer to mid-day in winter to cope with seasonal fluctuations. If sunset time were used to start foraging, we would expect activity to start at specific times relative to sunset. The internal rhythm indicating sunset time might itself be entrained to changes in light levels (Rosengren & Fortelius 1986). The timing of the foraging onset, however, would depend on the state of the ants’ internal rhythm and not on the ambient light levels. Finally, if light intensity were used to time activity, we would expect light levels to be constant at the time activity starts. Foraging onset should then approximately follow sunset times as light levels are highly correlated with time relative to sunset. Seasonal and random changes in light levels owing to cloud cover and terrain should, however, allow us to distinguish between an internal rhythm and a direct measurement of light intensity.
In this study, we first show how foraging onset varies in six nests at different times of the year. Second, we report a much more detailed examination of the dynamics of the foraging onset at a single nest for every 30 min change in sunset time throughout the year. Third, we carry out field experiments to modify ambient light intensity and determine its effect on the timing of the foraging onset.
2. Material and methods
(a). Study site and study species
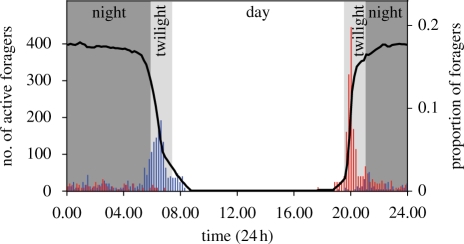
The foraging onset of the bull ant M. pyriformis (Smith) was studied in Canberra, Australia. Seven nests were identified, four at the Native Animal Enclosure located on the grounds of The Australian National University (35°16′50″ S, 149°7′50″ E) and three at the Mt Majura Nature Reserve (35°15′56″ S, 149°8′56″ E). Nests typically have a single entrance, with a circular opening in the soil without any elaborate mound-like structure. Workers leave and return to the nest individually with no evidence of recruitment. In summer, the majority of the workers leave the nest during the evening twilight (figure 1). Foragers leaving the nest are usually well directed and head straight to nearby Eucalyptus trees such as Eucalyptus macrorhyncha and Eucalyptus viminalis. During summer, foragers frequently returned to the nest with prey items such as flies, termites, beetles, moths, earwigs and ants, while during winter they feed on liquid food, harvested from tree sap and sugary exudates of sap-sucking insects. Workers continued to forage on a tree the entire night and returned to the nest in the morning twilight (figure 1).
Figure 1.
Daily activity rhythm of the primitive Australian ant, M. pyriformis, on a typical summer day. The black line indicates the number of active foragers. Bars indicate the proportion of outbound (red) and inbound (blue) foragers in 10 min bins. Sunrise occurs at the end of morning twilight and sunset occurs at the start of evening twilight. Twilight refers to the astronomical twilight that begins (or ends) when the centre of the Sun is at a depression angle of 18° above (or below) an ideal horizon.
(b). Astronomical data
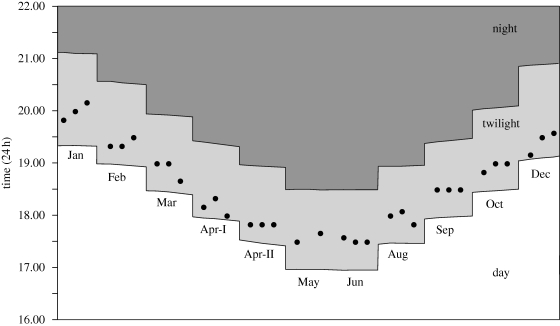
Astronomical data on sunset and twilight times were obtained from Geoscience Australia (http://www.ga.gov.au). Sunset is defined as the time in the evening when the upper edge of the Sun's disc coincides with an ideal horizon. The end of astronomical twilight is defined as the instant in the evening when the centre of the Sun is at a depression angle of 18° below an ideal horizon. The period between sunset and end of astronomical twilight is referred to as the evening twilight (also see Cronin et al. 2006; Welbergen 2008).
(c). Activity data
To study the temporal distribution of foraging onset in M. pyriformis, we set up a reference circle with a 30 cm radius around observation nests and counted the number of ants leaving the circle in 10 min bins. We used the time (10 min bin) at which the maximum number of foragers left the nest as a measure of foraging onset. For each 10 min recording interval, we recorded light intensities in photometric units (EXTECH: data logging light meter) and measured soil surface temperature (Sensortek BAT-12). We also recorded light intensities in radiometric units on some days (Warsash Scientific: ILT1700).
To address nest variability, we first monitored foraging bout activity once at six different nests at random times throughout the year. In order to achieve a more detailed understanding of the parameters influencing the timing of the foraging onset, we monitored the foraging bout activity at an additional nest over a full year. We monitored activity throughout the year whenever sunset time changed by 30 min. To account for dramatic weather conditions on these days (heavy rain, dust storms, etc.), we also recorded a day before and day after the specified date. This resulted in a total of 33 recordings for the whole year.
(d). Modifying light intensity
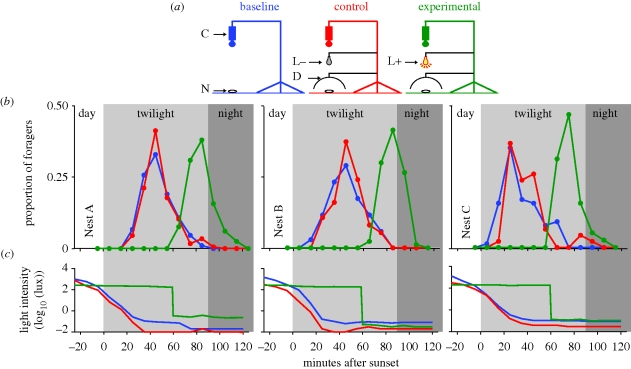
To test whether light levels were indeed critical for determining foraging onset, we experimentally increased light intensity at the usual time of foraging onset (figure 6). Two fluorescent light tubes were fixed over a dome-shaped diffuser to illuminate the nest entrance with approximately 250 lux (0.00015 w cm−2). The diffuser prevented the ants from viewing the lights as point sources. The diffuser hemisphere, measuring 30 cm in diameter, was placed above the nest entrance and held stationary 10 cm above the ground by strings fastened to a tripod. A circular hole at the top of the diffuser allowed us to record ant behaviour at the nest entrance. In the experimental condition, the light was ‘ON’ from 20 min before sunset up to 60 min after sunset. The light was then turned ‘OFF’ without disturbing the set-up. In the control condition, the same set-up was in place, but the light was ‘OFF’ for the entire period. In the baseline condition, we recorded activity patterns at the same nests using a camera mounted on a tripod. The number of ants exiting the 30 cm reference circle was counted in 10 min bins for all conditions. We tested each of the three conditions once at three nests, each in a pseudo-random order, ensuring the same nest was never tested on successive days. Ambient light intensity and surface temperature were measured both outside and underneath the diffuser during all tests.
Figure 6.
Modifying light intensity during evening twilight alters the foraging onset in ants at three nests (A–C). (a) The experimental set-up, (b) outbound activity and (c) light intensity in the baseline (blue), control (red) and experimental (green) conditions are shown. Activity was recorded by a camera (C) placed over the nest entrance (N). In the control condition, in addition, a dome (D) was placed above the nest entrance with lights OFF (L–). In the experimental condition, a dome was placed above the nest entrance with lights ON (L+) for 60 min after sunset.
(e). Analysis
For the first two experiments, we used a multivariate linear model to explain the effect of sunset time, surface temperature and light intensity on the peak outbound activity of ants. Model selection was carried out by comparing models that only differed in one parameter. The final model included only parameters that made a significant contribution (at the 5% level) to that final model.
We used a linear mixed model approach (REML), as implemented in the lme4 module of R (R Core Development Team 2008), to analyse the third experiment where we manipulated light levels. This was necessary as different nests were observed to have slightly different rhythms. We tested whether the time of foraging onset differed between conditions.
3. Results
(a). Multiple nests
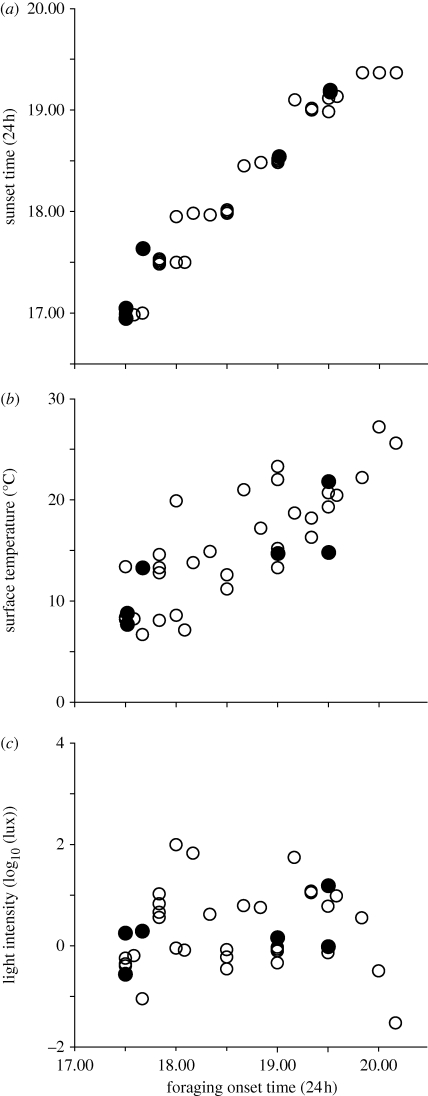
Over the entire year, the foraging onset of M. pyriformis occurred only during the evening twilight. Bouts of outgoing foragers lasted for 60 ± 15.5 min, with the majority of workers exiting 21.5 ± 10.9 min after sunset. Foraging onset was strongly predicted by sunset time (F1,3 = 571.7; p < 0.001; figure 2). There was, however, considerable variation in the exact timing of foraging bouts relative to sunset (2–33 min after sunset), leaving open the possibility that ants use a cue that is simply correlated with sunset time. Temperature and light intensity are two abiotic factors that change dramatically along both a diel scale and seasonally (figure 3) and could be used by ants to fine-tune the timing of foraging activity. The foraging onset occurred at a wide range of surface temperatures spanning from 7.7°C in winter to 21.8°C in the summer. If ants were selecting the temperature at which to become active, temperature at foraging onset should be relatively constant. This was clearly not the case (see also figure 5b). In addition, after adjusting for sunset time, there was no effect of temperature at sunset on the timing of foraging onset (F1,3 = 0.5723; p = 0.528).
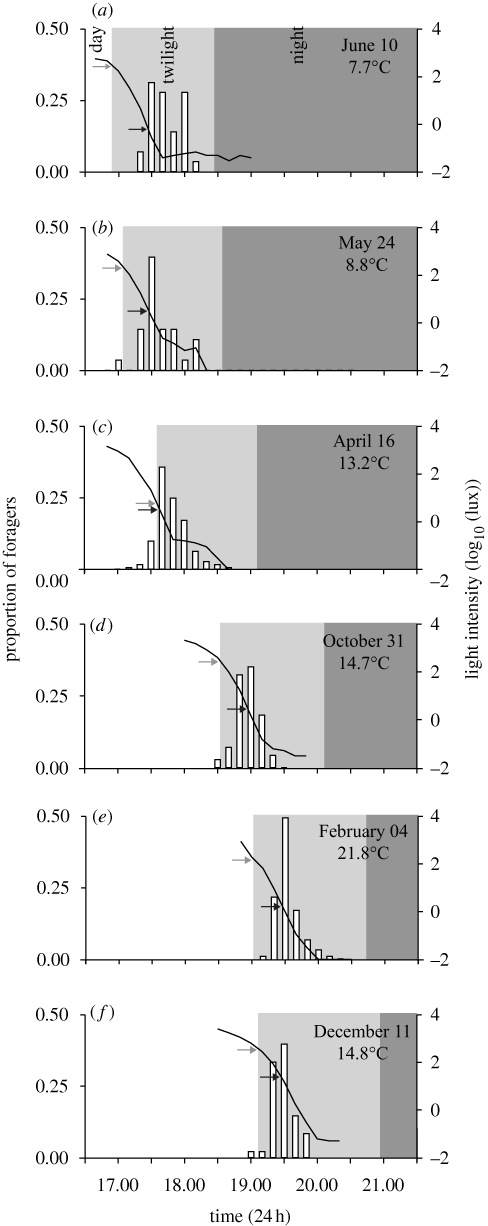
Figure 2.
Foraging bouts of M. pyriformis recorded from six nests at different times of the year. Sunset time corresponds to the start of twilight. Light intensity (black line), recorded in 10 min bins, is shown on the secondary y-axis. Grey arrows highlight light intensity at the time of sunset and black arrows indicate light intensity at the time of foraging onset. Foraging onset is defined as the time at which the maximum number of foragers left the nest. Sampling date and temperature at foraging onset are shown in each panel. Panels (a–f) are ordered according to increasing sunset time. (c) Nest was recorded on a mildly cloudy day, hence the lower light intensities at sunset time compared with the other days.
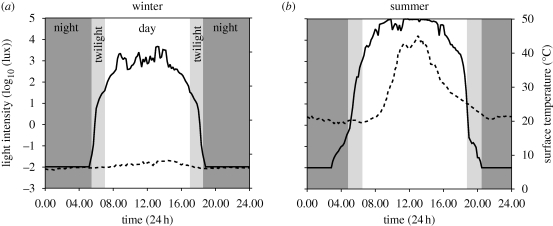
Figure 3.
Variation of light intensity and surface temperature at the same nest for (a) 1 day in winter (15 June 2009) and (b) 1 day in summer (7 January 2009). Solid line, light intensity; dotted line, surface temperature.
Figure 5.
Relation between time of foraging onset with (a) sunset time, (b) surface temperature and (c) light intensity. Data are from six nests (filled circles) and from a single nest (open circles).
Light intensity, though generally lower in winter than in summer, is theoretically constant at sunset time if topography, cloud cover and other atmospheric conditions are similar. Variation in light intensities during peak activity time was limited (mean ± s.d. = 1.25 ± 1.65 log10 (lux); mean = 0.0000176 w cm−2), suggesting that ants might time their activity to a particular light level. After adjusting for sunset time, there was indeed a significant effect of light intensity at sunset on the timing of foraging onset (F1,3 = 16.741; p = 0.02). The influence of light is evident in figure 2. For instance, on days where light intensity at sunset time was dimmer than usual (e.g. grey arrow, figure 2c), the foraging onset occurred, relative to sunset time, earlier than usual (black arrow, figure 2c). Similarly, on days where light intensity at sunset time was brighter than usual (e.g. grey arrow, figure 2a), the foraging onset occurred later than usual (black arrow, figure 2a).
(b). Single nest
Local light levels are affected by the local topography around the nest, and hence studying a single nest gives us the best chance to rigorously address the possible relationship between ambient light levels and timing of foraging onset. Regular sampling carried out over a full year at a single nest confirmed that foraging onset occurred only during the evening twilight and was strongly predicted by sunset time (F1,30 = 841.828; p < 0.001; figures 4 and 5a). Bouts of outgoing foragers lasted for 48.0 ± 18.3 min, with majority of the workers exiting the nest 25.9 ± 9.8 min after sunset. There was considerable variation in the timing of the foraging onset around sunset (3–48 min after sunset, figures 4 and 5a). Foraging onset occurred at a wide range of temperatures (6.7–27.2°C; figure 5b), spanning most of the temperature range at which these animals are normally active. If ants were indeed timing their foraging onset to a specific temperature, they would have had to start foraging at different times of the day during different seasons (night in summer and mid-day in winter), which did not happen. As a consequence, we see a clear correlation between temperature and foraging onset (figure 5b), suggesting that temperature does not determine the timing of foraging onset. This is confirmed by the fact that, after adjusting for sunset time, there was again no effect of temperature at sunset on the timing of foraging onset (F1,28 = 0.591; p = 0.4485). The exception was a single day in May when surface temperature fell below 6.6°C during the evening twilight and foraging stopped altogether. Light intensities at the time of foraging onset were consistently low (figure 5c), and there was no clear correlation between light intensity at foraging onset and timing of foraging onset, as would be expected if ants use light intensity as a decision criterion. There is, however, a clear effect of light intensity at sunset time on the timing of foraging onset (F1,30 = 6.9987; p = 0.013). The results of both these experiments (single and multiple nests) strongly suggest that the ants monitor ambient light intensity and begin their activity when light intensity has reached a certain level (mean ± s.d. = 0.99 ± 1.34 log10 (lux); mean = 0.00000888 w cm−2). To experimentally test this, we modified ambient light levels at three nest sites.
Figure 4.
Time of foraging onset of M. pyriformis at a single nest throughout a year. Foraging onset (filled circles) is defined as the time at which the maximum numbers of foragers left the nest. Sampling was carried out at every 30 min change in sunset time for three consecutive days. No activity occurred on the second recording day in May.
(c). Modifying light intensity
There was a significant difference in the time at which the foraging onset occurred between baseline, control (lights OFF) and experimental (lights ON) conditions (F2,4 = 168.99; p < 0.001; figure 6; see §2 for details). The foraging onset of ants from the baseline (absence of experimental set-up) and control conditions (experimental set-up with lights OFF) started at a similar time, indicating that the experimental set-up itself did not influence the foraging onset. In the experimental condition, where the lights were kept ON for 60 min past the normal foraging onset, not a single ant left the nest while the lights were on. When the lights were switched OFF, however, we observed ants scurrying close to the nest entrance within 5–10 s, following which they exited from the nest within a minute. The overall number of foragers did not differ between the three conditions.
4. Discussion
The Australian bull ant M. pyriformis begins its activity just after sunset throughout the year. Our results demonstrate that the ants prime their activity not to a certain time around sunset, but to a specific light intensity. If light intensity at sunset is brighter than normal, ants begin their activity later than usual (figure 2a) and if light intensity at sunset is darker than normal, they begin their activity earlier (figure 2c). Artificially increasing the light intensity at sunset time caused a delay in the ants' foraging onset (figure 6). However, as soon as the lights were turned off (60 min after sunset), the ants left the nest. The slight decrease in light intensity owing to the diffuser in the control condition did not cause the onset to occur earlier than usual. The expected change in onset time, however, was only about 10 min, and we may have missed this effect. It is also possible that a circadian rhythm exists in these ants that brings them close to the nest entrance at sunset, and light intensity determines the exact time at which foraging onset begins in the evening twilight. However, temperature, thought to regulate foraging traffic in most ants, failed to predict the timing of foraging onset in M. pyriformis.
The striking peaks of outbound activity that occur only during evening twilight in M. pyriformis are unique among ants (figures 1 and 4). However, there is some indication that such twilight activity may be common to other Myrmecia species that have been previously considered nocturnal. In Myrmecia desertorum, for instance, most foragers leave the nest between 17.30 and 18.00 in the winter and 18.00 and 20.00 in the summer, which was thought to be an adaptation for thermoregulation (Gray 1971). In light of our findings, it becomes apparent that the foraging onset in this congeneric species is closely related to sunset time in the evening twilight, similar to M. pyriformis.
(a). Why measure light levels?
Workers of M. pyriformis leave the nest in a narrow light window during the evening twilight. One of the reasons to start activity in this temporal niche, and not earlier or later, could be to avoid competition from other Myrmecia species. This seems unlikely as most of the competitive congeneric species hibernate during winter (P. Jayatilaka 2009, personal observation), yet M. pyriformis does not change the timing of its foraging onset. A second reason for beginning activity in the evening twilight could be to use the skylight polarization pattern for orientation, which is simplest when the Sun is at the horizon. However, this too is unlikely since both ants and other animals navigate using the complex polarization pattern that is available during the day (see Wehner 2001 and references therein). A third and a more likely possibility is that M. pyriformis avoids predators by starting activity during evening twilight. While this might explain their twilight emergence, it does not explain why these ants measure ambient light intensity rather than following a diurnal rhythm, which could relatively accurately predict sunset time (figures 4 and 5a). The fact that the ants actually measure light intensity suggests that light conditions are an important factor.
Our observations in different seasons at 10 nests indicate that the first task ants perform when they leave the nest is to navigate straight towards trees that are up to 20 m away. Having reached the trees, the ants continue to forage on them throughout the night before returning to the nest during morning twilight. As a consequence, the majority of ants carry out only one foraging trip per day, limiting the foraging efficiency of the colony.
Myrmecia ants are strongly visually oriented (Via 1977; Eriksson 1985), and this is supported by our findings that these ants use landmark information to navigate between the nest and their foraging trees. Individual ants establish idiosyncratic routes, which requires detailed visual position information (S. F. Reid 2009, personal observation). This suggests that the ants are forced to exit the nest in the evening twilight to use the little remaining light to find their precise route to the correct tree before nightfall. Although the visual system of these ants is adapted for low light intensities (Greiner et al. 2007), navigating from the nest to the tree beyond the evening twilight might prove difficult and time-consuming. Acquiring the necessary navigational information becomes increasingly difficult as night falls and light levels decrease. Delaying their exit from the nest thus results in degradation of the required visual information, and is reflected in animals walking slower and stopping longer at low light intensities compared with bright light conditions (S. F. Reid 2009, personal observation; see also Nørgaard et al. 2008). Coming out early, at higher light levels, on the other hand, would possibly increase the risk of predation. Both navigation and predation risk are hence probably dependent on light levels. This would explain why M. pyriformis measures light intensity directly to optimize the trade-off between predation risk and ease of navigation. The difficulty of navigating to the nest at night is also evident in another hymenopteran, the tropical bee, Megalopta genalis (Kelber et al. 2006). Unlike M. pyriformis, these bees carry out short foraging trips (2–34 min) in the astronomical twilight of morning and evening. Though bees returned to the nest in the morning and in the evening at about the same light intensities, they exited the nest in the morning at light intensities that were much dimmer than in the evening. This highlights the importance of requiring sufficient ambient light intensities to pinpoint the tiny nest entrance.
Many animals start their daily activity during the sunset period. While the activity patterns of animals during twilight have been studied in detail, the factors that trigger the activity itself are unknown. Bats have been shown to modify their emergence duration to the seasonal variation in twilight duration (Welbergen 2008). An indication that they also may be monitoring light intensity comes from findings that tropical bats emerge earlier relative to sunset time than their temperate counterparts (Jones & Rydell 1994). This is interesting since light levels in the tropics at a given time after sunset are lower than in temperate regions. In addition, recent evidence indicates that the emergence of flying foxes correlates with civil twilight, with the exact timing varying from being slightly early or late based on the level of cloud cover, which in turn affects ambient light intensity (J. Parsons & S. Robson 2009, personal communication). Even certain marine crustaceans such as mysid shrimps use light intensities to determine the daily extent of their vertical migration (Gal et al. 1999). Our findings in M. pyriformis thus raise the question whether other animals that begin activity in twilight also rely on direct measurements of light intensity to trigger their daily activity.
Acknowledgements
We greatly appreciate Jochen Zeil's support and help during all stages of this work. We thank Dinesh Rao, Richard Peters, Debjani Das and the two referees for their helpful comments on the manuscript. Many thanks to Piyankarie Jayatilaka for help with field work and to Paul Cooper for loaning equipment. We are grateful for the support from the Centre for Visual Sciences and the Australian Research Council under the ARC Centres of Excellence Programme. A.N. was supported by a postdoctoral fellowship from the Australian Research Council (DP0986606).
References
- Caveney S., Scholtz C. H., McIntyre P.1995Patterns of daily flight activity in onitine dung beetles (Scarabaeinae: Onitini). Oecologia 103, 444–452 (doi:10.1007/BF00328682) [DOI] [PubMed] [Google Scholar]
- Christian K. A., Morton S. R.1992Extreme thermophilia in a central Australian ant, Melophorus bagoti. Physiol. Zool. 65, 885–905 [Google Scholar]
- Cronin T. W., Warrant E. J., Greiner B.2006Celestial polarization patterns during twilight. Appl. Optics 45, 5582–5589 (doi:10.1364/AO.45.005582) [DOI] [PubMed] [Google Scholar]
- De Groot R. S.1983Origin, status and ecology of the owls in Galapagos. Ardea 71, 167–182 [Google Scholar]
- Dreisig H.1975Environmental control of the daily onset of luminescent activity in glowworms and fireflies (Coleoptera: Lampyridae). Oecologia 18, 85–99 [DOI] [PubMed] [Google Scholar]
- Duncan F. D., Crewe R. M.1994Field study on the foraging characteristics of a ponerine ant, Hagensia havilandi Forel. Insect Soc. 41, 85–98 (doi:10.1007/BF01240576) [Google Scholar]
- Eriksson E. S.1985Attack behaviour and distance perception in the Australian bulldog ant Myrmecia nigriceps. J. Exp. Biol. 119, 115–131 [Google Scholar]
- Fenn M. G. P., MacDonald D. W.1995Use of middens by red foxes: risk reverses rhythms of rats. J. Mammal. 76, 130–136 (doi:10.2307/1382321) [Google Scholar]
- Frederiksen R., Warrant E. J.2008Visual sensitivity in the crepuscular owl butterfly Caligo memnon and the diurnal blue morpho Morpho peleides: a clue to explain the evolution of nocturnal apposition eyes? J. Exp. Biol. 211, 841–851 [DOI] [PubMed] [Google Scholar]
- Gal G., Loew E. R., Rudstam L. G., Mohmmadian A. M.1999Ambient light levels determine the extent of diel vertical migration of many species including mysid shrimps. Can. J. Fish. Aquat. Sci. 56, 311–322 (doi:10.1139/cjfas-56-2-311) [Google Scholar]
- Gray B.1971Notes on the field behaviour of two ant species Myrmecia desertorum Wheeler and Myrmecia dispar (Clark) (Hymenoptera : Formicidae). Insect Soc. 18, 81–94 (doi:10.1007/BF02223114) [Google Scholar]
- Greenaway P.1981Temperature limits to trailing activity in the Australian arid-zone meat ant Iridomyrmex purpureus form viridiaeneus. Aus. J. Zool. 29, 621–630 (doi:10.1071/ZO9810621) [Google Scholar]
- Greiner B.2006Visual adaptations in the night-active wasp Apoica pallens. J. Comp. Neurol. 495, 255–262 (doi:10.1002/cne.20882) [DOI] [PubMed] [Google Scholar]
- Greiner B., Narendra A., Reid S. F., Dacke M., Ribi W. A., Zeil J.2007Eye structure correlates with distinct foraging-bout timing in primitive ants. Curr. Biol. 17, R879–R880 (doi:10.1016/j.cub.2007.08.015) [DOI] [PubMed] [Google Scholar]
- Hodgson E. S.1955An ecological study of the behavior of the leaf-cutting ant Atta cephalotes. Ecology 36, 293–304 (doi:10.2307/1933235) [Google Scholar]
- Hölldobler B., Möglich M.1980The foraging system of Pheidole militicida (Hymenoptera: Formicidae). Insect Soc. 27, 237–264 (doi:10.1007/BF02223667) [Google Scholar]
- Hölldobler B., Taylor R. W.1983A behavioral study of the primitive ant Nothomyrmecia macrops Clark. Insect Soc. 30, 381–401 [Google Scholar]
- Hölldobler B., Wilson E. O.1990The ants Cambridge, MA: The Belknap Press, Harvard University [Google Scholar]
- Horridge G. A.1978The separation of visual axes in apposition compound eyes. Phil. Trans. R. Soc. Lond. B 285, 1–59 (doi:10.1098/rstb.1978.0093) [DOI] [PubMed] [Google Scholar]
- Houston W. W. K., McIntyre P.1985The daily onset of flight in the crepuscular dung beetle Onitis alexis. Entomol. Exp. Appl. 39, 223–232 [Google Scholar]
- Johnsen S., Kelber A., Warrant E. J., Sweeney A. M., Widder W. A., Lee R. L., Hernández-Andrés J.2006Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. J. Exp. Biol. 209, 789–800 (doi:10.1242/jeb.02053) [DOI] [PubMed] [Google Scholar]
- Jones G., Rydell J.1994Foraging strategy and predation risk as factors influencing emergence time in echolocating bats. Phil. Trans. R. Soc. Lond. B 346, 445–455 (doi:10.1098/rstb.1994.0161) [Google Scholar]
- Kelber A., Warrant E. J., Pfaff M., Wallen R., Theobald J. C., Wcislo W. T., Raguso R. A.2006Light intensity limits foraging activity in nocturnal and crepuscular bees. Behav. Ecol. 17, 63–72 (doi:10.1093/beheco/arj001) [Google Scholar]
- Kronfeld-Schor N., Dayan T.2003Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 153–181 (doi:10.1146/annurev.ecolsys.34.011802.132435) [Google Scholar]
- McCluskey E. S.1958Daily rhythms in male harvester and Argentine ants. Science 128, 536–537 (doi:10.1126/science.128.3323.536) [DOI] [PubMed] [Google Scholar]
- McCluskey E. S.1965Circadian rhythms in male ants of five diverse species. Science 150, 1037–1039 (doi:10.1126/science.150.3699.1037) [DOI] [PubMed] [Google Scholar]
- McCluskey E. S.1992Periodicity and diversity in ant mating flights. Comp. Biochem. Physiol. 103, 241–243 (doi:10.1016/0300-9629(92)90574-A) [DOI] [PubMed] [Google Scholar]
- Nørgaard T., Nilsson D. E., Henschel J. R., Garm A., Wehner R.2008Vision in the nocturnal wandering spider Leucorchestris arenicola (Araneae: Sparassidae). J. Exp. Biol. 211, 816–823 (doi:10.1242/jeb.010546) [DOI] [PubMed] [Google Scholar]
- R Core Development Team 2008R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3-900051-07-0. See http://www.R-project.org [Google Scholar]
- Rosengren R.1977Foraging strategy of wood ants (Formica rufa group). II: Nocturnal orientation and diel periodicity. Acta Zool. Fenn. 150, 1–30 [Google Scholar]
- Rosengren R., Fortelius W.1986Light : dark induced activity rhythms in Formica ants (Hymenoptera: Formicidae). Entomol. Gener. 11, 221–228 [Google Scholar]
- Ruano F., Tinaut A., Soler J. J.2000High surface temperatures select for individual foraging in ants. Behav. Ecol. 11, 396–404 (doi:10.1093/beheco/11.4.396) [Google Scholar]
- Schoener T. W.1974Resource partitioning in ecological communities. Science 185, 27–39 (doi:10.1126/science.185.4145.27) [DOI] [PubMed] [Google Scholar]
- Somanathan H., Borges R. M., Warrant E. J.2008Visual ecology of Indian carpenter bees I: light intensities and flight activity. J. Comp. Physiol. A 194, 97–107 (doi:10.1007/s00359-007-0291-1) [DOI] [PubMed] [Google Scholar]
- Taylor F.1977Foraging behavior of ants: experiments with two species of myrmicine ants. Behav. Ecol. Sociobiol. 2, 147–167 [Google Scholar]
- Via S.1977Visually mediated snapping in the bulldog ant: a perceptual ambiguity between size and distance. J. Comp. Physiol. 121, 33–51 (doi:10.1007/BF00614179) [Google Scholar]
- Wehner R.2001Polarization vision: a uniform sensory capacity? J. Exp. Biol. 204, 2589–2596 [DOI] [PubMed] [Google Scholar]
- Welbergen J. A.2008Variation in twilight predicts the duration of the evening emergence of fruit bats from a mixed-species roost. Anim. Behav. 75, 1543–1550 (doi:10.1016/j.anbehav.2007.10.007) [Google Scholar]
- Ziv Y., Abramsky Z., Kotler B. P., Subach A.1993Interference competition and temporal and habitat partitioning in two gerbil species. Oikos 66, 237–244 (doi:10.2307/3544810) [Google Scholar]