Abstract
The spiny-finned teleost fishes (Acanthomorpha) include nearly one-third of all living vertebrate species and assume a bewildering array of bodyplans, but the macroevolutionary assembly of modern acanthomorph biodiversity remains largely unexplored. Here, I reconstruct the trajectory of morphological diversification in this major radiation from its first appearance in the Late Cretaceous to the Miocene using a geometric morphometric database comprising more than 600 extinct species known from complete body fossils. The anatomical diversity (disparity) of acanthomorphs is low throughout the Cretaceous, increases sharply and significantly in the wake of the Cretaceous–Palaeogene (K–P) extinction, and shows little change throughout subsequent Cenozoic intervals. This pattern of morphological diversification appears robust to two potential biasing factors: the ‘Lagerstätten effect’, and the non-random segregation of rare and common taxa along phenotypic axes. Dissecting the trajectory of acanthomorph radiation along phylogenetic lines reveals that the abrupt post-extinction increase in disparity is driven largely by the proliferation of trophically diverse modern groups within Percomorpha, a spiny-fin subclade containing more than 15 000 living species and identified as showing a substantially elevated diversification rate relative to background vertebrate levels. A major component of the Palaeogene acanthomorph radiation reflects colonization of morphospace previously occupied by non-acanthomorph victims of the K–P. However, other aspects of morphological diversification cannot be explained by this simple ecological release model, suggesting that multiple factors contributed to the prolific anatomical radiation of acanthomorphs.
Keywords: Acanthomorpha, adaptive radiation, biodiversity, ecological release, morphometrics, Teleostei
1. Introduction
The more than 18 000 species of spiny-finned fishes (Acanthomorpha) represent nearly one-third of living vertebrate biodiversity (Stiassny et al. 2004; Smith & Wheeler 2006). Modern examples range in size from among the smallest vertebrates (Watson & Walker 2004) to the largest ray-finned fishes in the sea (Nelson 2006), encompass bodyplans as divergent as those of flounders, pufferfishes, seahorses and anglerfishes, and include important targets of commercial fisheries like cod and tuna. Dissecting large-scale evolutionary patterns in acanthomorphs is a prerequisite for understanding the assembly of modern vertebrate diversity, and provides vital historical context for research in genomics, development, evolution, ecology and behaviour that targets acanthomorph model systems (Chen et al. 2004).
The oldest acanthomorph body fossils date to the early Late Cretaceous (eLK; Cenomanian, 100–94 Ma; all dates from Gradstein et al. 2004). The history of this clade is punctuated by the Cretaceous–Palaeogene (K–P) mass extinction, but the impact of this event on acanthomorph evolution remains little explored. Acanthomorph taxonomic diversity appears to have increased in the wake of the K–P (Patterson 1993b), drawing immediate comparisons to patterns reported for eutherian mammals and neoavian birds, two clades sometimes interpreted as having diversified in the Palaeogene to fill ecological roles cleared by extinction (Alroy 1999; Ericson et al. 2006).
Acanthomorphs present two major advantages over these terrestrial groups as a system for studying the evolutionary impacts of the K–P on a major vertebrate radiation. First, there is unambiguous evidence that the acanthomorph crown extends deep into the Cretaceous (Patterson 1993a,b), avoiding debates over divergence times that have overshadowed more central questions concerning the onset of trophic divergence and ecological dominance in birds and mammals (Alroy 1999). Second, and more importantly, many extinct acanthomorph species are known from intact skeletal remains, whereas the terrestrial groups are generally represented by fragmentary material. When coupled with research relating anatomical form to biomechanical function in modern fishes (e.g. Wainwright & Bellwood 2002), this fossil archive represents a potentially powerful tool for testing hypotheses of trophic diversification and ecological replacement.
Exploring acanthomorph radiation through an anatomical lens is a necessary complement to previous studies based on taxonomy alone (e.g. Patterson 1993b), because morphological diversity and taxonomic richness—and the evolutionary dynamics underlying them (Adams et al. 2009)—need not be closely linked (Foote 1996). Here, I apply geometric morphometrics to the rich body-fossil record of fishes in order to: (i) reconstruct the pattern of acanthomorph anatomical diversification over the 100 Myr history of the clade (including patterns within the hyperdiverse subclade Percomorpha, which contains more than 15 000 living species); (ii) identify intervals of acanthomorph history characterized by major shifts in morphological diversity; and (iii) test whether the K–P extinction shaped the trajectory of acanthomorph evolution in a manner consistent with the predictions of an ecological release model.
2. Material and methods
(a). Taxon sampling
The morphometric database assembled for this analysis comprises 1336 individual acanthomorph fossils from marine deposits assigned to 605 species ranging in age from eLK (Cenomanian) to Late Miocene (Messinian). A majority of specimens were examined and photographed in museum collections, but some examples were taken from published images (photographs or specimen drawings). The dagger symbol (†) indicates extinct taxa.
(b). Morphometric protocols
Landmarks were digitized using the software package tpsDig (Rohlf 2008a). Two classes of landmarks were used: fixed points marking discrete morphological features and sliding ‘semilandmarks’ designed to capture overall body shape (figure 1a). This scheme is similar to those applied by studies of modern acanthomorphs (e.g. Chakrabarty 2005; Young et al. 2009). The present analysis primarily aims to document patterns of anatomical diversification, but landmarks were selected to record ecologically or functionally characterized structures when possible (Wainwright & Bellwood 2002; Cooper & Westneat 2009). Whole-body morphology is not the most appropriate predictor of feeding ecology (e.g. Chakrabarty 2005). I have therefore investigated patterns of morphospace occupancy based on cranial landmark data alone (electronic supplementary material), with the caveat that this coarse approach is not as clear an indicator of trophic ecology as targeted analysis of jaw biomechanics and dental structure (Wainwright & Bellwood 2002).
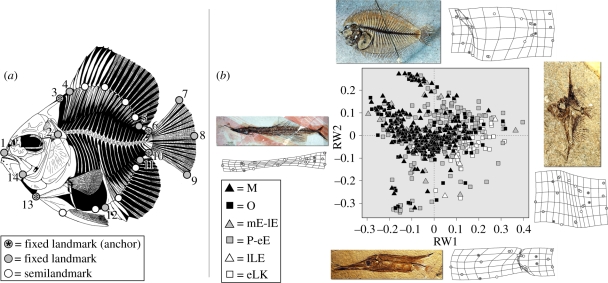
Figure 1.
Acanthomorph morphometrics. (a) Landmark scheme. Fixed landmarks are indicated by circles filled in grey and marked by numerals, while semilandmarks are marked by circles filled in white. Grey-filled circles containing an asterisk indicate fixed landmarks that serve as anchor points for intervening semilandmarks. Fixed landmarks record (moving clockwise, starting with the snout): 1, anterior tip of upper jaw (premaxilla); 2, joint between braincase and vertebral column; 3, posterodorsal tip of braincase (supraoccipital crest); 4, anterior insertion of dorsal fin; 5, posterior insertion of dorsal fin; 6, insertion of first ray of dorsal lobe of caudal fin; 7, distal tip of first principal ray of caudal fin (dorsal lobe); 8, posterior margin of caudal fin between dorsal and ventral lobes; 9, distal tip of first principal ray of caudal fin (ventral lobe); 10, insertion of first ray of ventral lobe of caudal fin; 11, posterior insertion of anal fin; 12, anterior insertion of anal fin; 13, ventral tip of pectoral girdle; 14, lower jaw joint. (b) Mean species values plotted on RW1 and RW2, which together account for over 62 per cent of overall variance. Symbols marking species indicate geological interval (eLK, early Late Cretaceous; lLK, late Late Cretaceous; P–eE, Palaeocene–early Eocene; mE–lE, Middle–Late Eocene; O, Oligocene; M, Miocene). Deformation grids indicate shapes found at extreme values along each axis, and fossils correspond to taxa approximating these morphologies. Higher axes given in the electronic supplementary material.
Fishes are prone to post-mortem contortion as muscle contraction induces curvature along the long axis of the body. Fossils showing this configuration were retrodeformed using the program tpsUtil (Rohlf 2008b). Procrustes-aligned coordinate data were subjected to relative warps (RW) analysis in tpsRelw (Rohlf 2007). Higher axes in multivariate ordinations often summarize noise, so analyses were restricted to RW that explain more than 5 per cent of overall variance (table 1). This study employs multivariate variance (sum of univariate variances) as a measure of disparity because it is relatively insensitive to differences in sample size (Ciampaglio et al. 2001). Additional details of morphometric analyses are given in the electronic supplementary material.
Table 1.
Significant morphospace axes, showing percentage of overall morphological variance each describes. Last two columns provide snapshots of extreme morphologies at opposite ends of each RW axis, visualized using deformation grids in the software package tpsRelw 1.45 (Rohlf 2007). These grids are shown for RW1 and RW2 in figure 1b and higher axes in the electronic supplementary material.
| RW | % variance | anatomical correlate (+ score) | anatomical correlate (− score) |
|---|---|---|---|
| 1. | 36.9 | deep body | slender body |
| 2. | 25.7 | long dorsal- and anal-fin bases | short dorsal- and anal-fin bases |
| 3. | 12.5 | deep body, small caudal fin | slender body, large caudal fin |
| 4. | 7.10 | short caudal peduncle | long caudal peduncle |
(c). Stratigraphic binning
Rather than stage-level intervals, I employ six composite bins of more comparable duration: (i) early Late Cretaceous (eLK; Cenomanian–Santonian, 16.1 Ma); (ii) late Late Cretaceous (lLK; Campanian–Maastrichtian, 18 Ma); (iii) Palaeocene–early Eocene (P–eE; Danian–Ypresian, 16.9 Ma); (iv) middle–late Eocene (mE–lE; Lutetian–Priabonian, 14.7 Ma); (v) Oligocene (O; Rupelian–Chattian, 10.9 Ma); and (vi) Miocene (M; Aquitanian–Messinian, 17.7 Ma).
3. Results
(a). The trajectory of anatomical radiation in acanthomorphs
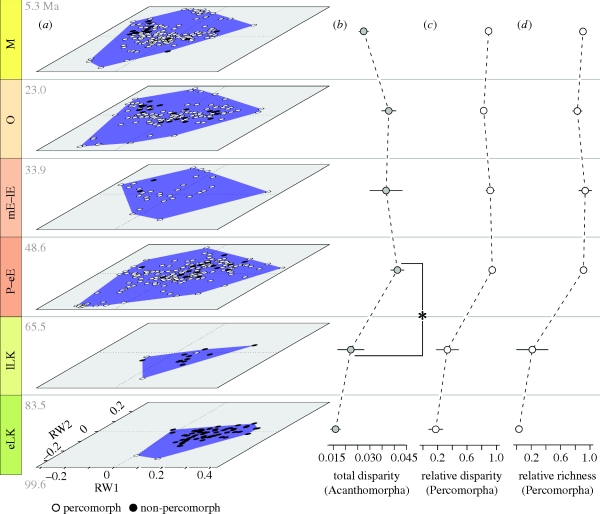
Cretaceous acanthomorphs are restricted to areas of morphospace with positive or weakly negative scores on RW1 (figure 2a,b). Taxa in this region are characterized by moderately deep bodies, and nearly all are anatomically primitive representatives of clades branching deep within acanthomorph phylogeny: lampridiforms (velifers and allies), polymixiids (beardfishes), incertae sedis paracanthopterygians, trachichthyoids (slimeheads) and holocentroids (squirrelfishes). Percomorpha (sensu Smith & Wheeler 2006) is the dominant living acanthomorph clade, but Cretaceous representatives of this group number only a handful of examples (Patterson 1993a,b; Arratia et al. 2004; figure 2d) that account for a minor fraction of overall acanthomorph morphological variety (partial disparity, Foote 1993; figure 2c).
Figure 2.
Patterns of diversification in acanthomorph teleosts. Abbreviations for stratigraphic intervals are those used in figure 1, with date estimates shown for the beginning and end of each bin. (a) Morphospace occupancy during acanthomorph history. White circles represent percomorphs, black circles indicate other acanthomorphs. (b) Acanthomorph disparity expressed as multivariate variance of significant RW axes (RW1–RW4). Error bars are ±1 s.d. generated from 10 000 bootstrap pseudoreplicates. Asterisk (*) marks a significant increase in disparity between the lLK and P–eE (LR = 8.390 × 107; Student's t-test: t = 1.89, d.f. = 207, p = 0.0313). (c) Percomorph contribution to overall acanthomorph disparity (percomorph partial disparity/acanthomorph disparity). Error bars are ±1 s.d. generated from 10 000 bootstrap pseudoreplicates. (d) Percomorph contribution to overall acanthomorph richness surveyed in this analysis. Error bars are 95 per cent binomial confidence intervals.
Two major differences immediately distinguish the Cenozoic history of acanthomorphs from this Mesozoic pattern, and these changes are fully realized in the first interval following the K–P. First, the P–eE and all subsequent Cenozoic bins are marked by substantially higher levels of morphological diversity than either Mesozoic interval. Second, percomorphs dominate the acanthomorph fauna throughout the Cenozoic, in terms of their fractional contribution to both disparity (figure 2c) and richness (figure 2d).
These two patterns are interrelated: the increase in acanthomorph disparity in the P–eE relative to the lLK is overwhelmingly driven by the appearance of new bodyplans within percomorphs. A pronounced excursion into regions of morphospace marked by strongly negative scores on RW1 reflects the evolution of slender body forms. New groups colonizing this region include the first representatives of several clades of pelagic predators: sphyraenids (baraccudas), carangids (jacks), gemplyids (snake mackerels), scombrids (tunas), gadiforms (cods), and †blochiids, †hemingwayids and †palaeorhynchids (extinct ‘billfishes’). With the exception of gadiforms, all of these groups branch from within Percomorpha (Smith & Wheeler 2006). Further proliferation along RW1 is accommodated by percomorph clades that have strongly attenuated bodies relative to stratigraphically older acanthomorphs: ophidiiforms (cusk eels), aulostomids (trumpetfishes) and fistulariids (cornetfishes).
Less pronounced expansion of acanthomorph morphospace also occurs along RW2 (figure 2a). This signals diversification in dorsal- and anal-fin insertion patterns, some of which appear related to the origin of novel locomotor strategies (e.g. Hove et al. 2001). As is the case on RW1, extreme morphologies on RW2 are dominated by percomorph subclades. Aulostomids and fistulariids, along with other syngnathiform (pipefishes and allies) groups and crown-clade tetraodontiforms (pufferfishes and allies), represent strong negative excursions along this axis that surpass the most extreme morphologies found in Mesozoic acanthomorphs. In addition to marking the appearance of clades with very short-based dorsal and anal fins, the P–eE also records the expansion of acanthomorphs into regions of morphospace with strongly positive scores on this axis. These groups are characterized by an elongated dorsal fin extending far anteriorly towards (or onto) the skull, and comprise stem and crown pleuronectiforms (flatfishes), luvarids (louvars), acanthurids (surgeonfishes) and lophiiforms (anglerfishes).
The gross pattern of acanthomorph morphospace occupation established by the close of the P–eE continues throughout the remainder of the Cenozoic with little change. The only conspicuous deviation is found in the mE–lE, where no sampled species show strongly negative scores on both RW1 and RW2. Syngnathiforms dominate this region of morphospace in other temporal bins, but examples from the mE–lE are too poorly preserved to be included in this analysis.
(b). Identifying major shifts in morphological diversity
Ratios of marginal likelihoods for variance (LR; Edwards 1992) were used to test for differences in anatomical diversity between successive stratigraphic bins (electronic supplementary material). For most intervals, there is no major shift in morphological diversity from the previous temporal bin. An exception is the transition between the lLK and P–eE (figure 2b), where an LR of 8.390 × 107 indicates a substantial increase in disparity in the wake of the K–P (LR ≥ 8 is taken as strong support for differences in variance; Royall 1997). This significant increase in morphological diversity is also reflected in standard statistical tests (Student's t-test: t = 1.89, d.f. = 207, p = 0.0313).
(c). Testing for ecological replacement in the aftermath of the K–P
Episodes of prolific diversification in the fossil record often follow mass extinction events (Erwin 2001; Krug et al. 2009). In such cases, rapid evolutionary radiation is often hypothesized to reflect the opportunistic refilling of functional roles once held by extinction victims (Jablonski 2008). Morphological radiation in acanthomorphs is focused in the aftermath of the K–P, raising the question of whether any aspects of diversification in this group might have been driven by ecological release.
Early surveys focusing on extinction intensity concluded that fishes were little affected by the K–P (Patterson & Smith 1989; MacLeod et al. 1997), but more recent analyses addressing patterns of selectivity indicate substantial changes in marine teleost faunas. Cavin & Martin (1995) and Cavin (2001) found that families of pelagic predators made up a disproportionate number of bony fish extinction victims, and genus-level investigations point to the extirpation of an ecological guild comprising large-bodied taxa whose jaw mechanics and gut contents indicate piscivory (Friedman 2009).
In order to test the hypothesis that some acanthomorph clades radiated to fill vacated ecological roles following the K–P, I have targeted the genera that fall outside the envelope of extinction survivors in at least one variant of the bivariate (jaw-closing mechanical advantage by body size) ecomorphospace presented in Friedman (2009). Of the 14 genera fitting this criterion, none are acanthomorphs, and 10 are represented by sufficiently intact material to be included in the morphometric analysis. In contrast to previous sections, I have excluded landmarks indicating the insertions of the dorsal and anal fins. This modification stems from the fact that most acanthomorphs, regardless of their ecology, are characterized by dorsal- and anal-fin insertions far more extensive (relative to overall body length) than non-acanthomorphs. I therefore take the primary signal for these traits to be phylogenetic in cases where acanthomorphs are contrasted with non-acanthomorphs. As a consequence, the RW axes shown in figure 3 do not summarize patterns of variation identical to those in previous ordinations (figs S1 and S2 in the electronic supplementary material).
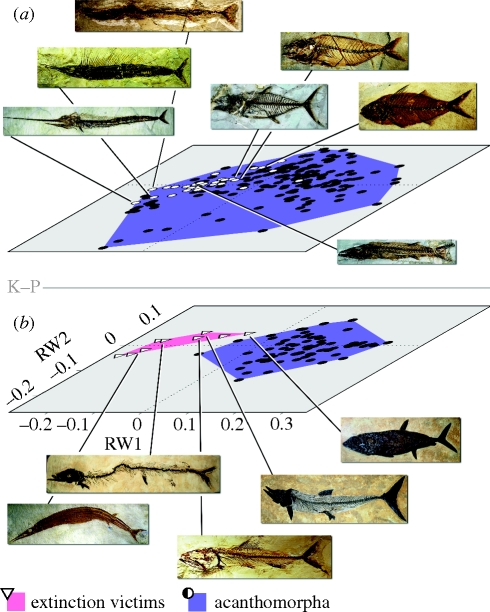
Figure 3.
Major aspects of acanthomorph diversification in the early Cenozoic are consistent with the refilling of morphospace vacated by non-acanthomorph victims of the end-Cretaceous extinction. Ordinations differ from those in figures 1b and 2a in excluding landmarks 4, 5, 11 and 12 (see explanation in text). (a) Acanthomorphs from the P–eE. Acanthomorphs belonging to predatory clades hypothesized to represent ecological replacements of non-acanthomorph victims (Cavin 2001; Friedman 2009; electronic supplementary material) are indicated by white circles. Images are, from left to right and top to bottom, representatives of †Rhamphognathidae, Pomatomidae, †Palaeorhynchidae, Carangidae, †Blochiidae, Scombridae, Sphyraenidae. (b) All Cretaceous acanthomorphs (eLK and lLK) plus non-acanthomorph victims of the K–P falling outside the morphological envelope of survivors in Friedman (2009). White triangles represent extinction victims. Images are, from left to right and top to bottom, representatives of †Pachyrhizodontidae, †Saurocephalidae, †Cimolichthyidae, †Aspidorhynchidae and †Enchodontidae. See the electronic supplementary material for full listing of victims and putative replacements.
Together, these non-acanthomorph taxa yield an estimate of the region of teleost morphoospace that was devastated by the K–P, which can be used to test whether acanthomorph diversification is consistent with the refilling of vacated functional roles. Pelagic predatory acanthomorph clades hypothesized to represent functional replacements were specified a priori, and include those families noted by Cavin (2001) and Friedman (2009), plus five additional groups: belonids (needlefishes), †mesogasterids and †rhamphognathids (putative atherinomorphs), †‘euzaphlegids’ (a heterogeneous assemblage that probably includes members of the scombroid stem), and gadiforms minus Bregmacerotidae (this family is excluded because its members are planktivores; Cohen 1986). Randomization tests were used to quantify similarity between extinction victims and these putative replacements.
The distributions of non-acanthomorph victims of the K–P and the pooled sample of Cretaceous acanthomorphs show no overlap (figure 3b). Extinction victims populate a narrow band of morphospace characterized by negative scores on RW1 and centred about zero on RW2. Morphologies in this region range from thunniform bodies equipped with large, high aspect-ratio caudal fins to slender forms with elongated rostra. By the close of P–eE, acanthomorph morphospace had expanded to encompass much of the region previously occupied by extinction victims, consistent with the hypothesis that some aspects of the acanthomorph radiation reflect ecological release. The envelope enclosing predatory acanthomorph clades corresponds closely with the field defined by victims of the K–P. Quantitative tests corroborate the pattern that is clear from visual inspection of the data: P–eE predatory acanthomorphs are much more similar to extinction victims than a randomly drawn sample of spiny-finned teleosts from that first Cenozoic interval (1000 realizations; mean empirical pairwise distance = 0.125, mean randomized pairwise distance = 0.238, p ≪ 0.01). A similar pattern emerges when only cranial landmarks are considered (electronic supplementary material).
4. Robustness of results to biases of the fossil record
(a). Non-random segregation of rare and common species along phenotypic axes
If rare species contribute more to measures of disparity than common ones, intervals yielding fewer fossils will appear to contain less morphological variety as a consequence of differential sampling. I have tested for a correlation between partial disparity (the contribution of an individual taxon to the measure of disparity at a more inclusive level; Foote 1993) and abundance within five acanthomorph faunas for which specimen counts are available, and found no consistent relationship between these variables (electronic supplementary material; cf. Deline 2009). Only one assemblage shows a significant correlation between abundance and partial disparity (Spearman rank-order correlation: r = 0.448, p = 0.0416), but this weak relationship is positive, and common species contribute relatively more to cumulative measures of disparity than do rare ones. This exercise suggests that uneven sampling over time is unlikely to be further exacerbated by covariation between abundance and morphology that systematically depresses the apparent disparity of intervals with poorer fossil records.
(b). The ‘Lagerstätten’ effect
Distortion introduced to temporal patterns of species richness by exceptional fossil deposits has long been recognized, and so-called ‘Lagerstätten effects’ (Raup 1972) undoubtedly underlie many prominent shifts in fish taxonomic diversity curves that arise from a direct reading of palaeontological data (Patterson & Smith 1987, 1989; Patterson 1993b; MacLeod et al. 1997; Hurley et al. 2007; for methods designed to determine whether such shifts reflect underlying evolutionary dynamics or are driven by variation in fossil record quality see Cavin & Forey 2007, Cavin 2010). The influence of such sites is rarely assessed in analyses of morphological diversification.
I have adopted two jack-knifing procedures to test for the effects of exceptional faunas on apparent patterns of morphological diversification. The first approach eliminated the single fossil assemblage in each stratigraphic bin with the greatest number of sampled acanthomorph species. The second approach removed from consideration all faunas that exceeded a threshold richness of 40 nominal actinopterygian fish species (the larger group containing acanthomorphs). This more severe procedure permitted the removal of multiple assemblages per temporal bin; an important allowance given that exceptionally fossiliferous deposits are often clustered stratigraphically (e.g. during sea-level highstands in the case of fishes; Cavin & Forey 2007; Cavin 2010). These deletion approaches have little overall effect on inferred patterns of morphological diversification, even though they reduce sampled acanthomorph species richness for several intervals by more than half (electronic supplementary material). Importantly, the most striking pattern to emerge from the analyses above—the increase in acanthomorph disparity between the P–eE and the Cretaceous—remains intact, despite the removal of the iconic early Eocene Bolca Lagerstätte under both approaches. Disparity trajectories assembled from all fossil sites and those based on a subset of assemblages are strongly correlated (Spearman r = 0.886 for both procedures; significance tests do not apply to whole : part comparisons), implying that the patterns of morphological diversification discussed in previous sections are not strongly distorted by exceptional fossil deposits.
5. Discussion
(a). The adaptive radiation of acanthomorph teleosts
The morphological diversification of acanthomorphs in the early Cenozoic was characterized by two interrelated themes of adaptive radiation: proliferation of new anatomies, and the exploitation of new ecological strategies (Schluter 2000). This significant increase in disparity coincides with a sharp rise in familial diversity (Patterson 1993b) and, perhaps more importantly, a major shift in the composition of marine fish faunas. A minor component of Cretaceous teleost assemblages in terms of relative richness (Patterson 1993a; Forey et al. 2003), acanthomorphs became taxonomic dominants in marine ecosystems by the end of the Palaeocene, within 10 Myr of the K–P (Bannikov & Parin 1997; Bonde 1997).
The morphological and taxonomic exuberance that characterizes the early Cenozoic history of acanthomorphs largely reflects the rapid appearance of trophically diverse modern groups within Percomorpha. Often referred to as the persistent ‘bush at the top’ (Nelson 1989) of the teleost tree of life, percomorph intrarelationships have proven difficult to dissect. Short internodes along the spine of percomorph molecular phylogenies (Li et al. 2008, 2009), coupled with high levels of incongruence between topologies reconstructed from different sequences (Chen et al. 2003), are consistent with the rapid radiation inferred from palaeontological data (cf. Poe & Chubb 2004). The significance of these patterns is amplified by the discovery that percomorphs show a greater increase in diversification rate relative to background levels across vertebrates than all other major clades except neoavian birds (Alfaro et al. 2009a,b).
(b). Extrinsic factors shaping patterns of acanthomorph morphological diversification
One major feature of the Palaeogene radiation of acanthomorphs—the origin of several clades of pelagic predators—appears related to the filling of vacated functional roles, but additional axes of morphological diversification are not clearly explained under this model. This might reflect the conservative approach applied here, which focuses on regions of morphospace that were devastated, rather than thinned, by extinction, combined with the limitations of a broadly framed landmark-based morphometric scheme in comparison to a targeted functional analysis. A mosaic pattern of turnover could remain an important but subtle mechanism underlying aspects of the acanthomorph radiation. Selection against higher trophic levels of the sort inferred for teleosts during the K–P (Cavin 2001; Friedman 2009) might yield particularly nuanced succession dynamics, because piscivorous fishes assume a range of morphologies and represent the most anatomically disparate diet class in some modern faunas (Chakrabarty 2005). Attempts to detect such a pattern with the present dataset are confounded by the fact that several piscivorous acanthomorph clades originating in the P–eE (e.g. flatfishes, anglerfishes) display highly specialized bodyplans that have no precedents among Cretaceous fishes, acanthomorphs or otherwise, highlighting the ecological limitations of the morphometric scheme applied here.
Another outstanding feature of the early Palaeogene radiation of acanthomorphs lies at the opposite end of the trophic spectrum, with the proliferation of clades with cranial structure implying precise biting, grazing and manipulation of benthic prey items (Bellwood 2003; Bellwood & Hoey 2004). The signal of these cranial innovations is swamped by postcranial variation in the whole-body analyses (figure 2), but is apparent as the colonization of a region of morphospace diagnosed by relatively deep suspensoria occurring in conjunction with small, anteriorly shifted jaws in ordinations that only consider cranial landmarks (electronic supplementary material). The major excursion into this area of morphospace during the P–eE is driven by several percomorph clades: crown tetraodontiforms (pufferfishes and allies), acanthurids (surgeonfishes), zanclids (Moorish idols) and sparids (porgies). Isolated crown tetraodontiform dentitions of probable Maastrichtian age (Gallo et al. 2009) are too fragmentary to be included in the present morphometric scheme, but indicate that elements of this morphological innovation lie in the lLK.
Unlike the case shown for pelagic predators, there is no obvious indication that the K–P cleared regions of morphospace later colonized by benthic grazers (Friedman 2009). The closest Mesozoic analogues of these percomorph groups appear to be †pycnodonts, an anatomically diverse clade of stem teleosts that survived the K–P and persisted until the mid-Eocene (Nursall 1996). Instead, the radiation of these particular percomorph clades has been linked (Bellwood & Wainwright 2002) to the ascendance of scleractinian-dominated reefs in the early Palaeogene (Kiessling 2008). The correlation between ecological association with reefs and elevated rates of diversification demonstrated for some modern acanthomorph clades (Alfaro et al. 2007, 2009a,b), combined with strong evidence for reefs as cradles of biodiversity throughout the Phanerozoic (Kiessling et al. 2010), lends weight to this hypothesis. However, potential decoupling of rates of taxonomic diversification and morphological change (cf. Adams et al. 2009) means that it remains uncertain whether reef clades likewise show elevated levels of phenotypic change. Future studies of reef-fish evolution should address this unsettled issue.
Unlike that of pelagic predators, the radiation of reef-associated acanthomorph clades clearly traces its roots into the Mesozoic. The lLK acanthomorph fauna from Nardò, Italy, is dominated by typical Cretaceous forms (lampridiforms, trachichthyoids, zeiforms and a possible paracanthopterygian; Patterson 1993a,b; Tyler et al. 2000; Taverne 2003) in terms of both richness and abundance, but, uniquely among Cretaceous marine sites, yields multiple articulated percomorph taxa (Patterson 1993a,b; Santini & Tyler 2003). These rare examples are plesiomorphic representatives of groups that today show strong associations with reefs (tetraodontiforms, syngnathiforms; Wainwright & Bellwood 2002). Intriguingly, the Nardò fish beds derive from a carbonate platform yielding prolific assemblages of †rudist bivalves (Schlüter et al. 2008), the primary structural contributors to Late Cretaceous reefs. The origin of these reef-associated percomorph clades in the Mesozoic, coupled with the first appearance of pelagic predatory groups in the aftermath of the K–P, suggests that the alignment of multiple factors, rather than any one event, contributed to the spectacular diversification of acanthomorphs between the lLK and the close of the P–eE (cf. Bellwood & Wainwright 2002).
(c). Timing the acanthomorph radiation: clocks and rocks, phylogenetic divergence and trophic differentiation
The results presented here reflect a time scale for acanthomorph evolution based on the stratigraphic distribution of body fossils. The dense fish otolith record, an independent check on the less complete record of intact skeletons, records a broadly similar picture of acanthomorph diversification (Patterson 1993a; Nolf 1995, 2003), but some molecular clock analyses deliver a very different timeline than either of these palaeontological archives. The origin of the acanthomorph crown has been estimated to lie between the Early Triassic (Yamanoue et al. 2006) and the Early Jurassic (Azuma et al. 2008), predating the oldest fossils of crown teleosts (Late Jurassic; Patterson 1993b). The absence of any pre-Cretaceous acanthomorphs is striking, especially as this interval contains some of the best-studied marine Lagerstätten of the Phanerozoic (Solnhofen, Cerin, Posidonia Shale, Oxford Clay, Monte San Giorgio; Bottjer et al. 2001). A major implication of this pattern is that acanthomorphs, if extant during this interval, were rare to the degree that they were ecologically insignificant.
It seems more likely that the absence of acanthomorphs from early–mid Mesozoic deposits is genuine rather than artefactual, and that the time scales advanced by some clock analyses are distorted by a reliance on mitogenomic data and calibrations that derive from previous molecular estimates or hypothetical biogeographical scenarios (see arguments in Hurley et al. 2007). A recent analysis of nuclear sequence data (Santini et al. 2009) does provide a timeline for acanthomorph evolution that closely matches palaeontological chronologies, but this congruence might be spurious. Calibrations used by that study within Acanthomorpha are generally credible, but many upper bounds applied outside of the clade are substantially underestimated (e.g. crown Sarcopterygii by 4 Myr, crown Actinopterygii by 108 Myr, crown Neopterygii by 59 Myr; Hurley et al. 2007; Friedman 2007).
Many debates on the timing of evolutionary radiations are unnecessarily confused by conflation of three separate aspects of diversification: (i) the phylogenetic splitting of clades; (ii) the onset of major anatomical and trophic divergence; and (iii) the ascendance to ecological dominance (see Alroy 1999). These major events are clearly staggered in the timeline of acanthomorph diversification reconstructed here. Important phylogenetic splits within Percomorpha occurred by the Late Cretaceous, but members of this clade remained rare throughout this interval relative to other acanthomorphs, with most examples displaying few derived characters convincingly linking them to specialized modern groups (Arratia et al. 2004). Major morphological (and presumably trophic) divergence appears confined to the very end of the Cretaceous and the early Palaeogene (figure 2a–c). Regardless of the absolute timing of phylogenetic splits, two outstanding faunal patterns remain intact: the taxonomic dominance of percomorphs within acanthomorphs (figure 2d) and the ascendance of acanthomorphs within marine faunas (Patterson 1993a; Bannikov & Parin 1997; Bonde 1997; Forey et al. 2003) are Cenozoic phenomena. This coherent time scale, coupled with an emerging phylogenetic framework (Smith & Wheeler 2006; Li et al. 2008, 2009), excellent fossil preservation and a renewed drive to place extinct forms relative to modern clades (e.g. Santini & Tyler 2003), make acanthomorphs a powerful system for further research targeting the factors underlying the evolution of modern vertebrate biodiversity.
Acknowledgements
I thank M. Brazeau, M. Coates, M. Foote, L. Sallan, P. Wagner, M. Westneat and two anonymous reviewers for helpful comments on various incarnations of this paper, and the collections managers, curators and research scientists at the many institutions visited during the course of this study for access to the specimens in their care. This research was supported by an EPA STAR Fellowship (award number FP916730), a National Science Foundation Graduate Research Fellowship (award number DGE-0228235), an Evolving Earth Grant, a grant from the Lerner-Grey Fund for Marine Research, and a Hinds Fund Grant from the University of Chicago.
References
- Adams D. C., Berns C. M., Kozak K. H., Wiens J. J.2009Are rates of species diversification correlated with rates of morphological evolution? Proc. R. Soc. B 276, 2729–2738 (doi:10.1098/rspb.2009.0543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro M. E., Santini F., Brock C. D.2007Do reefs drive diversification in marine teleosts? Evidence from the pufferfishes and their allies (Order Tetraodontiformes). Evolution 61, 2104–2126 (doi:10.1111/j.1558-5646.2007.00182.x) [DOI] [PubMed] [Google Scholar]
- Alfaro M. E., Brock C. D., Banbury B. L., Wainwright P. C.2009aDoes evolutionary innovation in pharyngeal jaws lead to rapid lineage diversification in labrid fishes? BMC Evol. Biol. 9, 255 (doi:10.1186/1471-2148-9-255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro M. E., Santini F., Brock C., Alamillo H., Dorburg A., Rabosky D. L., Carnevale G., Harmon L. J.2009bNine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc. Natl Acad. Sci. USA 106, 13 410–13 414 (doi:10.1073/pnas.0811087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroy J. A.1999The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation. Syst. Biol. 48, 107–118 (doi:10.1080/106351599260472) [DOI] [PubMed] [Google Scholar]
- Arratia G., Lòpez-Arbarello A., Prasad G. V. R., Parmar V., Kriwet J.2004Late Cretaceous–Paleocene percomorphs (Teleostei) from India—early radiation of perciformes. In Recent advances in the origin and early radiation of vertebrates (eds Arratia G., Wilson M. V. H., Cloutier R.), pp. 635–663 Munich, Germany: Verlag Dr. Friedrich Pfeil [Google Scholar]
- Azuma Y., Kumazawa Y., Miya M., Mabuchi K., Nishida M.2008Mitogenomic evaluation of the historical biogeography of cichlids toward reliable dating of teleostean divergences. BMC Evol. Biol. 8, 215 (doi:10.1186/1471-2148-8-215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannikov A. F., Parin N. N.1997The list of marine fishes from Cenozoic (Upper Paleocene–Middle Miocene) localities in southern European Russia and adjacent countries. J. Ichthyol. 37, 149–161 [Google Scholar]
- Bellwood D. R.2003Origins and escalation of herbivory in fishes: a functional perspective. Paleobiology 29, 71–83 (doi:10.1666/0094-8373(2003)029<0071:OAEOHI>2.0.CO;2) [Google Scholar]
- Bellwood D. R., Hoey A.2004Feeding in Mesozoic fishes: a functional perspective. In Mesozoic fishes 3—systematics, paleoenvironments and biodiversity (eds Arratia G., Tintori A.), pp. 639–649 Munich, Germany: Verlag Dr. Friedrich Pfeil [Google Scholar]
- Bellwood D. R., Wainwright P. C.2002The history and biogeography of fishes on coral reefs. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P. F.), pp. 5–32 Amsterdam, The Netherlands: Academic Press [Google Scholar]
- Bonde N.1997A distinctive fish fauna in the basal ash-series of the Fur/Ølst formation (Upper Paleocene, Denmark). Aarhus Geosci. 6, 33–48 [Google Scholar]
- Bottjer D. J., Etter W., Hagadorn J. W., Tang C. M.2001Exceptional fossil preservation: a unique view on the evolution of marine life New York, NY: Columbia University Press [Google Scholar]
- Cavin L.2001Effects of the Cretaceous–Tertiary boundary event on bony fishes. In Geological and biological effects of impact events (eds Buffetaut E., Koeberl C.), pp. 141–158 Berlin, Germany: Springer [Google Scholar]
- Cavin L.2010The Late Jurassic ray-finned fish peak of diversity: biological radiation or preservational bias? In Origin and phylogenetic interrelationships of teleosts (eds Nelson J. S., Schultze H.-P., Wilson M. V. H.), pp. 111–121 Munich, Germany: Verlag Dr. Friedrich Pfeil [Google Scholar]
- Cavin L., Forey P. L.2007Using ghost lineages to identify diversification events in the fossil record. Biol. Lett. 3, 201–204 (doi:10.1098/rsbl.2006.0602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavin L., Martin M.1995Les actinopterygians et la limite Crétacé–Tertiaire. Geobios M. S. 19, 183–188 [Google Scholar]
- Chakrabarty P.2005Testing conjectures about morphological diversity in cichlids of Lakes Malawi and Tanganyika. Copeia 2005, 359–373 (doi:10.1643/CG-04-089R2) [Google Scholar]
- Chen W.-J., Bonillo C., Lecointre G.2003Repeatability of clades as a criterion of reliability: a case study for molecular phylogeny of Acanthomorpha (Teleostei) with a larger number of taxa. Mol. Phylogenet. Evol. 26, 262–288 (doi:10.1016/S1055-7903(02)00371-8) [DOI] [PubMed] [Google Scholar]
- Chen W.-J., Ortí G., Meyer A.2004Novel evolutionary relationships among four fish model systems. Trends Genet. 20, 424–431 (doi:10.1016/j.tig.2004.07.005) [DOI] [PubMed] [Google Scholar]
- Ciampaglio C. N., Matthieu K., McShea D. W.2001Detecting changes in morphospace occupation patterns in the fossil record: characterization and analysis of measures of disparity. Paleobiology 27, 695–715 (doi:10.1666/0094-8373(2001)027<0695:DCIMOP>2.0.CO;2) [Google Scholar]
- Cohen D. M.1986Bregmacerotidae. In Fishes of the north-eastern Atlantic and the Mediterranean (eds Whitehead P. J. P., Bauchot M.-L., Hureau J.-C., Nielsen J., Tortonese E.), pp. 711–712 Paris, France: UNESCO [Google Scholar]
- Cooper W. J., Westneat M. W.2009Form and function of damselfish skulls: rapid and repeated evolution into a limited number of trophic niches. BMC Evol. Biol. 9, 24 (doi:10.1186/1471-2148-9-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deline B.2009The effects of rarity and abundance distributions on measurements of local morphological disparity. Paleobiology 35, 175–189 (doi:10.1666/08028.1) [Google Scholar]
- Edwards A. W. F.1992Likelihood: the expanded edition Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- Ericson P. G. P., et al. 2006Diversification of Neoaves: integration of molecular sequence data and fossils. Biol. Lett. 2, 543–547 (doi:10.1098/rsbl.2006.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin D. H.2001Lessons from the past: biotic recoveries from mass extinctions. Proc. Natl Acad. Sci. USA 98, 5399–5403 (doi:10.1073/pnas.091092698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote M.1993Contributions of individual taxa to overall morphological disparity. Paleobiology 19, 403–419 [Google Scholar]
- Foote M.1996Models of morphological diversification. In Evolutionary paleobiology (eds Jablonski D., Erwin D., Lipps J.), pp. 62–86 Chicago, IL: University of Chicago Press [Google Scholar]
- Forey P. L., Lu Y., Patterson C., Davies C. E.2003Fossil fishes from the Cenomanian (Upper Cretaceous) of Namoura, Lebanon. J. Syst. Palaeontol. 1, 230–330 (doi:10.1017/S147720190300107X) [Google Scholar]
- Friedman M.2007Styloichthys as the earliest coelacanth: implications for early osteichthyan interrelationships. J. Syst. Palaeontol. 5, 289–343 (doi:10.1017/S1477201907002052) [Google Scholar]
- Friedman M.2009Ecomorphological selectivity among marine teleost fishes during the end-Cretaceous extinction. Proc. Natl Acad. Sci. USA 106, 5218–5223 (doi:10.1073/pnas.0808468106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., de Carvalho M. S. S., Suto A. A.2009A possible occurrence of Diodontidae (Teleostei, Tetraodontiformes) in Upper Cretaceous of the Paraíba Basin, northeastern Brazil. Cret. Res. 30, 599–604 (doi:10.1016/j.cretres.2008.12.001) [Google Scholar]
- Gradstein F., Ogg J., Smith A.2004A geological time scale 2004 Cambridge, UK: Cambridge University Press [Google Scholar]
- Hove J. R., O'Bryan L. M., Gordon M. S., Webb P. W., Weihs D.2001Boxfishes (Teleostei: Ostraciidae) as a model system for fishes swimming with many fins: kinematics. J. Exp. Biol. 204, 1459–1471 [DOI] [PubMed] [Google Scholar]
- Hurley I. A., et al. 2007A new time-scale for ray-finned fish evolution. Proc. R. Soc. B 274, 489–498 (doi:10.1098/rspb.2006.3749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski D.2008Biotic interactions and macroevolution: extensions and mismatches across scales and levels. Evolution 62, 715–739 (doi:10.1111/j.1558-5646.2008.00317.x) [DOI] [PubMed] [Google Scholar]
- Kiessling W.2008Sample-standardized expansion and collapse of reef building in the Phanerozoic. Fossil Rec. 11, 7–18 (doi:10.1002/mmng.200700008) [Google Scholar]
- Kiessling W., Simpson C., Foote M.2010Reefs as cradles of evolution and sources of biodiversity in the Phanerozoic. Science 327, 196–198 (doi:10.1126/science.1182241) [DOI] [PubMed] [Google Scholar]
- Krug A. Z., Jablonksi D., Valentine J. W.2009Signature of the end-Cretaceous mass extinction in the modern biota. Science 323, 767–771 (doi:10.1126/science.1164905) [DOI] [PubMed] [Google Scholar]
- Li C., Lu G., Ortí G.2008Optimal data partitioning and a test case for ray-finned fishes (Actinopterygii) based on ten nuclear loci. Syst. Biol. 57, 519–539 (doi:10.1080/10635150802206883) [DOI] [PubMed] [Google Scholar]
- Li B., Dettaï A., Cruaud G., Couloux A., Desoutter-Meniger M., Lecointre G.2009RNF213, a new nuclear marker for acanthomorph phylogeny. Mol. Phyl. Evol. 50, 345–363 (doi:10.1016/j.ympev.2008.11.013) [DOI] [PubMed] [Google Scholar]
- MacLeod N., et al. 1997The Cretaceous–Tertiary biotic transition. J. Geol. Soc. Lond. 154, 265–292 (doi:10.1144/gsjgs.154.2.0265) [Google Scholar]
- Nelson G.1989Phylogeny of major fish groups. In The hierarchy of life (eds Fernholm B., Bremer K., Brundin L., Jörnvall H., Rutberg L., Wanntorp E.), pp. 325–336 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- Nelson J. S.2006Fishes of the world Hoboken, NJ: John Wiley & Sons [Google Scholar]
- Nolf D.1995Studies on fossil otoliths—the state of the art. In Recent developments in fish otolith research (eds Secor D. H., Dean J. M., Campana S. E.), pp. 513–544 Columbia, SC: University of South Carolina Press [Google Scholar]
- Nolf D.2003Fish otoliths from the Santonian of the Pyrenean fauna province, and an overview of all otolith-documented North Atlantic Late Cretaceous teleosts. Bull. Inst. Roy. Sci. Nat. Belg. Sci. Terr. 73, 155–173 [Google Scholar]
- Nursall J. R.1996Distribution and ecology of pycnodont fishes. In Mesozoic fishes—systematics and paleoecology (eds Arratia G., Vihol G.), pp. 115–124 Munich, Germany: Verlag Dr. Friedrich Pfeil [Google Scholar]
- Patterson C.1993aAn overview of the early fossil record of acanthomorphs. Bull. Mar. Sci. 52, 29–59 [Google Scholar]
- Patterson C.1993bOsteichthyes: Teleostei. In The fossil record, vol. 2 (ed. Benton M.), pp. 621–656 London, UK: Chapman & Hall [Google Scholar]
- Patterson C., Smith A. B.1987Is the periodicity of extinctions a taxonomic artefact? Nature 330, 248–251 (doi:10.1038/330248a0) [Google Scholar]
- Patterson C., Smith A. B.1989Periodicity in extinction: the role of systematics. Ecology 70, 802–811 (doi:10.2307/1941349) [Google Scholar]
- Poe S., Chubb A. L.2004Birds in a bush: five genes indicate explosive evolution of avian orders. Evolution 58, 404–415 [PubMed] [Google Scholar]
- Raup D. M.1972Taxonomic diversity during the Phanerozoic. Science 177, 1065–1071 (doi:10.1126/science.177.4054.1065) [DOI] [PubMed] [Google Scholar]
- Rohlf F. J.2007tpsRelw, v. 1.45 Stony Brook, NY: Department of Ecology & Evolution, SUNY Stony Brook [Google Scholar]
- Rohlf F. J.2008atpsDig, v. 2.11 Stony Brook, NY: Department of Ecology & Evolution, SUNY Stony Brook [Google Scholar]
- Rohlf F. J.2008btps Util, v. 1.40 Stony Brook, NY: Department of Ecology & Evolution, SUNY Stony Brook [Google Scholar]
- Royall R. M.1997Statistical evidence: a likelihood paradigm New York, NY: Chapman & Hall [Google Scholar]
- Santini F., Harmon L. J., Carnevale G., Alfaro M.2009Did genome duplication drive the origin of teleosts? A comparative study of diversification in ray-finned fishes. BMC Evol. Biol. 9, 194 (doi:10.1186/1471-2148-9-194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F., Tyler J. C.2003A phylogeny of the families of fossil and extant tetraodontiform fishes (Acanthomorpha, Tetraodontiformes), Upper Cretaceous to Recent. Zool. J. Linn. Soc. 139, 565–617 (doi:10.1111/j.1096-3642.2003.00088.x) [Google Scholar]
- Schluter D.2000The ecology of adaptive radiation Oxford, UK: Oxford University Press [Google Scholar]
- Schlüter M., Steuber T., Puente M.2008Chronostratigraphy of Campanian–Maastrichtian platform carbonates and rudist associations of Salento (Apulia, Italy). Cret. Res. 29, 100–114 (doi:10.1016/j.cretres.2007.04.005) [Google Scholar]
- Smith W. L., Wheeler W. C.2006Venom evolution widespread in fishes: a phylogenetic road map for the bioprospecting of piscine venoms. J. Hered. 97, 206–217 (doi:10.1093/jhered/esj034) [DOI] [PubMed] [Google Scholar]
- Stiassny M. L. J., Wiley E. O., Johnson G. D., de Carvalho M. R.2004Gnathostome fishes. In Assembling the tree of life (eds Cracraft J., Donoghue M. J.), pp. 410–429 Oxford, UK: Oxford University Press [Google Scholar]
- Taverne L.2003Les poissons crétacés de Nardò. 14°. Lissoberyx pugliensis sp. nov. (Teleostei, Beryciformes, Trachichthyidae). Boll. Mus. Civ. Stor. Nat. Verona Geol. Paleontol. Preistor. 27, 3–13 [Google Scholar]
- Tyler J. C., Bronzi P., Ghiandoni A.2000The Cretaceous fishes of Nardo 11°. A new genus and species of Zeiformes, Cretazeus rinaldii, the earliest record for the order. Boll. Mus. Civ. Stor. Nat. Verona Geol. Paleontol. Preistor. 24, 11–28 [Google Scholar]
- Wainwright P. C., Bellwood D. R.2002Ecomorphology of feeding in coral reef fishes. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P. F.), pp. 33–55 Amsterdam, The Netherlands: Academic Press [Google Scholar]
- Watson W., Walker H. J., Jr2004The world's smallest vertebrate, Schindleria brevipinguis, a new paedomorphic species in the family Schindleriidae (Perciformes: Gobioidei). Rec. West. Austr. Mus. 56, 139–142 [Google Scholar]
- Yamanoue Y., Miya M., Inoue J. G., Matsuura K., Nishida M.2006The mitochondrial genome of spotted green pufferfish Tetraodon nigroviridis (Teleostei: Tetraodontiformes) and divergence time estimation among model organisms in fishes. Genes Genet. Syst. 81, 29–39 (doi:10.1266/ggs.81.29) [DOI] [PubMed] [Google Scholar]
- Young K. A., Snoeks J., Seehausen O.2009Morphological diversity and the roles of contingency, changes and determinism in African cichlid radiations. PLoS One 4, e4740 (doi:10.1371/journal.pone.0004740) [DOI] [PMC free article] [PubMed] [Google Scholar]