Abstract
Weapons used in male fighting can be costly to males and are often reported to trade off with other characters such as wings or spermatogenic investment. This study investigated whether increased investment into weapons can generate evolutionary changes in mating strategy for armed males. Male flour beetles, Gnatocerus cornutus, have enlarged mandibles that are used in male–male competition. We subjected these weapons to 12 generations of bidirectional selection and found trade-offs between weapons and two other male characters: wing and testis size. In addition, probably as a consequence of the observed changes in investment, dispersal ability and ejaculatory volume differ significantly between the lines. This indicates that the exaggeration of a weapon can be associated with dispersal and ejaculatory strategies. Thus, altered investment into weapons can lead to correlated changes in life-history traits.
Keywords: exaggerated trait, resource allocation, sexual selection, sperm competition
1. Introduction
Male–male competition for mates is a key component of sexual selection and is responsible for the evolution of exaggerated traits that are used as weapons in male fights (Andersson 1994). Obvious examples include the antlers of deer and the horns and mandibles of beetles (Eberhard 1979; Andersson 1994; Emlen 1996, 2008; Shuster & Wade 2003). However, exaggerated traits can be developmentally costly for males because production of weapons is energetically and physiologically expensive (Emlen 2001; Okada & Miyatake 2009). Consistent with this, several recent studies have demonstrated intra-organism competition for resources between weapons and other somatic traits (Nijhout & Emlen 1998; Moczek & Nijhout 2004; Tomkins et al. 2004, 2005). Therefore, because investment in exaggerated traits can lead to trade-offs against other fitness-related traits, life-history strategies can be affected by the evolution of sexually selected traits (Hunt et al. 2004; Tomkins et al. 2004).
Many studies of male beetles have reported morphological trade-offs between weapons and sensory organs such as eyes and antennae (Nijhout & Emlen 1998; Emlen 2001), as well as between weapons and other traits related to mating success, including wings used in mate searching (Kawano 1995; Tomkins et al. 2005; Okada et al. 2007) and testes as a measure of male spermatogenic investment (Simmons & Emlen 2006). Such trade-offs indirectly suggest that dispersal and ejaculatory strategies can coevolve with the weapons used in armed male beetles (Emlen 2001; Simmons & Emlen 2006; Okada et al. 2007).
Here, we investigated correlated responses in testis and wing size to selection of male weapon size in armed male beetles. Males of the broad-horned flour beetle Gnatocerus cornutus usually fight for territories with their enlarged mandibles, but as an alternative tactic occasionally disperse in order to acquire a new territory (Okada & Miyatake 2010; Sasaki et al. in press). In addition, because females copulate with two or more males (Okada & Miyatake 2009), the resulting sperm competition should select an ejaculatory character (e.g. Parker 1990; Simmons et al. 1999). Since males must allocate available resources between testes, wings and mandibles, competition between the growth trajectories of these characters is predicted. Our previous work on this species established populations with different relative mandible sizes using bidirectional (up/down) artificial selection for mandible length (Okada & Miyatake 2009). In the present study, we first investigated correlated responses in morphological traits to artificial selection on mandible size. Subsequently, to support that these responses affected male mating strategy, we compared the fighting success, ejaculatory expenditure and dispersal behaviour of populations with different relative mandible sizes. Lastly, we discuss the mating strategy associated with exaggeration of the male weapon in G. cornutus.
2. Material and methods
(a). Beetles
The G. cornutus beetle culture has been maintained in the laboratory of the National Food Research Institute, Japan, for 50 years. The culture originated from adults collected in Miyazaki city (31°54′ N, 131°25′ E), Japan, in June 1957 (Okada et al. 2006). Beetles were reared on whole meal (Yoshikura Shokai, Tokyo, Japan) enriched with brewer's yeast (Asahi Beer, Tokyo, Japan) in a chamber (2400 × 2400 × 2400 cm; CC-T2000, Sanyo, Osaka, Japan) kept at 25°C and 60 per cent relative humidity.
(b). Artificial selection
The measurement of body parts and the selection method was described in detail by Okada & Miyatake (2009). Briefly, in each generation, the 12 males with the shortest mandibles were selected to propagate short-mandible lines (S line); the 12 males with the longest mandibles were selected to propagate the long-mandible line (L line); and 12 males were randomly selected to propagate the control line (C line). Females do not have mandibles and were chosen randomly from the stock culture. Two selection replicates were established from the stock culture for the short, long and control lines (L1, C1 and S1; L2, C2 and S2). Females were chosen randomly from within each line. Experiments were conducted on 12th generation adults, 15–20 days after emergence. Each individual was placed in one well of a 24-well tissue culture plate with food (1 g), as described above, until the following experiment, and thus did not interact with conspecifics.
(c). Correlated response in investments in spermatogeny and wings
Twenty virgin males of each line were randomly chosen for measurement, and the right and left testes were removed from the body using a fine forceps and carefully separated from the surrounding tissue in deionized water. The length (L) and width (W) of both testes were measured to the nearest 0.01 mm with a dissecting microscope monitoring system (VM-60, Olympus, Tokyo, Japan), and testis volume (mm3) was calculated on the assumption that the testis was oval, using the formula V = (πLW2)/6. The total volume was used for the testis size. Subsequently, we gripped the wing axillaries with the forceps and carefully removed the left wing from the body. We traced the area of the left wing (±0.01 mm2) using a dissecting microscope monitoring system. Lastly, body width was measured as an index of body size.
(d). Correlated response in fighting success, ejaculatory and dispersal behaviours
To investigate whether investments in a weapon were associated with success in male fighting, we staged 25 contests per replicate between L and S males (i.e. L1 versus S1 and L2 versus S2) in the following manner. To observe male–male interactions, we placed a paper filter (17 mm diameter) in a plastic container (17 mm diameter, 20 mm height) for the fight site. Two males were simultaneously introduced into the plastic container, and behaviour was observed for 30 min. The male that pushed his opponent out of the fighting arena and chased him was denoted the winner. The loser was the male that retreated from the fight site. For a more detailed description of the methods, see Okada et al. (2006). The difference in body size between contestants was less than 0.01 mm (the total range of the body width of males was less than 2%; Okada et al. 2006).
To examine the number of sperm stored by females, males of L1, C1 and S1 were paired with C1 females, and males of L2, C2 and S2 were paired with C2 females. Copulations took place in glass vials (15 mm diameter, 40 mm height) supplied with paper filters during the normal light phase. We observed 18 copulations per line. After mating, the female was immediately removed from the glass vial to prevent additional matings and stored at −80°C. The spermatheca was subsequently removed from the female body with fine forceps and carefully separated from the surrounding tissue. The spermatheca was then placed in a drop of deionized water on a glass slide, crushed and cut into small pieces. The sperm suspension was stirred with the forceps to distribute the sperm and air-dried on the glass slide. Sperm number was counted on the slide under an optical microscope (BX51, Olympus). Sperm numbers were log10 transformed for subsequent statistical analysis. Body width was measured as an index of the body size.
To examine which line of males had the greatest dispersal tendency, we placed a paper filter (50 mm diameter) in a glass container (50 mm diameter, 100 mm height) kept at 30°C and 60 per cent relative humidity for observation. Of 150 males of each line, each individual was introduced into the container, and the number of males dispersing from the container was observed every hour during scotophase under a red light for 8 h. We compared the male dispersal tendencies between selection regimes at 8 h after the introduction. Several previous studies indicated that the dispersal tendency in weapon-bearing male beetles is positively associated with the dispersal ability in the field (see Okada et al. 2007, 2008).
(e). Statistics
Sizes of mandible, wing and testis were analysed using a multivariate analysis of variance (MANOVA) with the selection regime (L, C or S) and replicate (1 and 2) as explanatory variables, and body size (body width) as a covariate. In addition, a univariate analysis of variance (ANOVA) within the MANOVA was used for the response of each trait to selection. We estimated the relative mean of each line at the mean of the covariate with the least-square mean (SPSS Inc. 2001). Student's t-test was used for comparison of the relative mean between selection regimes, correcting the significance level for multiple comparisons by the sequential Bonferroni method (Rice 1989). Total and among-selection regimes’ SSCP matrices from the MANOVA model can be used to infer phenotypic and genetic covariances, respectively (Morrison 1990). To calculate phenotypic and genetic correlations, we standardized the total and among-selection regimes’ SSCP matrices. The significance of the correlation was deduced using Fisher's r-to-Z transformation. Stored sperm number was analysed using ANOVA with the selection regime (L, C or S) as a fixed effect, replicate (1 and 2) as a random effect and body size as a covariate. To compare male dispersal tendencies between selection regimes at 8 h after the beginning of experiment, we applied a generalized linear model with the selection regime (L, C or S) and replicate (1 and 2) as explanatory variables, with binomial errors and a logit link function (Hardy & Field 1998; Pomfret & Knell 2006). If there was a significant effect of the selection regime, pairwise comparisons were performed between selection regimes correcting the significance level for multiple comparisons by the sequential Bonferroni method. We used a reduced model that removed non-significant interaction terms from the full model (Grafen & Hails 2002). All statistical analyses were carried out using SPSS for Windows v. 11 (SPSS Inc. 2001).
3. Results
(a). Correlated responses in investments in weapon, spermatogeny and wing size
For body size, the selection regime had no significant effect in the reduced model (selection regime: F2,116 = 0.284, p = 0.754; replicate: F1,116 = 1.605, p = 0.208). Thus, artificial selection on the absolute mandible length produced no correlated response in body size (n = 20 in each strain: L1, 1.23 ± 0.011 (mean ± s.e.); L2, 1.24 ± 0.011; C1, 1.23 ± 0.009; C2, 1.25 ± 0.010; S1, 1.23 ± 0.007; S2, 1.24 ± 0.013).
For mandible length, multiple comparisons showed that the relative line mean was significantly larger in the long selection regime than in the control and short selection regimes, and significantly smaller in the short selection regime than in the control (table 1). By contrast, multiple comparisons of testis size and wing size showed that the relative line means were significantly smaller in the long selection regime than in the control and short selection regimes, and significantly larger in the short selection regime than in the control (table 1). In MANOVA, the reduced model showed that the selection regime and body size had a significant effect on morphological traits (selection regime, Wilk's λ = 0.121, F6,226 = 70.490, p < 0.0001; replicate, Wilk's λ = 0.925, F3,113 = 1.465, p = 0.228; body size, Wilk's λ = 0.168, F3,113 = 186.776, p < 0.0001). Additionally, MANOVA univariate tests showed that the selection regime and body size had significant effects on each trait (mandible length: selection regime, F2,115 = 329.752, p < 0.0001; replicate, F1,115 = 3.689, p = 0.057; body size, F1,115 = 150.974, p < 0.0001; testis size: selection regime, F2,115 = 41.262, p < 0.0001; replicate, F1,115 = 0.384, p = 0.537; body size, F1,115 = 292.600, p < 0.0001; wing size: selection regime, F2,115 = 41.837, p < 0.0001; replicate, F1,115 = 0.412, p = 0.522; body size, F1,115 = 153.911, p < 0.0001). Additionally, there were negative genetic correlations between sizes of the mandible, testis and wing (table 2). These results indicate negative genetic trade-offs between the mandible, testis and wing.
Table 1.
Mean and adjusted line mean (± s.e.) of morphological traits in males of each line. Different letters indicate a significant difference among regimes at p < 0.05 by Student's t-test with the sequential Bonferroni method (SPSS Inc. 2001). Relative line mean is estimated using the least-square mean.
| trait | n | selection regime |
||
|---|---|---|---|---|
| long | control | short | ||
| mandible size (mm) | ||||
| replicate 1 | 20 | 0.45 ± 0.012 | 0.39 ± 0.010 | 0.27 ± 0.013 |
| replicate 2 | 20 | 0.45 ± 0.010 | 0.39 ± 0.011 | 0.27 ± 0.009 |
| relative line mean | 0.45 ± 0.0050a | 0.39 ± 0.0050b | 0.27 ± 0.0050c | |
| testis size ×10−3 (mm3) | ||||
| replicate 1 | 20 | 9.50 ± 0.24 | 9.74 ± 0.22 | 10.50±0.14 |
| replicate 2 | 20 | 9.71 ± 0.20 | 10.25 ± 0.17 | 10.55 ± 0.34 |
| relative line mean | 9.53 ± 0.08a | 9.94 ± 0.08b | 10.60 ± 0.08c | |
| wing size (mm2) | ||||
| replicate 1 | 20 | 4.74 ± 0.022 | 4.79 ± 0.027 | 4.89 ± 0.018 |
| replicate 2 | 20 | 4.76 ± 0.023 | 4.86 ± 0.025 | 4.89 ± 0.031 |
| relative line mean | 4.75 ± 0.012a | 4.82 ± 0.012b | 4.90 ± 0.012c | |
Table 2.
Total and among-selection regimes’ correlation matrices. Values in parentheses are s.e.
| mandible length | testis size | wing size | ||
|---|---|---|---|---|
| total | mandible length | — | ||
| (phenotypic correlation) | testis size | −0.0938 (±0.1049) | — | |
| wing size | 0.0897 (±0.1187) | 0.1167 (±0.1830) | — | |
| among-selection regime | mandible length | — | ||
| (genetic correlation) | testis size | −0.6249 (±0.0974)* | — | |
| wing size | −0.5656 (±0.0862)* | 0.4846 (±0.0639)* | — |
*p < 0.05 by Fisher's r-to-Z transformation.
(b). Correlated response in fighting success
In L1 versus S1 and L2 versus S2 male contests, L males were the winners significantly more frequently than S males (L1 versus S1: 19 versus 6 (winners), d.f. = 1, χ2 = 15.88, p < 0.0001; L2 versus S2: 20 versus 5, d.f. = 1, χ2 = 17, p < 0.0001). Therefore, there was a positive relationship between weapon investment and fighting success in this male.
(c). Correlated response in ejaculatory expenditure
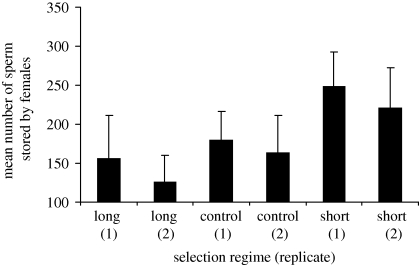
Figure 1 shows the numbers of sperm stored by females mating with males from each line. In ANOVA with the body size as a covariate, the covariate had no significant effect on sperm number (body size: F1,103 = 2.590, p = 0.111). In ANOVA without the covariate, the reduced model showed that the selection regime had a significant effect on the number of sperm stored (selection regime: F2,104 = 5.623, p = 0.0048; replicate: F1,104 = 0.323, p = 0.567). Multiple comparisons showed that the adjusted line mean estimated from this reduced model was significantly smaller in the long selection regimes than in the short selection regimes for the number of sperm stored.
Figure 1.
Mean (± s.e.) number of sperm stored by females of the 12th generation of each line. n = 18 in each line.
(d). Correlated response in dispersal behaviour
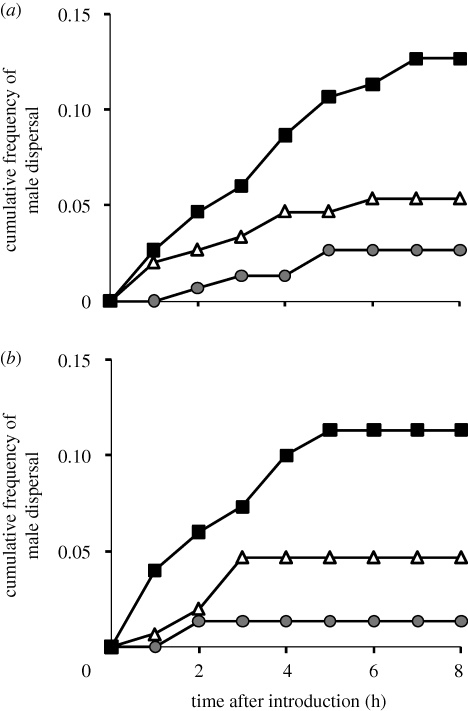
Figure 2 shows the dispersal tendency for each line. The reduced model revealed that the selection regime had a significant effect on dispersal tendency at 8 h after the introduction (selection regime: d.f. = 2, χ2 = 26.803, p < 0.0001; replicate: d.f. = 1, χ2 = 0.483, p = 0.487). In each pairwise comparison, after correcting for multiple comparisons with the reduced model, the dispersal tendency was significantly lower in the long selection regime than in the control and short selection regimes, and significantly greater in the short selection regime than in the control (α = 0.05; Rice 1989; L and C, selection regime: d.f. = 1, χ2 = 4.128, p = 0.0442; replicate: d.f. = 1, χ2 = 0.449, p = 0.503; L and S, selection regime: d.f. = 1, χ2 = 25.405, p < 0.0001; replicate: d.f. = 1, χ2 = 0.427, p = 0.514; C and S, selection regime: d.f. = 1, χ2 = 9.716, p = 0.0018; replicate: d.f. = 1, χ2 = 0.196, p = 0.658).
Figure 2.
Cumulative dispersal tendency of males of the 12th generation of each line for each time interval. (a) replicate 1, (b) replicate 2. n = 150 in each line. Grey circles, long; white triangles, control; black squares, short.
4. Discussion
Our study demonstrated that selection on weapon size generated correlated responses in testis and wing size (i.e. associations between weapons and these characters). As a result, the relative sizes of testes and wings were significantly smaller in L lines than in C and S lines, and in the C lines than in the S lines (table 1). In addition, there were negative genetic correlations between sizes of mandible, testes and wings (table 2). These results suggest genetic trade-offs between investments in weapon, testes and wings in this male, similar to findings at the phenotypic level in several horned beetles (Kawano 1995; Emlen 2001; Simmons & Emlen 2006). However, there were only two replicate lines in each experimental selection treatment and the effective population size (i.e. 12 individuals of each sex) per generation within each line was not so large. Thus, it might be possible that the limited sample size and the effective population size may confound or undermine the results, due to inbreeding effects or genetic drift.
Similar to morphological associations, the number of sperm stored by females was significantly greater when they mated with S line males compared with L line males, and male dispersal tendency was significantly lower in the L lines compared with the C and S lines, and significantly greater in the S lines than in the C lines. Weapon exaggeration can therefore not only have significant effects on the evolution of morphology, but also on male behavioural traits. These results suggest a genetic basis to the reduction in dispersal ability and ejaculatory expenditure owing to the enlargement of the male weapon. Therefore, dispersal and ejaculatory strategies may be associated with the exaggeration of weapons in weapon-bearing males.
Males normally guard territories and mates with their weapons (Okada et al. 2006), and there was a positive relationship between weapon investment and fighting success in this beetle. As a result, males with long mandibles have higher remating rates with the same mate. Because these males will therefore face a lower risk of sperm competition, they are unlikely to need to inseminate each female with more sperm per copulation. By contrast, the reduction in mandible investment will decrease the success in male fighting. Males disperse to new territories, which may or may not contain other males, when they fail to guard their territories (Okada & Miyatake 2010; Sasaki et al. in press). Because the females copulate with two or more males (Okada & Miyatake 2009), these dispersing males will face a higher risk of sperm competition from guarding males compared with males with long mandibles. Males generally increase their ejaculatory expenditure when the risk of sperm competition is higher (e.g. Parker 1990; Gage 1994; Hosken 1997; Simmons et al. 1999; Hosken & Ward 2001). Males with short mandibles potentially need to invest more in testes and wings for mating success. Therefore, this trade-off between the weapon, spermatogenesis and dispersal characters may occur as a result of selection on the mating strategy.
The optimal investment rate of the three traits may be influenced by ecological factors such as population density. A high-density population will experience more intraspecific competition for territories (Kokko & Rankin 2006; Pomfret & Knell 2008). The costs of defending females or territories may become greater than the benefits, if many rival males exist (Kokko & Rankin 2006). At high densities, the cost may be larger for males with longer mandibles. Additionally, females are likely to encounter and mate with a large number of males when density is high, leading to high levels of sperm competition (only if there was no sperm displacement). Males probably compete via scramble competition for matings, and high fitness will be related to the ability to find females and success in sperm competition (Pomfret & Knell 2008). Indeed, there is strong theoretical and empirical evidence that sperm competition selects for males that invest heavily in sperm production (Parker 1998; Simmons 2001). Therefore, higher densities may favour males with short mandibles and large wings and testes, and this selection may also affect the genetic trade-off between the three traits.
Two additional explanations could also account for the trade-off between the weapon and spermatogenic characters. First, males with large mandibles might preferentially invest fewer sperm in each female. This strategic response might evolve if these males experience a high remating rate and a high risk of sperm depletion. In this case, a difference in sperm numbers would arise purely as a result of asymmetries in mating rates, even when the short- and large-mandibled males have the same rates of spermatogenesis. Second, it is possible that the control females might preferentially retain the sperm of short- rather than large-mandibled males. This would explain differences in sperm numbers even if males from different lines transfer ejaculates of similar size.
In conclusion, this study showed genetic trade-offs between the weapon, spermatogenesis and dispersion characters in an armed male beetle. Based on this resource allocation trade-off, the exaggeration of a male weapon can generate evolutionary changes in dispersal and ejaculatory strategies in G. cornutus males. Therefore, exploring how weapons and other somatic traits are genetically and developmentally associated can be a powerful aid in our understanding of the evolution of the life-history strategy of weapon-bearing males.
Acknowledgements
We thank Drs David Hosken and Tommaso Pizzari and two anonymous reviewers for valuable comments. This study was partially supported by Research Fellowships for Young Scientists (JSPS 205869 to T.Y. and 195563 to K.O.) and by a Grant-in-Aid for Scientific Research (KAKENHI 19370011) to T.M., both from Japanese Ministry of Education, Science, Sports and Culture.
References
- Andersson M.1994Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- Eberhard W. G.1979The function of horns in Podischnus agenor (Dynastinae) and other beetles. In Sexual and reproductive competition in insects (eds Blum M. S., Blum N. A.), pp. 231–258 New York, NY: Academic Press [Google Scholar]
- Emlen D. J.1996Artificial selection on horn body-length size allometry in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Evolution 50, 1219–1230 (doi:10.2307/2410662) [DOI] [PubMed] [Google Scholar]
- Emlen D. J.2001Costs and the diversification of exaggerated animal structures. Science 291, 1534–1536 (doi:10.1126/science.1056607) [DOI] [PubMed] [Google Scholar]
- Emlen D. J.2008The evolution of animal weapons. Annu. Rev. Ecol. Syst. Evol. 39, 387–413 (doi:10.1146/annurev.ecolsys.39.110707.173502) [Google Scholar]
- Gage M. J. G.1994Associations between body size, mating pattern, testis size and sperm lengths across butterflies. Proc. R. Soc. Lond. B 258, 247–254 (doi:10.1098/rspb.1994.0169) [Google Scholar]
- Grafen A., Hails R.2002Modern statistics for the life sciences. Oxford, UK: Oxford University Press [Google Scholar]
- Hardy I. C. W., Field S. A.1998Logistic analysis of animal contests. Anim. Behav. 56, 787–792 (doi:10.1006/anbe.1998.0833) [DOI] [PubMed] [Google Scholar]
- Hosken D. J.1997Sperm competition in bats. Proc. R. Soc. Lond. B 264, 385–392 (doi:10.1098/rspb.1997.0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken D. J., Ward P. J.2001Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13 (doi:10.1046/j.1461-0248.2001.00198.x) [Google Scholar]
- Hunt J., Bussiere L. F., Jennions M. D., Brooks R.2004What is genetic quality? Trends Ecol. Evol. 19, 329–333 (doi:10.1016/j.tree.2004.03.035) [DOI] [PubMed] [Google Scholar]
- Kawano K.1995Horn and wing allometry and male dimorphism in giant rhinoceros beetles (Coleoptera, Scarabaeidae) of tropical Asia and America. Ann. Entomol. Soc. Am. 88, 92–99 [Google Scholar]
- Kokko H., Rankin D. J.2006Lonely hearts or sex in the city? Density-dependent effects in mating systems. Phil. Trans. R. Soc. B 361, 319–334 (doi:10.1098/rstb.2005.1784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczek A. P., Nijhout H. F.2004Trade-offs during the development of primary and secondary sexual traits in a horned beetle. Am. Nat. 163, 184–191 (doi:10.1086/381741) [DOI] [PubMed] [Google Scholar]
- Morrison D. F.1990Multivariate statistical methods, 3rd edn New York, NY: McGraw-Hill [Google Scholar]
- Nijhout H. F., Emlen D. J.1998Competition among body parts in the development and evolution of insect morphology. Proc. Natl Acad. Sci. USA 95, 3685–3689 (doi:10.1073/pnas.95.7.3685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Miyatake T.2009Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus cornutus. Anim. Behav. 77, 1057–1065 (doi:10.1016/j.anbehav.2009.01.008) [Google Scholar]
- Okada K., Miyatake T.2010Effect of losing on male fights of broad horned flour beetle, Gnatocerus cornutus. Behav. Ecol. Sociobiol. 64, 361–369 (doi:10.1007/s00265-009-0852-0) [Google Scholar]
- Okada K., Miyanoshita A., Miyatake T.2006Intra-sexual dimorphism in male mandibles and male aggressive behavior in the broad-horned flour beetle Gnatocerus cornutus (Coleoptera: Tenebrionidae). J. Insect Behav. 19, 457–467 (doi:10.1007/s10905-006-9038-z) [Google Scholar]
- Okada K., Nomura Y., Miyatake T.2007Relations between allometry, male–male interactions and dispersal in a sap beetle, Librodor japonicus. Anim. Behav. 74, 749–755 (doi:10.1016/j.anbehav.2006.09.020) [Google Scholar]
- Okada K., Miyatake T., Nomura Y., Kuroda K.2008Fighting, dispersing, and sneaking: body-size dependent mating tactics by male Librodor japonicus beetles. Ecol. Entomol. 33, 269–275 (doi:10.1111/j.1365-2311.2007.00965.x) [Google Scholar]
- Parker G. A.1990Sperm competition games: sneaks and extra-pair copulations. Proc. R. Soc. Lond. B 242, 127–133 (doi:10.1098/rspb.1990.0115) [Google Scholar]
- Parker G. A.1998Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 3–54 London, UK: Academic Press [Google Scholar]
- Pomfret J. C., Knell R. J.2006Sexual selection and horn allometry in the dung beetle Euoniticellus intermedius. Anim. Behav. 71, 567–576 (doi:10.1016/j.anbehav.2005.05.023) [Google Scholar]
- Pomfret J. C., Knell R. J.2008Crowding, sex ratio and horn evolution in a South African beetle community. Proc. R. Soc. B 275, 315–321 (doi:10.1098/rspb.2007.1498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W. R.1989Analyzing tables of statistical tests. Evolution 43, 223–225 (doi:10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- Sasaki T., Okada K., Kajiwara T., Miyatake T.In press On the optimal duration of memory of losing a conflict—a mathematical model approach. J. Biol. Dynam. (doi:10.1080/17513750903161036) [DOI] [PubMed] [Google Scholar]
- Shuster S. M., Wade M. J.2003Mating system and strategies Princeton, NJ: Princeton University Press [Google Scholar]
- Simmons L. W.2001Sperm competition and its evolutionary consequences in the insects Princeton, NJ: Princeton University Press [Google Scholar]
- Simmons L. W., Emlen D. J.2006Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346–16 351 (doi:10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W., Tomkins J. L., Hunt J.1999Sperm competition games played by dimorphic male beetles. Proc. R. Soc. Lond. B 266, 145–150 [Google Scholar]
- SPSS Inc 2001SPSS for Windows version 11.0 Chicago, IL: SPSS Inc [Google Scholar]
- Tomkins J. L., Radwan J., Kotiaho J. S., Tregenza T.2004Genic capture and resolving the lek paradox. Trends Ecol. Evol. 19, 323–328 (doi:10.1016/j.tree.2004.03.029) [DOI] [PubMed] [Google Scholar]
- Tomkins J. L., Kotiaho J. S., LeBas N. R.2005Phenotypic plasticity in the developmental integration of morphological trade-offs and secondary sexual trait compensation. Proc. R. Soc. B 272, 543–551 (doi:10.1098/rspb.2004.2950) [DOI] [PMC free article] [PubMed] [Google Scholar]