Abstract
Many animals use the spectral distribution of light to guide behaviour, but whether they have colour vision has been debated for over a century. Our strong subjective experience of colour and the fact that human vision is the paradigm for colour science inevitably raises the question of how we compare with other species. This article outlines four grades of ‘colour vision’ that can be related to the behavioural uses of spectral information, and perhaps to the underlying mechanisms. In the first, even without an (image-forming) eye, simple organisms can compare photoreceptor signals to locate a desired light environment. At the next grade, chromatic mechanisms along with spatial vision guide innate preferences for objects such as food or mates; this is sometimes described as wavelength-specific behaviour. Here, we compare the capabilities of di- and trichromatic vision, and ask why some animals have more than three spectral types of receptors. Behaviours guided by innate preferences are then distinguished from a grade that allows learning, in part because the ability to learn an arbitrary colour is evidence for a neural representation of colour. The fourth grade concerns colour appearance rather than colour difference: for instance, the distinction between hue and saturation, and colour categorization. These higher-level phenomena are essential to human colour perception but poorly known in animals, and we suggest how they can be studied. Finally, we observe that awareness of colour and colour qualia cannot be easily tested in animals.
Keywords: colour vision, phototaxis, colour preference, colour learning, colour categorization, chromaticity
1. Introduction
Colour science is founded on human perception, even in the definition of ‘light’ as the human-visible part of the electromagnetic spectrum (electronic supplementary material, box; Wyszecki & Stiles 1982). An anonymous late 18th century work, attributed to John Elliot (1786) by Mollon (1987), was perhaps first to recognize that light is part of a wider spectrum. Similarly, the 18th century saw a realization that plant and animal colours do not exist simply for human pleasure (Sprengel 1793; von Frisch 1943). When insects and Daphnia were found to see a broader spectrum than humans, biologists such as Lubbock (1882, 1888) started to call ultraviolet and infrared radiation light (Lubbock 1882). Similarly, interest in animal senses led to the first tests of animal colour vision (Lubbock 1888).
Despite the modern understanding of our place in nature, the emphasis on human cognition, and even culture, makes the attribution of colour vision to other species controversial (Hess 1910; von Frisch 1912; Thompson et al. 1992; Stoerig 1998; O'Regan & Noe 2001; Skorupski & Chittka in press). In his pioneering studies, von Frisch (1913, 1914) trained bees and fish to colour stimuli; he had an operational definition of colour vision as ‘the ability to discriminate two uniform lights irrespective of their relative intensities’ (i.e. by their spectral composition; Wyszecki & Stiles 1982). However, von Frisch's method partly accounts for the view that learning is fundamental to ‘true’ colour vision, as distinct from ‘wavelength-specific behaviours’, which are not modified by experience (Wigglesworth 1976; Menzel 1979; Goldsmith 1991). One rationale for this distinction is that learning requires a neural representation of colour. Similarly, Skorupski & Chittka (in press) argue that vision ‘entails the identification of shapes, sizes and locations of objects in the world’, which may imply an internal representation of the physical stimulus; it follows that tests based on training to objects of defined shapes and transfer to objects of different shapes are required to demonstrate vision. Stoerig and others, who study primate blindsight,1 propose a still stronger criterion that distinguishes between vision and sight, and requires awareness or sensation of colour (Stoerig & Cowey 1997; Heywood et al. 1998; Stoerig 1998). This criterion puts the subject more or less beyond the reach of study on non-human species.
By comparison with other sensory faculties, the debate about colour vision is unusual. For hearing, olfaction or polarization vision, sensitivity to a physical stimulus is the criterion, which is established in simple behavioural tests. Argument about definitions is often best left to aficionados, but the criteria for colour vision raise questions about the behavioural uses of spectral information, the underlying neural mechanisms and even the sensory experience of other species (Nagel 1974). At a more practical level, these issues are relevant to the question of how biologically important colour stimuli such as communication signals are seen by their intended receivers (Guilford & Dawkins 1991).
Clearly, the capabilities of a sensory system depend on how it serves behaviour. Animals from many phyla have some type of colour vision, which suggests a general utility for spectral information. Such widespread occurrence is perhaps surprising, because in natural conditions chromatic signals (electronic supplementary material, box) are weak and noisy compared with achromatic signals (i.e. of low bandwidth; Ruderman et al. 1998; van Hateren et al. 2002). This might imply that investment in multiple photoreceptors and additional neural processing would not pay off. In practice, spectral information is used in two main contexts: the illumination spectrum (rather than simple intensity) often drives phototaxes, and reflectance spectra are used to identify the material properties of objects (Brainard & Maloney 2004).
We suggest four main grades of colour vision that relate to the uses of spectral information and may correspond to successive levels of complexity in the underlying mechanisms. This scheme risks placing humans at the top of a scala naturae, but is perhaps justified by the insight into colour that our experience and visual ecology gives. More simply, the terms and concepts from colour science are widely applied to animals (figure 1; electronic supplementary material, box; Endler 1992; Bennett et al. 1994).
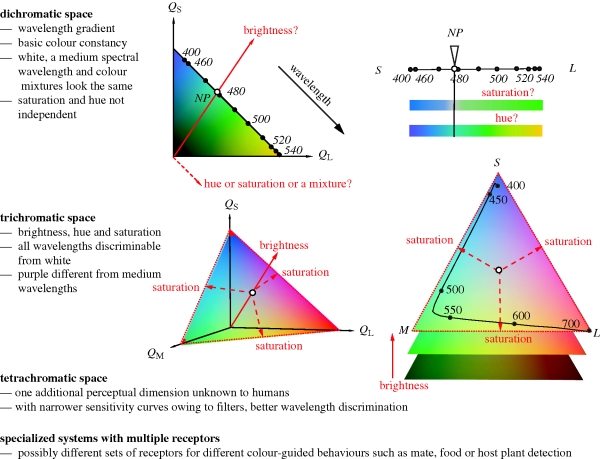
Figure 1.
Colour spaces (receptor spaces, left side; see also electronic supplementary material, box) and chromatic spaces (right side) of dichromats and trichromats illustrating the relationship between the human percepts of hue, saturation and brightness and the physiological receptor axes. As dichromatic colour space only has two dimensions, hue, saturation and brightness are not independent. A tetrachromatic space (probably possessed by birds and fish) may theoretically add a fourth dimension to colour (akin to hue or saturation), but the physical interpretation and ecological significance of this are not obvious. While organisms such as butterflies and stomatopods have many more than four types of spectral receptors, it is not clear how they are used. To our knowledge, there is no evidence for ‘pentachromatic’ or higher-dimensional colour vision. QS, QM, QL are quantum catches of receptors sensitive to short, medium and long wavelengths, and S, M and L in chromatic spaces depict hypothetical colours eliciting signals in the respective receptor only. NP indicates the neutral point where a spectral light appears the same to the dichromat as broad-spectrum white light.
2. Levels of colour vision
(a). Photokinesis and phototaxis: use of colour without spatial vision
In water, the ambient spectrum depends upon depth and water quality. For example, Daphnia move to yellowish water, probably because it is rich in algae. An early test of animal colour vision by Lubbock (1888) showed that Daphnia phototaxis is sensitive to the light spectrum, rather than intensity alone. Lubbock concluded that Daphnia have colour vision, but cautioned that it would be ‘impossible to prove that they actually perceive colours’. The fact that Daphnia magna has four spectral types of photoreceptors (Smith & Macagno 1990) nicely illustrates how such ‘simple’ animals may have complex spectral coding (§2b(ii)).
Colour-guided phototaxis like Daphnia's is common to many animals, and as well as unicellular organisms (e.g. Menzel 1979; Spudich & Bogomolni 1984; Steverding & Troscianko 2003). Theoretically, image-forming eyes are not needed for non-directional responses (‘kineses’), though even single-celled organisms often have some types of directionality (Jékely 2009). All that are needed are two directionally sensitive photoreceptors with different spectral sensitivities and an opponent interaction to compare their responses. Sometimes, temporal comparison of signals from two spectral types of receptors translates into directional information, as when scanning bees and ants discriminate the solar from the anti-solar half of the sky (Wehner 1989). Similarly, a nematode that lacks spatial resolution may achieve a colour-guided phototaxis by comparing the colour signal with a set preference value (Croll 1966; also Menzel 1979). These studies show that information about the illumination spectrum is useful to the simplest systems, which lack a focused image and have minimal processing capabilities. More advanced colour vision could have evolved from such origins.
(b). Innate preferences for coloured objects and the dimensionality of colour vision
Compared with light sources, reflecting objects are generally a much richer source of information: there are far more ‘kinds’ of object than lights. Here, an eye that produces a focused image on a retina is virtually essential (Land & Nilsson 2002). Some caterpillar stemmata come close to having the minimal 2-pixel colour image, which requires four photoreceptors (Ichikawa & Tateda 1982; Nilsson 2009). The widespread use of colour vision in relatively simple animals is striking, and consistent with the idea that chromatic signals allow object recognition with simpler neural mechanisms than are needed for form vision (Marr 1982; Mather 2006).
Innate colour-sensitive responses to objects, sometimes referred to as wavelength-specific behaviours (§2c), are therefore found where recognition needs to be robust to environmental light changes (Campenhausen 1986; Osorio & Vorobyev 2005), such as for mate and host recognition. For example, female fiddler crabs (Uca mjoebergi) prefer yellow (long-wavelength reflecting) claws to white or grey, with both real and dummy males (Detto 2007). Similarly, the attraction of male glow-worms (Lampyris noctiluca) to 550 nm (green) light, which resembles female bioluminescence, is reduced by addition of shorter wavelengths (450 nm; Booth et al. 2004). This evidence for an opponent interaction is perhaps surprising because one might expect detection of bioluminescence to place a premium on absolute sensitivity (i.e. total photon catch). Even here, one must assume that that signal reliability more than offsets the cost owing to the increased noise in chromatic mechanisms (figure 2; electronic supplementary material, box).
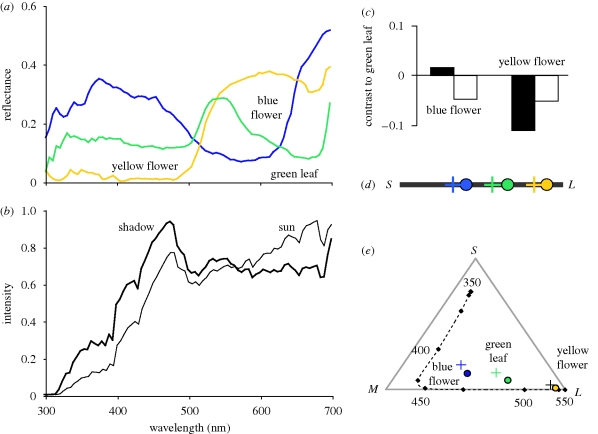
Figure 2.
(a) The spectrum reflected from flowers and green vegetation changes under changing illumination, e.g. (b) sun and shady spots, and leads to (c) changes in achromatic contrast that make object recognition difficult (black bars, shadow; white bars, sun). However, (d) even in dichromatic (but more obvious in trichromatic) colour space, all colours shift in the same direction. With colour constancy, colour shifts become even smaller (adapted from Kelber & Roth 2006). (d,e) Filled coloured circles show colour loci of objects in sunny spots, and crosses show colour loci of objects in the shade. For details of colour space diagrams, see figure 1.
To investigate the mechanisms underlying these behaviours, it is useful to start with knowledge of photoreceptor spectral sensitivities. One can then specify receptor responses for a given stimulus spectrum, and hence infer how these signals and opponent mechanisms are used in behaviour (Kelber et al. 2003). The simplest task is to identify receptor inputs to achromatic behaviours (figures 1 and 2; electronic supplementary material, box); for example, honeybee escape and dorsal light responses are controlled by UV receptors, and the optomotor response is controlled by green receptors (Menzel 1979; Srinivasan & Lehrer 1988). Similarly, one can identify inputs to chromatic mechanisms: those we have described for fireflies and fiddler crabs probably involve two spectral types of photoreceptors. Likewise, fruitflies (Dacus) and aphids searching for a site to lay eggs use an opponent mechanism to guide their flight towards surfaces that reflect long-wavelength light (Moericke 1949; Kelber 2001). Most studies of behaviours that implicate a single ‘perceptual dimension’ (or neural mechanism) suggest that this is either purely chromatic or purely achromatic. It is an open question whether the dimension ever lies on a mixed chromatic and achromatic axis (figure 1).
An apparent weakness of many spectrally selective behaviours is that by comparing outputs of just two receptors (sensitive to long and short wavelengths) they simply prefer either short or long wavelengths, rather than a particular peak value. For example, many insects prefer yellow to green when actually searching for green leaves, which have a reflectance peak at 550 nm (figure 2a; Prokopy & Owens 1983). Papilio butterflies avoid this shortcoming by using short-, medium- and long-wavelength receptors to give a ‘green –(red + blue)’ chromatic signal when egg-laying. This allows them to find green leaves, avoiding both longer and shorter wavelengths (Lythgoe 1979; Kelber 1999).
Papilio's oviposition behaviour is interesting in that, although relying on three receptor types, it is explained by a single chromatic interaction, and hence is dichromatic (electronic supplementary material, box; Kelber 1999). In contrast, when Papilio butterflies choose nectar sources (i.e. flowers), they are tetrachromatic (figure 1; Koshitaka et al. 2008). But even tetrachromacy falls short of the theoretical capability of these butterflies, which have up to eight spectral types of receptors (§2b).
(i). Dichromacy, trichromacy and colour constancy
Behavioural studies typically test whether a species (or behaviour) has colour vision as opposed to being colour-blind (i.e. monochromatic; electronic supplementary material, box). That is, they test whether the animal can separate the spectral composition of a stimulus from its overall intensity. At a minimum, this requires two dimensions of colour—typically one achromatic and one chromatic—and is said to be dichromatic (Jacobs 1981; Kelber et al. 2003). However, many animals have more than two spectral receptors. What then are the consequences of increasing receptor numbers and the (potential) dimensionality of colour vision?
The signal of a single receptor type can give reliable information about a light source, but not a reflecting object. This is why deep-sea fish looking at bioluminescent objects can manage with a pure rod retina (Douglas & Partridge 1997), and why the finding that glow-worms use a colour signal came as a surprise (Booth et al. 2004; §2b). Reflecting objects change luminance with illumination colour and thus cannot be detected reliably under changing illumination (e.g. Campenhausen 1986).
The three basic dimensions of colour recognized by trichromatic humans—hue, saturation and brightness—are probably ecologically meaningful in that they give different types of information about pigmentation, lustre and other material qualities. Thus, the difference between dichromatic and trichromatic (or higher) systems may be comparable to that between dichromacy and ‘colour-blindness’ (i.e. monochromacy). A dichromatic eye generally cannot separate the three dimensions of colour and is especially likely to confound changes in wavelength (roughly ‘hue’) from spectral purity (‘saturation’; figure 1; Wachtler et al. 2004; Roth et al. 2007). An obvious example is that at the dichromatic ‘neutral point’, achromatic (spectrally flat) light matches a certain monochromatic wavelength (Jacobs & Yolton 1969); consequently, dichromats tend to fail tests of discrimination of certain colours from all shades of grey (Roth et al. 2007). This ambiguity in the causes of signals (e.g. confusion of spectral peak and spectral purity) may well explain why, as we have mentioned, both innate and learnt dichromatic responses are commonly directed to either short or long wavelengths.
Notwithstanding their limitations, it is worth noting that simple systems may achieve colour constancy, simply by receptor adaptation to the mean light intensity—known as a von Kries mechanism (figure 2; Foster & Nascimento 1994). This allows the animal, with minimal neural processing, to recognize an object in sunlight or shade and even in a patchy light environment (e.g. Osorio et al. 1997; Kelber & Roth 2006). By comparison, the huge variation of illumination intensity across natural images makes the equivalent achromatic process—lightness constancy—relatively ineffective (Robilotto & Zaidi 2004). Indeed, it has been suggested that colour vision evolved, in the first place, to gain constancy (Campenhausen 1986; Maximov 2000). Though human colour constancy involves high-level mechanisms (Bloj et al. 1999), we are aware of no tests of animal colour constancy that convincingly exclude von Kries-type mechanisms (but see Lotto & Wicklein 2005).
(ii). Why have more than three receptors?
One might argue that trichromacy is ideal for colour vision: not only does it give separate dimensions of hue, saturation and brightness (figure 1), but it has long been observed that three components are adequate for coding most variation in natural spectra with opsin-based pigments (Barlow 1982; Maloney 1986; Kelber et al. 2003). Indeed, there is no good explanation of why many animals have more than three types of spectral receptors (Barlow 1982; Vorobyev 2003; Osorio & Vorobyev 2008). Why do birds have five cone types (and tetrachromatic colour vision), butterflies have up to eight types of receptors (Koshitaka et al. 2008) and stomatopod crustaceans up to sixteen (Osorio et al. 1997; Cronin & Marshall 2004)? Possible answers are benefits of narrowly tuned receptors for colour discrimination and colour constancy (Osorio et al. 1997; Vorobyev 2003), or that different sets of receptors are used for different behaviours, perhaps to give multiple specialized di- or trichromatic systems. The latter idea is attractive, but evidence is weak. Possible examples come from butterflies, such as the swallowtail Papilio xuthus, which has eight spectral types of receptors, but appears to use only four types for colour discrimination (Koshitaka et al. 2008). Similarly, as mentioned above, Papilio aegeus seems to use only three of its receptor types (probably also eight in total) for choosing oviposition substrates (Kelber 1999).
Potentially very strong driving forces for the evolution of additional receptors are sexual selection and sexual drive (Boughman 2002), leading to coevolution with colour signals, perhaps as a Fisherian runaway process. Although the question of the coevolution of senses and colour signals has a long history (Allen 1879; Cronin 1991), there is little evidence for photoreceptor spectral sensitivities (or any other mechanism of colour vision) evolving in response to sexual or other communication signals, rather than colour signals ‘exploiting’ general-purpose mechanisms (e.g. Seehausen et al. 2008). Once again, the evolutionary variation in receptor sensitivities of butterflies may provide an exception to this generalization, as there is some evidence that this is driven by sexual signalling (Frentiu & Briscoe 2008). In Nymphalidae, there is a correlation between the occurrence of new UV-reflecting wing pigments and a duplicated gene for UV pigment in Heliconius (Briscoe et al. in press). Similarly, the East Asian form of the small white butterfly Pieris rapae crucivora is sexually dimorphic, with the males displaying higher UV reflectance on the wing, and the males have enhanced UV sensitivity, possibly to aid sex discrimination (Wakakuwa et al. 2005). By comparison, neither coloration nor receptor sensitivities are sexually dimorphic in the European race Pieris rapae rapae (Giraldo & Stavenga 2007).
In summary, innate behaviour towards coloured objects relying on image-forming eyes and colour-opponent mechanisms allows for reliable recognition of mates, food and hosts, and is a very common type of colour vision.
(c). Colour, learning and cognition
Thirty years after Lubbock's study of Daphnia, von Frisch and his collaborators had difficulty convincing contemporaries that animals have colour vision, or a ‘colour sense’ (German ‘Farbensinn’; Hess 1910, 1913; von Frisch 1913, 1914). von Frisch’s (1912) first experiments asked how fish change body colour depending on the background, but his main work tested learned associations with food by bees and fish (von Frisch 1913, 1914). As we have said, von Frisch used an operational definition: if subjects behaved differently towards a stimulus of one colour, for instance blue, compared with any shade of grey, they were said to see blue as a colour different from grey. Honeybees and fish (minnows, Phoxinus phoxinus) can indeed discriminate blue from grey, which requires a chromatic mechanism.
Despite the initial scepticism, von Frisch's method established the paradigm for animal colour vision, which may account for the distinction from wavelength-specific behaviour,2 a term used when there is no evidence for learning (Wigglesworth 1976; Menzel 1979; Jacobs 1981; Goldsmith 1991). More concretely, Goldsmith (1991) argues that learning gives ‘evidence for a perceptual dimension of colour’, and continues, ‘until our concepts are expanded by new experimental findings, this distinction (between wavelength specific behaviour and colour vision) should remain useful’. What is at stake is the idea that ‘vision’ is a cognitive phenomenon, with an internal representation of the world (Marr 1982; Skorupski & Chittka in press). Goldsmith (1991) implies that the ability to learn an association between any colour and food requires a representation of a chromatic dimension; perhaps like the map of colour space reported in the monkey primary visual cortex (Xiao et al. 2007). The idea that bees have a representation of colour that is independent of a specific motor system is also supported by evidence that they use a common system in several contexts: for finding flowers, and to identify nest sites and navigational landmarks (Cheng et al. 1986; Somanathan et al. 2008). This observation accords with the idea that humans have a sensation of colour independent of its context.
Learning is, of course, more relevant in some contexts than others; as we have mentioned (§2b), Papilio learns flower colours for nectar feeding, but not host plant colours for egg laying. Given the predictability of leaf colours and the fact that reproductive success is measured only after the female has died, colour learning might not be expected in oviposition behaviour. However, females of the small white butterfly P. rapae learn to associate the colour of the oviposition substrate with the chemicals that tell her she has landed on the correct host (Traynier 1984). Conversely, flower-visiting insects do have innate preferences that help them to find a first nectar reward (e.g. Scherer & Kolb 1987; Giurfa et al. 1995; Goyret et al. 2008). The colour preference may be part of an innate ‘flower template’, but, in experiments, most species approach objects of the preferred colour, independent of shape and other typical flower features, indicating a representation of colour that is independent of other features. Along with the ability to learn arbitrary colours, evidence of this kind has been argued to point to the existence of an internal representation of a colour continuum (Menzel 1979; Goldsmith 1991; Skorupski & Chittka in press). Nonetheless, evidence for—and the definition of—representation is controversial in cognitive science (O'Regan & Noe 2001; Webb 2006), and we leave the reader to judge the relevance of the experimental evidence to this question.
To conclude, from an ecological point of view, colour learning adds flexibility in the choice of coloured objects, an adaptation to changing availability of food sources, hosts and nest sites in a variable environment, while innate preferences allow for efficient coding of objects that are likely to be rather constant and optimal during an animal's lifetime (such as conspecifics or green leaves for egg laying).
(d). Perception of large colour differences, colour categories and colour appearance
Human colour vision entails much more than discrimination. We group colours into a few distinct categories that divide the perceptual space given by receptors or low-level opponent mechanisms into a small number of well-defined regions (figure 1; electronic supplementary material, fig. S1b; Berlin & Kay 1969; Harnad 1987). Also, we recognize qualities of colour, designated by the terms hue, saturation and ‘brightness’ (electronic supplementary material, box). It is argued that such concepts require language (and are determined by linguistic convention; Davidoff 2001; Fagot et al. 2006), but one can easily imagine that similar abilities could be beneficial to species without language (Poralla & Neumeyer 2006). Unfortunately, little is known about how other species perceive supra-threshold colour differences or classify colour. For instance, we do not know of any evidence that hue and saturation are qualitatively distinct (electronic supplementary material, box; Wyszecki & Stiles 1982). Similarly, colour categorization is controversial (Harnad 1987; Davidoff 2001; Osorio 2009). We now look at how such issues can be addressed experimentally (electronic supplementary material, fig. S1).
A traditional way to study neural mechanisms in colour psychophysics is based on multi-stage models (zone theory; Wyszecki & Stiles 1982), whereby low-level mechanisms (e.g. photoreceptors and chromatic opponent neurons) set discrimination thresholds, which provide a basic metric of colour difference (i.e. the just-noticeable difference, jnd; Kelber et al. 2003; Poralla & Neumeyer 2006; Ham & Osorio 2007; Osorio 2009). Simple models predict that the magnitude of the perceived supra-threshold stimuli should scale linearly (or logarithmically) with the discrimination threshold (Schrödinger 1920; Stevens 1957; Ham & Osorio 2007). Higher-level mechanisms then reveal themselves in deviations from the predictions of such models (electronic supplementary material, fig. S1; Poralla & Neumeyer 2006; Baddeley et al. 2007; Osorio 2009): for instance, the existence of categorical boundaries (in the continuum of colour space), representation of hue and saturation as distinct perceptual dimensions or unique hues (electronic supplementary material, fig. S1 and box).
As long ago as 1965, Shepard observed that ignorance of discrimination thresholds in species such as pigeons was a major impediment to work on avian colour learning and cognition (Shepard 1965; Osorio 2009). It is now possible to derive a metric based on the jnd either by direct psychophysical tests or by modelling based on receptor responses (Vorobyev & Osorio 1998; Kelber et al. 2003; Goldsmith & Butler 2005). Thus, in goldfish, generalization from single colours gives no evidence for categorical boundaries, but fish trained to single or multiple colours prefer or learn certain wavelengths preferentially (which may be evidence for ‘focal’ colours that could be ideal exemplars of a category; Poralla & Neumeyer 2006).
Given that birds have good colour discrimination and, seemingly, advanced cognition, it is not surprising that they should provide evidence for colour categorization. With pigeons (Columba livia), Wright & Cumming (1971; electronic supplementary material, fig. S3d) avoided the need to use a metric based on the discrimination threshold. They used a match-to-sample procedure to test categorization of monochromatic lights, in which birds learnt to respond to a light that matched an example wavelength (512, 572 or 655 nm). In the tests, the sample wavelength was novel and birds had to choose between the two nearest training lights. This identified two wavelengths, 540 and 600 nm, that were equally likely to be matched to 512/572 or 572/655 nm standards. To move from a measure of similarity to evidence of categorization, the standard lights were blue-shifted by approximately 20 nm (to 473, 555 and 633 nm). Crucially, there was no corresponding shift in the ‘ambiguous’ wavelengths, suggesting that they lie at categorical boundaries.
Poultry chicks learn colour remarkably accurately: for example, equivalent to a wavelength range of about ±2 nm for monochromatic light, even without differential training (Osorio et al. 1999; Osorio 2009). The ability to divide the colour continuum finely makes it possible to explore how these birds deal with suprathreshold stimuli, for instance by categorization. There is evidence that week-old chicks form colour categories based on experience of multiple colours (electronic supplementary material, fig. S1; Jones et al. 2001; Osorio et al. 2009). Specifically, when they are trained with food to two rewarded colours (e.g. yellow and red, or blue and green), intermediate colours (e.g. orange or turquoise) are treated as similar (i.e. food sources) to the rewarded colours, but colours beyond the limits set by the known rewarded colours are excluded. This suggests that the chicks place the known rewarded colours as categorical ends of a line in their colour space. The ability to recognize a region with sharp boundaries in the perceptual space accords with the conventional criterion for categorical perception (Harnad 1987).
Other than in birds, and the limited evidence from fish, there is little convincing evidence for colour categorization in other species, including non-human primates (Fagot et al. 2006). Application of suitable tests may well change this view.
3. Conclusion: seeing the world from an animal's point of view
The ultimate aim of the study of non-human senses is to understand the world from the animal's point of view (Nagel 1974; Guilford & Dawkins 1991). Sensory ecology starts by asking how low-level systems, such as sense organs, are matched to natural signals; but similar principles apply to any mechanism (Lythgoe 1979). Colour science raises questions about the behavioural uses of spectral information in nature, and the underlying neural mechanisms. Conversely, the study of visual ecology helps to understand the selective pressures under which colour vision has evolved in humans and other lineages. At a practical level, we want to relate the terminology of human colour vision to animal studies (electronic supplementary material, box; Bennett et al. 1994).
There are common features that seem to reflect shared ecological needs of colour vision. Examples include the widespread occurrence of two to four spectral receptor types (but see §2b). Similarly, the distinction between chromatic and achromatic (or luminance) mechanisms, which was originally made for primates, probably applies widely (Boynton & Kaiser 1968; Hempel de Ibarra et al. 2002; Gegenfurtner & Kiper 2003; Osorio & Vorobyev 2005). Broadly speaking, these phenomena are interpreted in terms of both the nature of the physical stimuli and their behavioural uses. Colour is useful for recognizing objects and assigning a value to them; for instance, as food or mates. This is presumably why colour is so salient to us and so prominent in biological communication signals.
As we move beyond simple tests of the existence of colour vision and arguments about its definition, we need to study how animals use spectral information in natural contexts and to relate these to how they perceive and learn about their world (Vorobyev et al. 2001; Roth et al. 2007; Skorupski & Chittka in press).
Acknowledgements
This commentary text has partly been inspired by the ideas put forward by Skorupski & Chittka (in press), and we thank them for discussion. A.K. thanks the Swedish Research Council for ongoing financial support.
Endnotes
Blindsight refers to the ‘residual vision’ after lesion of the visual cortex while leaving other visual pathways intact.
The term wavelength-specific behaviour is ambiguous because it is also applied to behaviour guided by achromatic mechanisms, which normally involve only one type of receptor (Menzel 1979).
References
- Allen G.1879The colour sense: its origin and development. London, UK: Trubner & Company [Google Scholar]
- Baddeley R. J., Osorio D., Jones C. D.2007Generalization of color by chickens: experimental observations and a Bayesian model. Am. Nat. 169, S27–S41 (doi:10.1086/510142) [DOI] [PubMed] [Google Scholar]
- Barlow H. B.1982What causes trichromacy? A theoretical analysis using comb-filtered spectra. Vis. Res. 22, 635–643 (doi:10.1016/0042-6989(82)90099-2) [DOI] [PubMed] [Google Scholar]
- Bennett A. T. D., Cuthill I. C., Norris K.1994Sexual selection and the mismeasure of color. Am. Nat 144, 848–860 (doi:10.1086/285711) [Google Scholar]
- Berlin B., Kay P.1969Basic color terms: their universality and evolution Berkeley, CA: University of California Press [Google Scholar]
- Bloj M. G., Kersten D., Hurlbert A. C.1999Perception of three-dimensional shape influences colour perception through mutual illumination. Nature 402, 877–879 (doi:10.1038/47245) [DOI] [PubMed] [Google Scholar]
- Booth D., Stewart A. J. A., Osorio D.2004Colour vision in the glow-worm Lampyris noctiluca (L.) (Coleoptera: Lampyridae): evidence for a green–blue chromatic mechanism. J. Exp. Biol 207, 2373–2378 (doi:10.1242/jeb.01044) [DOI] [PubMed] [Google Scholar]
- Boughman J. W.2002How sensory drive can promote speciation. Trends Ecol. Evol. 17, 571–577 (doi:10.1016/S0169-5347(02)02595-8) [Google Scholar]
- Boynton R. M., Kaiser P. K.1968Vision: the additivity law made to work for heterochromatic photometry with bipartite fields. Science 161, 366–368 (doi:10.1126/science.161.3839.366) [DOI] [PubMed] [Google Scholar]
- Brainard D. H., Maloney L. T.2004Perception of color and material properties in complex scenes. J. Vis. 4, ii–iv (doi:10.1167/4.9.i) [DOI] [PubMed] [Google Scholar]
- Briscoe A. D., et al. In press Positive selection of a duplicated ultraviolet-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl Acad. Sci. USA [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenhausen C. V.1986Photoreceptors, lightness constancy and color vision. Naturwissenschaften 76, 82–83 [DOI] [PubMed] [Google Scholar]
- Cheng K., Collett T. S., Wehner R.1986Honeybees learn the colours of landmarks. J. Comput. Physiol. 159, 69–73 (doi:10.1007/BF00612497) [Google Scholar]
- Croll N. A.1966The phototactic response and spectral sensitivity of Chroiviadorina viridis (Nematoda, Chromadorida) with a note on the nature of the paired pigment spots. Nematologica 12, 610–614 [Google Scholar]
- Cronin H.1991The ant and the peacock: altruism and sexual selection from Darwin to today Cambridge, UK: Cambridge University Press [Google Scholar]
- Cronin T. W., Marshall J.2004The visual world in mantis shrimps. The crustacean nervous systems (ed. Prete F.), pp. 239–268 Cambridge, MA: MIT Press [Google Scholar]
- Davidoff J.2001Language and perceptual categorisation. Trends Cogn. Sci. 5, 382–387 (doi:10.1016/S1364-6613(00)01726-5) [DOI] [PubMed] [Google Scholar]
- Detto T.2007The fiddler crab Uca mjoebergi uses colour vision in mate choice. Proc. R. Soc. B 274, 2785–2790 (doi:10.1098/rspb.2007.1059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. H., Partridge J. C.1997On the visual pigments of deep-sea fish. J. Fish Biol. 50, 68–85 (doi:10.1111/j.1095-8649.1997.tb01340.x) [Google Scholar]
- Endler J. A.1992Signals, signal conditions, and the direction of evolution. Am. Nat. 139, s125–s153 (doi:10.1086/285308) [Google Scholar]
- Fagot J., Goldstein J., Davidoff J., Pickering A.2006Cross-species differences in color categorization. Psychon. Bull. Rev. 13, 275–280 [DOI] [PubMed] [Google Scholar]
- Foster D. H., Nascimento S. M. C.1994Relational colour constancy from invariant cone-excitation ratios. Proc. R. Soc. Lond. B 257, 115–121 (doi:10.1098/rspb.1994.0103) [DOI] [PubMed] [Google Scholar]
- Frentiu F. D., Briscoe A. D.2008A butterfly's eye view of birds. BioEssays 30, 1151–1162 (doi:10.1002/bies.20828) [DOI] [PubMed] [Google Scholar]
- Gegenfurtner K. R., Kiper D. C.2003Color vision. Ann. Rev. Neurosci. 26, 181–206 (doi:10.1146/annurev.neuro.26.041002.131116) [DOI] [PubMed] [Google Scholar]
- Giraldo M. A., Stavenga D. G.2007Sexual dichromism and pigment localization in the wing scales of Pieris rapae butterflies. Proc. R. Soc. B 274, 97–102 (doi:10.1098/rspb.2006.3708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurfa M., Nunez J., Chittka L., Menzel R.1995Colour preferences of flower-naïve honeybees. J. Comput. Physiol. A 177, 247–259 [Google Scholar]
- Goldsmith T. H.1991The evolution of visual pigments and colour vision. In The perception of colour (ed. Gouras P.), pp. 62–89 London, UK: Macmillan [Google Scholar]
- Goldsmith T. H., Butler B. K.2005Color vision of the budgerigar (Melopsittacus undulatus): hue matches, tetrachromacy, and intensity discrimination. J. Comput. Physiol. A 191, 933–951 (doi:10.1007/s00359-005-0024-2) [DOI] [PubMed] [Google Scholar]
- Goyret J., Pfaff M., Raguso R. A., Kelber A.2008Why do Manduca sexta feed from white flowers? Innate and learnt colour preferences in a hawkmoth. Naturwissenschaften 95, 569–576 (doi:10.1007/s00114-008-0350-7) [DOI] [PubMed] [Google Scholar]
- Guilford T., Dawkins M. S.1991Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14 (doi:10.1016/S0003-3472(05)80600-1) [Google Scholar]
- Ham A. D., Osorio D.2007Colour preferences and colour vision in poultry chicks. Proc. R. Soc. B 274, 1941–1948 (doi:10.1098/rspb.2007.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnad S.1987Psychophysical and cognitive aspects of categorical perception: a critical overview. In Categorical perception: the groundwork of cognition (ed. Harnad S.), pp. 1–25 New York, NY: Cambridge University Press [Google Scholar]
- Hempel de Ibarra N., Giurfa M., Vorobyev M.2002Discrimination of coloured patterns by honeybees through chromatic and achromatic cues. J. Comput. Physiol. A 188, 503–512 [DOI] [PubMed] [Google Scholar]
- Hess C.1910Über den angeblichen Nachweis von Farbensinn bei Fischen. Pflugers Arch. Gesamte Physiol. Menschen Tiere 134, 1–14 (doi:10.1007/BF01692539) [Google Scholar]
- Hess C.1913Experimentelle Untersuchungen über den angeblichen Farbensinn der Bienen. Zool. Jahrb. Jena Abt. allg. Zool. 34, 81–106 [Google Scholar]
- Heywood C. A., Kentridge R. W., Cowey A.1998Cortical color blindness is not ‘blindsight for color’. Conscious. Cogn. 7, 410–423 (doi:10.1006/ccog.1998.0364) [DOI] [PubMed] [Google Scholar]
- Ichikawa T., Tateda H.1982Distribution of colour receptors in the larval eyes of four species of Lepidoptera. J. Comput. Physiol. 149, 317–324 (doi:10.1007/BF00619147) [Google Scholar]
- Jacobs G. H.1981Comparative color vision. New York, NY: Academic Press [Google Scholar]
- Jacobs G. H., Yolton R. L.1969Dichromacy in the ground squirrel. Nature 223, 414–415 (doi:10.1038/223414a0) [DOI] [PubMed] [Google Scholar]
- Jékely G.2009Evolution of phototaxis. Phil. Trans. R. Soc. B 364, 2795–2808 (doi:10.1098/rstb.2009.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. D., Osorio D., Baddeley R. J.2001Colour categorization by domestic chicks. Proc. R. Soc. Lond. B 268, 2077–2084 (doi:10.1098/rspb.2001.1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelber A.1999Ovipositing butterflies use a red receptor to see green. J. Exp. Biol. 202, 2619–2630 [DOI] [PubMed] [Google Scholar]
- Kelber A.2001Receptor based models for spontaneous colour choices in flies and butterflies. Ent. Exp. Appl. 99, 231–244 (doi:10.1023/A:1019244619503) [Google Scholar]
- Kelber A., Roth L. S. V.2006Nocturnal colour vision—not as rare as we might think. J. Exp. Biol. 209, 781–788 (doi:10.1242/jeb.02060) [DOI] [PubMed] [Google Scholar]
- Kelber A., Vorobyev M., Osorio D.2003Colour vision in animals—behavioural tests and physiological concepts. Biol. Rev. 78, 81–118 (doi:10.1017/S1464793102005985) [DOI] [PubMed] [Google Scholar]
- Koshitaka H., Kinoshita M., Vorobyev M., Arikawa K.2008Tetrachromacy in a butterfly that has eight varieties of spectral receptors. Proc. R. Soc. B 275, 947–954 (doi:10.1098/rspb.2007.1614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M. F., Nilsson E.2002Animal eyes Oxford, UK: Oxford University Press [Google Scholar]
- Lotto R. B., Wicklein M.2005Bees encode behaviourally significant spectral relationships in complex scenes to resolve stimulus ambiguity. Proc. Natl Acad. Sci. USA 102, 16 870–16 874 (doi:10.1073/pnas.0503773102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbock J.1882Ants, bees and wasps London, UK: Kegan Paul [Google Scholar]
- Lubbock J.1888On the senses, instincts and intelligence of animals with special reference to insects London, UK: Kegan Paul [Google Scholar]
- Lythgoe J. N.1979The ecology of vision Oxford, UK: Clarendon [Google Scholar]
- Maloney L. T.1986Evaluation of linear models of surface spectral reflectance with small numbers of parameters. J. Opt. Soc. Am. A 3, 1673–1683 (doi:10.1364/JOSAA.3.001673) [DOI] [PubMed] [Google Scholar]
- Marr D.1982Vision New York, NY: Freeman [Google Scholar]
- Mather G.2006Foundations of perception Hove, UK: Psychology Press [Google Scholar]
- Maximov V. V.2000Environmental factors which may have led to the appearance of colour vision. Phil. Trans. R. Soc. B 355, 1239–1242 (doi:10.1098/rstb.2000.0675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R.1979Spectral sensitivity and color vision in invertebrates. In Handbook of sensory physiology, vol. VII/6A (ed. Autrum H.), pp. 503–580 Comparative Physiology and Evolution of Vision in Invertebrates.Berlin, Germany: Springer [Google Scholar]
- Moericke V.1949Über den Farbensinn der Pfirsichblattlaus (Myzodes persicae Sulz.). J. Pest Sci. 22, 139–140 [Google Scholar]
- Mollon J. D.1987John Elliot MD (1747–1787). Nature 329, 19–20 (doi:10.1038/329019a0) [DOI] [PubMed] [Google Scholar]
- Nagel T.1974What is it like to be a bat? Phil. Rev. 83, 435–450 (doi:10.2307/2183914) [Google Scholar]
- Nilsson E.2009The evolution of eyes and visually guided behaviour. Phil. Trans. R. Soc. B 364, 2833–2847 (doi:10.1098/rstb.2009.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan J. K., Noe A.2001What it is like to see: a sensorimotor theory of perceptual experience. Synthese 129, 79–103 (doi:10.1023/A:1012699224677) [Google Scholar]
- Osorio D.2009Colour generalization by birds. In Cognitive biology: evolutionary and developmental perspectives on mind, brain, and behavior (eds Tommasi L., Nadel L., Peterson M. A.), pp. 129–146 Cambridge, MA: MIT Press [Google Scholar]
- Osorio D., Vorobyev M.2005Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B 272, 1745–1752 (doi:10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D., Vorobyev M.2008A review of the evolution of animal colour vision and visual communication signals. Vis. Res 48, 2042–2051 (doi:10.1016/j.visres.2008.06.018) [DOI] [PubMed] [Google Scholar]
- Osorio D., Marshall N. J., Cronin T. W.1997Stomatopod photoreceptor spectral tuning as an adaptation for colour constancy in water. Vis. Res. 37, 3299–3309 (doi:10.1016/S0042-6989(97)00136-3) [DOI] [PubMed] [Google Scholar]
- Osorio D., Miklosi A., Gonda Z.1999Visual ecology and perception of coloration patterns by domestic chicks. Evol. Ecol 13, 673–689 (doi:10.1023/A:1011059715610) [Google Scholar]
- Osorio D., Ham A. D., Gonda Z., Andrew R. J.2009Sensory generalization and learning about novel colours by poultry chicks. Quart. J. Exp. Psychol. 62, 1249–1256 [DOI] [PubMed] [Google Scholar]
- Poralla J., Neumeyer C.2006Generalization and categorization of spectral colors in goldfish. II. Experiments with two and six wavelengths. J. Comput. Physiol. A 192, 469–479 (doi:10.1007/s00359-005-0082-5) [DOI] [PubMed] [Google Scholar]
- Prokopy R. J., Owens E. D.1983Visual detection of plants by herbivorous insects. Ann. Rev. Entomol. 28, 337–364 (doi:10.1146/annurev.en.28.010183.002005) [Google Scholar]
- Robilotto R., Zaidi Q.2004Limits of lightness identification for real objects under natural viewing conditions. J. Vis. 4, 779–797 (doi:10.1167/4.9.9) [DOI] [PubMed] [Google Scholar]
- Roth L. S. V., Balkenius A., Kelber A.2007Colour perception in a dichromat. J. Exp. Biol. 210, 2795–2800 (doi:10.1242/jeb.007377) [DOI] [PubMed] [Google Scholar]
- Ruderman D. R., Cronin T. W., Chiao C. C.1998Statistics of cone responses to natural images: implications for visual coding. J. Opt. Soc. Am. A 15, 2036–2045 (doi:10.1364/JOSAA.15.002036) [Google Scholar]
- Scherer C., Kolb G.1987The influence of color stimuli on visually controlled behavior in Aglais urticae L. and Pararge aegeria L. (Lepidoptera). J. Comput. Physiol. A 161, 891–898 (doi:10.1007/BF00610230) [Google Scholar]
- Schrödinger E.1920Grundlinien einer Theorie der Farbenmetrik im Tagessehen. Ann. Phys. 368, 481–520 (doi:10.1002/andp.19203682202) [Google Scholar]
- Seehausen O., et al. 2008Speciation through sensory drive in cichlid fish. Nature 455, 620–626 (doi:10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- Shepard R. N.1965Approximation to uniform gradients of generalization by monotone transformations of scale. In Stimulus generalization (ed. Mostofsky D.), pp. 94–110 Stanford, CA: Stanford University Press [Google Scholar]
- Skorupski P., Chittka L. Is colour cognitive? Opt. Laser Technol. In press. ( doi:10.1016/j.optlastec.2008.12.015) [Google Scholar]
- Smith K. C., Macagno E. R.1990UV photoreceptors in the compound eye of Daphnia magna (Crustacea, Branchiopoda). A fourth spectral class in single ommatidia. J. Comput. Physiol. A 166, 597–606 [DOI] [PubMed] [Google Scholar]
- Somanathan H., Borges R. M., Warrant E. J., Kelber A.2008Nocturnal carpenter bees learn landmark colours in starlight. Curr. Biol. 18, R996–R997 (doi:10.1016/j.cub.2008.08.023) [DOI] [PubMed] [Google Scholar]
- Sprengel C. K.1793Das entdeckte Geheimnis der Natur im Bau und in der Befruchtung der Blumen. Berlin, Germany: Vieweg [Google Scholar]
- Spudich J. L., Bogomolni R. A.1984Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature 312, 509–513 (doi:10.1038/312509a0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M. S., Lehrer M.1988Spatial acuity of honeybee vision and its spectral properties. J. Comput. Physiol. A 162, 159–172 (doi:10.1007/BF00606081) [Google Scholar]
- Stevens S. S.1957On the psychophysical law. Psychol. Rev. 64, 153–181 (doi:10.1037/h0046162) [DOI] [PubMed] [Google Scholar]
- Steverding D., Troscianko T.2003On the role of blue shadows in the visual behaviour of tsetse flies. Proc. R. Soc. Lond. B 271(Suppl. 3), S16–S17 (doi:10.1098/rsbl.2003.0121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoerig P.1998Wavelength information processing versus color perception: evidence from blindsight and color-blind sight. In Color vision: perspectives from different disciplines (eds Backhaus W. G. K., Kliegl R., Werner J. S.), pp. 131–147 Berlin, Germany: DeGruyter [Google Scholar]
- Stoerig P., Cowey A.1997Blindsight in man and monkey. Brain 120, 535–559 (doi:10.1093/brain/120.3.535) [DOI] [PubMed] [Google Scholar]
- Thompson E., Palacios A. G., Varela F. J.1992Ways of coloring: comparative color vision as a case study for cognitive science. Behav. Brain Sci. 15, 1–26 [Google Scholar]
- Traynier R. M. M.1984Associative learning in the ovipositional behaviour of the cabbage butterfly, Pieris rapae. Physiol. Entomol. 9, 465–472 (doi:10.1111/j.1365-3032.1984.tb00789.x) [Google Scholar]
- van Hateren J. H., Rüttiger L., Sun H., Lee B. B.2002Processing of natural temporal stimuli by macaque retinal ganglion cells. J. Neurosci. 22, 9945–9960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Frisch K.1912Über farbige Anpassung bei Fischen. Zool. Jahrb. Abt. allg. Zool. Physiol. Tiere 32, 209–214 [Google Scholar]
- von Frisch K.1913Weitere Untersuchungen über den Farbensinn der Fische. Zool. Jahrb. Abt. allg. Zool. Physiol. Tiere 34, 43–68 [Google Scholar]
- von Frisch K.1914Der Farbensinn und Formensinn der Biene. Zool. Jahrb. Abt. allg. Zool. Physiol. Tiere 35, 1–188 [Google Scholar]
- von Frisch K.1943Christian Konrad Sprengel's flower theory 150 years ago and today. Naturwissenschaften 31, 223–229 (doi:10.1007/BF01482236) [Google Scholar]
- Vorobyev M.2003Coloured oil droplets enhance colour discrimination. Proc. R. Soc. Lond. B 270, 1255–1261 (doi:10.1098/rspb.2003.2381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M., Osorio D.1998Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M., Marshall N. J., Osorio D., Hempel de Ibarra N., Menzel R.2001Colourful objects through animal eyes. Color Res. Appl. 26, S214–S216 (doi:10.1002/1520-6378(2001)26:1+<::AID-COL45>3.0.CO;2-A) [Google Scholar]
- Wachtler T., Dohrmann U., Hertel R.2004Modeling color percepts of dichromats. Vis. Res. 44, 2843–2855 (doi:10.1016/j.visres.2004.06.016) [DOI] [PubMed] [Google Scholar]
- Wakakuwa M., Stavenga D. G., Arikawa K.2005Sexual dimorphism of short-wavelength photoreceptors in the small white butterfly, Pieris rapae crucivora. J. Neurosci. 25, 5935–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B.2006Transformation, encoding and representation. Curr. Biol 6, R184–R185 (doi:10.1016/j.cub.2006.02.034) [DOI] [PubMed] [Google Scholar]
- Wehner R.1989The hymenopteran skylight compass: matched filtering and parallel coding. J. Exp. Biol. 146, 63–85 [Google Scholar]
- Wigglesworth V. B.1976Insects and the life of man, ch. 11, pp. 127–137 The Contributions of Sir John Lubbock to Insect Physiology London, UK: Chapman & Hall [Google Scholar]
- Wright A. A., Cumming W. W.1971Color-naming functions for the pigeon. J. Exp. Anal. Behav. 15, 7–17 (doi:10.1901/jeab.1971.15-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszecki G., Stiles W. S.1982Color science: concepts and methods, quantitative data and formulae, 2nd edn New York, NY: Wiley [Google Scholar]
- Xiao Y., Casti A., Xiao J., Kaplan E.2007Hue maps in primate striate cortex. Neuroimage 35, 771–786 (doi:10.1016/j.neuroimage.2006.11.059) [DOI] [PMC free article] [PubMed] [Google Scholar]