Abstract
Nest microclimate can have strong effects that can carry over to later life-history stages. We experimentally cooled the nests of tree swallows (Tachycineta bicolor). Females incubating in cooled nests reduced incubation time and allowed egg temperatures to drop, leading to extended incubation periods. We partially cross-fostered nestlings to test carry-over effects of cooling during incubation on nestling innate constitutive immunity, assessed through bacteria killing ability (BKA) of blood. Nestlings that had been cooled as eggs showed a lower ability to kill bacteria than control nestlings, regardless of the treatment of their foster mother. However, there was no effect of treatment of rearing females on nestling BKA in control nestlings, even though cooled females made significantly fewer feeding visits than did control females. This suggests that the effect of cooling occurred during incubation and was not due to carry-over effects on nestling condition. Nestlings that were exposed to experimental cooling as embryos had lower residual body mass and absolute body mass at all four ages measured. Our results indicate that environmental conditions and trade-offs experienced during one stage of development can have important carry-over effects on later life-history stages.
Keywords: bacteria killing ability, carry-over effects, egg temperature, experimental cooling, incubation investment
1. Introduction
Optimal energetic investment in each life-history stage is governed by trade-offs within a stage and across other stages (Stearns 1992). In particular, birds have been an excellent model group for investigating life-history tradeoffs because they have generally distinct reproductive stages: egg production, incubation and nestling rearing. Investment in one life-history stage can have effects on subsequent life-history stages within a current reproduction bout (Heaney & Monaghan 1996; Reid et al. 2000; Hanssen et al. 2005; de Heij et al. 2006; Pérez et al. 2008). Here, we experimentally increase investment in incubation through experimental cooling to assess the consequences of reproductive investment in one stage on subsequent life-history stages. We chose to modify nest microclimate, as investment in incubation is particularly sensitive to fluctuations in environmental conditions (Conway & Martin 2000; Cresswell et al. 2003; Ardia et al. 2009).
The consequences of incubation conditions can be significant for developing embryos. For example, periodic cooling of zebra finch (Taeniopygia guttata) eggs has been shown to reduce embryonic growth efficiency (Olson et al. 2006), and suboptimal incubation conditions affect hatchling mass and growth in zebra finches (Gorman et al. 2005) and lead to reduced growth in blue tits (Parus caeruleus; Nilsson et al. 2008) and body condition in Northern lapwings (Vanellus vanellus; Larsen et al. 2003). Previously, we experimentally heated nests of the tree swallow (Tachycineta bicolor) during incubation and found that (i) breeding females increase investment during incubation by increasing time spent incubating and raising egg temperatures (Ardia et al. 2009), and (ii) heating nests during incubation leads to direct and indirect carry-over effects on nestling mass and condition (Pérez et al. 2008).
In this study, we reverse the direction of our manipulation and cool nests during incubation. We first examine how experimental cooling affects incubation investment by examining changes in time spent incubating and in egg temperature. Maintenance of proper egg temperature is critical as egg temperature levels affect rewarming costs for females (Reid et al. 2002; Voss et al. 2006) and developmental conditions for embryos (Olson et al. 2006). A proper consideration of egg temperature may reflect incubation investment more effectively than time spent incubating on its own, as the temperature of eggs reflects the actual energetic transfer of heat. We predicted that experimental cooling will lead to lower-quality developmental conditions, reflected in both decreased time spent incubating and decreased egg temperatures, with the consequence of extending the incubation period.
We then examined how modifying incubation conditions would affect nestling quality and parental behaviour. To separate the direct effects of the cooling treatment on offspring from the indirect effects that may arise from effects of our manipulation on breeding females, we partially cross-fostered nestlings between paired cooled and control nests. In addition to examining nestling condition and female behaviour, we examined how our manipulation affected innate constitutive immunity, reflected in bacteria killing ability (BKA) of blood, a first defence against microbial pathogens (Millet et al. 2007). We predicted that decreased incubation investment would have carry-over effects on nestling immunity, even in cooled embryos raised as nestlings by control females. In addition, we predicted that offspring cooled as embryos would maintain lower residual body mass (i.e. body condition) than control nestlings, regardless of the experimental treatment of the female that fed them during the nestling period.
2. Material and methods
(a). General field methods and nest cooling experiment
The experiment was conducted from May to July 2007 in Amherst, MA (42°22′ N, 72°31′ W), and followed the monitoring procedure and feeding observation protocol of Ardia et al. (2009; see the electronic supplementary material). Females were allocated to two treatment groups that were balanced for clutch initiation date and clutch size: cooled nests and control nests. Balance between treatments persisted during the nestling period, including brood size. For each cooled nest, we installed a Peltier thermoelectric cooling device (All Electronics Corporation, Van Nuys, CA) connected to a 115 amp-hour deep cycle marine battery (Kirkland Signature, Issaquah, WA) stored in a plastic tub beneath the nest-box on incubation day 6. In order to remove heat from the heat sink, fans were installed on the heated side of the Peltier devices. The cooling device operated for 15 minutes of every hour. The Peltier devices were placed in three-sided protective wooden shelters, which were installed on all boxes before the breeding season, after removing the adjacent side of the nest-box to facilitate air flow from Peltier devices. Control nests also had sides removed and received Peltier devices, in which only the fans operated so as to mimic any potential disturbance associated with placement of coolers and noise disturbance from fans. Coolers were removed on incubation day 10. All females were recaptured 12–13 days after clutch completion (2–3 days after removal of coolers) to measure change in body mass. Insect availability was recorded using a 2 m aerial insect sampler powered by a Robbins and Myers 1650 r.p.m. (12.95 m s−2) 1/12 HP motor (Dayton, OH; McCarty & Winkler 1999) to collect daily samples of aerial insect abundance during the breeding season (additional information in the electronic supplementary material).
Eighteen pairs of nests with the same hatch date were subjected to a split-nest partial cross-fostering experiment. On nestling day 3 (hatching = day 0), we cross-fostered nestlings such that each nest had a combination of its own chicks and those from the other nest. For more information on cross-fostering, see the electronic supplementary material. Chicks were individually marked using coloured nail polish applied to the toes on the day of hatching. Nestling measurements were taken on days 1, 4, 7, 10 and 13 after hatching using a digital balance for mass (±0.1 g) and digital calipers for skull length (±0.01 mm). Beginning on nestling day 4, the tarsus was measured (±0.01 mm), and on day 7 wing length to the tip of the longest pin feather was measured (±0.5 mm).
(b). Incubation behaviour and egg temperature
Once a breeding attempt was discovered, a datalogger (Thermochron iButton DS1921, Dallas Semiconductor, Dallas, TX; accuracy ±1.0°C) was placed in each nest cup adjacent to the eggs, so as to avoid interfering with heat transfer among eggs. This datalogger was set to record temperature at 4 min intervals in order to monitor incubation behaviour of females (more information in the electronic supplementary material). Incubation behaviour was characterized as percentage of time spent incubating eggs and was analysed for three periods: (i) a 48 h period on incubation days 4–5, prior to allocating them to treatment groups; (ii) a 96 h period on incubation days 6–10 during the cooling (or control) treatment; and (iii) a 48 h period on incubation days 10–12, post-treatment. The program Rhythm (1.0) was used to determine off-bouts after a visual rechecking of the output, with a minimum off-bout duration of 5 min and a minimum off-bout change in temperature of 4°C (Cooper & Mills 2005). Total time spent incubating (%) was calculated by subtracting the time consumed by off-bouts from the total period under observation.
Egg temperature was measured by placing an artificial egg in nests for a 24 h period between incubation days 9–10, the last day of the cooling treatment. A 13-mm-long plastic egg (Berenice's Crafts, www.berenicecrafts.com) was filled with wire-pulling lubricant (ClearGlide, Ideal Industries, Sycamore, IL). This fluid closely mimics the thermal properties of an egg (M. A. Voss 2005, unpublished data). In the centre of each plastic egg, the probe of a HOBO U12 type-T thermocouple thermometer (Onset Corporation, Bourne, MA) was placed. Two measures of incubation egg temperature were measured: average temperature during on-bouts and average temperature during off-bouts (Ardia et al. 2009).
(c). Bacteria killing ability
From day 13 nestlings, we collected blood from the brachial artery into heparinized microcapillary tubes and stored the blood on ice until returning to the laboratory; all samples were run within 90 min of blood collection (Matson et al. 2006). Just prior to assays, samples were diluted 1 : 20 (in CO2-independent media; Gibco no. 18045, Carlsbad, CA). A standard number (approx. 150) of colony-forming units (CFUs) of Escherichia coli (ePower Microorganisms no. 0483E7, MicroBio-Logics, St Cloud, MN; ATCC no. 8739) was added to each sample (ratio 1 : 10). Plasma–bacteria mixtures were then incubated for 45 min at 41°C, and plated in duplicate onto tryptic-soy agar using sterile technique. We used two agar-filled Petri dishes inoculated with diluted bacteria as positive controls and two agar-filled plates swabbed with a sterile bacteria spreader as negative controls. All plates were incubated at 41°C overnight. To quantify BKA, total CFUs on each plate were counted and the average of duplicates was divided by the average of the positive control replicates for that assay run. No negative controls contained CFUs.
(d). Statistical analyses
Before beginning analyses, variables were tested for assumptions of normality using Shapiro–Wilk's W (all variables W ≥ 0.98, p ≥ 0.23). Effect of cooling treatment on internal nest-box temperature was assessed by comparing the change in temperature following placement of cooler with the difference in temperature between paired boxes prior to placement of cooling devices, owing to slight variation in temperature among nest-boxes at the site (Ardia et al. 2006). Change in incubation behaviour (percentage of time incubating, on-bout and off-bout duration) was tested by examining change in incubation behaviour over time (pre-treatment, treatment, post-treatment) using repeated-measures ANOVA (SAS 1988), with stage of incubation period as the repeated measure for each female. General linear models (PROC GLMs; SAS 1988) were used to examine factors affecting incubation period and egg temperatures. For all models, the following covariates were included: clutch initiation date, clutch size, average ambient temperature, insect availability and female age (SY versus ASY). We examined factors affecting change in body mass and feeding behaviour using GLMs with the following covariates: brood size, clutch initiation date, average temperature in the previous 24 h, and initial body mass prior to cooling treatment. The direct and indirect effects of our cooling treatment on nestling body mass, condition and BKA were assessed using a general linear mixed model (PROC MIXED; SAS 1988), with nest of origin and nest of rearing as random factors, and the following fixed effects: clutch initiation date, average temperature in the previous 24 h, clutch size and female age (both mother and foster mother). For mixed models, we used Satterthwaite denominator degrees of freedom. For all models, all two-way interaction terms were included initially in each model and then removed sequentially by highest p-value for those interactions with p > 0.20; removal of interactions did not change the significance of main effects. Except where noted, means are reported as least-square means (LSM) with standard errors, which are calculated to include the effects of covariates. Differences were considered statistically significant at p < 0.05.
3. Results
(a). Effects on incubation behaviour and egg temperature
Experimental cooling reduced nest-box temperatures relative to control boxes (average change in temperature 5.9°C ± 0.4, n = 18). Cooled boxes had lower internal temperatures than control boxes (t34 = 32.1, p < 0.001; mean internal temperature ± s.d., °C: cooled boxes 12.7 ± 1.5, maximum 19.7, minimum 3.3, n = 18; control boxes 18.6 ± 1.0, maximum 20.2, minimum 12.7, n = 18).
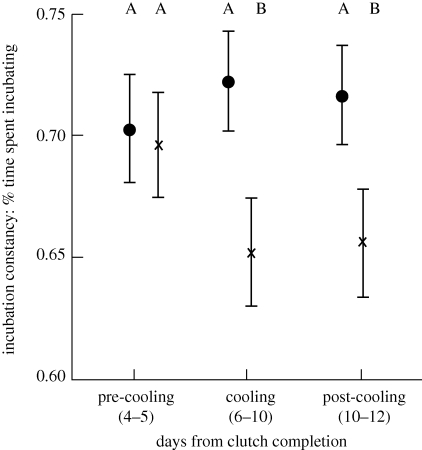
Cooled females reduced the time spent incubating following experimental cooling, while control females showed no change in time spent incubating (repeated measures GLM, effect of cooling: F2,64 = 7.8, p < 0.001; figure 1). Females subjected to experimental cooling continued to show reduced time incubating even after the cooling treatment was removed, while control females maintained similar incubation time over the same period (repeated measures GLM, interaction between time and cooling treatment: F2,64 = 8.4, p < 0.001; figure 1). Clutch initiation date, clutch size, residual body mass, ambient temperature, insect availability and female age had no effect on how females responded to the cooling treatment (all F1,29 < 2.5, p > 0.11).
Figure 1.
The effect of experimental cooling of nest-boxes on time spent incubating of female tree swallows. Values represent LSM correcting for covariates; error bars represent standard error. Letters refer to significant differences between groups. Control treatment, n = 17; cooled treatment, n = 18. Black dot, control; cross, cooled.
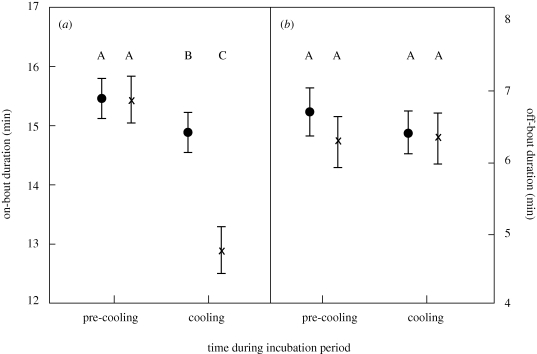
Experimental cooling also changed the average duration of on-bouts, leading to shorter on-bouts in cooled females (repeated measures GLM: F1,29 = 11.4, p = 0.002; figure 2), but there was no change in off-bout duration (repeated measures GLM: F1,29 = 1.3, p = 0.26; figure 2). Clutch initiation date, clutch size, residual body mass, ambient temperature, insect availability and female age had no effect on changes in on-bouts and off-bouts in response to the cooling treatment (all F1,29 < 2.7, p > 0.11). The decrease in on-bouts in response to cooling led to an increase in the total number of incubation bouts per day in cooled females (46.6 versus 41.7).
Figure 2.
The effect of experimental cooling of nest-boxes on (a) on-bout and (b) off-bout durations during incubation of female tree swallows. Values represent LSM correcting for covariates; error bars represent standard error. Letters refer to significant differences between groups. Control treatment, n = 17; cooled treatment, n = 18. Black dot, control; cross, cooled.
Embryonic development conditions, measured as egg temperatures, changed in response to experimental cooling. Cooled females maintained lower average on-bout egg temperatures (temperature ± s.e., °C: control, 35.8 ± 0.21; cooled, 34.5 ± 0.32; GLM: F1,29 = 26.9, p < 0.001) and off-bout egg temperatures (temperature ± s.e., °C: control, 30.7 ± 0.42; cooled, 29.2 ± 0.41; GLM: F1,30 = 43.2, p < 0.001). Cooled females with higher residual body mass (body condition) were less affected by cooling, as reflected in higher average on-bout egg temperatures (interaction between treatment and residual body mass: β = −0.13, F1,28 = 5.2, p < 0.03), but there was no effect of residual body mass on off-bout egg temperatures (F1,29 = 1.1, p = 0.27). Ambient temperature had no effect on on-bout egg temperatures (F1,28 = 1.2, p = 0.28), but off-bout egg temperatures were higher when ambient temperatures were warmer (F1,29 = 4.5, p = 0.04). Clutch initiation date, clutch size, insect availability and female age (all F1,28 < 2.2, p > 0.15) had no effect on changes in on-bouts and off-bouts in response to the cooling treatment.
Experimental cooling extended incubation period (days ± s.e.; control, 14.1 ± 0.18; cooled, 14.9 ± 0.21; GLM: F1,30 = 13.9, p = 0.001).
Females incubating in cooled boxes lost absolute body mass between the incubation and the early nestling period, while control females did not (change in body mass, g: control females, 0.25 ± 0.17, n = 17; cooled females, −1.8 ± 0.99, n = 18; F1,34 = 11.9, p = 0.001, partial r2 = 0.39). Initial body mass was retained as a covariate in the model predicting change in body mass (β = −0.37, F1,34 = 9.1, p = 0.004, partial r2 = 0.28); all other covariates were dropped from the model. We found the same trend when we compared change in residual body mass, a measure of body condition. Cooled females lost body condition between day 3 of incubation and the end of the incubation period, while control females showed no change in body condition (change in residual body mass, g: control, −0.10 ± 0.84, n = 17; cooled, −1.41 ± 1.96, n = 18; F1,35 = 7.2, p = 0.01, partial r2 = 0.22).
(b). Effect of cooling during the nestling period
By pairing nests for cross-fostering and feeding observations, we were able to minimize environmental variation between treatment groups. First, we used a paired-samples t-test to test whether paired nests differed in their feeding rates. Control females made 3.27 ± 0.80 s.e. more feeding visits per 50 min observation to 8-day-old nestlings and 3.17 ± 1.25 s.e. more to 13-day-old nestlings than did cooled females (day 8: t17 = 4.1, p = 0.001, n = 18; day 13: t16 = 2.5, p = 0.02, n = 17; average feeding visits per observation: day 8, control 7.9, cooled 4.6; day 13, control 8.3, cooled 5.1). Control and cooled males showed no difference in feeding rate (day 8: t17 = 1.3, p = 0.19; day 13: t16 = 0.8, p = 0.45). We then tested factors affecting the total number of parental feeding visits (female + male) by combining ages and including nest identity as a random factor. Nestlings raised in nests cooled during incubation received fewer visits (F1,66 = 5.3, p = 0.02). Feeding rates increased as ambient temperatures increased (F1,66 = 4.4, p = 0.03) and in larger broods (F1,66 = 4.8, p = 0.03). There was no difference in feeding between days 8 and 13 (F1,66 = 0.5, p = 0.48) and no effect of female age (F1,66 = 0.7, p = 0.41) or clutch initiation date (F1,66 = 1.6, p = 0.21).
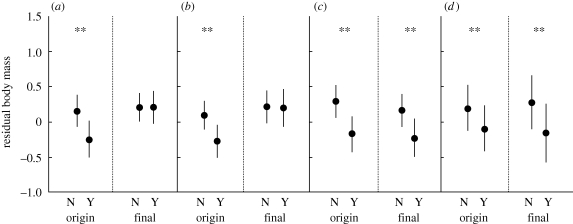
A mixed model GLM comparing the effects of cooling treatment on the box of origin and the box of rearing revealed direct effects of cooling on nestling residual body mass. Nestlings that were exposed to experimental cooling as embryos had lower residual body mass and absolute body mass on days 4 and 7 (figure 3; table A1 in the electronic supplementary material). There was also an indirect effect of cooling on nestlings through absolute body mass; nestlings fed by females that were experimentally cooled during the incubation period were smaller in body mass on days 10 (mass ± s.e., g: control, 20.1 ± 0, n = 66; cooled, 18.9 + 0.43, n = 70) and 13 (control, 20.5 ± 0.04, n = 66; cooled, 19.4 ± 0.06, n = 70), with a slight difference at day 7 (table A1 in the electronic supplementary material).
Figure 3.
Effect of experimental cooling on the residual body mass of nestling tree swallows following cross-fostering manipulation intended to separate the direct and indirect effects of cooling nest-boxes during incubation. Residual body mass calculated as the residuals of a regression of head–bill length on body mass. Origin refers to treatment experienced during the incubation period. Rearing refers to the treatment experienced by rearing female. N = not cooled (control), Y = cooled. **p < 0.05. (a) Day 4, (b) day 7, (c) day 10 and (d) day 13. See text for more detail on model construction.
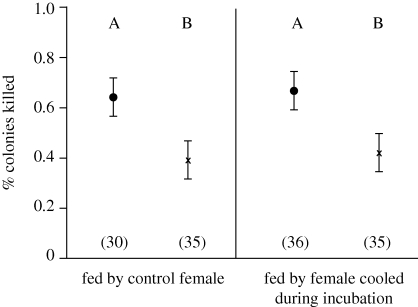
Experimental cooling had only direct effects on innate immunity of nestlings, assessed through BKA. Nestlings that experienced experimental cooling as embryos had lower ability to kill bacteria (mixed GLM treatment at origin: F1,34.2 = 26.2, p < 0.001; figure 4), while the treatment experienced by the rearing female had no effect (treatment at final nest: F1,36.3 = 0.88, p = 0.34; figure 4). This effect is observed while controlling for nestling residual body mass (F1,36.7 = 1.48, p = 0.23) and parental feeding rate (F1,38.2 = 1.85, p = 0.18), neither of which predicted nestling BKA. In addition, incubation period had no direct effect on innate immunity (F1,37.8 = 1.67, p = 0.20). Clutch initiation date, clutch size and female age also had no effect on BKA of nestlings (all F < 1.00, p > 0.36).
Figure 4.
Effect of experimental cooling on the ability of nestling tree swallow blood to kill E. coli measured as the percentage of colonies killed relative to controls. Values represent LSM correcting for covariates; error bars represent standard error. Letters refer to significant differences between groups. See text for more detail on laboratory methods and model construction. Black dot, control; cross, cooled.
4. Discussion
Increasing energetic costs during incubation revealed effects during incubation and significant carry-over effects on the nestling stage. Experimental cooling led to changes in incubation behaviour and lower-quality embryonic development conditions, as reflected in egg temperatures and time spent incubating, while nestlings cooled as embryos had lower innate immunity, reflected in BKA, and reduced body condition. These results indicate that the influence of environmental conditions and parental investment on developmental conditions in one life stage can have profound effects on later offspring phenotype.
Breeding female tree swallows responded to increased incubation costs by modifying incubation investment. Incubation investment can be parcelled into three general components, all of which were affected by our manipulation: total time spent incubating, the distribution of that time into incubation bouts and the actual egg temperature maintained during incubation. Our results support the growing evidence that incubation is costly and investment in incubation is probably constrained by a mix of strategic adjustment and energetic constraint (Cresswell et al. 2003; de Heij et al. 2006; Ardia & Clotfelter 2007).
First, we found that cooled females reduced time spent incubating relative to controls, consistent with results from our previous work, where wing feather clipping led to decreased incubation (Ardia & Clotfelter 2007) and experimental heating led to increased incubation (Ardia et al. 2009). Reduction of incubation time can reduce costs by decreasing the total time spent maintaining development conditions, but the cost of rewarming eggs may increase owing to increased time off eggs (Biebach 1986; Reid et al. 2000), especially if the number of total incubation bouts changes. Second, we found that cooled females had shorter on-bouts than did control females, but not longer off-bouts. Decreased on-bouts in cooled females suggest that bout duration is determined by an energy threshold and when that threshold is reached a bout ends (Haftorn & Ytreberg 1988; Weathers & Sullivan 1989; Chaurand & Weimerskirch 1994; Reid et al. 1999). Similar off-bouts between control and cooled females are consistent with this interpretation, as similar time feeding may be required if similar energy levels are expended. In addition, off-bout duration may also be constrained in order to minimize rewarming costs (Vleck 1981; Biebach 1986). A consequence of reducing on-bout duration is that cooled females increased the total number of incubation bouts over the day, increasing costs to females through a greater number of rewarming events and to embryos through greater irregularity in developmental conditions.
Lastly, we found that cooled females maintained lower egg temperatures during on-bouts, suggesting that increased costs of maintaining egg temperatures led females to maintain lower egg temperature than controls. In addition, egg temperatures during off-bouts were lower in cooled nests, probably because of lower starting points owing to lower on-bout temperatures and increased thermal loss owing to cooler nest temperatures. These results provide further demonstration of egg temperature as a parallel and independent pathway for females to modify incubation investment (Ardia & Clotfelter 2007; Ardia et al. 2009). Cooled females faced modified resource allocation trade-offs and responded by decreasing embryonic development conditions. The use of egg temperature to modify incubation investment is probably a mix of strategy and constraint. Cooled females with greater body condition reduced egg temperatures less than other cooled females. This suggests that, assuming condition is reflective of quality, higher-quality females are better able to deal with our manipulation, as a simple energetic trade-off would result in a negative correlation between condition and egg temperature. This strategic adjustment is consistent with our previous work, where higher quality individuals were less affected by feather clipping (Ardia & Clotfelter 2007). However, our results also show evidence of energetic constraints as females subjected to the cooling treatment continued to show a reduction in time spent incubating after the cooling apparatus was removed and had reduced feeding effort during the nestling period, both of which suggest a reduction in energetic reserves. However, without additional manipulations we cannot rule out a strategic adjustment by females in response to a perceived reduction in offspring quality that may have occurred during the manipulation period.
For developing embryos, egg temperature has the strongest effect on embryonic development as incubating parents provide the majority of heat needed to fuel metabolism and development. Here, we report that cooling extends incubation periods by almost a full day compared with control nests. Across species, lower egg temperatures are correlated with longer incubation periods (Martin 2002). Within a species, reducing temperatures leads to extended incubation periods. For example, artificial incubation of chicken eggs (38°C versus 35–36°C) leads to extended incubation periods (1–2 days of 21 days) and reduced body weight (Mortola 2006). Extended incubation can lead to decreased size at hatch and reduced yolk reserves (D. R. Ardia 2008, unpublished data), an additional cost of lowering developmental temperatures.
Most importantly, we found evidence for both direct and indirect effects of cooling on offspring quality. Nestlings that were incubated in cooled nests showed reduced innate constitutive immunity, reflected in a reduced ability to kill a strain of E. coli. Partial cross-fostering revealed that this pattern was due to the direct effect of cooling, since nestlings cooled as embryos (regardless of the treatment group of the female that fed them as nestlings) had lower BKA. BKA gives insight into the ability of individuals to kill invasive bacteria and represents a general integrative measure of cellular immune components (Millet et al. 2007). Thus, reduced innate immunity suggests a significant reduction in ‘immunocompetence’ and reflects a significant potential consequence of reduced developmental conditions. This is an important result because variation in egg temperature (and thus developmental conditions) is an important component of life-history variation within and among species (Martin et al. 2007; Martin & Schwabl 2008). Our study is the first, to our knowledge, to show carry-over effects of cooling in wild birds. Domestic chickens cooled as embryos show reduced haematocrit and oxygen carrying ability of blood (Black & Burggren 2004) and increased myonuclei (Hammond et al. 2007) as chicks.
The mechanism by which modifying developmental temperature affects innate immunity is unclear. Even though cooling also led to lower body condition and body mass, there was no direct link between condition and innate immunity in individual chicks, suggesting that the effect of cooling is developmental and physiological, rather than simply energetic. Little is known about the ontogeny of immunity in altricial birds (Apanius 1998; Ardia & Schat 2008). A partial cross-fostering experiment in tree swallows with no temperature manipulation revealed that BKA is best predicted by nest of origin, indicating a strong heritable or maternal-effects link (Morrison et al. 2009). Preliminary investigations modifying developmental temperatures during incubation in the laboratory suggest that innate immunity is limited even in full-term embryos (A. Ackerman & D. Ardia 2009, unpublished data), implying that the direct effects of cooling are manifested as the immune system develops after hatch. Timing of cooling has been shown to be critical in affecting other physiological traits in precocial birds (Nichelmann 2004; Tzschentke 2008). It is important to note that we report effects from a single strain of bacteria. Although BKA shows strong patterns of variation with other life-history traits (Matson et al. 2006; Buehler et al. 2008; Rubenstein et al. 2008), it is not clear how the ability to kill one strain relates to other strains and species of microbes. A contributing factor to the pattern we report here could be differences in brood competition that might arise owing to size differences caused by cooling. Average body mass and variation in body mass were kept the same in each nest, but differences in competitive ability may have still occurred. If this is the case, then it is probably a contributory factor but cannot alone explain the differences we report, as growth and condition are not the strongest predictors of innate immunity (Morrison et al. 2009). More research is needed to understand the ontogeny of innate immunity, especially under controlled conditions.
Nestlings that were incubated as eggs in cooled nests were in lower body condition and smaller mass throughout the nestling period, regardless of the treatment group of the female that reared them (i.e. their mother or foster mother). Lower energy reserves from hatching owing to sub-optimal development conditions could have handicapped nestlings. Later in the nestling period, nestlings fed by cooled mothers (even those who experienced control conditions as embryos) had lower body mass. Here we report that the indirect effect on nestlings of cooling their nest of rearing is revealed later in the nestling period. Parental provisioning rates have a significant effect on nestling mass and growth rate later in the nestling period (Henderson & Hart 1993; Ricklefs et al. 1994). These results suggest that cooling nest-boxes served to reduce female condition during the incubation period, thus altering the trade-off between self-maintenance and parental investment later in the nestling period, leading to reduction in nestling condition. Our results of direct (nest of origin) and indirect (nest of rearing) effects on older nestlings are in contrast to results from our heating experiment, where only nest of rearing effects were found in older nestlings (Pérez et al. 2008).
The difference in the effect of cooling versus heating could be due to the role of egg temperature in driving embryonic development (Hepp et al. 2006; Olson et al. 2006; Martin et al. 2007). For example, Olson et al. (2006) found that cooled zebra finch (Poephilia gutatta) eggs produced smaller embryos that grew less efficiently, which increased the duration of incubation period. Cooled eggs in their study consumed more yolk over the same developmental period than control eggs, and yolk consumption was proportional to temperature. This reduced growth efficiency may have carried over to the nestling period and led to possible trade-offs between growth rate and BKA, as differences in body mass disappear later in the nestling stage, similar to the effects of heating (Pérez et al. 2008).
Our results demonstrate clearly that embryonic conditions experienced during incubation can affect nestling quality and condition throughout nestling development. As incubating females modify their investment based on environmental conditions, the consequences to offspring are significant and, our results indicate, are manifested in later stages. Thus, as parents adjust life-history trade-offs in a single stage in response to changes in conditions and strategies, the long-term effects on their offspring may be important. The findings of this study are also important to our understanding of life-history evolution. It is becoming increasingly clear that parental condition at one reproductive stage can have cascading consequences for future stages (Gorman & Nager 2004; Naguib & Gil 2005), and our results are consistent with this idea. They reveal that conditions experienced in one life-history stage (incubation) can carry over into future life-history stages, such as the nestling period. If the stages of reproduction are indeed linked in potentially complex ways, then the ramifications of environmental variations and developmental conditions that were originally thought to affect only a single stage may be much wider than previously appreciated. Our results reveal that microclimate can have strong effects on offspring condition, either directly on development or indirectly by influencing the investment trade-offs of parents. In light of potential global climate change in the near future, it is essential that the reproductive and demographic ramifications of local temperature variation be studied (Martin et al. 2007).
Acknowledgements
Our work was conducted with the approval of the Institutional Animal Care and Use Committees of Franklin & Marshall College and Amherst College.
We thank Alyssa Ackerman, Michael Gleicher and Shaylon Stolk for essential field assistance; Elizabeth Rice, Ben Taft and Natasha Korobov for assistance in erecting nest-boxes; and Jim Brassord, Bob Shea and Ron Hebert for logistical support. Funding for the research was provided by Hackman Summer Scholars Program at Franklin & Marshall College and the Axel Schupf '57, Phyllis Mofson '84 and Webster Funds of Amherst College.
References
- Apanius V.1998Ontogeny of immune function. In Avian growth and development (eds Starck J., Ricklefs R. E.), pp. 203–222 New York, NY: Oxford University Press [Google Scholar]
- Ardia D. R., Clotfelter E. D.2007Individual quality and age affect responses to an energetic constraint in a cavity-nesting bird. Behav. Ecol. 18, 259–266 (doi:10.1093/beheco/arl078) [Google Scholar]
- Ardia D. R., Schat K. A.2008Ecoimmunology. In Avian immunology (eds Davison F., Kaspers B., Schat K. A.), pp. 421–441 London, UK: Academic Press [Google Scholar]
- Ardia D. R., Perez J. H., Clotfelter E. D.2006Nest box orientation affects internal temperature and nest site selection by Tree Swallows. J. Field Ornithol. 77, 339–344 (doi:10.1111/j.1557-9263.2006.00064.x) [Google Scholar]
- Ardia D. R., Perez J. H., Chad E. K., Voss M. A., Clotfelter E. D.2009Temperature and life history: experimental heating leads female tree swallows to modulate egg temperature and incubation behavior. J. Anim. Ecol. 78, 4–13 (doi:10.1111/j.1365-2656.2008.01453.x) [DOI] [PubMed] [Google Scholar]
- Biebach H.1986Energetics of rewarming a clutch in starlings (Sturnus vulgaris). Physiol. Zool. 59, 69–75 [Google Scholar]
- Black J. L., Burggren W. W.2004Acclimation to hypothermic incubation in developing chicken embryos (Gallus domesticus): II. Hematology and blood O2 transport. J. Exp. Biol. 207, 1553–1561 (doi:10.1242/jeb.00910) [DOI] [PubMed] [Google Scholar]
- Buehler D. M., Piersma T., Matson K., Tieleman B. I.2008Seasonal redistribution of immune function in a migrant shorebird: annual-cycle effects override adjustments to thermal regime. Am. Nat. 172, 783–796 (doi:10.1086/592865) [DOI] [PubMed] [Google Scholar]
- Chaurand T., Weimerskirch H.1994Incubation routine, body mass regulation and egg neglect in the Blue Petrel Halobaena caerulea. Ibis 136, 285–290 (doi:10.1111/j.1474-919X.1994.tb01097.x) [Google Scholar]
- Conway C. J., Martin T. E.2000Evolution of passerine incubation behavior: influence of food, temperatures, and nest predation. Evolution 54, 670–685 (doi:10.1111/j.0014-3820.2000.tb00068.x) [DOI] [PubMed] [Google Scholar]
- Cooper C. B., Mills H.2005New software for quantifying incubation behavior from time-series recordings. J. Field Ornithol. 76, 352–356 [Google Scholar]
- Cresswell W., Holt S., Reid J. M., Whitfield D. P., Mellanby R. J.2003Do energetic demands constrain incubation scheduling in a biparental species? Behav. Ecol. 14, 97–102 (doi:10.1093/beheco/14.1.97) [Google Scholar]
- de Heij M. E., van den Hout P. J., Tinbergen J. M.2006Fitness cost of incubation in great tits (Parus major) is related to clutch size. Proc. R. Soc. B 273, 2353–2361 (doi:10.1098/rspb.2006.3584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman H. E., Nager R. G.2004Prenatal developmental conditions have long-term effects on offspring fecundity. Proc. R. Soc. Lond. B 271, 1923–1928 (doi:10.1098/rspb.2004.2799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman H. E., Orr K. J., Adam A., Nager R. G.2005Effects of incubation conditions and offspring sex on embryonic development and survival in the zebra finch (Taeniopygia guttata). Auk 122, 1239–1248 (doi:10.1642/0004-8038(2005)122[1239:EOICAO]2.0.CO;2) [Google Scholar]
- Haftorn S., Ytreberg N. J.1988Incubation rhythm in the pied flycatcher Ficedula hypoleuca. Fauna Norveg. Ser. C. Cinclus 11, 71–88 [Google Scholar]
- Hammond C. L., Simbi B. H., Stickland N. C.2007In ovo temperature manipulation influences embryonic motility and growth of limb tissues in the chick (Gallus gallus). J. Exp. Biol. 210, 2667–2675 (doi:10.1242/jeb.005751) [DOI] [PubMed] [Google Scholar]
- Hanssen S. A., Hasselquist D., Folstad I., Erikstad K. E.2005Cost of reproduction in a long-lived bird: incubation effort reduces immune function and future reproduction. Proc. R. Soc. B 272, 1039–1046 (doi:10.1098/rspb.2005.3057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney V., Monaghan P.1996Optimal allocation of effort between reproductive phases: the trade-off between incubation costs and subsequent brood rearing capacity. Proc. R. Soc. Lond. B 263, 1719–1724 (doi:10.1098/rspb.1996.0251) [Google Scholar]
- Henderson I. G., Hart P. J. B.1993Provisioning, parental investment and reproductive success in Jackdaws Corvus monedula. Ornis Scand. 24, 142–148 (doi:10.2307/3676364) [Google Scholar]
- Hepp G. R., Kennamer R. A., Johnson M. H.2006Maternal effects in wood ducks: incubation temperature influences incubation period and neonate phenotype. Funct. Ecol. 20, 307–314 [Google Scholar]
- Larsen V. A., Lislevand T., Byrkjedal I.2003Is clutch size limited by incubation ability in northern lapwings. J. Anim. Ecol. 71, 784–792 (doi:10.1046/j.1365-2656.2003.00751.x) [Google Scholar]
- Martin T. E.2002A new view of avian life-history evolution tested on an incubation paradox. Proc. Natl Acad. Sci. 269, 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. E., Schwabl H.2008Variation in maternal effects and embryonic development rates among passerine species. Phil. Trans. R. Soc. B 363, 1663–1674 (doi:10.1098/rstb.2007.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. E., Auer S. K., Bassar R. D., Niklison A. M., Lloyd P.2007Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution 61, 2558–2569 (doi:10.1111/j.1558-5646.2007.00204.x) [DOI] [PubMed] [Google Scholar]
- Matson K. D., Tieleman B. I., Klasing K. C.2006Capture stress and the bactericidal competence of blood and plasma in five species of tropical birds. Physiol. Biochem. Zool. 79, 556–564 (doi:10.1086/501057) [DOI] [PubMed] [Google Scholar]
- McCarty J. P., Winkler D. W.1999Relative importance of environmental variables in determining the growth rates of nestling tree swallows Tachycineta bicolor. Ibis 141, 286–296 (doi:10.1111/j.1474-919X.1999.tb07551.x) [Google Scholar]
- Millet S., Bennet J., Lee K. A., Hau M., Klasing K. C.2007Quantifying and comparing constitutive immunity across avian species. Dev. Comp. Immunol. 31, 188–201 (doi:10.1016/j.dci.2006.05.013) [DOI] [PubMed] [Google Scholar]
- Morrison E., Ardia D. R., Clotfelter E. D.2009Cross-fostering reveals sources of variation in innate immunity and hematocrit in nestling tree swallows. J. Avian Biol. 40, 573–578 (doi:10.1111/j.1600-048X.2009.04910.x) [Google Scholar]
- Mortola J. P.2006Metabolic response to cooling temperatures in chicken embryos and hatchlings after cold incubation. Comp. Biochem. Physiol. A Mol. Integrat. Physiol. 145, 441–448 (doi:10.1016/j.cbpa.2006.07.020) [DOI] [PubMed] [Google Scholar]
- Naguib M., Gil D.2005Transgenerational effects on body size caused by early developmental stress in zebra finches. Biol. Lett. 1, 95–97 (doi:10.1098/rsbl.2004.0277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichelmann M.2004Perinatal epigenetic temperature adaptation in avian species: comparison of turkey and muscovy duck. J. Thermal Biol. 29, 613–619 (doi:10.1016/j.jtherbio.2004.08.032) [Google Scholar]
- Nilsson J. F., Stjernman M., Nilsson J. A.2008Experimental reduction of incubation temperature affects both nestling and adult blue tits Cyanistes caeruleus. J. Avian Biol. 39, 553–559 (doi:10.1111/j.0908-8857.2008.04199.x) [Google Scholar]
- Olson C. R., Vleck C. M., Vleck D.2006Periodic cooling of bird eggs reduces embryonic growth efficiency. Physiol. Biochem. Zool. 79, 927–936 (doi:10.1086/506003) [DOI] [PubMed] [Google Scholar]
- Pérez J. H., Ardia D. R., Chad E. K., Clotfelter E. D.2008Experimental heating reveals nest temperature affects nestling condition in tree swallows (Tachycineta bicolor). Biol. Lett. 4, 468–471 (doi:10.1098/rsbl.2008.0266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. M., Monaghan P., Ruxton G. D.1999The effect of clutch cooling rate on starling, Sturnus vulgaris, incubation strategy. Anim. Behav. 58, 1161–1167 (doi:10.1006/anbe.1999.1241) [DOI] [PubMed] [Google Scholar]
- Reid J. M., Monaghan P., Ruxton G. D.2000Resource allocation between reproductive phases: the importance of thermal conditions in determining the cost of incubation. Proc. R. Soc. Lond. B 267, 37–41 (doi:10.1098/rspb.2000.0963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. M., Ruxton G. D., Monaghan P., Hilton G. M.2002Energetic consequences of clutch temperature and clutch size for a uniparental intermittent incubator: the starling. Auk 119, 54–61 (doi:10.1642/0004-8038(2002)119[0054:ECOCTA]2.0.CO;2) [Google Scholar]
- Ricklefs R. E., Shea R. E., Choi I. H.1994Inverse relationship between functional maturity and exponential growth rate of avian skeletal muscle—a constraint on evolutionary response. Evolution 48, 1080–1088 (doi:10.2307/2410368) [DOI] [PubMed] [Google Scholar]
- Rubenstein D. R., Parlow A. F., Hutch C. R., Martin L. B.2008Environmental and hormonal correlates of immune activity in a cooperatively breeding tropical bird. Gen. Comp. Endocrinol. 159, 10–15 (doi:10.1016/j.ygcen.2008.07.013) [DOI] [PubMed] [Google Scholar]
- SAS 1988SAS/STAT user's guide Cary, NC: SAS Institute [Google Scholar]
- Stearns S. C.1992The evolution of life histories Oxford, UK: Oxford University Press [Google Scholar]
- Tzschentke B.2008Monitoring the development of thermoregulation in poultry embryos and its influence by incubation temperature. Comput. Electron. Agricult. 64, 61–71 (doi:10.1016/j.compag.2008.05.003) [Google Scholar]
- Vleck C. M.1981Hummingbird incubation—female attentiveness and egg temperature. Oecologia 51, 199–205 (doi:10.1007/BF00540601) [DOI] [PubMed] [Google Scholar]
- Voss M. A., Hainsworth F. R., Ellis-Felege S. N.2006Use of a new model to quantify compromises between embryo development and parental self-maintenance in three species of intermittently incubating passerines. J. Thermal Biol. 31, 453–460 (doi:10.1016/j.jtherbio.2006.03.002) [Google Scholar]
- Weathers W. W., Sullivan K. A.1989Nest attentiveness and egg temperature in the yellow-eyed junco. Condor 91, 628–633 (doi:10.2307/1368113) [Google Scholar]