Abstract
Relationships between structure and function are a primary focus in biology, yet they are most often considered within individual species. Sexually dimorphic communication behaviours and the morphology of associated structures can vary widely, even among closely related species, and these traits provide an ideal opportunity to investigate the evolution of structure–function patterns. Using nine Anolis lizard species, we addressed a series of questions regarding sex differences in and the evolution of relationships between extension of the throat fan (dewlap) and morphology of the muscles and cartilage controlling it. The main results indicated that within species, males displayed the dewlap more often than females and consistently exhibited larger associated structures. These data are consistent with work in other vertebrates in which corresponding sex differences in reproductive morphology and behaviour have been documented. Across species, however, we found no evidence that the rate of dewlap extension evolved in association with dewlap morphology. Thus, we provide an example of traits that, when considered in a phylogenetic framework, exhibited limited associations between behaviour and morphology, perhaps as the result of constraints imposed by the ecological contexts in which different species occur.
Keywords: communication, dewlap, morphology, neuromuscular traits, phylogeny
1. Introduction
The vast diversity of communication behaviours and the morphology of structures associated with them provide an ideal opportunity to study mechanistic relationships between structure and function across animal taxa. Approaches that have been especially valuable in elucidating structure–function relationships include comparisons between males and females (e.g. Nottebohm & Arnold 1976; Breedlove & Arnold 1980) and for seasonally breeding animals, comparisons within sexes between times of reproductive activity and quiescence (e.g. Ball et al. 2004). Most mechanistic studies have explored these relationships within a single species, with the majority using model rodent or songbird species (reviewed by Cooke et al. 1998).
Exceptions to these single-species studies exist, however, including particularly intriguing examples from songbirds (MacDougall-Shackleton & Ball 1999; Brenowitz & Beecher 2005). Evolutionary relationships exist between measures of song complexity and the volumes of forebrain song nuclei (Devoogd et al. 1993; Székely et al. 1996; Brenowitz 1997). Similarly, associations between vocalizations and morphological features of the peripheral vocal organ are exhibited in diverse avian species (Suthers et al. 1999; Suthers & Zollinger 2004).
Research to date has provided extremely valuable information on the interactions among neural and endocrine traits and behaviour. Yet, to determine the generality of structure–function patterns in communication, it is necessary to examine these patterns in additional taxa. In this study, we took advantage of diversity in a sexually dimorphic display behaviour across a group of lizards. Using species in the genus Anolis (anoles), we investigated the evolution of the muscles and cartilage that support the extension of a colourful gular fan (dewlap). This structure is displayed when the ceratohyoid (CH) muscles on each side of the throat contract, causing the second ceratobranchial cartilage (hereafter ‘cartilage’) to extend (Wade 2005). In the vast majority of anole species, the dewlap is substantially larger in males than in females, and it is used frequently by males (but infrequently by females) during courtship and aggression (Jenssen 1977). It may also be used to deter pursuit by predators (Leal & Rodriguez Robles 1997) or in species recognition (Nicholson et al. 2007). Pronounced variation in dewlap size and use exists among anole species, as well as in the degree of sexual dimorphism in both structure and function, suggesting that the muscle and cartilage involved in dewlap extension may vary among and within species as well.
The only anole in which relationships between dewlap extension and morphology of these dewlap components has been investigated is A. carolinensis, a species used extensively in laboratory studies. These animals have been the focus of behavioural studies since the 1880s (Monks 1881), and detailed descriptions of the neural, endocrine and muscular components that influence dewlap extension are available (Wade 2005). In this species, two lines of evidence suggest that associations exist between dewlap structure and function. First, numerous features of the mechanical system that extends the dewlap (including muscle fibre size, cartilage length and motoneuron somata) are larger in males, which display often, compared with females, which do not (O'Bryant & Wade 1999). Second, the rate of dewlap extension is positively correlated with CH fibre size in adult male anoles (Neal & Wade 2007). However, evolutionary relationships between dewlap use and the musculature and cartilage that support this structure have not yet been examined.
Anoles are an excellent system for this type of analysis. Approximately 400 species exist in the genus Anolis, exhibiting extensive variation in a broad range of morphological, ecological and behavioural traits (Losos 2009). Many species of anoles are readily observed in the field, and individuals of both sexes perform easily quantified, sex- and species-specific display behaviours. Also, the existence of a robust anole phylogeny (Nicholson et al. 2005) allows for the investigation of evolutionary relationships between structural and functional traits far more rigorously than is possible within A. carolinensis (or other single-species model systems).
In this study, we examined dewlap-associated morphological traits across nine species of anoles that display a range of dewlap size and use (figures 1 and 2). Geographical and taxonomic sampling were focused around two species that exhibit relative sexual monomorphism in dewlap size: A. valencienni from Jamaica, in which both sexes have substantial dewlaps (Hicks & Trivers 1983), and A. bahoruocoensis from the Dominican Republic, in which both sexes have diminutive dewlaps (Fitch & Henderson 1987; Orrell & Jenssen 1998). We also studied two additional Jamaican species, four other species from the Dominican Republic, and A. carolinensis from the southeast US, all of which show varying degrees of greater sexual dimorphism than A. bahoruocoensis and A. valencienni. In each species, we measured cartilage length and cross-sectional area, area of CH muscle fibres and CH muscle height. We also measured snout–vent length (SVL) and mass as controls for body size. We used cross-sectional areas of the trachea and the fibres of the genioglossus (GG; a muscle involved in tongue extension) as procedural controls; they were measured in the same tissue sections as the dewlap-associated traits, but are not involved in dewlap extension.
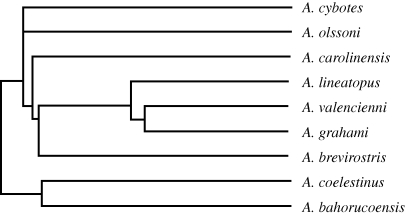
Figure 1.
Phylogenetic relationships among species. Ultrametric tree from the phylogeny in Nicholson et al. (2005), pruned to include only the species in this study.
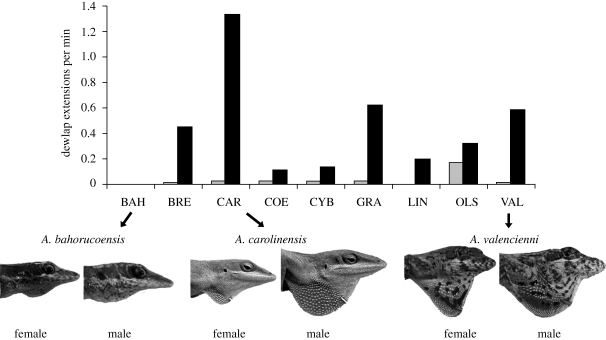
Figure 2.
Sexual dimorphism in dewlap use and size. Average dewlap display rates from Johnson (2007). BAH, A. bahorucoensis; BRE, A. brevirostris; CAR, A. carolinensis; COE, A. coelestinus; CYB, A. cybotes; GRA, A. grahami; LIN, A. lineatopus; OLS, A. olssoni; VAL, A. valencienni. Photographs represent the range of sexual dimorphism and dewlap sizes across these species. Dewlaps were fully extended using forceps. Grey shaded box, female; black shaded box, male.
We addressed four questions regarding structure–function relationships. First, we examined whether consistent differences exist among species and between sexes. Second, we used behavioural data on dewlap use, primarily from Johnson (2007), to determine whether the rate of dewlap extension has evolved in association with dewlap morphology. Third, we asked whether sexual dimorphism in morphological traits has evolved in association with sexual dimorphism in dewlap use. Finally, we examined relationships among the evolution of the morphological traits themselves.
2. Material and methods
(a). Morphology
We collected specimens of three species from the north central coast of Jamaica: A. grahami and A. lineatopus from the grounds of Circle B Farm in Priory (18°26.104′ N, 77°14.469′ W), and A. valencienni from the grounds of Discovery Bay Marine Laboratory in Discovery Bay (18°28.14′ N, 77°24.90′ W). In southwest Dominican Republic, we obtained specimens of five species: A. brevirostris, A. coelestinus, A. cybotes and A. olssoni on the grounds of Coralsol Beach Resort near Bauruco (18°03.45′ N, 71°06.75′ W), and A. bahorucoensis near the montane town of Polo (18°07.59′ N, 71°16.12′ W). We collected A. carolinensis from the Barataria Preserve of Jean Lafitte National Historical Park in Marrero, LA, USA (29°47.22′ N, 90°06.53′ W). All specimens were captured during the Anolis summer breeding season.
We captured all animals (10–11 females and 11–13 males per species) by noose or hand between 08.00 and 18.30 h, except A. valencienni, A. bahorucoensis and A. olssoni, which we caught between 21.15 and 01.00 h or 04.30 and 06.00 h while they were sleeping. We placed each individual in an air-filled plastic bag until processing.
We measured SVL, mass and length of the cartilage under the dewlap. Animals were then rapidly decapitated (average time from capture to euthanasia = 82 min), and a portion of the throat including the CH muscles was immediately frozen on dry ice. Tissues were transported on dry ice to Michigan State University and stored at −80°C. At the time of tissue collection, we confirmed that each animal was in breeding condition. Each male had large, vascularized testes, and each female had at least one yolking follicle and/or oviductal egg.
We sectioned frozen throat tissues at 20 µm and stained the tissues with haemotoxylin and eosin. Using Scion (NIH) Image software, we measured the cross-sectional area of 25 arbitrarily selected fibres in the CH and GG muscles (following O'Bryant & Wade 1999; Neal & Wade 2007). Values were obtained from both the left and right sides within the middle third of the rostro-caudal extent of the muscle. We quantified the cross-sectional area of cartilage and trachea, as well as CH height (estimated by the minor axis of the ellipse that best approximates the muscle shape) in five tissue sections in the middle third of the muscles. Statistical analyses employed an average of each measure for each individual.
To determine whether the species differ in qualitative aspects of throat morphology, we examined preserved specimens of one adult male and female of each species. These specimens were borrowed from the Museum of Comparative Zoology and were collected between ca 1953 and 1983 near our collection sites. To confirm the presence of all muscle and cartilage components of the dewlap extension system, we carefully peeled back the skin at the throat, visually examined the tissues underneath and exposed the cartilage for photographs.
(b). Behaviour
To determine whether the morphology of the dewlap and related structures is associated with functional use of the dewlap across species, we conducted analyses using field behavioural data on the rate of dewlap extension for each sex and species. Behavioural and morphological data for A. carolinensis were collected from the same individuals; for all other species, behavioural and morphological variables were assessed in different individuals. Data for seven of the eight Caribbean species in this study (all except A. brevirostris, for which behavioural data have not been published) are reported in Johnson (2007) and Johnson et al. (in press). For all species, observations were conducted between 06.00 and 19.00 h, and never during inclement weather.
For each Caribbean species except A. brevirostris, all adult lizards within 1 or 2 approximately 500 m2 plots were captured and given a unique mark. Focal observations were conducted on marked lizards for an average of 65 h on 30–95 animals per species. Each observation lasted 20 min for most species, but for the more cryptic A. bahorucoensis and A. valencienni, observations lasted up to 180 min. All behaviours were recorded, including dewlap extensions. Each lizard was observed for a maximum of five periods (or, for the two cryptic species, a maximum of 5 h), and behaviour rates were averaged across observations for each individual for statistical analyses. Observations of A. brevirostris (10.4 h of observation on 18 animals) and mainland A. carolinensis from the US (91 h on 58 animals) were similar, except that unmarked animals were observed.
(c). Statistical analyses
To determine whether species, sex or their interaction differed among the morphological traits, we conducted a multivariate analysis of variance (MANOVA), followed by simple main effect tests (Jaccard 1997) to interpret species × sex interactions. Simple main effect tests allow the analysis of one factor by considering the influence of the second factor. This approach reduces type I error across each set of comparisons by reducing the number of contrasts considered (Jaccard 1997).
We calculated an index of sexual dimorphism using the Lovich & Gibbons (1992) ‘two-step’ ratio (Smith 1999) following Stephens & Wiens (2009) to further examine differences between males and females in these species. This index scales among species of different sizes, and is appropriate for use in phylogenetically informed analyses (Smith 1999; Stephens & Wiens 2009). The index consists of the difference between a value of 1 and the ratio of the average size of the larger sex to the average size of the smaller sex. If males were larger than females on a trait, by convention the ratio was positive.
We used a series of phylogenetically informed analyses to determine whether morphological traits evolved in association with each other and/or with dewlap extension rates. These analyses were conducted using the anole phylogeny in Nicholson et al. (2005), pruned to include only the species in this study, with branch lengths made proportional to time using the program r8 s (Sanderson 2003). We calculated independent contrasts (Felsenstein 1985) for all traits using the program IDC (Revell 2006). Most contrasts were adequately standardized (following Garland et al. 1992). However, contrasts for male SVL, male mass, female mass and female GG fibre size were correlated with contrast length (the sum of the corrected branch lengths subtending the contrasted nodes (Felsenstein 1985)). These contrasts were therefore recalculated using logarithmically transformed branch lengths.
To examine relationships among morphological traits across the nine species, we calculated uncentred correlations (i.e. correlations that assume that the mean of each variable is zero, analogous to forcing a regression through the origin (as in Garland et al. 1992)) on independent contrasts for each trait. p-values were obtained by forcing the regression of each pair of traits through the origin. Because males and females differ dramatically in both size and shape (Butler & Losos 2002), we analysed data from the two sexes separately.
Finally, to determine whether a relationship exists among species in the morphological traits associated with dewlap use and dewlap extension rate, we performed a regression for each sex where contrasts for dewlap extension rate were the dependent variable and contrasts of cartilage length, cross-sectional area of the cartilage, CH height and CH fibre size were the independent variables. To determine whether relationships exist among the sexual dimorphism of these traits, we performed a similar regression using contrasts of sexual dimorphism indices (described above).
3. Results
(a). Sex and species differences in morphology
Qualitative examination of gross throat morphology in preserved museum specimens (table S1 in the electronic supplementary material) revealed that across the nine species, males and females have the same cartilage and muscular components controlling dewlap movement. These features are generally larger in males, but the degree to which males exhibit larger features than females varies across species, particularly for the cartilage (figure S1 in the electronic supplementary material).
Using quantitative measures of morphological traits (table 1), we found significant differences between sexes (male > female), among species and an interaction between them (MANOVA, Wilks' λ-test; sex: F8,143 = 111.0, p < 0.001, partial η2 = 0.86; species: F64,831 = 20.7, p < 0.001, partial η2 = 0.50; species × sex: F64,831 = 6.7, p < 0.001, partial η2 = 0.26). Univariate analysis of variance (ANOVA) followed by simple main effect tests revealed that within each species, all dewlap-associated traits were larger in males than in females, except for CH fibre size in A. bahorucoensis and A. valencienni, the two anoles previously described as having relatively monomorphic dewlaps (table 2; Hicks & Trivers 1983; Fitch & Henderson 1987; Orrell & Jenssen 1998). Importantly, the sex difference in the CH muscle fibres is not simply associated with larger male size, as for eight of the nine species GG fibre size did not differ between the sexes (p for all species >0.07; table 2). For A. brevirostris, the one species in which the sexes differ, this trait is on average only 21 per cent larger in males (GG fibre size: F1,176 = 5.4, p = 0.021), substantially smaller than the 200 per cent sex difference observed in CH fibre area (F1,179 = 22.1, p < 0.001). To further investigate the role of body size on the evolution of dewlap-associated traits, we used analysis of covariance (ANCOVA) with SVL as a covariate. We found that SVL was a significant covariate for each of the four dewlap-associated traits (all F6,155 > 49, all p < 0.001), but that all species and sex differences reported above remain significant (all p < 0.01), indicating that differences in dewlap-associated traits among species and between males and females exist independently of body size differences.
Table 1.
Morphological traits across nine anole species. (Means with standard deviations are shown. SVL, snout–vent length; GG, genioglossus muscle; and CH, ceratohyoid muscle.)
| sex | n | measures associated with dewlap use |
||||
|---|---|---|---|---|---|---|
| cartilage length (mm) | cartilage area (mm2) | CH depth (mm) | CH fibre size (µm2) | |||
| A. bahorucoensis | M | 13 | 12.8 (2.1) | 0.034 (0.005) | 0.19 (0.04) | 597 (193) |
| F | 11 | 8.1 (0.8) | 0.011 (0.002) | 0.10 (0.02) | 439 (101) | |
| A. brevirostris | M | 12 | 17.8 (1.9) | 0.062 (0.014) | 0.32 (0.08) | 846 (170) |
| F | 10 | 9.8 (1.4) | 0.012 (0.005) | 0.10 (0.04) | 409 (120) | |
| A. carolinensis | M | 12 | 25.5 (2.6) | 0.102 (0.018) | 0.46 (0.04) | 961 (107) |
| F | 10 | 12.9 (3.2) | 0.038 (0.007) | 0.25 (0.02) | 612 (93) | |
| A. coelestinus | M | 13 | 22.4 (3.8) | 0.064 (0.012) | 0.32 (0.07) | 811 (212) |
| F | 10 | 13.9 (2.0) | 0.023 (0.002) | 0.15 (0.05) | 397 (69) | |
| A. cybotes | M | 12 | 32.4 (6.3) | 0.176 (0.041) | 0.50 (0.08) | 1035 (321) |
| F | 10 | 15.8 (2.9) | 0.042 (0.011) | 0.22 (0.07) | 551 (134) | |
| A. grahami | M | 12 | 25.6 (2.3) | 0.091 (0.011) | 0.39 (0.10) | 1165 (229) |
| F | 10 | 13.5 (1.5) | 0.020 (0.005) | 0.18 (0.03) | 448 (84) | |
| A. lineatopus | M | 12 | 32.8 (1.3) | 0.154 (0.028) | 0.57 (0.08) | 1075 (278) |
| F | 10 | 13.7 (2.5) | 0.024 (0.008) | 0.18 (0.04) | 531 (142) | |
| A. olssoni | M | 12 | 27.6 (4.5) | 0.089 (0.019) | 0.39 (0.07) | 1054 (314) |
| F | 10 | 8.8 (1.8) | 0.016 (0.005) | 0.16 (0.02) | 372 (131) | |
| A. valencienni | M | 11 | 30.0 (3.5) | 0.106 (0.029) | 0.41 (0.08) | 1057 (191) |
| F | 10 | 23.5 (0.8) | 0.045 (0.006) | 0.31 (0.03) | 736 (102) | |
| body size | procedural controls | |||||
| sex | n | SVL (mm) | mass (g) | trachea (mm2) | GG fibre size (µm2) | |
| A. bahorucoensis | M | 13 | 41.4 (2.8) | 1.31 (0.31) | 0.29 (0.05) | 1043 (304) |
| F | 11 | 36.5 (2.0) | 0.93 (0.18) | 0.26 (0.03) | 1126 (170) | |
| A. brevirostris | M | 12 | 44.0 (3.2) | 2.63 (0.43) | 0.35 (0.04) | 1221 (241) |
| F | 10 | 40.7 (1.3) | 1.86 (0.22) | 0.26 (0.04) | 1002 (207) | |
| A. carolinensis | M | 12 | 63.4 (2.5) | 5.57 (0.76) | 0.41 (0.04) | 917 (139) |
| F | 10 | 52.1 (3.0) | 3.22 (0.46) | 0.31 (0.03) | 1008 (127) | |
| A. coelestinus | M | 13 | 63.5 (5.2) | 5.85 (1.36) | 0.64 (0.17) | 1301 (257) |
| F | 10 | 49.8 (1.0) | 2.74 (0.33) | 0.36 (0.07) | 1143 (197) | |
| A. cybotes | M | 12 | 60.9 (6.3) | 6.65 (2.07) | 1.02 (0.25) | 1320 (271) |
| F | 10 | 48.0 (5.5) | 3.79 (1.08) | 0.57 (0.16) | 1198 (316) | |
| A. grahami | M | 12 | 59.7 (5.0) | 5.79 (1.69) | 0.54 (0.10) | 1236 (182) |
| F | 10 | 46.0 (2.3) | 2.41 (0.50) | 0.29 (0.04) | 1097 (176) | |
| A. lineatopus | M | 12 | 59.4 (3.1) | 5.14 (0.59) | 0.65 (0.16) | 1138 (172) |
| F | 10 | 44.3 (2.4) | 2.06 (0.37) | 0.32 (0.06) | 1119 (115) | |
| A. olssoni | M | 12 | 42.9 (1.9) | 1.19 (0.28) | 0.20 (0.03) | 855 (179) |
| F | 10 | 40.0 (3.4) | 0.81 (0.29) | 0.18 (0.02) | 828 (204) | |
| A. valencienni | M | 11 | 62.0 (4.4) | 4.09 (1.06) | 0.44 (0.09) | 1282 (287) |
| F | 10 | 54.7 (3.5) | 2.70 (0.56) | 0.29 (0.01) | 1022 (112) | |
Table 2.
Simple main effect tests comparing males and females within each species. (F-values shown. Italic font indicates p < 0.05; bold font indicates p < 0.01; italic and bold font indicate p < 0.001. CLEN, cartilage length; CARE, cartilage area; CHHT, CH muscle height; CHF, CH muscle fibre area; SVL, snout–vent length; TRA, trachea area; GGF, GG muscle fibre area.)
| species | dewlap-associated measures |
body size |
procedural controls |
|||||
|---|---|---|---|---|---|---|---|---|
| CLEN | CARE | CHHT | CHF | SVL | MASS | TRA | GGF | |
| A. bahorucoensis | 14.2 | 12.5 | 11.9 | 3.4 | 10.5 | 1.0 | 0.7 | 0.9 |
| A. brevirostris | 41.6 | 54.4 | 65.0 | 22.1 | 4.5 | 3.7 | 3.6 | 5.4 |
| A. carolinensis | 102.7 | 94.5 | 57.0 | 19.6 | 54.1 | 36.9 | 4.3 | 0.9 |
| A. coelestinus | 45.7 | 38.3 | 51.1 | 27.1 | 85.6 | 81.8 | 47.3 | 3.3 |
| A. cybotes | 171.9 | 367.7 | 113.0 | 40.1 | 69.6 | 54.2 | 110.5 | 2.6 |
| A. grahami | 94.6 | 89.3 | 57.8 | 70.7 | 95.5 | 85.4 | 37.4 | 1.6 |
| A. lineatopus | 136.4 | 235.3 | 149.2 | 50.2 | 75.3 | 49.4 | 37.2 | 0.2 |
| A. olssoni | 219.0 | 108.7 | 72.3 | 64.6 | 2.8 | 0.8 | 0.2 | 0 |
| A. valencienni | 31.3 | 52.8 | 7.1 | 2.3 | 17.7 | 12.3 | 4.6 | 0.5 |
(b). Evolution of morphological traits
Variation in the pattern of display behaviours is not explained by variation in any of the dewlap-associated morphological traits, for either males or females (regression with independent contrasts; males: F4,8 = 0.35, p = 0.83, r2 = 0.26; females: F4,8 = 1.93, p = 0.27, r2 = 0.66). Analysis of sexual dimorphism indices (ratios of male : female measures) of these traits also revealed that the degree of sex difference in morphology is unrelated to dimorphism in dewlap extension rate (regression with independent contrasts; F4,8 = 0.24, p = 0.90, r2 = 0.19).
Measures of dewlap-associated traits (cartilage length and area, CH height and fibre size) were correlated with each other in both sexes (table 3). Interestingly, measures of body size were associated with dewlap-associated traits in females but not males.
Table 3.
Uncentred correlations among morphological traits across nine species. (Values were obtained separately for the two sexes; males are above the diagonal, females below. Significant (p < 0.05) correlations are in italics. Abbreviations as in table 2.)
| CLEN | CARE | CHHT | CHF | SVL | MASS | TRA | GGF | |
|---|---|---|---|---|---|---|---|---|
| CLEN | — | 0.886 | 0.901 | 0.728 | 0.523 | 0.442 | 0.530 | 0.178 |
| CARE | 0.878 | — | 0.917 | 0.620 | 0.511 | 0.543 | 0.724 | 0.259 |
| CHHT | 0.895 | 0.948 | — | 0.774 | 0.570 | 0.572 | 0.597 | 0.110 |
| CHF | 0.892 | 0.830 | 0.915 | — | 0.405 | 0.477 | 0.415 | 0.065 |
| SVL | 0.922 | 0.903 | 0.888 | 0.780 | — | 0.920 | 0.677 | 0.458 |
| MASS | 0.603 | 0.770 | 0.571 | 0.428 | 0.757 | — | 0.849 | 0.610 |
| TRA | 0.485 | 0.646 | 0.382 | 0.295 | 0.500 | 0.826 | — | 0.727 |
| GGF | 0.540 | 0.506 | 0.318 | 0.382 | 0.434 | 0.647 | 0.821 | — |
4. Discussion
(a). Sex differences in morphology and behaviour
The present data indicate that across species, male anoles generally possess larger dewlaps (defined by cartilage length) than females, and males use their dewlaps far more frequently than females (figure 2). Morphology of associated muscles and cartilage is also enhanced in males (tables 1 and 2). These results are consistent with previous work in laboratory-housed A. carolinensis, in which males extend their dewlaps far more frequently than females and have larger CH fibres and motoneurons that innervate the CH (O'Bryant & Wade 1999). Further, within male A. carolinensis, CH fibre size is positively correlated with dewlap extension rate (Neal & Wade 2007).
Similar associations between morphology and communication behaviour, in which structures used predominantly by one sex are much larger in that sex, exist across vertebrate taxa. Zebra finches (Taeniopygia guttata) are perhaps the most dramatic example; males sing but females do not, and the regions of the brain involved in song production and muscles of the vocal organ are much larger in males than in females (Arnold 1997; Wade 2001). In African clawed frogs (Xenopus laevis) and midshipmen fish (Porichthys notatus), males also vocalize during courtship, and the muscles associated with sound production and the neurons innervating them are larger in males than in females (Sassoon & Kelley 1986; Kelley et al. 1988; Bass 1990). Together, these studies provide strong support for the generality of the relationship between the size of neural and muscular structures and the frequency of their use. However, they cannot provide information on causation; larger structures might facilitate or result from enhanced behavioural performance, or the two factors may not be directly related (associations may exist from relationships with a mediating factor). ‘Training effects’, in which increased muscle use causes growth, are commonly observed in mammalian studies, but studies on squamates have not demonstrated similar plasticity in skeletal muscles in response to exercise (reviewed by Eme et al. 2009). Controlled experiments in anole lizards would be required to determine whether such a principle applies to the dewlap. However, CH fibres in adult males are no larger during the breeding season (when males display frequently) than the non-breeding season (when they do not) (Neal & Wade 2007).
(b). Species differences in morphology and behaviour
Individual species such as those described above have served as excellent model systems for mechanisms of behaviour, yet to more fully understand the evolution of such traits and the generality of structure–function relationships, investigations among taxa are necessary. While comparing distantly related species is informative (Brenowitz 1997), it can be difficult to identify behavioural measures appropriate for each of them. Indeed, even closely related species can exhibit extensive variation in communication-associated morphology and behaviour. Anolis lizards provide a system in which variation in communication traits allows for powerful tests of structure–function relationships, yet their behaviours and morphologies are similar enough to permit direct comparison across species.
Although we generally found consistent sex differences in dewlap-associated morphologies and frequency of their use within anole species, phylogenetic comparative analyses did not reveal such relationships across the nine species. In particular, we found no evidence for the association of dewlap morphology and use across species in regression analyses. This discrepancy is especially noticeable with comparison of the two species in which males and females have relatively monomorphic dewlaps (Hicks & Trivers 1983; Fitch & Henderson 1987; Orrell & Jenssen 1998). The sexes do not differ in the size of the CH muscle fibres in either A. valencienni or A. bahorucoensis (table 2), yet males and females differ dramatically in their dewlap use in the former but not the latter (figure 2). This pattern suggests that, across species and sexes, the size of CH fibres is directly associated with the size of the dewlap (cartilage length; table 3) but can be unrelated to the degree to which the muscle is used. Therefore, while a general pattern between dewlap morphology and use exists, some morphological components may have evolved independently of the rate of dewlap use.
Together, the present results nicely parallel findings from the bird song literature, in which some mechanistic traits involved in communication are conserved across taxa, while species-specific morphologies allow species-specific signal patterns. For example, phylogenetic studies of oropendolas and caciques show that while many characteristics of bird song are conserved (Price & Lanyon 2002), particular components of song organization and structure have evolved in lineage-specific patterns (Price & Lanyon 2004). Further, while the syrinx is responsible for producing song across all species, different species have evolved distinct structures and motor patterns that allow them to produce particular acoustic features (Suthers et al. 1999; Suthers & Zollinger 2004).
(c). Ecological contexts of communication behaviours
Species-specific patterns of morphology and behaviour may result from a variety of ecological factors. In the case of anoline dewlaps, one possibility is that species with differing population densities may experience different rates of social interaction, which would probably include dewlap use. However, no relationship between density and dewlap use exists in this dataset (Johnson et al. in press).
Another potential influence is predation rate, as use of the colourful dewlaps presumably makes anoles more susceptible to visually oriented predators. Yet, the one mainland species (A. carolinensis) that presumably encounters the highest predation risk (as predators are generally more abundant and diverse on mainlands) displays the highest rate of dewlap use. This pattern suggests indirect support for the pursuit-deterrence hypothesis, which states that signals given by prey when they detect a predator cause the predator to stop its pursuit (Caro 1995), and raises the possibility that the relatively high display rate of A. carolinensis is the result of increased predation risk. This idea is consistent with results from a study comparing populations of A. sagrei in differing predation regimes (Vanhooydonck et al. 2009).
Additionally, varying sexual selection across species may result in different signals conveyed by dewlap extension. Males of species that experience more competition, and thus stronger sexual selection pressure, are generally predicted to exhibit greater sexual size dimorphism (SSD) and to evolve honest signals of fighting ability (Andersson 1994). High SSD anoles might then be expected to exhibit associations between fighting ability and dewlap morphology and/or behaviour. However, Lailvaux & Irschick (2007) found that dewlap size was a significant predictor of the winner of male conflicts in species with low, but not high, SSD. Further, dewlap rate was not associated with SSD (Lailvaux & Irschick 2007), a finding consistent with our results, as A. bahorucoensis, A. brevirostris, A. olssoni and A. valencienni are considered low SSD (Butler & Losos 2002) and did not exhibit consistently different dewlap rates from the high SSD species (figure 2). Sexual selection does not appear to explain variation in dewlap behaviour, but it may play a role in dewlap-associated musculature, as the two species in which CH fibres were equivalent between the sexes (A. bahorucoensis and A. valencienni) are both low SSD. This pattern could suggest that species with more intense sexual selection have evolved larger CH muscles in response to the need to communicate fighting ability, but does not explain the sexual dimorphism in musculature in the other two low SSD species.
The habitats in which signals are performed are also likely to influence the evolution of communication traits. Among anoles, species in high visibility habitats display more frequently than those in low visibility habitats (Johnson et al. in press). However, this pattern does not explain the variation among female dewlap use, as in A. olssoni (a species that occurs in tall grasses with very low visibility), females appear to display more frequently than those of the other species (figure 2). Nicholson et al. (2007) also found that anole species in the same structural habitat have evolved very different dewlap morphologies, but suggest that different light environments may explain the evolution of this variation. Anole species in sunny habitats extend their dewlaps more frequently than those in shade (Ord & Martins 2006), but light conditions appear to be unrelated to the evolution of dewlap colour and signal detectablity (Fleishman et al. 2009). Additional field studies are necessary to determine which ecological contexts are critical influences in the evolution of both the behaviours and their underlying morphological structures.
(d). Influence of body size on dewlap evolution
In analyses of any morphological trait, one might expect larger species (or the larger sex) to have correspondingly evolved larger structures. Therefore, when comparing morphologies across species, it is important to consider body size. Among the anole species, dewlap-associated morphological traits were generally positively correlated for both sexes (table 3), indicating that as larger dewlaps have evolved, the underlying structures have also increased in size.
However, our two measures of body size (SVL and mass) exhibited different relationships with dewlap-associated traits for males and females (table 3). Across the nine species, larger male body size was correlated with one procedural control (trachea size), but none of the dewlap-associated traits. In females, however, body size (SVL in particular) was positively correlated with all four measures of dewlap-associated structures. This pattern may be the result of differential selective pressures for the sexes, with natural selection an important evolutionary forces for both sexes, but sexual selection an important evolution force for males only (Vanhooydonck et al. 2009). Dewlaps are traits used predominantly by males during courtship and in territorial competitions with other males (Jenssen 1977). As such an important component of communication, the pressures to evolve effective signals in a particular environment while maintaining a structure size that does not overly handicap the animal may be stronger forces driving the evolution of dewlap size than would be predicted by an allometric relationship with body size. Females, in contrast, generally use their dewlaps rarely, and almost never in reproductive contexts (Jenssen et al. 2000; Losos 2009). Thus, there may be no selective pressure for females to evolve dewlap-associated traits beyond the minimum required size for dewlap extension, with little or no pressure from sexual selection to evolve larger CH muscles. Because the contexts in which females use their dewlap have been little studied (Nunez et al.1997; Jenssen et al. 2000), and equivocal data exist regarding the importance of the male dewlap in female mate choice (Tokarz 1995) and male territory defence (Tokarz et al. 2003; Vanhooydonck et al. 2005), this hypothesis remains to be evaluated.
In summary, structure–function relationships have long been a focus of studies on behavioural mechanisms. Our analyses of anole dewlaps demonstrate that while morphology and function are often associated, such that commonly used structures have generally evolved larger sizes, the generality of these patterns is limited by species-specific differences that are probably constrained by the ecological contexts in which the structures are used. While evolutionary and mechanistic studies of behaviour typically use different methodologies and address different questions, increasing studies that combine the two approaches can eventually lead to an integrative understanding of the relationships among structural and functional traits.
Acknowledgements
All procedures were performed in accordance with the guidelines of the Michigan State University Institutional Animal Care and Use Committee.
We thank T. Hsieh, R. Cohen and J. Vandecar for valuable field assistance, J. Caton for tissue sectioning and three anonymous reviewers for helpful comments on earlier versions of this manuscript. We also appreciate the support of Circle B Farm, Discovery Bay Marine Laboratory and K. Gaynor in Jamaica; Coralsol Beach Resort in the Dominican Republic; and Jean Lafitte National Park, Barataria, and D. Horton in Louisiana. This work was supported by a Michigan State University Provost Office Postdoctoral Fellowship to M.A.J. and NSF IOS-0742833 to J.W.
References
- Andersson M.1994Sexual selection Princeton, NJ: Princeton University Press [Google Scholar]
- Arnold A. P.1997Sexual differentiation of the zebra finch song system: positive evidence, negative evidence, null hypothesis, and a paradigm shift. J. Neurobiol. 33, 572–584 (doi:10.1002/(SICI)1097-4695(19971105)33:5<572::AID-NEU6>3.0.CO;2-1) [PubMed] [Google Scholar]
- Ball G. F., Auger C. J., Bernard D. J., Charlier T. D., Sartor J. J., Riters L. V., Balthazart J.2004Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann. N. Y. Acad. Sci. 1016, 586–610 (doi:10.1196/annals.1298.043) [DOI] [PubMed] [Google Scholar]
- Bass A. H.1990Sounds from the intertidal zone-vocalizing fish. Bioscience 40, 249–258 (doi:10.2307/1311261) [Google Scholar]
- Breedlove S. M., Arnold A. P.1980Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science 210, 564–566 (doi:10.1126/science.7423210) [DOI] [PubMed] [Google Scholar]
- Brenowitz E. A.1997Comparative approaches to the avian song system. J. Neurobiol. 33, 517–531 (doi:10.1002/(SICI)1097-4695(19971105)33:5<517::AID-NEU3>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- Brenowitz E. A., Beecher M. D.2005Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci. 28, 127–132 (doi:10.1016/j.tins.2005.01.004) [DOI] [PubMed] [Google Scholar]
- Butler M. A., Losos J. B.2002Multivariate sexual dimorphism, sexual selection, and adaptation in Greater Antillean Anolis lizards. Ecol. Monogr. 72, 541–559 [Google Scholar]
- Caro T. M.1995Pursuit-deterrence revisited. Trends Ecol. Evol. 10, 15–29 (doi:10.1016/S0169-5347(00)89207-1) [DOI] [PubMed] [Google Scholar]
- Cooke B., Hegstrom C. D., Villeneuve L. S., Breedlove S. M.1998Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 19, 323–362 (doi:10.1006/frne.1998.0171) [DOI] [PubMed] [Google Scholar]
- Devoogd T. J., Krebs J. R., Healy S. D., Purvis A.1993Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc. R. Soc. Lond. B 254, 75–82 (doi:10.1098/rspb.1993.0129) [DOI] [PubMed] [Google Scholar]
- Eme J., Owerkowicz T., Gwalthney J., Blank J. M., Rourke B. C., Hicks J. W.2009Exhaustive exercise training enhances aerobic capacity in American alligator (Alligator mississippiensis). J. Comp. Physiol. B 179, 921–931 (doi:10.1007/s00360-009-0374-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J.1985Phylogenies and the comparative method. Am. Nat. 125, 1–15 (doi:10.1086/284325) [Google Scholar]
- Fitch H. S., Henderson R. W.1987Ecological and ethological parameters in Anolis bahorucoensis, a species having rudimentary development of the dewlap. Amphibia–Reptilia 8, 69–80 (doi:10.1163/156853887X00063) [Google Scholar]
- Fleishman L. J., Leal M., Persons M. H.2009Habitat light and dewlap color diversity in four species of Puerto Rican anoline lizards. J. Comp. Physiol. A 195, 1043–1060 (doi:10.1007/S00359-009-0478-8) [DOI] [PubMed] [Google Scholar]
- Garland T., Harvey P. H., Ives A. R.1992Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32 [Google Scholar]
- Hicks R. A., Trivers R. L.1983The social behavior of Anolis valencienni. In Advances in herpetology and evolutionary biology (eds Rhodin A. G. J., Miyata K.), pp. 570–595 Cambridge, MA: Museum of Comparative Zoology [Google Scholar]
- Jaccard J.1997Interaction effects in factorial analysis of variance Thousand Oaks, CA: Sage Publications [Google Scholar]
- Jenssen T. A.1977Evolution of anoline display behavior. Am. Zool. 17, 203–215 [Google Scholar]
- Jenssen T. A., Orrell K. S., Lovern M. B.2000Sexual dimorphisms in aggressive signal structure and use by a polygynous lizard Anolis carolinensis. Copeia 2000, 140–149 (doi:10.1643/0045-8511(2000)2000[0140:SDIASS]2.0.CO;2) [Google Scholar]
- Johnson M. A.2007Behavioral ecology of Caribbean Anolis lizards: a comparative approach St Louis, MO, USA: Washington University [Google Scholar]
- Johnson M. A., Revell L. J., Losos J. B.In press Behavioral convergence and adaptive radiation: effects of habitat use on territorial behavior in Anolis lizards. Evolution (doi:10.1111/j.1558-5646.2009.00881.x) [DOI] [PubMed] [Google Scholar]
- Kelley D. B., Fenstemaker S., Hannigan P., Shih S.1988Sex differences in the motor nucleus of cranial nerve IX–X in Xenopus laevis: a quantitative Golgi study. J. Neurobiol. 19, 413–429 (doi:10.1002/neu.480190503) [DOI] [PubMed] [Google Scholar]
- Lailvaux S. P., Irschick D. J.2007The evolution of performance-based male fighting ability in Caribbean Anolis lizards. Am. Nat. 170, 573–586 (doi:10.1086/521234) [DOI] [PubMed] [Google Scholar]
- Leal M., Rodriguez Robles J. A.1997Signalling displays during predator–prey interactions in a Puerto Rican anole, Anolis cristatellus. Anim. Behav. 54, 1147–1154 (doi:10.1006/anbe.1997.0572) [DOI] [PubMed] [Google Scholar]
- Losos J. B.2009Lizards in an evolutionary tree: ecology and adaptive radiation of anoles Berkeley, CA: University of California Press [Google Scholar]
- Lovich J. E., Gibbons J. W.1992A review of techniques for quantifying sexual size dimorphism. Growth Develop. Aging 56, 269–281 [PubMed] [Google Scholar]
- MacDougall-Shackleton S. A., Ball G. F.1999Comparative studies of sex differences in the song-control system of songbirds. Trends Neurosci. 22, 432–436 (doi:10.1016/S0166-2236(99)01434-4) [DOI] [PubMed] [Google Scholar]
- Monks S. P.1881A partial biography of the green lizard. Am. Nat. 15, 96–99 (doi:10.1086/272750) [Google Scholar]
- Neal J. K., Wade J.2007Courtship and copulation in the adult male green anole: effects of season, hormone and female contact on reproductive behavior and morphology. Behav. Brain Res. 177, 177–185 (doi:10.1016/j.bbr.2006.11.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K. E., Glor R. E., Kolbe J. J., Larson A., Hedges S. B., Losos J. B.2005Mainland colonization by island lizards. J. Biogeogr. 32, 929–938 (doi:10.1111/j.1365-2699.2004.01222.x) [Google Scholar]
- Nicholson K. E., Harmon L. J., Losos J. B.2007Evolution of Anolis lizard dewlap diversity. PLoS ONE 2, e274 (doi:10.1371/journal.pone.0000274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F., Arnold A. P.1976Sexual dimorphism in vocal control areas of the songbird brain. Science 4261, 211–213 (doi:10.1126/science.959852) [DOI] [PubMed] [Google Scholar]
- Nunez S. C., Jenssen T. A., Ersland K.1997Female activity profile of a polygynous lizard (Anolis carolinensis): evidence of intersexual asymmetry. Behaviour 134, 205–223 (doi:10.1163/156853997X00458) [Google Scholar]
- O'Bryant E. L., Wade J.1999Sexual dimorphisms in a neuromuscular system regulating courtship in the green anole lizard: effects of season and androgen treatment. J. Neurobiol. 40, 202–213 (doi:10.1002/(SICI)1097-4695(199908)40:2<202::AID-NEU6>3.0.CO;2-A) [DOI] [PubMed] [Google Scholar]
- Ord T. J., Martins E. P.2006Tracing the origins of signal diversity in anole lizards: pylogenetic approaches to inferring the evolution of complex behaviour. Anim. Behav. 71, 1411–1429 (doi:10.1016/j.anbehav.2005.12.003) [Google Scholar]
- Orrell K. S., Jenssen T. A.1998Display behavior of Anolis bahorucoensis: an anole with a diminuitive dewlap. Carib. J. Sci. 34, 113–125 [Google Scholar]
- Price J. J., Lanyon S. M.2002Reconstructing the evolution of complex bird song in the oropendolas. Evolution 56, 1514–1529 [DOI] [PubMed] [Google Scholar]
- Price J. J., Lanyon S. M.2004Patterns of song evolution and sexual selection in the oropendolas and caciques. Behav. Ecol. 14, 485–497 (doi:10.1093/beheco/arh040) [Google Scholar]
- Revell L. J.2006. IDC: a program for the calculation of independent contrasts. See http://anolis.oeb.harvard.edu/~liam/programs/
- Sanderson M. J.2003r8 s: inferring absolute rates of evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 (doi:10.1093/bioinformatics/19.2.301) [DOI] [PubMed] [Google Scholar]
- Sassoon D., Kelley D. B.1986The sexually dimorphic larynx of Xenopus laevis: development and androgen regulation. Am. J. Anat. 177, 457–472 (doi:10.1002/aja.1001770404) [DOI] [PubMed] [Google Scholar]
- Smith R. J.1999Statistics of sexual size dimorphism. J. Hum. Evol. 36, 423–459 (doi:10.1006/jhev.1998.0281) [DOI] [PubMed] [Google Scholar]
- Stephens P. R., Wiens J. J.2009Evolution of sexual size dimorphisms in emydid turtles: ecological dimorphism, Rensch's rule, and sympatric divergence. Evolution 63, 910–925 (doi:10.1111/j.1558-5646.2008.00597.x) [DOI] [PubMed] [Google Scholar]
- Suthers R. A., Zollinger S. A.2004Producing song: the vocal apparatus. Ann. N. Y. Acad. Sci. 1016, 109–129 (doi:10.1196/annals.1298.041) [DOI] [PubMed] [Google Scholar]
- Suthers R. A., Goller F., Pytte C.1999The neuromuscular control of birdsong. Phil. Trans. R. Soc. Lond. B 354, 927–939 (doi:10.1098/rstb.1999.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely T., Catchpole C. K., Devoogd A., Marchl Z., Devoogd T. J.1996Evolutionary changes in a song control area of the brain (HVC) are associated with evolutionary changes in song repertoire among European warblers (Sylviidae). Proc. R. Soc. Lond. B 263, 607–610 (doi:10.1098/rspb.1996.0091) [Google Scholar]
- Tokarz R. R.1995Mate choice in lizards: a review. Herpetol. Monogr. 9, 17–40 (doi:10.2307/1466994) [Google Scholar]
- Tokarz R. R., Paterson A. V., McMann S.2003Laboratory and field test of the functional significance of the male's dewlap in the lizard Anolis sagrei. Copeia 2003, 502–511 (doi:10.1643/00-170) [Google Scholar]
- Vanhooydonck B., Herrel A. Y., van Damme R., Irschick D. J.2005Does dewlap size predict male bite performance in Jamaican Anolis lizards? Funct. Ecol. 19, 38–42 (doi:10.1111/j.0269-8463.2005.00940.x) [Google Scholar]
- Vanhooydonck B., Herrel A., Meyers J. J., Irschick D. J.2009What determines dewlap diversity in Anolis lizards? An among-island comparison. J. Evol. Biol. 22, 293–305 (doi:10.1111/j.1420-9101.2008.01643.x) [DOI] [PubMed] [Google Scholar]
- Wade J.2001Zebra finch sexual differentiation: the aromatization hypothesis revisited. Microsc. Res. Tech. 54, 354–363 (doi:10.1002/jemt.1148) [DOI] [PubMed] [Google Scholar]
- Wade J.2005Current research on the behavioral neuroendocrinology of reptiles. Horm. Behav. 48, 451–460 (doi:10.1016/j.yhbeh.2005.02.006) [DOI] [PubMed] [Google Scholar]