Abstract
Phenological dynamics are controlled by environmental factors, disturbance regimes and species interactions that alter growth or mortality risk. Ecosystem engineers can be a key source of disturbance, yet their effects on the phenologies of co-occurring organisms are virtually unexplored. We investigated how the abundance of a dominant ecosystem engineer, spawning sockeye salmon (Oncorhynchus nerka), alters the emergence phenology of stream insects. In streams with high densities of salmon, peak insect emergence occurred in early July, immediately prior to salmon spawning. By contrast, peak insect emergence in streams with low densities of salmon was weeks later and more protracted. The emergence of specific taxa was also significantly related to salmon density. A common rearing experiment revealed that differences in emergence timing are maintained in the absence of spawning salmon. We hypothesize that these patterns are probably driven by predictable and severe disturbance from nest-digging salmon driving local adaptation and being a trait filter of insect emergence. Thus, salmon regulate the timing and duration of aquatic insect emergence, a cross-ecosystem flux from streams to riparian systems.
Keywords: ecosystem engineer, disturbance regime, bioturbation, marine-derived nutrients
1. Introduction
Seasonal dynamics of communities are controlled by a combination of ecological and evolutionary forces (e.g. Van Noordwijk et al. 1995). The phenology of an organism can consist of plastic responses to cues (e.g. Laurila et al. 1998) or be a locally adapted trait (e.g. Van Noordwijk et al. 1995). According to theory, the evolution of phenology is controlled by the conflict between advantages of further growth versus the risk of mortality (Ludwig & Rowe 1990; Iwasa & Levin 1995). Thus, organisms can mediate growth potential of other species; for example, shrimp populations have evolved so that their offspring will hatch during the spring phytoplankton bloom (Koeller et al. 2009). Alternatively, ecosystem engineers that change abiotic drivers can indirectly influence phenology; for example, beaver dams warm wetlands, altering the emergence phenology of an amphibian (Skelly & Freidenburg 2000). Furthermore, organisms that cause mortality through predation or disturbance would directly modify risk. Although there are many examples of predation driving phenology; for example, fish predation pressure causes earlier maturity of their zooplankton prey (e.g. Stibor 1992), the evolutionary effects of biotic sources of disturbance are virtually unexplored. We would anticipate that organisms which strongly alter either growth resources or mortality risk have the potential to strongly influence the phenology of co-occurring organisms.
Anadromous and semelparous Pacific salmon (Oncorhynchus spp.) are a critical structuring component of coastal freshwater ecosystems through cumulative effects as sources of nutrients and energy, and as a source of physical disturbance to spawning habitats (Schindler et al. 2003; Moore 2006; Janetski et al. 2008). Pacific salmon grow large through foraging in the marine ecosystem, and then migrate to relatively confined freshwaters where they cease feeding, spawn and die (Quinn 2005). Their carcasses and excreta can fertilize food webs (e.g. Chaloner et al. 2004; Tiegs et al. 2008), and their nest-digging can disturb benthic communities (Peterson & Foote 2000; Moore et al. 2004; Lessard & Merritt 2006; Moore & Schindler 2008; Honea & Gara 2009; Monaghan & Milner 2009). These previous studies have focused on how salmon impact the populations or growth of stream organisms. For example, previously we found that salmon nest-digging is strongly negatively associated with aquatic invertebrate seasonal abundance in spawning streams; in fact many lotic insects were virtually absent during salmon spawning seasons in streams with high salmon densities (Moore & Schindler 2008). Given that spawning salmon strongly alter resources for growth and mortality risk in a seasonally predictable fashion; they may be a likely driver of the phenology of stream organisms. To our knowledge, there has only been one study that has explicitly examined how spawning salmon impact the phenology of coastal organisms—Ben-David (1997) found that lactation timing in mink corresponded tightly to the seasonality of salmon carcass availability, upon which they feed.
We investigated how the phenology and abundance of an ecosystem engineer alters the phenology of a suite of co-occurring species. Specifically, we examined the relationship between spawning salmon and the emergence phenology of benthic stream insects. We tested the hypothesis that aquatic insects emerge into terrestrial adults prior to salmon spawning, minimizing their risk from this predictable and severe disturbance. We observed that high densities of spawning sockeye salmon (Oncorhynchus nerka) strongly alter the timing of aquatic insect emergence, thereby changing the seasonality of aquatic-terrestrial coupling.
2. Material and methods
(a). Study system
We examined the relationship between spawning sockeye salmon and the phenology of benthic insects in streams of the Wood River system of southwestern Alaska, USA (59°24′ N, 158°53′ W). This 2600 km2 river system is a spawning and nursery habitat for anadromous sockeye salmon (O. nerka). Sockeye represent more than 99 per cent of the spawning salmon in the study streams. An average of 1.1 million sockeye spawn in the streams, rivers and beaches of this system every year (Baker et al. 2006). This watershed has no logging, agriculture, roads or other major direct sources of human land-use change.
In the many streams of this system, salmon enter to spawn in mass in mid-July, peaking in August and finishing in September. Salmon generally start spawning within 2 days of entering streams. The phenology of this spawning varies among streams, but is consistent among years for a given stream. For example, the average standard deviation of entry dates for streams in this area is approximately 2 days (n = 5, University of Washington Alaska Salmon Program 2009, unpublished data). Spawning sockeye dig large nests (approx. 2 m2 and 20 cm deep), and previous studies in this system have shown that this nest-digging can disturb large proportions (up to 100%) of spawning habitats (Moore 2006) impacting benthic communities (Moore & Schindler 2008). In this study we focus on aquatic insects. Their life cycle starts as an egg, frequently deposited in off-channel habitats (with fewer salmon), which lasts from several hours to several months, after which they hatch into a small larvae or nymph (Merritt & Cummins 1996). It is likely that these early phases are more resistant than later (and larger) stages to disturbance, such as that from nest-digging salmon, because of their small size and ability to persist in the interstices of stream substrates. For example, previous studies have found small-sized benthic invertebrates to be more resistant to disturbance from flooding in streams (e.g. Townsend et al. 1997).
We focused our study on 10 streams that flow into lakes Nerka and Aleknagik. Streams were selected to span a natural gradient in salmon density, influenced by environmental factors such as access (e.g. shallow reaches at the entrance of streams) and spawning habitat (e.g. substrate size and stream gradient). Streams are fed by a combination of groundwater, snow-melt and rain. Linear intersite distances between streams ranged from approximately 1 to 43 km. We quantified water temperatures in the study streams using a combination of Hobo data loggers (Onset Co.) and i-buttons (Thermochron). To control for seasonal differences in temperature monitoring across streams, we focused on temperatures during July and August. To verify that this index of stream temperature is indicative of the annual temperature regime that would be experienced by stream invertebrates; we collected stream temperatures over an entire annual cycle in five of our study streams using Hobo data loggers (Onset Co.) (September 2007–September 2008) and estimated cumulative degree-days. Annual cumulative degree-day was strongly and positively correlated with average July and August temperature (r2 = 0.74).
(b). Benthic insect phenology
We monitored insect emergence phenology from June to September, which represents the bulk of the ice-free period. Aquatic life stages of insects were collected every 11.3 ± 4.3 days (mean ± 1 s.d.) from June to September using a Surber sampler (0.5 mm mesh; sampling to a depth of 10 cm). We collected 4.8 ± 1.3 Surber samples at each stream at each sampling event for a total of 1063 samples, distributed over four summers from 2002 to 2005. Each sample was taken from a different sampling location in the stream. Samples were preserved in 70 per cent ethanol. Invertebrates were sorted and identified to the taxonomic resolution shown in the electronic supplementary material, table S1 (Merritt & Cummins 1996). For each taxa in each sample, the body length of 5–10 random individuals was measured using an ocular micrometer. We also noted the life stage of each individual (e.g. pupal stages and dark wing pads (dwp) indicating late instars). These data were used to estimate emergence timing using three different approaches, as outlined below.
We also directly quantified emergence timing in two of the study streams (N-4, low salmon; Pick, high salmon) using traps that captured insects as they emerged into their terrestrial stages (Francis et al. 2006). We monitored emergence continuously from 24 June to 10 September in 2004. Emergence traps were boxes (0.5 × 0.5 × 0.2 m; length × width × height) made of hardware cloth, secured above the stream bed with rebar, and covered with 1 mm mesh mosquito netting. These traps were secured flush with the water surface, and were open on the bottom side, allowing insects emerging from the stream to enter the trap. A suspended translucent plastic plate (345 cm2) coated with Tangle-trap Insect Trap Coating was attached to the underside of the roof of the emergence trap to catch the emerged insects. Emergence was generally quantified over 48 h periods.
(c). Common rearing experiment
We used a common rearing experiment to examine if observed in situ differences in emergence phenology among streams persisted in the absence of nest-digging salmon. We collected live insects with a Surber sampler from three source streams, N-4 (low salmon), Pick (high salmon) and Hansen (high salmon) and allowed them to grow and emerge in mesocosms. We focused this experiment on chloroperlidae stoneflies and Cinygmula spp. mayflies given their abundance across the different study streams. A subsample of 15 insects per taxa were stocked in each mesocosm. Insects were collected prior to salmon entering the streams to spawn. The resultant insect densities were on the lower end of densities observed in streams. Starting sizes were significantly different across the different streams for Cingymula spp. (mean ± 1 s.d. of length was 6.06 ± 1.5 for Hansen and 4.4 ± 0.9 for N-4; two-sample t-test, t178 = 8.07, p < 0.001) but not for chloroperlidae (mean ± 1 s.d. of length was 6.24 ± 1.3 for Hansen and 5.84 ± 1.4 for N-4; two-sample t-test, t29 = 0.73, p = 0.47).
Mesocosms were plastic totes (68 l; 61 × 40 × 41.9 cm) with a bottom layer of rinsed cobble from the adjacent lake to provide substrate. These mesocosms were covered with 1 mm mesh mosquito netting to retain emerging insects. Mesocosms were fed with water that was a mixture from Lake Aleknagik and a groundwater spring. Water temperature in each tote was measured every several days with a hand-held thermometer, temperatures ranged from 6 to 14°C and showed no variation among mesocosms. Mesocosms were placed on the beach of Lake Aleknagik, thus subjected to ambient air temperatures and light regimes, and different source populations were randomly assigned among totes. There were six replicates for each of three source streams, for a total of 18 mesocosms. A grizzly bear destroyed three of the mesocosms during the early phases of the experiment (two from Pick Creek and one from N-4). This loss of replication for Pick Creek compromised statistical power. Thus, analyses were constrained to insects from N-4 and Hansen Creek. This experiment was run from 19 June to 24 August, 2004, and the mesocosms were checked every 2–4 days for emerging insects.
(d). Statistical analyses
We estimated in situ emergence timing based on benthic samples via three techniques. When possible, we estimated the mean and standard deviation of emergence timing for each taxa for each stream using all of these three indices. However, there were cases where sample sizes were insufficient to estimate one or more of these indices of emergence for a given taxon. Normal distributions were used to describe the emergence patterns of insects, fit to the observed data by using the nonlinear function fitting in Systat (v. 12.0). Data from all years of the study were pooled in the analyses.
(i). Size trajectories
We used size trajectories of benthic insects as an index of emergence timing. Average emergence timing was estimated as the day when the average-sized individual reached the estimated size of emergence. We estimated size of emergence as the average body length of observed pupae (or dark wing pad individuals) for that taxon and stream. When we did not observe individuals that exhibited signs of metamorphosing, we used the strong relationship between maximum size observed and pupae size to extrapolate size at emergence. We estimated the average date of emergence as the intersection between the average size of emergence (as described above) and the linear regression between date and body length of benthic individuals. The linear regression on date and body length was restricted to individuals from dates prior to when individuals started to reach the size of emergence threshold, and excluded smaller individuals from the next generation. In order to estimate variance around the estimated average date of emergence, we ran the regression 500 times on bootstrapped data for each taxa and stream, estimating average emergence for each bootstrap. The lowest 5 per cent bootstrap value was used as a lower confidence interval of emergence timing.
(ii). Abundance of upcoming metamorphosis
We fit normal distributions of emergence timing based on cumulative observations of pupation or observations of individuals in the last instar (e.g. dwp for Baetis) for each stream. In this index, peak emergence represents the date when the number of emerging individuals from a given taxa is the highest.
(iii). Proportion of upcoming metamorphosis
We fit normal distributions of emergence timing based on cumulative proportions of pupation or observations of individuals in the last instar (e.g. dwp). In this index, peak emergence represents the date when 50 per cent of individuals of a population have emerged.
The three indices of emergence timing were strongly related across all taxa and streams (emergencenumber = 0.57 × emergencesize + 4.3, r2 = 0.73; emergencenumber = 0.70 × emergenceproportion + 9.1, r2 = 0.66). Using the regression parameters, we converted all estimates from methods 1 and 3 above to be equivalent to the second (i.e. based on abundance of emerging individuals). The emergence timing from all indices were averaged for each taxa and stream. For each stream, we estimated the seasonal flux of aquatic insects by summing the distributions of estimated emergence for all taxa in a stream, with taxa being weighted by their biomass. We used these distributions to calculate the date of 5 and 95 per cent emergence for each stream.
We used general linear models (GLM; Systat 12.0) to assess potential factors influencing emergence timing for the entire community and for each specific taxon (estimated as described above). Factors included stream temperature, stream total phosphorus (TP) concentration during spring (Moore et al. 2007), salmon density and taxa. Akaike's Information Criterion, corrected for small sample sizes (AICc), was used to assess model parsimony (Burnham & Anderson 1998). Akaike importance weights for parameters (ω) were also calculated, an index of the relative importance of individual parameters (Burnham & Anderson 1998). Salmon densities were estimated based on the annual surveys of total count of spawning sockeye (live and dead; Rogers & Schindler 2008) from 2001 to 2005 and spawning area estimates from Marriott (1964).
The data from the direct measurement of insect flux and the common rearing experiment were analysed by fitting cumulative normal distributions to the cumulative proportion emerged for each mesocosm using the nonlinear function fitting (Systat 12.0).
3. Results
(a). Community patterns
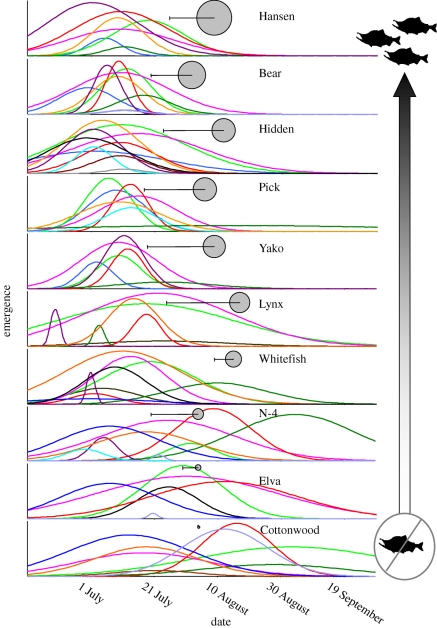
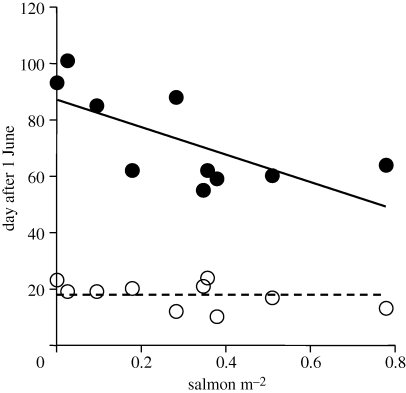
Study streams varied widely in salmon density, with a 5-year average density ranging from 0.81 to less than 0.01 salmon m−2. Patterns of insect emergence varied dramatically among these streams (figure 1). The average emergence timing across all taxa in the five streams with high densities of salmon was in early July (6–9 July). By contrast, peak insect emergence in streams with lower densities of salmon was weeks later (23 July–7 August). Furthermore, in streams with high densities of salmon, all abundant taxa emerged in the narrow window of early- to mid-July, where 90 per cent of the emerging biomass of insects occurred over a 40-day period (figure 2). This emergence window corresponded to the period immediately prior to the entry of spawning salmon to these streams (figure 1). By contrast, in streams with low densities of salmon, emergence of different taxa was spread across the summer period, ranging from early July to late August, where 90 per cent of emerging insect biomass occurred over a 70-day window (figure 2). Specifically, the date of 5 per cent emergence was not significantly related to salmon density (p > 0.15), but the date of 95 per cent of emergence was significantly negatively related to salmon density (r2 = 0.47, n = 10, p = 0.03).
Figure 1.
Seasonal patterns of aquatic insect emergence in 10 streams spanning a gradient in salmon density. Streams are ordered from top to bottom by descending spawning salmon densities. Each curve represents the estimated emergence timing of an insect taxon. Curves were normalized such that the height of each curve was relative to the rank abundance of the taxa for each stream. Different coloured curves represent different taxa (Plecopterans: bright green = Chloroperlidae, dark green = Zapada spp., aqua blue = Isoperla spp.; Dipterans: pink = Chironomidae, Trichopterans: orange = all Trichopterans; Ephemeropterans: black = Ephemerella inermis, royal blue = Baetis bicaudatus, red = Cinygmula spp., purple = Drunella doddsi, brown = Acentrella turbidae, grey = Ameletus spp., lavender = Epeorus grandis; see electronic supplementary material, table S1). Grey circles represent timing and magnitude of spawning sockeye salmon. The diameter of the circle is proportional to the average density of spawning salmon, and the centre of the circle is the median date of peak salmon density. The left error bar portrays the median date of entry of spawning salmon.
Figure 2.
Relationship between salmon density and timing of total insect emergence. Open circles represent the date at which 5 per cent of the insect biomass had emerged and closed circles indicate the date when 95 per cent of insect biomass had emerged. The solid line shows the significant negative relationship between stream salmon density and date of 95 per cent emergence. The dashed line indicates the average date of 5 per cent emergence, as this slope was not significant.
A combination of factors explained variation in the community-wide patterns of emergence across streams (electronic supplementary material, table S2). First, insect emergence was significantly earlier in streams with higher salmon density (n = 74, salmon, r2 = 0.21, p < 0.0001). In addition, insect emergence was significantly earlier in streams that were warmer (n = 74, temperature, r2 = 0.07, p = 0.021). Furthermore, different taxa had significantly different emergence timing (n = 74, taxa, r2 = 0.37, p = 0.001). In addition, insect emergence was significantly earlier in streams with higher phosphorus levels (n = 74, TP, r2 = 0.10, p = 0.004). When these different factors were combined in a GLM, the most parsimonious model was one that included all four of these factors (n = 74, salmon density, stream temperature, TP and taxa; AICc = 578.6, ΔAICc = 0); together, these three factors explained 64 per cent of the variance in insect emergence timing. The other model that received substantial empirical support (ΔAICc = 1.7) included the factors of salmon density, TP and taxa (r2 = 0.61). We note that all predictor variables were weakly correlated with each other (all pairwise comparisons r2 < 0.05). Models that included interaction terms between the factors were substantially less parsimonious (electronic supplementary material, table S2). Inclusion of all parameters received substantial support from the data, but salmon was the most important parameter according to Akaike importance weights (ω; electronic supplementary material, table S2).
(b). Taxon-specific patterns
Patterns of emergence for specific taxa were also strongly but differently influenced by salmon density (figure 1). Taxa that emerged early in streams with few salmon tended not to exhibit temporal shifts in emergence timing in streams with higher densities of salmon. For example, both Baetis bicaudatus and Drunella doddsi emerged in late June and early July across all streams where it was found (figure 1). By contrast, taxa that emerged late in streams with few salmon either exhibited phenological shifts in streams with many salmon (e.g. Cingymula spp.) or were found in exceedingly low densities in streams with many salmon (e.g. Nemouridae spp.; figure 1). We could estimate emergence timing for three taxonomic groups that were common to all 10 study streams: Cinygmula spp. mayflies, chloroperlidae stoneflies and chironomids.
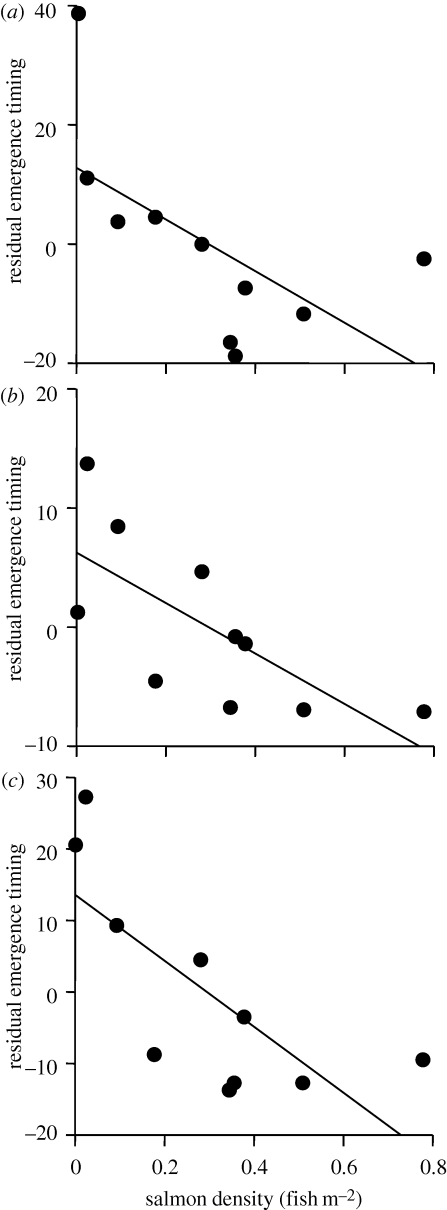
Cingymula spp. mayflies emerged significantly earlier in streams with higher densities of salmon and higher concentrations of TP (figure 3a), and the models that received substantial empirical support were those that included salmon and TP (n = 10, ΔAICc = 0, r2 = 0.74) or only salmon (n = 10, ΔAICc = 0.48, r2 = 0.50). These mayflies emerged roughly a month earlier in streams with high salmon densities. Stream temperature explained only 17.4 per cent of the observed variance in Cingymula spp. emergence timing and was not a significant explanatory factor alone or in concert with other factors (electronic supplementary material, table S2). According to Akaike's ω for parameters, salmon was 5.4 times more plausible than temperature and 1.4 times more plausible than TP in explaining emergence timing of these taxa (electronic supplementary material, table S2).
Figure 3.
Relationships between salmon density and emergence phenology of three insect groups. Each point represents the estimated average emergence timing for a stream. The y-axis is the residual emergence timing after regressing emergence timing with stream temperature (i.e. larger number indicate later emergence). (a) Chloroperlidae; (b) Chironomidae; (c) Cinygmula.
Peak emergence of chironomids was also earlier in streams with higher densities of salmon; the only model with substantial empirical support was one with only salmon (n = 10, ΔAICc = 0.0, r2 = 0.50). Models that included temperature and TP received considerably less empirical support (n = 10, ΔAICc > 4.9 for all; electronic supplementary material, table S2). As a predictor parameter, salmon was 12.3 times more plausible than temperature and 6.8 times more plausible than TP based on Akaike's ω (electronic supplementary material, table S2).
Chloroperlidae also emerged earlier in streams with higher densities of salmon, the only model with substantial empirical support only included salmon as a factor (n = 9, ΔAICc = 0, r2 = 0.39). TP alone exhibited some empirical support (n = 9, ΔAICc = 2.1, r2 = 0.24), and other models including temperature received considerably less support (electronic supplementary material, table S2). Salmon was 5.4 times more plausible than temperature and 2.3 times more plausible than TP based on Akaike's ω (electronic supplementary material, table S2).
(c). Direct measurement of insect flux from two focal streams
Emergence traps that directly measured the flux of emerging insects also revealed differences in emergence phenology between a stream with high density (Pick Creek) and a stream with low density (N-4 Creek) of salmon (electronic supplementary material, figure S1 and table S3). Specifically, mayfly emergence rates were highest in early July in Pick Creek (high salmon), while rates were highest in late August in N-4 (low salmon). Dipteran (predominantly chironomids) emergence was dispersed across the season in Pick Creek, and peaked in late August in N-4. Patterns of caddisfly emergence were more similar across the two streams; however, peak emergence of caddisflies in Pick Creek was in late June, while the peak was around two weeks later in N-4 (early- to mid-July). Stonefly emergence was highest in early- to mid-July in Pick Creek, while their emergence was low and dispersed across the season in N-4. Insects emerged statistically earlier in the stream with many salmon when compared with the stream with few salmon, as confidence intervals for mean emergence date did not overlap across streams (electronic supplementary material, table S3). These emergence patterns also confirmed the patterns we observed using indirect methods, there was a strong positive relationship between the estimated emergence using direct (emergence traps) and indirect methods (size trajectories and pupation) (r2 = 0.69, p = 0.01).
(d). Common rearing experiment
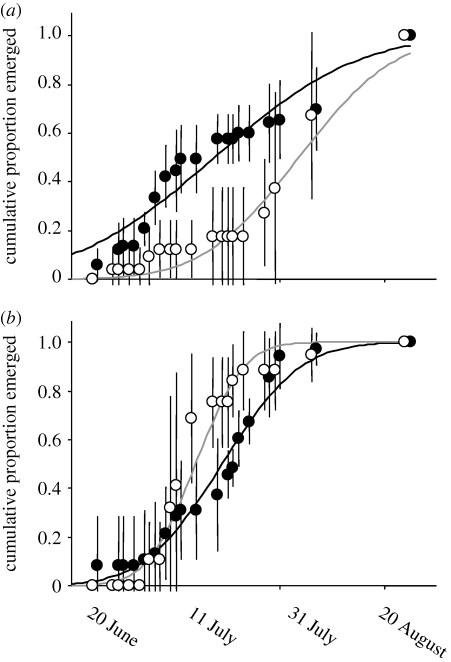
A common rearing experiment revealed that differences in emergence timing among streams were maintained in the absence of spawning salmon (figure 4; electronic supplementary material, table S4). In the common rearing experiment, Cinygmula spp. mayflies from Hansen Creek (high salmon) emerged on 18 July on average, more than two weeks earlier than those from N-4 (low salmon). The direction of this pattern is consistent with that observed in situ from stream populations, where Hansen Cinygmula spp. emerged on 9 July and N-4 Cinygmula spp. emerged on 9 August. In the common rearing experiment, chloroperlidae from the two streams emerged at fairly similar timing, with N-4 chloroperlidae emerging slightly before Hansen chloroperlidae (5 days). Patterns of in situ emergence of chloroperlidae were also fairly similar between N-4 and Hansen (figure 1); after correcting for differences in stream temperature, in situ emergence of Hansen chloroperlidae was 6 days later than those in N-4.
Figure 4.
Emergence timing from the common rearing experiment. Shown are the cumulative proportion emerged of (a) Cinygmula spp. and (b) chloroperlidae, averaged across replicates, ±1 s.d. The curves represent the best-fit cumulative normal distributions. Open circles, N-4 (low salmon); closed circles, Hansen (high salmon).
4. Discussion
The phenology of organisms is controlled by a combination of abiotic and biotic factors. The most likely abiotic driver of stream insect emergence phenology is water temperature—previous studies have demonstrated that warmer water leads to earlier emergence (e.g. Harper & Peckarsky 2006). In this study, temperature did explain some of the variation in emergence timing, with warmer streams having earlier emergence for most taxa (electronic supplementary material, table S2). Total phosphorus also explained some of the variation in emergence timing, with more nutrient-rich streams having earlier emergence for most taxa (electronic supplementary material, table S2).
Across all analyses, salmon density was a factor in all of the top models and had the highest Akaike importance weight in every aspect of this study (electronic supplementary material, table S2). In streams with high densities of salmon, insects emerged in early July, leaving the stream immediately prior to the onset of salmon spawning (figure 1). By contrast, peak insect emergence in streams with low densities of salmon was weeks later, in late July to early August. The duration of insect emergence was truncated in streams with high densities of salmon, the period that contained 90 per cent of emerging insect biomass was approximately 30 days shorter in streams with high salmon densities (figure 2). Salmon density was significantly related to large shifts in phenology for the entire community, for coarse taxonomic groups such as chironomids, and for single taxa (Cinygmula spp.; figures 1 and 3). These strong relationships between salmon density and aquatic insect emergence raises the question: what is the mechanism of relationship between salmon density and aquatic insect emergence (figure 1)? Below, we discuss several possible mechanisms.
Given the large body of research on the importance of predation in controlling phenology (e.g. Laurila et al. 1998), it may be tempting to hypothesize that the observed patterns are driven by predation by adult salmon. However, salmon do not feed as spawning adults (Quinn 2005), so predation by adult sockeye cannot be driving insect phenology.
Salmon could induce changes in insect phenology because of their effects on stream productivity and growth rates of insects. Spawning salmon can increase concentrations of key limiting nutrients such as phosphorus through excretion and decomposition, and much research has investigated how these nutrients fertilize stream food webs (Janetski et al. 2008). This bottom-up fertilization could increase insect growth and, consequently, advance insect phenology. However, during salmon spawning (when nutrient concentrations are elevated), we previously found that bioturbation from salmon nest-digging decreased algal abundance, thereby decreasing available food for grazing benthic insects (Moore & Schindler 2008). After salmon spawning, nutrient concentrations decrease to background levels (Moore et al. 2007)—salmon density explained little of the observed stream-to-stream variance in spring TP concentrations (n = 10, r2 = 0.05, p = 0.52). TP was present in some of the top models (but never replaced salmon density) for analyses on the total community and for Cinygmula, but not for other taxa (electronic supplementary material, table S2). While some invertebrate taxa feed directly on salmon carcasses (e.g. Winder et al. 2005), the vast majority of insects were not observed in these streams with high densities of salmon when carcasses were available, during or after salmon spawning (Moore & Schindler 2008). For these reasons, it seems unlikely that fertilization from salmon is the primary mechanism by which salmon influence insect phenology.
Emergence may be a plastic response to increased risk (e.g. Peckarsky et al. 2002), perhaps insects are responding to cues from salmon. Given that insects predominantly emerge prior to salmon entering the stream to spawn (figure 1), the cue would need to be from the previous year of spawners. If this were the case, interannual variation in salmon density could translate into interannual variation in emergence timing. The most extreme interannual variation in salmon densities was in N-4, with peak annual salmon densities that ranged from 0.002 salmon m−2 during 2003 to 0.16 salmon m−2 during 2004. Despite this change, insects did not exhibit interannual variation in emergence timing (electronic supplementary material, figure S2); evidence against emergence plasticity.
Direct mortality from salmon nest-digging could drive earlier emergence if late emerging individuals are killed by salmon nest-digging. Salmon nest-digging dislodges and kills benthic invertebrates (Peterson & Foote 2000; Monaghan & Milner 2009) and may make them more vulnerable to predation by stream-dwelling fishes (Scheuerell et al. 2007). This nest-digging can directly control insect abundance; in a previous study that was performed in the subset of the same streams (Moore et al. 2004), we found that excluding salmon from small plots in spawning areas led to a three times increase in densities of Chironomidae relative to reference plots. However, these exclusions did not alter the densities of other insect taxa such as mayflies, which disappeared from streams prior to salmon spawning (even in locations where salmon were excluded from spawning), indicating that seasonal mortality from salmon nest-digging does not directly drive emergence timing for many taxa. Furthermore, phenological differences across salmon densities persisted even when data analyses were restricted to size trajectory-based estimates of emergence; this method of estimating emergence would not be influenced by direct mortality from salmon spawning. In addition, the common rearing experiment indicated that the observed differences in emergence timing for specific taxa (Cingymula spp.) persisted in the absence of direct disturbance from nest-digging salmon (figure 4). Therefore, direct mortality by nest-digging salmon probably contributes to observed differences in phenology across streams in some (i.e. Chironomidae), but not most taxa.
Salmon nest-digging could cause selection for earlier emergence timing of aquatic insects. Predictable, severe and frequent disturbances are strong selective agents on life histories (Lytle 2002; Lytle & Poff 2004) and high densities of spawning salmon fit all three of those categories (Moore 2006). Insects that emerge prior to salmon spawning would avoid this potentially dangerous period, with their eggs or young persisting through this disturbance. Our data support the notion that eggs and the smaller larval instars are less vulnerable to salmon disturbance, as has been previously observed for flood disturbance (e.g. Townsend et al. 1997). We hypothesize that high densities of spawning salmon drives local adaptation of insect emergence phenology, and if insect species cannot adapt, this biotic disturbance acts as a trait filter (Poff 1997) that excludes late-emerging taxa. Consistent with this hypothesis, insect taxa that emerged late in streams with few salmon either exhibited a shift in emergence timing (i.e. local adaptation in phenology) or were absent from streams with high densities of salmon (i.e. trait filter). Taxa that emerged early in streams with few salmon did not exhibit these shifts in phenology (figure 1). These results build upon several previous studies suggesting that the life histories of benthic invertebrates may mediate the impact of nest-digging; stream invertebrates with rapid life cycles (such as chironomids) can recover rapidly following salmon disturbance (e.g. Chaloner et al. 2004; Lessard & Merritt 2006).
Our data strongly suggest that salmon nest-digging drives insect phenology. However, local adaptation of insect phenology to salmon is predicated on two conditions. First, emergence timing needs to be controlled by variable and heritable traits. This assumption is supported by previous research on insects (e.g. Mousseau & Roff 1989). Second, gene flow between populations must be relatively low so that local adaptation is not swamped. Although this depends on species (and their dispersal abilities), many stream insects do exhibit fine-scale genetic structure associated with limited gene flow (Bohonak & Jenkins 2003). Furthermore, we acknowledge that the taxonomic resolution of insects that exhibited a phenological shift was not to the species level. For example, the Cinygmula genus is poorly described, and the specimens we collected did not allow for identification by taxonomic experts. Thus, we cannot be certain that phenological shifts are occurring within a single species. Finally, our common rearing experiment provided evidence that phenological differences persisted in the absence of spawning salmon. However, this experiment does not control for maternal effects or other differences across source populations that were manifested during the earlier phases of the insects' lives. For example, starting sizes of Cinygmula from Hansen were larger than N-4, thus these taxa were already on different life-history trajectories.
If nest-digging salmon are indeed driving the evolution of aquatic insect life histories, then our study represents an example of how an ecosystem engineer can drive life-history evolution. There are few examples of how ecosystem engineers, through changing abiotic conditions, drive evolution of other species. For example, Stinchcombe & Schmitt (2006) showed that oak tree leaf litter, through modifying soil microclimate, impacts evolution of an understory annual plant. Our study represents an alternative pathway, how bioturbation by an ecosystem engineer drives evolution of life histories. Ecosystem engineers do not only modify their own evolutionary trajectory (e.g. Odling-Smee et al. 1996), but also other species they impact (e.g. Thayer 1979).
Through influencing aquatic insect phenology, salmon modify the timing and duration of resource fluxes from streams to riparian ecosystems (figure 2). While this resource subsidy is shorter in duration in streams with many salmon, it was concentrated during the period prior to salmon spawning when salmon resources are not readily available for riparian consumers. Emerging aquatic insects can be an important source of prey for a variety of riparian consumers, such as bats, spiders, lizards and birds (Baxter et al. 2005). Thus, salmon, through changing insect phenology, may indirectly alter the behaviour, distributions and populations of riparian consumers. In this and many coastal ecosystems; salmon migrate, spawn and die in a pulse that is concentrated in time—our study shows that these regular migrations set the rhythms of stream communities. While there is growing concern that climate change can disrupt coupling of organisms' phenologies (e.g. Winder & Schindler 2004), our study suggests that human activities that decrease the abundance of dominant species could also have cascading phenological consequences.
Acknowledgements
This is a contribution from the Alaska Salmon Program of the University of Washington, supported by the Gordon and Betty Moore Foundation, the National Science Foundation, the Alaska salmon processors, and the UW School of Aquatic and Fishery Sciences.
We would like to thank numerous people who assisted with fieldwork, including Matt Baker, Amanda Barg, Chris Boatright, Curtis Brock, Jackie Carter, Justin Fox, Tessa Francis, Gordon Holtgrieve, Sue Johnson, Wendy Palen, Laura Payne, Casey Ruff and Mark Scheuerell. Sorting of benthic invertebrate samples was greatly assisted by Justin Fox, Adam Goodwin, Karen Knirsken, Peter Lisi, Greg Osborn, Casey Ruff and Jared Willis. We also thank Wease Bollman of Rihron, Robert Wisseman of Aquatic Biology Associated, Inc., and Luke Jacobus for their insect identification services. Earlier drafts of this manuscript were improved by two anonymous reviewers, Mark Novak, Ann-Marie Osterback and Corey Phillis.
References
- Baker T. T., Fair L. F., Clark R. A., Hasbrouck J. J.2006Review of Salmon Escapement Goals in Bristol Bay, 2006. Fisheries Manuscript No. 06-05, Anchorage, Alaska [Google Scholar]
- Baxter C. V., Fausch K. D., Saunders W. C.2005Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw. Biol. 50, 201–220 [Google Scholar]
- Ben-David M.1997Timing of reproduction in wild mink: the influence of spawning Pacific salmon. Can. J. Zool. 75, 376–382 (doi:10.1139/z97-047) [Google Scholar]
- Bohonak A. J., Jenkins D. G.2003Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecol. Lett. 6, 783–796 (doi:10.1046/j.1461-0248.2003.00486.x) [Google Scholar]
- Burnham K. P., Anderson D. R.1998Model selection and multimodel inference New York, NY: Springer [Google Scholar]
- Chaloner D. T., Lamberti G. A., Merritt R. W., Mitchell N. L., Ostrom P. H., Wipfli M. S.2004Variation in responses to spawning Pacific salmon among three south-eastern Alaska streams. Freshw. Biol. 49, 587–599 (doi:10.1111/j.1365-2427.2004.01213.x) [Google Scholar]
- Francis T. B., Schindler D. E., Moore J. W.2006Aquatic insects play a minor role in dispersing salmon-derived nutrients into riparian forests in southwestern Alaska. Can. J. Fish. Aquat. Sci. 63, 2543–2552 (doi:10.1139/F06-144) [Google Scholar]
- Harper M. P., Peckarsky B. L.2006Emergence cues of a mayfly in a high-altitude stream ecosystem: potential response to climate change. Ecol. Appl. 16, 612–621 (doi:10.1890/1051-0761(2006)016[0612:ECOAMI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Honea J. M., Gara R. I.2009Macroinvertebrate community dynamics: strong negative response to salmon redd construction and weak response to salmon-derived nutrient uptake. J. N. Am. Benthol. Soc. 28, 207–219 (doi:10.1899/08-030.1) [Google Scholar]
- Iwasa Y., Levin S. A.1995The timing of life history events. J. Theor. Biol. 172, 33–42 (doi:10.1006/jtbi.1995.0003) [Google Scholar]
- Janetski D. J., Chaloner D. T., Tiegs S. D., Lamberti G. A.2008Pacific salmon effects on stream ecosystems: a quantitative synthesis. Oecologia 159, 583–595 (doi:10.1007/s00442-008-1249-x) [DOI] [PubMed] [Google Scholar]
- Koeller P., et al. 2009Basin-scale coherence in phenology of shrimps and phytoplankton in the North Atlantic Ocean. Science 324, 791–793 (doi:10.1126/science.1170987) [DOI] [PubMed] [Google Scholar]
- Laurila A., Kujasola J., Ranta E.1998Predator-induced changes in life history in two anuran tadpoles: effects of predator diet. Oikos 83, 307–317 (doi:10.2307/3546842) [Google Scholar]
- Lessard J. L., Merritt R. W.2006Influence of marine-derived nutrients from spawning salmon on aquatic insect communities in southeast Alaskan streams. Oikos 113, 334–343 (doi:10.1111/j.2006.0030-1299.14512.x) [Google Scholar]
- Ludwig D., Rowe L.1990Life-history strategies for energy gain and predator avoidance under time constraints. Am. Nat. 135, 686–707 (doi:10.1086/285069) [Google Scholar]
- Lytle D. A.2002Flash floods and aquatic insect life-history evolution: evaluation of multiple models. Ecology 83, 370–385 [Google Scholar]
- Lytle D. A., Poff N. L.2004Adaptation to natural flow regimes. Trends Ecol. Evol. 19, 94–100 (doi:10.1016/j.tree.2003.10.002) [DOI] [PubMed] [Google Scholar]
- Marriott R. A.1964Stream catalog of the Wood River lake system, Bristol Bay, Alaska Washington, DC: United States Fish and Wildlife Service [Google Scholar]
- Merritt R. W., Cummins K. W.1996An introduction to the aquatic insects of North America, 3rd ed.Dubuque, IA: Kendall Hall Publishing Company [Google Scholar]
- Monaghan K. A., Milner A. M.2009Effect of anadromous salmon redd construction on macroinvertebrate communities in a recently formed stream in coastal Alaska. J. N. Am. Benthol. Soc. 28, 153–166 (doi:10.1899/08-071.1) [Google Scholar]
- Moore J. W.2006Animal ecosystem engineers in streams. BioScience 56, 237–246 (doi:10.1641/0006-3568(2006)056[0237:AEEIS]2.0.CO;2) [Google Scholar]
- Moore J. W., Schindler D. E.2008Biotic disturbance and benthic community dynamics in salmon-bearing streams. J. Anim. Ecol. 77, 275–284 (doi:10.1111/j.1365-2656.2007.01336.x) [DOI] [PubMed] [Google Scholar]
- Moore J. W., Schindler D. E., Scheuerell M. D.2004Disturbance of freshwater habitats by anadromous salmon in Alaska. Oecologia 139, 298–308 (doi:10.1007/s00442-004-1509-3) [DOI] [PubMed] [Google Scholar]
- Moore J. W., Schindler D. E., Carter J. L., Fox J., Griffiths J., Holtgrieve G. W.2007Biotic control of stream fluxes: spawning salmon drive nutrient and matter export. Ecology 88, 1278–1291 (doi:10.1890/06-0782) [DOI] [PubMed] [Google Scholar]
- Mousseau T. A., Roff D. A.1989Adaptation to seasonality in a cricket: patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution 43, 1483–1496 (doi:10.2307/2409463) [DOI] [PubMed] [Google Scholar]
- Odling-Smee F. J., Laland K. N., Feldman M. W.1996Niche construction. Am. Nat. 147, 641–648 (doi:10.1086/285870) [Google Scholar]
- Peckarsky B. L., McIntosh A. R., Taylor B. W., Dahl J.2002Predator chemicals induce changes in mayfly life history traits: a whole-stream manipulation. Ecology 83, 612–618 [Google Scholar]
- Peterson D. P., Foote C. J.2000Disturbance of small-stream habitat by spawning sockeye salmon in Alaska. Trans. Am. Fish. Soc. 129, 924–934 (doi:10.1577/1548-8659(2000)129<0924:DOSSHB>2.3.CO;2) [Google Scholar]
- Poff N. L.1997Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J. N. Am. Benthol. Soc. 16, 391–409 (doi:10.2307/1468026) [Google Scholar]
- Quinn T. P.2005The behavior and ecology of pacific salmon and trout Bethesda, MD: American Fisheries Society [Google Scholar]
- Rogers L. A., Schindler D. E.2008Asynchrony in population dynamics of sockeye salmon in southwest Alaska. Oikos 17, 1578–1586 [Google Scholar]
- Scheuerell M. D., Moore J. W., Schindler D. E., Harvey C. J.2007Varying effects of anadromous sockeye salmon on the trophic ecology of two species of resident salmonids in southwest Alaska. Freshw. Biol. 52, 1944–1956 (doi:10.1111/j.1365-2427.2007.01823.x) [Google Scholar]
- Schindler D. E., Scheuerell M. D., Moore J. W., Gende S. M., Francis T. B., Palen W. J.2003Pacific salmon and the ecology of coastal ecosystems. Front. Ecol. Environ. 1, 31–37 [Google Scholar]
- Skelly D. K., Freidenburg L. K.2000Effects of beaver on the thermal biology of an amphibian. Ecol. Lett. 3, 483–486 (doi:10.1046/j.1461-0248.2000.00186.x) [Google Scholar]
- Stibor H.1992Predator induced life-history shifts in a freshwater cladoceran. Oecologia 92, 162–165 (doi:10.1007/BF00317358) [DOI] [PubMed] [Google Scholar]
- Stinchcombe J. R., Schmitt J.2006Ecosystem engineers as selective agents: the effects of leaf litter on emergence time and early growth in Impatiens capensis. Ecol. Lett. 9, 258–270 (doi:10.1111/j.1461-0248.2005.00872.x) [DOI] [PubMed] [Google Scholar]
- Thayer C. W.1979Biological bulldozers and the evolution of marine benthic communities. Science 203, 458–461 (doi:10.1126/science.203.4379.458) [DOI] [PubMed] [Google Scholar]
- Tiegs S. D., Chaloner D. T., Levi P., Rüegg J., Tank J. L., Lamberti G. A.2008Timber harvest transforms ecological roles of salmon in southeast Alaska rain forest streams. Ecol. Appl. 18, 4–11 (doi:10.1890/07-0655.1) [DOI] [PubMed] [Google Scholar]
- Townsend C. R., Dolédec S., Scarsbrook M. R.1997Species traits in relation to temporal and spatial heterogeneity in streams: a test of habitat templet theory. Freshw. Biol. 37, 367–387 (doi:10.1046/j.1365-2427.1997.00166.x) [Google Scholar]
- Van Noordwijk A. J., McCleery R. H., Perrins C. M.1995Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 64, 451–458 [Google Scholar]
- Winder M., Schindler D. E.2004Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85, 2100–2106 (doi:10.1890/04-0151) [Google Scholar]
- Winder M., Schindler D. E., Moore J. W., Johnson S. P., Palen W. J.2005Do bears facilitate transfer of salmon resources to aquatic macroinvertebrates? Can. J. Fish. Aquat. Sci. 62, 2285–2293 (doi:10.1139/f05-136) [Google Scholar]