Abstract
The hypothalamic–pituitary–adrenal (HPA) axis is a neuroendocrine system that regulates the circulating levels of vital glucocorticoid hormones. The activity of the HPA axis is characterized not only by a classic circadian rhythm, but also by an ultradian pattern of discrete pulsatile release of glucocorticoids. A number of psychiatric and metabolic diseases are associated with changes in glucocorticoid pulsatility, and it is now clear that glucocorticoid responsive genes respond to these rapid fluctuations in a biologically meaningful way. Theoretical modelling has enabled us to identify and explore potential mechanisms underlying the ultradian activity in this axis, which to date have not been identified successfully. We demonstrate that the combination of delay with feed-forward and feedback loops in the pituitary–adrenal system is sufficient to give rise to ultradian pulsatility in the absence of an ultradian source from a supra-pituitary site. Moreover, our model enables us to predict the different patterns of glucocorticoid release mediated by changes in hypophysial-portal corticotrophin-releasing hormone levels, with results that parallel our experimental in vivo data.
Keywords: neuroendocrine regulation, glucocorticoid hormones, ultradian pulsatility, mathematical modelling, numerical continuation
1. Introduction
Frequency of coding of intercellular signals is a well-accepted mode of communication between neurons. More than this, however, it is actually a common mechanism of communication across a broad range of both inter- and even intra-cellular systems (Goldbeter 1996). Even an organism as primitive as the slime mould (Dictyostelium discoideum) only aggregates in response to external pulses of cyclic AMP delivered with a periodicity of 5 minutes and not to constant stimuli or frequencies greater than every 2 minutes (Darmon et al. 1975).
In mammals, the endocrine system is one of the major signalling systems to use frequency encoding. In addition to the vital metabolic hormone insulin (Lang et al. 1979), the best described endocrine systems that signal through ultradian rhythms are found in the hypothalamic–pituitary neuroendocrine pathways. Pulsatile gonadotropin-releasing hormone (GnRH) release results in the concordant release of LH pulses from the pituitary (Belchetz et al. 1978; Clarke 2002), while modulation of GnRH pulse frequency can produce differential LH and FSH secretion (Wildt et al. 1981) via regulation of LH beta and FSH beta mRNA expression (Papavasiliou et al. 1986). Interactions between hypothalamic somatostatin and growth-hormone-releasing hormone neuronal systems result in episodic release of growth hormone (GH) (Plotsky & Vale 1985), which is in turn an important factor in mediating GH-dependent gene expression (Waxman et al. 1995).
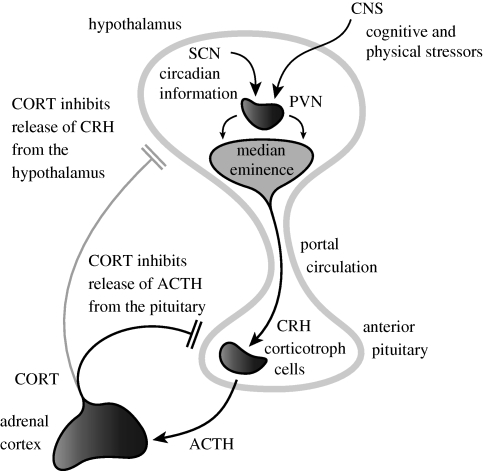
Another system that is characterized by an ultradian rhythm is the hypothalamic–pituitary–adrenal (HPA) axis (figure 1). This stress-responsive neuroendocrine system is extremely well adapted to respond to homeostatic challenge. The HPA axis governs the circulating levels of vital glucocorticoid hormones (CORT), which in turn have major regulatory effects on the cardiovascular, metabolic, cognitive and immunological state of the animal (Chrousos 1995; de Kloet et al. 2005; McEwen 2007). The central regulator of this axis—the paraventricular nucleus (PVN) of the hypothalamus—is a major relay for afferent information from limbic areas of the central nervous system that can detect cognitive or emotional stressors, and also from brain stem structures that detect more physical stressors such as inflammation or hypotension (Ulrich-Lai & Herman 2009). The PVN also receives a major input from the hypothalamic suprachiasmatic nucleus (SCN) that coordinates the body's circadian rhythms (Reppert & Weaver 2002). The corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) containing parvocellular neurons in the PVN project to the median eminence of the hypothalamus from where they release CRH and AVP into the hypothalamic–pituitary portal circulation (Engler et al. 1989; Ixart et al. 1991). The CRH and AVP pass along this vascular route to access their receptors on corticotroph cells in the anterior pituitary. These cells in turn are activated by occupation of their CRH and AVP receptors to release corticotrophin (ACTH) into the general circulation through which it accesses the glucocorticoid-secreting cells in the cortex of the adrenal gland. It is these cells that synthesize and release the final product of HPA activation—the glucocorticoid hormones. The final link in this circuit is that physiological levels of glucocorticoid hormones themselves feedback in a negative manner predominantly on the pituitary gland—but also at the level of the PVN and hippocampus—to inhibit further release of ACTH (Jones et al. 1977; Dallman et al. 1987).
Figure 1.
Regulation of HPA axis activity. The hypothalamic PVN receives circadian inputs from the SCN and homeostatic/stress inputs from the brain stem and limbic areas. The PVN projects to the median eminence where it releases CRH into the portal circulation. This passes to corticotrophs in the anterior pituitary which release ACTH from pre-formed granules into the venous circulation. This ACTH reaches the adrenal cortex where it activates the synthesis and secretion of CORTisol (in man) or CORTicosterone (in the rodent). CORT in turn feeds back to inhibit the release of ACTH from the anterior pituitary, and to a lesser extent, CRH from the hypothalamus.
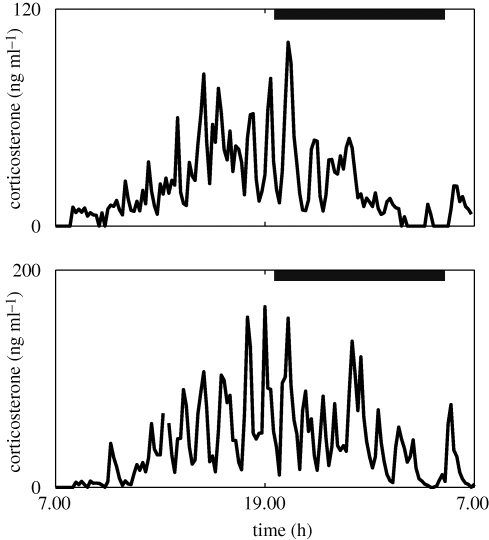
The HPA axis has a unique pattern of activity. Levels are low during the periods of sleep inactivity and increase in anticipation of waking, peaking in the morning in man (Weitzman et al. 1971) and evening in the rodent (Dallman et al. 1978), with the resultant classic circadian rhythm. This rhythm, however, is not made up of a simple smooth change in hormone levels over the 24 hours. The circadian changes of glucocorticoids are a result of changes in the activity of an underlying ultradian rhythm (Veldhuis et al. 1989; Jasper & Engeland 1991; Windle et al. 1998a; Spiga et al. 2007). Glucocorticoids are actually released from the adrenal gland in discrete pulses that result in rapidly changing levels of hormone, both in the blood and within the tissues (figure 2). It is in fact the changes in pulse amplitude, and to a lesser extent frequency, that make up the circadian rhythm (Lui et al. 1987; Iranmanesh et al. 1989; Veldhuis et al. 1989, 1990; Windle et al. 1998b) and the changes of HPA activity that occur in response to altered physiological and pathological conditions. This pulsatility of glucocorticoid secretion is also an important factor in determining the responsivity of the HPA axis to stress (Windle et al. 1998a; Lightman et al. 2008) and the transcriptional responses of glucocorticoid responsive genes (Stavreva et al. 2009).
Figure 2.
Experimental data demonstrating the ultradian glucocorticoid rhythm underlying the classic circadian profile. Levels of blood corticosterone were recorded over a 24 h period in two individual male Sprague–Dawley rats. Blood samples were collected every 10 min using an automated blood sampling system. Grey bars indicate the dark phase (19.15–05.15 h). Adapted from Spiga et al. (2007).
We have good evidence that the SCN determines the circadian activity of the HPA axis by modulating the inhibitory gain to the PVN; however, we have no idea of the mechanism responsible for the regulation of ultradian activity. Although it is often simply presumed that there must be some sort of hypothalamic pulse generator, there is no good evidence for its existence. The only supportive data come from studies with cultured explants of the macaque hypothalamus (Mershon et al. 1992) and from rat median eminence (Ixart et al. 1991) that show episodic release of CRH. The relevance of this is unclear, particularly in the absence of cyclic feedback of inhibitory signals from circulating glucocorticoids that are now known to have rapid inhibitory effects even on basal HPA activity (Atkinson et al. 2008). Indeed, there is good evidence for the lack of importance of a pulsatile CRH signal for this ultradian rhythm from studies in sheep that have had surgical disconnection of the hypothalamus from the pituitary. These animals still maintain pulsatile cortisol secretion, despite their loss of a normal response to stress (Engler et al. 1990). This clearly shows that even in the absence of the stress-activatable hypothalamic input, the ultradian rhythm of cortisol secretion is maintained.
Understanding the mechanisms underlying ultradian HPA activity is very important. It is becoming increasingly clear that glucocorticoid responsive genes respond to these rapid fluctuations in a biologically meaningful way (Stavreva et al. 2009) and that a number of psychiatric and metabolic diseases are associated with changes in cortisol pulsatility (Young et al. 2004, 2007). Motivated by recent accounts of feed-forward and feedback loops supporting robust oscillations in a number of biological contexts (Stricker et al. 2008; Tsai et al. 2008; Tigges et al. 2009), we hypothesized that the pituitary–adrenal system (which contains a positive delayed feed-forward connection between ACTH and CORT (Papaikonomou 1977), as well as negative nonlinear feedback of CORT on ACTH mediated by the glucocorticoid receptor (GR) (Drouin et al. 1992)) could support ultradian oscillations in the absence of a hypothalamic pulse generator. To address this hypothesis, we considered a deterministic theoretical model characterizing the principal interactions between the anterior pituitary and the adrenal cortex (see figure 1, and also figure S7 in the electronic supplementary material). We employed a powerful mathematical technique called numerical continuation (Kuznetsov 1995; Engelborghs et al. 2001; Krauskopf et al. 2007)—enabling us to systematically characterize how the behaviour of the system depends on the parameters of the system (see the electronic supplementary material for more details)—to explain the mechanisms giving rise to natural oscillatory rhythms in the HPA axis.
2. Results and discussion
The aim of model development for the HPA axis was to elucidate whether—using biologically motivated approximations of each of the main compartments of the axis—the system could support ultradian glucocorticoid fluctuations in a similar manner to those observed experimentally, and to explore mechanisms by which these could occur. For this purpose, we adapted a recently proposed model (Gupta et al. 2007) using ordinary differential equations (ODEs) that provided a compromise between analytical tractability and biological plausibility. This approach allowed for the integration of experimentally determined parameter values (where known), while permitting a theoretical analysis using a simplified model with the potential for refinement using experimental data. One of the key assumptions made during the modelling process was that the rapid inhibition of hypothalamic CRH by glucocorticoids is not an important factor. This relates back to the fact that the anterior pituitary is the major site for glucocorticoid feedback (Keller-Wood & Dallman 1984) and the relatively slow effect of glucocorticoids on CRH gene transcription (Ma et al. 1997). Specifically, the model uses linear mass action kinetics to describe the dynamic levels of ACTH, GR and CORT, and incorporates a delay term to account for the well-known delay in the CORT response to ACTH that results from the lack of releasable pools of CORT and the need to synthesize the hormone for release (see the electronic supplementary material for more details).
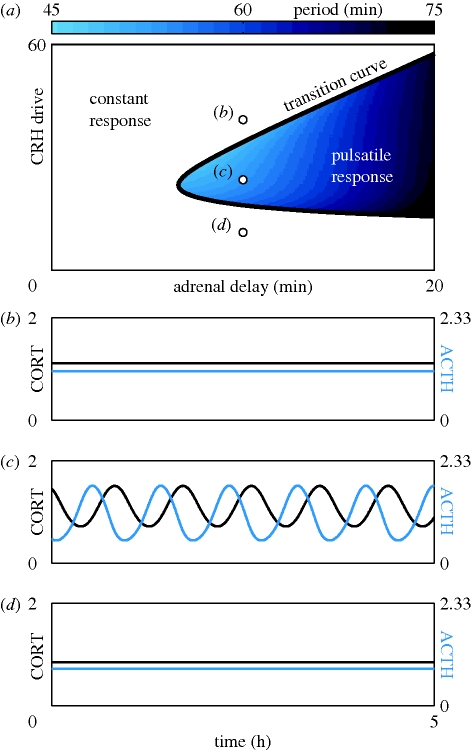
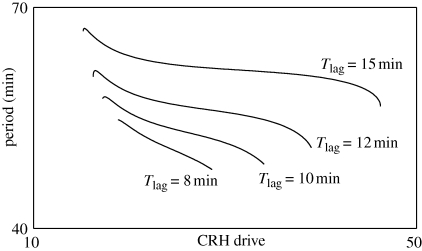
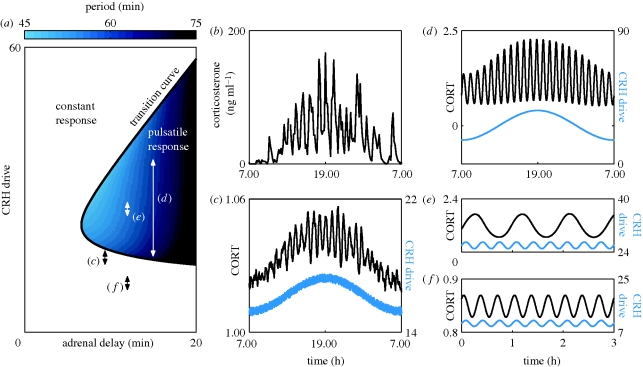
Using numerical simulations and continuation methods (see the electronic supplementary material for more details), we determined a range of values in both CRH drive and delay for which ultradian activity was observed in ACTH and CORT (figure 3a,c). It is important to stress that these ultradian pulses are an intrinsic property of the pituitary–adrenal system, since they occur in response to a constant level of CRH drive. Furthermore, the ultradian period of the pulses (figure 4 and the colour bar in figure 3a) is consistent with previous experimental studies, which have reported an interpulse interval range between 47.2 and 54.6 min (Windle et al. 1998a). Interestingly, our simulations demonstrated that only intermediate values of the CRH drive resulted in ultradian pulses, while high or low CRH drive resulted in a constant response in ACTH and CORT levels (figure 3a,b,d).
Figure 3.
Response of the pituitary–adrenal system to constant CRH drive. Units of all hormone levels are arbitrary. (a) Different combinations of constant CRH drive and delay can lead to two qualitatively different responses. On one side of the transition curve, the pituitary–adrenal system responds with constant levels in ACTH and CORT. On the other side of the transition curve, the pituitary–adrenal system responds with pulsatile fluctuations in the levels of ACTH and CORT, despite the fact that the CRH drive is constant. In the region of pulsatile response, the frequency of the pulses is indicated by the colour bar. (b–d) Model predictions for ACTH (blue) and CORT (black). Each time series was computed with the same delay (10 min), but different levels of constant CRH drive, as indicated by the three points in (a).
Figure 4.
Period of CORT pulses inside the pulsatile region. Period of ultradian CORT rhythm computed for different values of the adrenal delay Tlag (min) and different levels of CRH drive (arb. units). For all four values of the delay, we observe ultradian pulses with a physiological period. See also the colour bar in figure 3a.
Experimental data demonstrate significant changes in the amplitude of ultradian activity over the course of a 24 hour period (figure 5b). Theoretically, we considered the effect of circadian modulation of the PVN by the SCN by driving the pituitary–adrenal system with a circadian (a period of 24 hours) CRH input. Our numerical results parallel experimental observations (Windle et al. 1998b), whereby the amplitude increases markedly (and the frequency increases slightly) during the high-drive CRH input (figure 5a,d). Perhaps most significantly, when we included stochastic effects as well as a circadian modulation of the CRH drive, we observed so-called noise-induced coherent oscillations (NICOs) (Wiesenfeld & Moss 1995; Gammaitoni et al. 1998) for values of the CRH drive close to (but below) the transition curve (beyond which ultradian pulses were observed in the noise-free scenario). These NICOs closely resembled the experimental data (figure 5b,c) providing evidence for the hypothesis that feed-forward and feedback interactions within the pituitary–adrenal system are the foundation of ultradian activity observed experimentally (see also figure S8 in the electronic supplementary material for more examples).
Figure 5.
Response of the pituitary–adrenal system to circadian and ultradian patterns of CRH drive. Units of all hormone levels are arbitrary. (a) Different combinations of constant CRH drive and delay can lead to two qualitatively different responses. On one side of the transition curve, the pituitary–adrenal system responds with constant levels in ACTH and CORT. On the other side of the transition curve, the pituitary–adrenal system responds with pulsatile fluctuations in the levels of ACTH and CORT, despite the fact that the CRH drive is constant. In the region of pulsatile response, the frequency of the pulses is indicated by the colour bar. (b) Experimental data demonstrating an increase in pulse amplitude during the circadian peak. Adapted from Spiga et al. (2007). (c) Model prediction for a noisy circadian CRH drive close to (but below) the pulsatile region, as indicated by the corresponding arrow in (a). Response demonstrates NICOs during the peak of the circadian CRH drive. Computed with a delay of 9.4 min. (d) Model prediction for a circadian CRH drive in the pulsatile region, as indicated by the corresponding arrow in (a). Response demonstrates increased pulse amplitude during the peak of the circadian CRH drive. Computed with a delay of 15 min. (e) Model prediction for ultradian pulses of CRH drive in the pulsatile region, as indicated by the corresponding arrow in (a). Response demonstrates a frequency in CORT governed by the pituitary–adrenal system and not by the frequency of the CRH forcing. Computed with a delay of 12 min. (f) Model prediction for ultradian pulses of CRH drive in the region of constant response, as indicated by the corresponding arrow in panel (a). Response demonstrates a frequency in CORT that is governed by the frequency of the CRH forcing. Computed with a delay of 12 min.
We also considered the effect of ultradian CRH pulses on the response of the pituitary–adrenal system. Experimental work has reported a pulsatile pattern of CRH release from the median eminence of the hypothalamus in the rat, with a mean frequency of three pulses per hour (Ixart et al. 1991). Our numerical work shows that the pituitary–adrenal system responds to ultradian CRH pulses differently depending on the precise level of these pulses. If their level lies within the region of constant response (figure 5a), then the pituitary–adrenal system responds with pulses of CORT at the same frequency as the driving CRH pulses (figure 5f). Alternatively, if the level of the CRH pulses lies within the region of pulsatile response (figure 5a), then the pituitary–adrenal system responds with pulses of CORT at a frequency governed by the intrinsic properties of the pituitary–adrenal system (figure 5e).
Finally, we illustrate how this theoretical approach to understanding the ultradian glucocorticoid rhythm can aid the planning of both experimental and clinical trials. One very important area of clinical medicine that has been linked to both over- and under-activity of the HPA axis is the mood disorders. Depression, in particular, has been consistently associated with significant elevations of HPA activity (Holsboer 2001; Pariante 2003), and many studies have shown that this increased activity is associated with a diminution of sensitivity to the negative feedback by endogenous glucocorticoids. This has been demonstrated by data showing a blunting of endogenous glucocorticoid inhibition following the administration of the synthetic glucocorticoid dexamethasone, or an inhibition of the ACTH response in the dexamethasone-CRH test (Nemeroff 1996; Holsboer 2000; Pariante & Miller 2001; Pariante 2004). Furthermore, glucocorticoid secretion patterns of transgenic mice with reduced GR resemble those patterns seen in subjects with major depression (Pepin et al. 1992). Thus, the use of GR antagonists clearly has great potential as a therapeutic strategy in treating patients with mood disorders linked to HPA axis dysfunction.
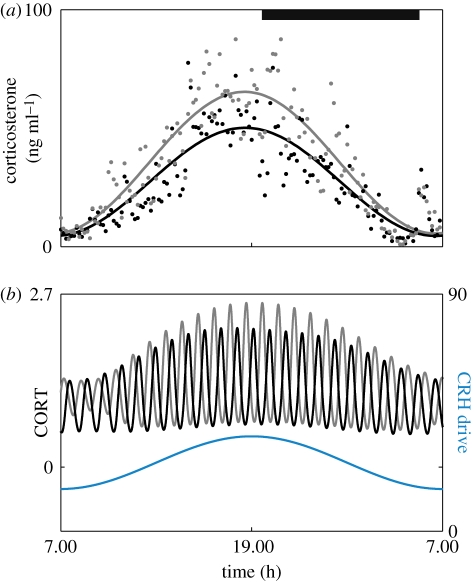
The model we employ here is the first to incorporate the dynamics of the GR in the anterior pituitary (Gupta et al. 2007), and therefore provides an ideal platform to investigate the effects that GR antagonists/agonists have on the dynamics of endogenous glucocorticoid secretion. Model results demonstrate that infusion of a GR antagonist (such as Org 34850) increases the amplitude of the ultradian glucocorticoid rhythm during the peak of the circadian CRH drive (figure 6b). Furthermore there is a minor increase in ultradian frequency under the influence of a GR antagonist. These theoretical observations are consistent with experimental studies on the rat (Spiga et al. 2007), where following 5 days of treatment with the GR antagonist Org 34850, mean corticosterone levels were elevated over the 24 hour cycle (figure 6a). Furthermore, this general elevation was the result of an underlying increase in both the amplitude and frequency of the ultradian pulses. In the same study, analysis of the corticosterone rhythm revealed that Org 34850 had its greatest effect during the peak of the circadian rhythm.
Figure 6.
Effect of subchronic treatment with a GR antagonist on the 24 h corticosterone profile. (a) Data points represent mean levels of blood corticosterone measured from individual male Sprague–Dawley rats injected twice a day for 5 days with either the GR antagonist Org 34850 (10 mg kg−1, subcut., n = 7, grey dots) or VEH (5% mulgofen in 0.9% saline, 1 ml kg−1, subcut., n = 7, black dots). Blood samples were recorded over a 24 h period and collected every 10 min using an automated blood sampling system. Also shown are curves numerically fitted to the two datasets, demonstrating an increase in amplitude during the circadian peak under the influence of Org 34850. Grey bar represents the dark phase (19.15–05.15 h). Adapted from Spiga et al. (2007). (b) Model simulations show the response of the system to circadian CRH both with (grey) and without (black) a GR antagonist. Infusion of a GR antagonist increases the amplitude of the ultradian glucocorticoid rhythm during the peak of the circadian CRH drive together with a minor increase in ultradian frequency (grey). Computed with a delay of 15 min.
Biological systems use rhythmic activity in many time domains, from rapid electrical oscillations in the central nervous system to daily, monthly or even yearly hormone rhythms. Many hormones are also secreted in ultradian patterns, which are important for the maintenance of tissue responsiveness and the avoidance of receptor downregulation. The mechanisms underlying many of these rhythms have been very unclear, and in this paper we have been able to show that relatively simple feed-forward and feedback interactions between the pituitary and adrenal cortex are sufficient to account for the glucocorticoid rhythms we observe experimentally. These oscillations will of course be modified by the gain from the CRH and AVP input to the pituitary, which in turn can be modified by the activity of suprapituitary feedback mediated through both the GR and the mineralocorticoid receptor. This theoretical approach, which simply depends upon systems having delayed feed-forward and feedback pathways, could also provide the basis for understanding ultradian rhythmicity in many other biological systems.
Acknowledgements
The authors acknowledge financial support from the EPSRC via grant EP/E032249/1, and also financial support from The Wellcome Trust via grant 074112/Z/04/Z. We thank Francesca Spiga for supplying experimental data. Useful discussions with Mohit Adhikari and Oscar Benjamin are also gratefully acknowledged.
References
- Atkinson H. C., Wood S. A., Castrique E. S., Kershaw Y. M., Wiles C. C., Lightman S. L.2008Corticosteroids mediate fast feedback of the rat hypothalamic–pituitary–adrenal axis via the mineralocorticoid receptor. Am. J. Physiol. Endocrinol. Metab. 294, E1011–E1022 (doi:10.1152/ajpendo.00721.2007) [DOI] [PubMed] [Google Scholar]
- Belchetz P. E., Plant T. M., Nakai Y., Keogh E. J., Knobil E.1978Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202, 631–633 (doi:10.1126/science.100883) [DOI] [PubMed] [Google Scholar]
- Chrousos G. P.1995The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 332, 1351–1362 (doi:10.1056/NEJM199505183322008) [DOI] [PubMed] [Google Scholar]
- Clarke I. J.2002Two decades of measuring GnRH secretion. Reprod. Suppl. 59, 1–13 [PubMed] [Google Scholar]
- Dallman M. F., Engeland W. C., Rose J. C., Wilkinson C. W., Shinsako J., Siedenburg F.1978Nycthemeral rhythm in adrenal responsiveness to ACTH. Am. J. Physiol. 235, R210–R218 [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Akana S. F., Cascio C. S., Darlington D. N., Jacobson L., Levin N.1987Regulation of ACTH secretion: variations on a theme of B. Recent Prog. Horm. Res. 43, 113–173 [DOI] [PubMed] [Google Scholar]
- Darmon M., Brachet P., Da Silva L. H.1975Chemotactic signals induce cell differentiation in Dictyostelium discoideum. Proc. Natl Acad. Sci. USA 72, 3163–3166 (doi:10.1073/pnas.72.8.3163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E. R., Joëls M., Holsboer F.2005Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475 (doi:10.1038/nrn1683) [DOI] [PubMed] [Google Scholar]
- Drouin J., Sun Y. L., Tremblay S., Lavender P., Schmidt T. J., de Léan A., Nemer M.1992Homodimer formation is rate-limiting for high affinity DNA binding by glucocorticoid receptor. Mol. Endocrinol. 6, 1299–1309 (doi:10.1210/me.6.8.1299) [DOI] [PubMed] [Google Scholar]
- Engelborghs K., Luzyanina T., Samaey G.2001DDE-BIFTOOL v. 2.00: a Matlab package for bifurcation analysis of delay differential equations. Technical Report TW-330, Department of Computer Science, K.U. Leuven, Leuven, Belgium [Google Scholar]
- Engler D., Pham T., Fullerton M. J., Ooi G., Funder J. W., Clarke I. J.1989Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep. I. Effect of an audiovisual stimulus and insulin-induced hypoglycaemia. Neuroendocrinology 49, 367–381 (doi:10.1159/000125141) [DOI] [PubMed] [Google Scholar]
- Engler D., Pham T., Liu J. P., Fullerton M. J., Clarke I. J., Funder J. W.1990Studies of the regulation of the hypothalamic–pituitary–adrenal axis in sheep with hypothalamic-pituitary disconnection. II. Evidence for in vivo ultradian hypersecretion of proopiomelanocortin peptides by the isolated anterior and intermediate pituitary. Endocrinology 127, 1956–1966 (doi:10.1210/endo-127-4-1956) [DOI] [PubMed] [Google Scholar]
- Gammaitoni L., Hänggi P., Jung P., Marchsoni F.1998Stochastic resonance. Rev. Mod. Phys. 70, 223–288 (doi:10.1103/RevModPhys.70.223) [Google Scholar]
- Goldbeter A.1996Biochemical oscillations and cellular rhythms: the molecular basis of periodic and chaotic behaviour Cambridge, UK: Cambridge University Press [Google Scholar]
- Gupta S., Aslakson E., Gurbaxani B. M., Vernon S. D.2007Inclusion of the glucocorticoid receptor in a hypothalamic pituitary adrenal axis model reveals bistability. Theor. Biol. Med. Model. 4, 8 (doi:10.1186/1742-4682-4-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F.2000The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23, 477–501 (doi:10.1016/S0893-133X(00)00159-7) [DOI] [PubMed] [Google Scholar]
- Holsboer F.2001Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J. Affect. Disord. 62, 77–91 (doi:10.1016/S0165-0327(00)00352-9) [DOI] [PubMed] [Google Scholar]
- Iranmanesh A., Lizarralde G., Johnson M. L., Veldhuis J. D.1989Circadian, ultradian and episodic release of beta-endorphin in men and its temporal coupling with cortisol. J. Clin. Endocrinol. Metab. 68, 1019–1026 (doi:10.1210/jcem-68-6-1019) [DOI] [PubMed] [Google Scholar]
- Ixart G., Barbanel G., Nouguier-Soulé J., Assenmacher I.1991A quantitative study of the pulsatile parameters of CRH-41 secretion in unanesthetized free-moving rats. Exp. Brain Res. 87, 153–158 [DOI] [PubMed] [Google Scholar]
- Jasper M. S., Engeland W. C.1991Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am. J. Physiol. 261, R1257–R1268 [DOI] [PubMed] [Google Scholar]
- Jones M. T., Hillhouse E. W., Burden J. L.1977Dynamics and mechanics of corticosteroid feedback at the hypothalamus and anterior pituitary gland. J. Endocrinol. 73, 405–417 (doi:10.1677/joe.0.0730405) [DOI] [PubMed] [Google Scholar]
- Keller-Wood M. E., Dallman M. F.1984Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 5, 1–24 (doi:10.1210/edrv-5-1-1) [DOI] [PubMed] [Google Scholar]
- Krauskopf B., Osinga H. M., Galán-Vioque J.(eds)2007Numerical continuation methods for dynamical systems: path following and boundary value problems Berlin, Germany: Springer [Google Scholar]
- Kuznetsov Y. A.1995Elements of applied bifurcation theory Berlin, Germany: Springer [Google Scholar]
- Lang D. A., Matthews D. R., Peto J., Turner R. C.1979Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N. Engl. J. Med. 301, 1023–1027 [DOI] [PubMed] [Google Scholar]
- Lightman S. L., et al. 2008The significance of glucocorticoid pulsatility. Eur. J. Pharmacol. 583, 255–262 (doi:10.1016/j.ejphar.2007.11.073) [DOI] [PubMed] [Google Scholar]
- Lui J. H., Kazer R. R., Rasmussen D. D.1987Characterization of the twenty-four hour secretion patterns of adrenocorticotropin and cortisol in normal women and patients with Cushing's disease. J. Clin. Endocrinol. Metab. 64, 1027–1035 [DOI] [PubMed] [Google Scholar]
- Ma X. M., Levy A., Lightman S. L.1997Rapid changes of heteronuclear RNA for arginine vasopressin but not for corticotropin releasing hormone in response to acute corticosterone administration. J. Neuroendocrinol. 9, 723–728 (doi:10.1046/j.1365-2826.1997.00646.x) [DOI] [PubMed] [Google Scholar]
- McEwen B. S.2007Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (doi:10.1152/physrev.00041.2006) [DOI] [PubMed] [Google Scholar]
- Mershon J. L., Sehlhorst C. S., Rebar R. W., Liu J. H.1992Evidence of a corticotrophin-releasing hormone pulse generator in the macaque hypothalamus. Endocrinology 130, 2991–2996 (doi:10.1210/en.130.5.2991) [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B.1996The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol. Psychiatry 1, 336–342 [PubMed] [Google Scholar]
- Papaikonomou E.1977Rat adrenocortical dynamics. J. Physiol. 265, 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavasiliou S. S., Zmeili S., Khoury S., Landefeld T. D., Chin W. W., Marshall J. C.1986Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone alpha and beta subunits in male rats. Proc. Natl Acad. Sci. USA 83, 4026–4029 (doi:10.1073/pnas.83.11.4026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante C. M.2003Depression, stress and the adrenal axis. J. Neuroendocrinol. 15, 811–812 [DOI] [PubMed] [Google Scholar]
- Pariante C. M.2004Glucocorticoid receptor function in vitro in patients with major depression. Stress 7, 209–219 (doi:10.1080/10253890500069650) [DOI] [PubMed] [Google Scholar]
- Pariante C. M., Miller A. H.2001Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry 49, 391–404 (doi:10.1016/S0006-3223(00)01088-X) [DOI] [PubMed] [Google Scholar]
- Pepin M. C., Pothier F., Barden N.1992Antidepressant drug action in a transgenic mouse model of the endocrine changes seen in depression. Mol. Pharmacol. 42, 991–995 [PubMed] [Google Scholar]
- Plotsky P. M., Vale W.1985Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science 230, 461–463 (doi:10.1126/science.2864742) [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R.2002Coordination of circadian timing in mammals. Nature 418, 935–941 (doi:10.1038/nature00965) [DOI] [PubMed] [Google Scholar]
- Spiga F., Harrison L. R., Wood S. A., Atkinson H. C., MacSweeney C. P., Thomson F., Craighead M., Grassie M., Lightman S. L.2007Effect of the glucocorticoid receptor antagonist Org 34850 on basal and stress-induced corticosterone secretion. J. Neuroendocrinol. 19, 891–900 (doi:10.1111/j.1365-2826.2007.01605.x) [DOI] [PubMed] [Google Scholar]
- Stavreva D. A., et al. 2009Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat. Cell Biol. 11, 1093–1102 (doi:10.1038/ncb1922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker J., Cookson S., Bennett M. R., Mather W. H., Tsimring L. S., Hasty J.2008A fast, robust and tunable synthetic gene oscillator. Nature 456, 516–519 (doi:10.1038/nature07389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges M., Marquez-Lago T. T., Stelling J., Fussenegger M.2009A tunable synthetic mammalian oscillator. Nature 457, 309–312 (doi:10.1038/nature07616) [DOI] [PubMed] [Google Scholar]
- Tsai T. Y., Choi Y. S., Ma W., Pomerening J. R., Tang C., Ferrell J. E., Jr2008Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science 321, 126–129 (doi:10.1126/science.1156951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai Y. M., Herman J. P.2009Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409 (doi:10.1038/nrn2647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis J. B., Iranmanesh A., Lizarralde G., Johnson M. L.1989Amplitude modulation of a burst-like mode of cortisol secretion subserves the circadian glucocorticoid rhythm. Am. J. Physiol. 257, E6–E14 [DOI] [PubMed] [Google Scholar]
- Veldhuis J. B., Iranmanesh A., Johnson M. L., Lizarralde G.1990Amplitude, but not frequency, modulation of adrenocorticotropin secretory bursts gives rise to the nyctohemeral rhythm of corticotropic axis in man. J. Clin. Endocrinol. Metab. 71, 452–463 (doi:10.1210/jcem-71-2-452) [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Ram P. A., Park S. H., Choi H. K.1995Intermittent plasma growth hormone triggers tyrosine phosphorylation and nuclear translocation of a liver-expressed, Stat 5-related DNA binding protein. Proposed role as an intracellular regulator of male-specific liver gene transcription. J. Biol. Chem. 270, 13 262–13 270 [DOI] [PubMed] [Google Scholar]
- Weitzman E. D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T. F., Hellman L.1971Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J. Clin. Endocrinol. Metab. 33, 14–22 (doi:10.1210/jcem-33-1-14) [DOI] [PubMed] [Google Scholar]
- Wiesenfeld K., Moss F.1995Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature 373, 33–36 (doi:10.1038/373033a0) [DOI] [PubMed] [Google Scholar]
- Wildt L., Häusler A., Marshall G., Hutchison J. S., Plant T. M., Belchetz P. E., Knobil E.1981Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109, 376–385 (doi:10.1210/endo-109-2-376) [DOI] [PubMed] [Google Scholar]
- Windle R. J., Wood S. A., Shanks N., Lightman S. L., Ingram C. D.1998aUltradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139, 443–450 (doi:10.1210/en.139.2.443) [DOI] [PubMed] [Google Scholar]
- Windle R. J., Wood S. A., Lightman S. L., Ingram C. D.1998bThe pulsatile characteristics of hypothalamo-pituitary–adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology 139, 4044–4052 (doi:10.1210/en.139.10.4044) [DOI] [PubMed] [Google Scholar]
- Young E. A., Abelson J., Lightman S. L.2004Cortisol pulsatility and its role in stress regulation and health. Front. Neuroendocrinol. 25, 69–76 (doi:10.1016/j.yfrne.2004.07.001) [DOI] [PubMed] [Google Scholar]
- Young E. A., Ribeiro S. C., Ye W.2007Sex differences in ACTH pulsatility following metyrapone blockade in patients with major depression. Psychoneuroendocrinology 32, 503–507 (doi:10.1016/j.psyneuen.2007.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]