Abstract
Aposematic prey advertise their toxicity using conspicuous visual signals that predators quickly learn to avoid. However, it is advantageous for predators not to simply avoid toxic prey, but to learn about the amount of toxin that prey contain, and include them in their diets when the nutritional gains are high relative to the costs of ingesting the toxin. Therefore, when foraging on a defended prey population where individuals vary in their toxin concentration, predators should learn to use cues which distinguish prey with different levels of toxicity in order to include less defended individuals in their diets. In this experiment, we found that European starlings (Sturnus vulgaris) could learn to use a bitter taste to predict the amount of toxin that individual prey contained, and use that information to preferentially ingest less toxic prey to maximize their nutrient intake relative to the amount of toxin ingested. Our results suggest that bitter tastes could evolve as reliable signals of toxicity, and can help to explain why many toxins taste bitter. They also highlight the need to develop new mathematical simulations of the evolution of prey defences which incorporate the adaptive decision-making processes underlying nutrient and toxin management.
Keywords: denatonium, quinine, toxin regulation, avian, aposematism, mimicry
1. Introduction
Many animals possess defensive toxins, which are advertised to predators using conspicuous coloration and other warning signals, such as sounds and odours (Cott 1940; Rothschild & Haskell 1966; Edmunds 1974). This defensive strategy is known as aposematism and is widespread across the animal kingdom (Poulton 1890; Cott 1940; Mappes et al. 2005). The predominant view is that aposematism is an effective defence strategy because naive predators can rapidly learn to associate warning signals with the noxious effects of the toxins that they signal, and reduce their attack rates on them accordingly (Gittleman & Harvey 1980; Roper & Redston 1987; Alatalo & Mappes 1996). Indeed, for more than 100 years, the theoretical framework for aposematism and mimicry (where sympatric species share the same warning pattern) has been developed based upon the speed and durability of avoidance learning in naive predators (Müller 1879; Fisher 1930; Guilford 1990; Speed 1993a, 1999).
However, an alternative view is that predators should not simply learn to avoid toxic prey, since this would reduce their opportunities for gaining nutrients. Aposematic prey contain valuable nutrients as well as toxins, and could potentially be profitable sources of food (Sherratt et al. 2004; Barnett et al. 2007; Skelhorn & Rowe 2007). Therefore, predators should learn about the amount of toxin that aposematic prey contain in order that they can include them in their diets when it is profitable to do so (Skelhorn & Rowe 2007). Perhaps surprisingly, little is known about what animals learn about toxic prey, or how they trade-off the benefits of eating nutrients with the costs of eating of toxins (e.g. Yearsley et al. 2006; Torregrossa & Dearing 2009). However, given that predators are often long-lived and are naive for a relatively short period of their lives, it is essential to know how educated animals learn to regulate their intake of toxic prey in order to fully understand the selection pressures acting on both prey defences and warning signals.
In an aposematic prey population, individuals often vary in the amount of toxin that they contain, with some possessing higher toxin concentrations than others (e.g. Brower et al. 1967; de Jong et al. 1991). Although it is impossible to say what proportion of insect defence chemicals are unpalatable, many toxins commonly used by defended insects taste bitter (Eisner & Meinwald 1966; Blum 1981; Pasteels et al. 1983), which can lead to them being rejected by predators (Järvi et al. 1981; Wiklund & Järvi 1982; Brower & Calvert 1985; Sillén-Tullberg 1985; Gamberale-Stille & Guilford 2004; Skelhorn & Rowe 2006a,b; Halpin et al. 2008a,b). However, since the distastefulness of bitter-tasting toxins often increases with toxin concentration (Schafer et al. 1983), the bitter taste of toxic prey might also allow predators to learn about the toxins contained in prey and preferentially reject individuals with higher quantities of toxin. Because predators appear to have a limit to the amount of toxin that they are willing to ingest (Barnett et al. 2007; Skelhorn & Rowe 2007), it would be advantageous for them to be able to discriminate between individuals that vary in their toxin concentration and preferentially ingest the less defended individuals. This would enable them to gain more nutrients from a defended prey population for a given amount of toxin ingested.
Our experiment provides a crucial test of whether distastefulness can be used by predators as a signal of prey toxicity, and whether they can learn to use taste cues to strategically manage their intake of toxic prey. Using an established protocol of European starlings (Sturnus vulgaris) foraging on insect larvae (Skelhorn & Rowe 2006c, 2007, 2009; Barnett et al. 2007), we can independently manipulate the toxin concentration and the level of distastefulness of prey. Defended prey can be injected with different concentrations of quinine solution, a toxin that can cause an emetic response in humans and birds at high concentrations (Alcock 1970; Bateman & Dyson 1986), but at low concentrations is used widely as an aversant in learning experiments across a range of taxa (e.g. Schoenbaum et al. 1998; Darmaillacq et al. 2004). Although quinine is distasteful, starlings cannot taste it when it is injected into mealworms because they swallow the mealworms whole (Skelhorn & Rowe 2006c, 2007). However, starlings can detect the noxious effects of quinine post-ingestion, and can readily learn to associate the effects of quinine with reliable colour cues, and reduce their intake of quinine-injected mealworms (Skelhorn & Rowe 2006c, 2007; Barnett et al. 2007). Distastefulness can be manipulated independently by coating prey with Bitrex, a bitter-tasting substance that starlings find unpleasant, but is not toxic (Skelhorn & Rowe 2009). Therefore, for the first time to our knowledge, we are able to disassociate the toxicity and distastefulness of prey in a realistic foraging setting. Our experiment tests whether predators learn to use distastefulness to discriminate between prey that vary in their toxin concentrations, and whether they use this information to optimize their intake of toxic prey.
2. Material and methods
(a). Subjects and housing
Ten adult European starlings (five males and five females) were caught in Northumberland and held under an English Nature licence (no. 20062404). Birds were housed individually in wire cages measuring 45 × 75 × 45 cm, with a drawer at the bottom that could be pulled in and out. They were subject to a 10 L : 14 D cycle using florescent lights, and temperatures were maintained at 16–17°C (see Skelhorn & Rowe 2006c for further details). Water was provided ad libitum, as were Zoofood Pheasant breeder pellets and fruit except during training and experimenting when short periods of food deprivation were necessary. At the end of the experiment, birds were returned to a free-flight aviary before being released back into the wild.
(b). Preparation of artificial prey
The prey were mealworms (Tenebrio moloitor) selected to be of similar length (approx. 20 mm). We manipulated the toxin concentration of prey by injecting different concentrations of quinine sulphate solution through the mouthparts of a mealworm using a hypodermic needle. We produced mildly defended prey by injecting mealworms with 0.02 ml of 1 per cent quinine sulphate solution, and moderately defended prey by injecting mealworms with 0.02 ml of 3 per cent quinine sulphate solution. Our previous studies find no evidence that birds taste bitter chemicals when they are injected into mealworms using this method (Skelhorn & Rowe 2006c, 2009).
In order to manipulate the taste of the defended prey independently of their toxin concentration, we coated them with 0.02 ml of one of two Bitrex solutions to create mildly distasteful prey (two drops of 2% Bitrex solution diluted to 100 ml with water) or moderately distasteful prey (eight drops of 2% Bitrex solution diluted to 100 ml with water). Starlings do not reduce their consumption of mealworms coated with these solutions, and it has no measurable toxic effects (Skelhorn & Rowe 2009).
We also gave the birds undefended mealworms in our experimental trials, which were injected and coated with 0.02 ml of water, and were entirely palatable.
(c). General procedure
The experiment had four distinct and consecutive phases: training (2 days); learning (5 days); toxin manipulation (2 days); and, simultaneous choices (2 days; see the electronic supplementary material). In all phases, every subject was given a single trial on each day. Birds were food deprived for 2 h before being moved in their cages behind a white curtain erected in the same room. The bottom of the curtain was level with the drawer in the base of the cage, this visually isolated subjects from both the experimenter and conspecifics, and allowed the experimenter to present prey to the birds without being seen. Birds' behaviour was monitored using video cameras connected to television screens. Each bird had 5 min to acclimatize behind the curtain before the start of a trial.
(d). Training trials
In this phase, birds were trained to eat sequentially presented mealworms (which had not been experimentally manipulated). Birds received two trials of 18 prey presentations, in which the drawer of the cage was pulled out, a Petri dish containing a mealworm was placed in the drawer, and the drawer replaced. Subjects were given 1 min to attack the mealworm before the drawer was pulled out and the Petri dish and the mealworm were removed. If the mealworm was attacked and eaten, the Petri dish was removed immediately. Mealworms were presented every 3 min. In the second trial, all subjects readily attacked the prey and ate all 18 mealworms presented.
(e). Learning trials
All birds were given five learning trials in which they received a sequence of six undefended prey and 12 defended prey (six mildly defended and six moderately defended mealworms). Undefended and defended prey were made visually discriminable by placing purple and green paper discs under the Petri dishes, but mildly and moderately defended prey were visually identical (the colour which signalled defended mealworms was balanced across subjects). Unique prey sequences were produced for each trial for each bird, where prey type was randomized across trial in order that birds could not use temporal cues to determine the palatability of prey.
Before the start of these trials, birds were assigned to one of two experimental groups: the reliable group (three males and two females) and the unreliable group (two males and three females). These two groups differed in whether or not Bitrex reliably predicted the concentration of quinine in the defended prey. Therefore, birds in the reliable group were given six mealworms that were moderately toxic and moderately distasteful, and six mealworms that were mildly toxic and mildly distasteful. However, birds in the unreliable group received six moderately toxic mealworms where half were moderately distasteful and half were mildly distasteful, and six mildly toxic prey where half the mealworms were moderately distasteful and the other half were mildly distasteful. Therefore, the level of distastefulness in the unreliable group could not be used to predict the concentration of toxin in the mealworms. The unreliable group acted as a control to ensure that any discrimination between mildly and moderately defended prey by birds in the reliable group was owing to differences in toxin concentration rather than the level of distastefulness. We recorded the numbers of each prey type attacked and eaten during each training trial.
(f). Toxin manipulation trials
At the end of the training trials, subjects received two toxin manipulation trials on consecutive days. These trials followed the same protocol as the training trials, however, prior to the start of each trial, birds received three additional prey presentations. The additional presentations took the same form as experimental presentations (birds had 1 min to eat each prey item, and prey items were presented every 3 min), and the third additional presentation was made 3 min before the start of the experimental trial. In these presentations, we manipulated the birds' toxin burden, by giving them three mealworms that either contained 0.02 ml of water or 0.02 ml of 3 per cent quinine sulphate solution. When quinine is presented in this way, birds are known to detect its noxious effects (but not its taste) during the experimental trial (Skelhorn & Rowe 2007). These trials tested how birds used what they had learned about the prey to manage their intake of quinine when their toxin burden was raised. Mealworms used in these three pre-feeding presentations did not have any colour or taste associated with them. Three birds in each group received the water-injected mealworms on the first day and quinine-injected mealworms on the second day, while for the other birds the order of the trials was reversed. We recorded the numbers of each prey type attacked and eaten during each trial. We then performed a series of simultaneous choice trials to determine what cues birds were using to make their foraging decisions (see the electronic supplementary material).
3. Results
(a). Learning trials
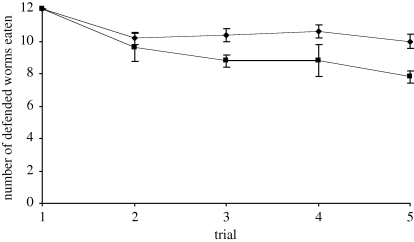
Birds in both groups readily learned to discriminate between quinine-injected and water-injected mealworms. All the birds invariably attacked and ate all the water-injected mealworms in every daily trial, but as expected, learned to reduce the total number of quinine-injected mealworms ingested in each trial over the five days, and ate fewer defended prey in trial 5 compared with trial 1 (Wilcoxon test: reliable group Z = 2.04, p = 0.041, n = 5; unreliable group Z = 2.04, p = 0.041, n = 5; figure 1).
Figure 1.
The mean number (±s.e.m.) of defended mealworms eaten in each of the five training trials (filled squares, unreliable; filled diamonds, reliable).
There was no difference in the total amount of quinine ingested by each group during the learning trials (reliable group, mean = 20.5 ± 0.33 mg; unreliable group, mean = 19.1 ± 0.67 mg; Mann–Whitney test: U = 5.5, p = 0.142, n = 10), indicating that birds were defending a specific toxin burden (see also Skelhorn & Rowe 2007). However, birds in the reliable group ate significantly more defended prey than birds in the unreliable group (Mann–Whitney test: U = 5, p = 0.012, n = 10; figure 1). This can be explained by examining the birds' decision-making behaviour in more detail, and comparing the numbers of moderately and mildly toxic prey that were eaten. Birds in the reliable group attacked all 12 of the quinine-injected mealworms that were presented, and used the taste of Bitrex to preferentially ingest more of the mildly toxic than the moderately toxic prey (Wilcoxon test: Z = 2.06, p = 0.039, n = 5; figure 2). By contrast, birds in the unreliable group were unable to use taste to discriminate among the defended prey (they did not taste-reject prey, and ate equal numbers of mildly and moderately distasteful prey (Wilcoxon test: Z = 0.272, p = 0.785, n = 5)), and consequently ate equal numbers of moderately toxic and mildly toxic mealworms (Wilcoxon test: Z = 1.22, p = 0.223, n = 5; figure 2). Therefore, reliable taste cues enabled birds to eat more of the defended prey and maximize their nutritional intake for the same amount of toxin ingested.
Figure 2.
The mean number (±s.e.m.) of mildly defended mealworms (filled bars) and moderately defended mealworms (open bars) eaten across the five training trials for birds in each experimental group.
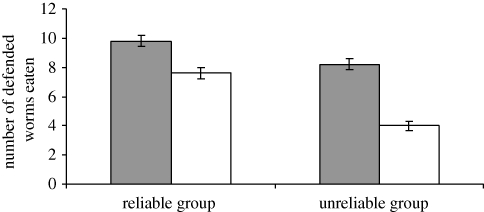
(b). Toxin manipulation trials
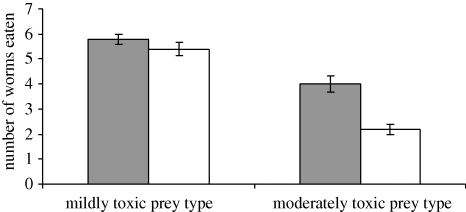
Birds in both groups ate significantly fewer defended mealworms in the trial when they had been pre-fed with quinine compared with when they had been pre-fed with water (Wilcoxon test: reliable group Z = 2.041, p = 0.041, n = 5; unreliable group Z = 2.06, p = 0.039, n = 5; figure 3), although birds in the unreliable group reduced their consumption more than those in the reliable group (Mann–Whitney test: Z = 2.47, p = 0.013, n = 10; figure 3). This difference can again be explained by examining their consumption of mildly and moderately toxic prey. Birds in the reliable group reduced their consumption of moderately toxic mealworms more than their consumption of mildly toxic mealworms when pre-fed with quinine (Wilcoxon test: Z = 2.070, p = 0.038, n = 5; figure 4). Therefore, they had not only learned to use taste cues to predict preys' toxin concentration, but they could use taste to reduce their ingestion of the moderately toxic prey relative to the mildly toxic prey when their toxin burden increased. However, birds in the unreliable group reduced their ingestion of both moderately and mildly toxic worms equally (Wilcoxon test: Z = 1.134, p = 0.257, n = 5). As in the learning phase, no bird in this group showed any taste-rejection behaviour, and there was no preference for mildly distasteful prey (Wilcoxon test: Z = 1.142, p = 0.180, n = 5). The results of our simultaneous choice trials show that all birds had learned to associate preys' coloration with their defences, and that in the absence of colour cues, birds could not detect chemical cues prior to attack (see the electronic supplementary material).
Figure 3.
The mean number (±s.e.m.) of defended mealworms eaten after being pre-fed with water-injected worms (filled bars) and after being pre-fed with quinine-injected worms (open bars) by birds in each experimental group.
Figure 4.
The mean number (±s.e.m.) of mildly defended mealworms and moderately defended mealworms eaten by birds in the reliable group after being pre-fed with water-injected worms (filled bars) and after being pre-fed with quinine-injected worms (open bars).
4. Discussion
This study, to our knowledge, provides the first evidence that predators learn to associate variation in a bitter taste with different levels of toxin in order to both manage their toxin burdens, and optimize their nutrient intake. We found that when taste was a reliable predictor of toxicity, birds learned about the toxin concentrations of their prey, and used taste to selectively-reject moderately toxic prey. This allowed birds given prey with reliable taste cues to consume more toxic prey items than birds given unreliable taste cues, while maintaining the same toxin burden. Consequently, the presence of reliable bitter taste cues allowed birds to obtain more nutrients from the defended prey population.
Previous studies of taste-rejection behaviour in birds have implicitly assumed that the rejection behaviour is instinctive or that the bitter taste itself is a feeding deterrent (e.g. Skelhorn & Rowe 2006a; Halpin et al. 2008a,b). However, by manipulating the association between bitter taste and toxicity, we are able to show instead that the taste-rejection of distasteful prey depends upon a learned association between taste and toxicity. Indeed, we found no evidence that the starlings rejected prey on the basis of taste unless it was a reliable indicator of toxicity. Furthermore, our data clearly show that birds in the reliable group learned the relative toxicity of mildly and moderately distasteful prey, since when their toxin burdens were artificially increased they reduced their consumption of moderately defended prey more than mildly defended prey.
It is well established that animals use taste to detect toxins in the foods that they eat, and that they can learn to associate pre-ingestive cues (e.g. the colour, odour or taste) with the ill effects of toxins in order to reduce their intake of toxic foods (e.g. Darmaillacq et al. 2004; Glendinning 2007). Indeed, conditioned taste aversion (CTA), where animals learn to associate a taste with the post-ingestive effects of a toxin, is perhaps one of the most well-studied learning paradigms (e.g. Bernstein 1999 for review). CTA studies primarily focus on the acquisition of the aversion by investigating the specific cues which are associated with toxicity or the speed or strength of the learned avoidance. By contrast, our approach demonstrates that birds use learned information about toxins in food in a naturalistic foraging task, and shows that what birds learn about toxic food affects the foraging strategies they employ to regulate their nutritional intake. What is clear from our data is that animals do not just learn to reduce their consumption of toxic food, but rather learn to select foods to maximize their nutrient intake relative to the amount of toxin that they are willing to ingest. Although long-term data suggest that free-ranging herbivores are able to select plants that are relatively high in nutrients and low in toxins (Moore & Foley 2005), this is, to our knowledge, the first demonstration of the way that a reliable gustatory signal could mediate this foraging strategy.
Knowing how predators learn to use distasteful cues to forage on toxic prey potentially changes the way in which we think about and study the evolution of prey defences and warning signals. First, our study may help to explain why many defence chemicals are often bitter tasting (Eisner & Meinwald 1966; Blum 1981; Pasteels et al. 1983). Reliable taste signals allow predators to selectively reject individuals that have invested more heavily in toxins, which would increase their chance of surviving predatory attacks, and benefit more from their investment in costly toxins. Conversely, individuals that invested less in toxins would be less likely to survive attack. Clearly it would benefit less defended individuals to dishonestly signal their level of toxicity. However, if predators have evolved bitter taste receptors in order to detect toxins, their bitter taste will provide inherently reliable information about the toxin concentration of the individuals that possess them. This will promote investment in chemical defences, which has been a major challenge to evolutionary theories of aposematism (Brower et al. 1967; Guilford 1994), and make distasteful toxins more evolutionarily stable than those that cannot be detected upon attack.
We are therefore left with the question: if distasteful toxins can evolve as reliable signals of toxicity, why have warning signals in other sensory modalities evolved (Rowe 1999; Rowe & Guilford 1999)? In contrast to distastefulness, warning signals in other sensory modalities, such as colours, odours, sounds and behavioural displays can all be perceived by predators prior to attack. Therefore these signals can provide information to predators that could reduce either the probability of an attack, or the probability of being damaged through more cautious handling by the predator (Sillén-Tullberg 1985; Rowe & Guilford 1999). Intriguingly, our experiment suggests that birds use colour signals if they provide the best information about toxin ingestion (i.e. if there are no reliable taste signals), or if it allows them to preferentially select more palatable prey. In addition, we might also expect that colour signals reduce the attack probability once a predator's maximum toxin burden is reached, since the predator no longer needs to discriminate between prey with different toxin concentrations. Selection on aposematic coloration is therefore likely to depend upon the variability in prey toxin concentrations and the amount of toxin the predator is willing to ingest, and not just the predator's ability to associate the coloration with the toxin. While there have been a few recent studies of the role of toxin regulation in the evolution of prey defences (Sherratt 2003; Sherratt et al. 2004; Barnett et al. 2007; Skelhorn & Rowe 2007), little is known about the cognitive and physiological processes underlying toxin regulation and decision-making when foraging on toxic prey. Therefore, this finding opens a novel avenue for understanding the evolutionary dynamics of warning coloration and prey defences more generally.
Finally, our findings demonstrate an important role for ‘educated predators’ in the evolution of prey defences. The theoretical foundations of aposematism and mimicry rest predominantly upon learning algorithms (Müller 1879; Fisher 1930; Guilford 1990; Speed 1993a, 1999), which are tested empirically by measuring the acquisition behaviour of naive predators (e.g. Gittleman & Harvey 1980; Alatalo & Mappes 1996; Rowe et al. 2004; Rowland et al. 2007). However, predators will not always be naive, and are likely to spend more of their lives as educated rather than naive predators. Educated predators will have learned to use different signals to predict prey toxicity, and use this knowledge in their foraging decisions, which will generate different selection pressures on warning signals. However, if we can understand this and integrate it with what we know about avoidance behaviour in naive predators, we might start to fully understand the variety and complexity of prey defences.
Acknowledgements
All deprivation periods and experimental procedures were in accordance with Home Office regulations and ASAB ethical guidelines.
This work was supported by a BBSRC Research grant (BB/D003 245/1), and a Lloyds Tercentenary Foundation Fellowship awarded to J. S. We would like to thank Lin Hedgcock and Michelle Waddle for husbandry, the Centre for Behaviour and Evolution's Weekly Meetings, for thought-provoking discussions and Jeri Wright for helpful comments on our manuscript.
References
- Alatalo R. V., Mappes J.1996Tracking the evolution of warning signals. Nature 382, 708–710 (doi:10.1038/382708a0) [Google Scholar]
- Alcock J.1970Punishment levels and the response of Black-capped Chickadees (Parus atricapillus) to three kinds of artificial seeds. Anim. Behav. 18, 592–599 (doi:10.1016/0003-3472(70)90057-6) [Google Scholar]
- Barnett C. A., Bateson M., Rowe C.2007State-dependent decision making: educated predators strategically trade off the costs and benefits of consuming aposematic prey. Behav. Ecol. 18, 645–651 (doi:10.1093/beheco/arm027) [Google Scholar]
- Bateman D. N., Dyson E. H.1986Quinine toxicity. Adverse Drug React. Toxicol. Rev. 5, 215–233 [PubMed] [Google Scholar]
- Bernstein I. L.1999Taste aversion learning: a contemporary perspective. Nutrition 15, 229–234 (doi:10.1016/S0899-9007(98)00192-0) [DOI] [PubMed] [Google Scholar]
- Bezzerides A. L., McGraw K. J., Parker R. S., Husseni J.2007Elytra colour as a signal of chemical defense in the Asian ladybird beetle Harmonia axyridis. Behav. Ecol. Sociobiol. 61, 1401–1408 (doi:10.1007/s00265-007-0371-9) [Google Scholar]
- Blum M. S.1981Chemical defences of arthropods. New York, NY: Academic Press [Google Scholar]
- Brower L. P., Calvert W. H.1985Foraging dynamics of bird predators on overwintering monarch butterflies in Mexico. Evolution 39, 852–868 (doi:10.2307/2408685) [DOI] [PubMed] [Google Scholar]
- Brower L. P., Brower J. V. Z., Corvino J. M.1967Plant poisons in a terrestrial food chain. Proc. Natl Acad. Sci. USA 57, 893–898 (doi:10.1073/pnas.57.4.893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J., Mueller K. L., Hoon M. A., Adler E., Feng L., Guo W., Zuker C. S., Ryba N. J. P.2000T2Rs function as bitter taste receptors. Cell 100, 703–711 (doi:10.1016/S0092-8674(00)80706-0) [DOI] [PubMed] [Google Scholar]
- Cott H. B.1940Adaptive coloration in animals London, UK: Methuen [Google Scholar]
- Darmaillacq A.-S., Dickel L., Chichery M.-P., Agin V., Chichery R.2004Rapid taste aversion learning in adult cuttlefish Sepia officinalis. Anim. Behav. 68, 1291–1298 (doi:10.1016/j.anbehav.2004.01.015) [Google Scholar]
- de Jong P. W., Holloway G. J., Brakefield P. M., Vos H.1991Chemical defense in the ladybird beetles (Coccinellidae). II. Amount of reflex fluid, the alkaloid adaline and individual variation in defense in 2-spot ladybirds (Adalia bipunctata). Chemoecology 2, 15–19 [Google Scholar]
- Dong D., Jones G., Zhang S.2009Dynamic evolution of bitter taste receptor genes in vertebrates. BMC Evol. Biol. 9, 12 (doi:10.1186/1471-2148-9-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds M.1974Defence in animals Harlow, UK: Longman [Google Scholar]
- Eisner T., Meinwald J.1966Defensive secretions of arthropods. Science 153, 1341–1350 (doi:10.1126/science.153.3742.1341) [DOI] [PubMed] [Google Scholar]
- Fink L. S., Brower L. P.1981Birds can overcome the cardenolide defence of monarch butterflies in Mexico. Nature 291, 67–70 (doi:10.1038/291067a0) [Google Scholar]
- Fisher R. A.1930The genetical theory of natural selection. Oxford, UK: Clarendon Press [Google Scholar]
- Gamberale-Stille G., Guilford T.2004Automimicry destabilizes aposematism: predator sample-and-reject behaviour may provide a solution. Proc. R. Soc. Lond. B 271, 2621–2625 (doi:10.1098/rspb.2004.2893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittleman J., Harvey P. H.1980Why are distasteful prey not cryptic? Nature 286, 149–150 (doi:10.1038/286149a0) [Google Scholar]
- Glendinning J. I.2007How do predators cope with chemically defended food? Biol. Bull. 213, 252–266 [DOI] [PubMed] [Google Scholar]
- Guilford T.1990The evolution of aposematism. In Insect defences: adaptive mechanisms and strategies of prey and predators (eds Evans D. L., Schmidt J. O.), pp. 23–61 Albany, NY: State of New York Press [Google Scholar]
- Guilford T.1994‘Go-slow’ signalling and the problem of automimicry. J. Theor. Biol. 170, 311–316 (doi:10.1006/jtbi.1994.1192) [Google Scholar]
- Halpin C., Skelhorn J., Rowe C.2008aNaïve predators and selection for rare conspicuous defended prey: the initial evolution of aposematism revisited. Anim. Behav. 75, 771–781 (doi:10.1016/j.anbehav.2007.06.009) [Google Scholar]
- Halpin C., Skelhorn J., Rowe C.2008bBeing conspicuous and defended: selective benefits for the individual. Behav. Ecol. 19, 1012–1017 (doi:10.1093/beheco/arn069) [Google Scholar]
- Järvi T., Sillén-Tullberg B., Wiklund C.1981The cost of being aposematic. An experimental study of predation on larvae of Papilio machaon by the great tit Parus major. Oikos 36, 267–272 (doi:10.2307/3544623) [Google Scholar]
- Leimar O., Enquist M., Sillén-Tullberg B.1986Evolutionary stability of aposematic coloration and prey unprofitability: a theoretical analysis. Am. Nat. 128, 469–490 (doi:10.1086/284581) [Google Scholar]
- Mappes J., Marples N., Endler J. A.2005The complex business of survival by aposematis. Trends. Ecol. Evol. 20, 598–603 (doi:10.1016/j.tree.2005.07.011) [DOI] [PubMed] [Google Scholar]
- Moore B. D., Foley W. J.2005Tree use by koalas in a chemically complex landscape. Nature 435, 488–490 (doi:10.1038/nature03551) [DOI] [PubMed] [Google Scholar]
- Müller F.1879Ituna and Thyridia: a remarkable case of mimicry in butterflies. Trans. Entomol. Soc. Lond. 1879, 20–29 [Google Scholar]
- Pasteels J. A., Gregoire J. C., Rowell-Rahter M.1983The chemical ecology of defence in arthropods. Annu. Rev. Entomol. 28, 263–289 (doi:10.1146/annurev.en.28.010183.001403) [Google Scholar]
- Poulton E. B.1890The colours of animals: their meaning and use especially considered in the case of insects London, UK: Keghan, Paul, Trench, Trubner and Co. Ltd [Google Scholar]
- Roper T. J., Redston S.1987Conspicuousness of distasteful prey affects the strength and durability of one-trial avoidance learning. Anim. Behav. 35, 739–747 (doi:10.1016/S0003-3472(87)80110-0) [Google Scholar]
- Rothschild M., Haskell P. T.1966Stridulation of the garden tiger moth Arctia caja L. audible to the human ear. Proc. R. Entomol. Soc. Lond. A 41, 167–170 (doi:10.1111/j.1365-3032.1966.tb00337.x) [Google Scholar]
- Rowe C.1999Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921–931 (doi:10.1006/anbe.1999.1242) [DOI] [PubMed] [Google Scholar]
- Rowe C., Guilford T.1999The evolution of multimodal warning displays. Evol. Ecol. 13, 655–671 (doi:10.1023/A:1011021630244) [Google Scholar]
- Rowe C., Lindström L., Lyytinen A.2004The importance of pattern similarity between Müllerian mimics in predator avoidance learning. Proc. R. Soc. Lond. B 271, 407–413 (doi:10.1098/rspb.2003.2615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland H. M., Ihalainen E., Lindström L., Mappes J., Speed M. P.2007Co-mimics have a mutualistic relationship despite unequal defences. Nature 448, 64–67 (doi:10.1038/nature05899) [DOI] [PubMed] [Google Scholar]
- Schafer E. W., Bowels W. A., Hurlbut J.1983The acute oral toxicity, repellency, and hazard potential of 998 chemicals to one or more species of wild and domestic birds. Arch. Environ. Contam. Toxicol. 12, 355–382 (doi:10.1007/BF01059413) [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Chiba A. A., Gallagher M.1998Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat. Neurosci. 1, 155–159 (doi:10.1038/407) [DOI] [PubMed] [Google Scholar]
- Sherratt T. N.2003State-dependent risk-taking by predators in systems with defended prey. Oikos 103, 93–100 (doi:10.1034/j.1600-0706.2003.12576.x) [Google Scholar]
- Sherratt T. N., Speed M. S., Ruxton G. D.2004Natural selection on unpalatable species imposed by state-dependent foraging behaviour. J. Theor. Biol. 228, 217–226 (doi:10.1016/j.jtbi.2003.12.009) [DOI] [PubMed] [Google Scholar]
- Sillén-Tullberg B.1985Higher survival of an aposematic than of a cryptic form of a distasteful bug. Oecologia 67, 411–415 (doi:10.1007/BF00384948) [DOI] [PubMed] [Google Scholar]
- Skelhorn J., Rowe C.2006aAvian predators taste-reject aposematic prey on their level of chemical defence. Biol. Lett. 2, 348–350 (doi:10.1098/rsbl.2006.0483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelhorn J., Rowe C.2006bTaste-rejection by predators can explain the evolution of unpalatability. Behav. Ecol. Sociobiol. 60, 550–555 (doi:10.1007/s00265-006-0199-8) [Google Scholar]
- Skelhorn J., Rowe C.2006cPredator avoidance learning of prey with secreted or stored defences and the evolution of insect defences. Anim. Behav. 72, 835–842 [Google Scholar]
- Skelhorn J., Rowe C.2007Predators' toxin burdens influence their strategic decisions to eat toxic prey. Curr. Biol. 17, 1479–1483 (doi:10.1016/j.cub.2007.07.064) [DOI] [PubMed] [Google Scholar]
- Skelhorn J., Rowe C.2009Distastefulness as an anti-predator defence strategy. Anim. Behav. 78, 761–766 (doi:10.1016/j.anbehav.2009.07.006) [Google Scholar]
- Speed M. P.1993aMuellerian mimicry and the psychology of predation. Anim. Behav. 45, 571–580 (doi:10.1006/anbe.1993.1067) [Google Scholar]
- Speed M. P.1993bWhen is mimicry good for predators? Anim. Behav. 46, 1246–1248 (doi:10.1006/anbe.1993.1321) [Google Scholar]
- Speed M. P.1999Batesian, quasi-Batesian or Müllerian mimicry? Theory and data in mimicry research. Evol. Ecol. 13, 755–776 (doi:10.1023/A:1010871106763) [Google Scholar]
- Torregrossa A.-M., Dearing M. D.2009Nutritional toxicology of mammals: regulated intake of secondary compounds. Funct. Ecol. 23, 48–56 (doi:10.1111/j.1365-2435.2008.01523.x) [Google Scholar]
- Wiklund C., Järvi T.1982Survival of distasteful insects after being attacked by naïve birds: a reappraisal of the theory of aposematic coloration evolving through individual selection. Evolution 36, 998–1002 (doi:10.2307/2408077) [DOI] [PubMed] [Google Scholar]
- Yearsley J. M., Villalba J. J., Gordon I. J., Kyriazakis I., Speakman J. R., Tolkamp B. J., Illius A. W., Duncan A. J.2006A theory of associating food types with their postingestive consequences. Am. Nat. 167, 705–716 (doi:10.1086/502805) [DOI] [PubMed] [Google Scholar]