Abstract
Bradoriids are small bivalved marine arthropods that are widespread in rocks of Cambrian to Early Ordovician age. They comprise seven families and about 70 genera based on shield (‘carapace’) morphology. New bradoriid specimens with preserved soft-part anatomy of Kunmingella douvillei (Kunmingellidae) are reported from the Early Cambrian Chengjiang Lagerstätte of China together with, for the first time to our knowledge, a second bradoriid species with preserved soft parts, Kunyangella cheni (Comptalutidae). Kunmingella douvillei has a 10-segmented limb-bearing body with uniramous ninth and tenth appendages and a series of homogeneous, apparently (proximal parts not preserved) unspecialized post-antennal biramous limbs with setose leaf-shaped exopods. Each endopod consists of five podomeres. A presumed penultimate instar of Ky. cheni preserves remnants of three head and two trunk appendages, and the adult is reconstructed as having four head appendages. This material allows testing of the affinity of the Bradoriida. Kunmingella is identified as a stem crustacean in character-based analyses, through both morphological comparisons and cladistic reconstructions. Global parsimony analysis recovers a monophyletic Bradoriida as the sister group to crown crustaceans.
Keywords: Arthropoda, Bradoriida, Cambrian, Chengjiang Lagerstätte, China, exceptional preservation
1. Introduction
Bradoriida are a prolific group of Cambrian and Early Ordovician marine arthropods that form an important component of the Cambrian ecosystem (Williams et al. 2007). They had a worldwide distribution and occur in most of the major Cambrian lagerstätten (Hou et al. 1996, 2002, 2004; Siveter et al. 1996; Siveter & Williams 1997; Shu et al. 1999). They first appear in the fossil record slightly earlier than trilobites of the Early Cambrian Parabadiella Biozone in south China (Hou et al. 2002), and using bivalved shield (i.e. ‘carapace’) morphology alone have been resolved into at least seven families (Williams et al. 2007). In having a bivalved shield, bradoriids resemble the ostracod crustaceans, a group that ranges from at least the Ordovician to Recent (Salas et al. 2007; Siveter 2008; Williams et al. 2008). Ostracods are the most prolific arthropods represented in the fossil record and the taxon to which bradoriids were traditionally assigned (e.g. Sylvester-Bradley 1961). Elucidating the systematic affinities of the Bradoriida is hampered by limited evidence of their soft anatomy, which until now has been known only from Kunmingella douvillei (Mansuy 1912) from the Lower Cambrian of China (Hou et al. 1996; Shu et al. 1999) and from one specimen of an undescribed species from the Middle Cambrian of Australia (Hinz-Schallreuter 1993). The previously documented anatomical evidence suggested that Kunmingella—and by implication bradoriids in general—is not an ostracod; that it probably lies at a level low on the stem line of Crustacea (Hou et al. 1996; Shu et al. 1999); and that it is not closely related to the Phosphatocopida, the other widespread group of Cambrian bivalved arthropods that were also once thought to be ostracods (e.g. Müller 1979; cf. Siveter et al. 2001, 2003a; Maas et al. 2003).
Shield morphology is a poor means of establishing the affinity of bivalved arthropods because the bivalved shield is a feature prone to evolutionary convergence (Walossek 1993, p. 112). Even before the soft parts of bradoriids were known, Jones & Mckenzie (1980) had already suggested that the group represented an artificial taxon. As Siveter et al. (2003b, 2007) and Siveter (2008) have indicated with respect to ostracods, viable affinity of such bivalved fossil material can only be tested by reference to specimens with soft-part anatomy preserved. Newly discovered specimens described here of the bradoriid Km. douvillei (Kunmingellidae) and Kunyangella cheni (Comptalutidae) from Yunnan Province, southern China, provide a much expanded dataset by which to assess bradoriid palaeobiology. Our new morphological data reveal the adult Km. douvillei to possess a 10-segmented body with the last three trunk segments, together with the associated appendages, extending beyond the posterior margin of the shield, a long antenna inserted posterior of the eye and uniramous ninth and tenth appendages. In addition, we show that Ky. cheni bears at least five pairs of appendages. We use these data, in conjunction with those reported by Hou et al. (1996) and Shu et al. (1999), to examine the systematic affinity of two bradoriid families, and thereby examine the phylogenetic relationships of the group itself. Kunmingellids are known from the Cambrian of China and Siberia, and comptalulitids are documented from the Cambrian of China, central Asia, Siberia and Australia (Jones & Laurie 2007; Williams et al. 2007).
2. Material and methods
As with other exceptionally preserved fossils of the Chengjiang Konservat-Lagerstätte (Hou et al. 2004), the material described here is preserved as two-dimensional specimens or, more rarely, with weak three dimensionality. The taphonomic pathway involves pyrite replacement, later pseudomorphed by iron oxides, within clay-rich host sediment (Gabbott et al. 2004). Specimens were prepared mechanically using fine needles. Images were captured using a Canon five-dimensional DSLR Camera attached to Leitz Aristophot and Nikon Multiphot macrophotographic equipment. All material was collected from the Lower Cambrian of the Chengjiang and the Haikou (Kunming) areas, Yunnan Province, and is deposited in the Yunnan Key Laboratory for Palaeobiology (YKLP), Yunnan University, Kunming, China.
3. Systematic palaeontology
Phylum: Arthropoda, Subphylum and Class uncertain.
Order: Bradoriida Raymond, 1935?
Remarks. As there is no information on the soft-part anatomy of the type genus Bradoria Matthew, 1899, herein Kunmingella and Kunyangella are tentatively assigned to the Bradoriida.
Family: Kunmingellidae Huo & Shu, 1985
Genus: Kunmingella Huo, 1956
2002 Kunmingella Huo, 1956; Hou et al., p. 358 (q.v. for full synonymy).
2007 Kunmingella Huo, 1956; Zhang, p. 113
Diagnosis. Kunmingellids with adults having five limb-bearing segments in both the head and trunk; uniramous antenna inserted posterior of the eye; biramous second to eighth appendages, each bearing a leaf-shaped exopod; and uniramous ninth and tenth appendages. Shield with a broad anterodorsal lobe; posterior lobe elongate, straight to weakly crescent shaped, tapering, extends from just behind valve centre to immediately in front of the posterior cardinal corner. Broad, shallow depression occurs between anterodorsal and posterior lobes, deepest mid-dorsally. A low, narrow latero-admarginal ridge is entire between cardinal corners. Valves smooth to finely granulose.
Species: Kunmingella douvillei (Mansuy, 1912).
2002 Kunmingella douvillei (Mansuy, 1912); Hou et al., p. 359, figs 11a–k, 12a–j, 13a–h (q.v. for full synonymy).
2004 Kunmingella douvillei (Mansuy, 1912); Hou et al., p. 114, fig. 16.13
2007 Kunmingella douvillei (Mansuy, 1912); Zhang, p. 115, pl. 1, figs 1–13, pl. 2, figs 1–18, pl. 5, figs 1–11, text-figs 3A, 5A.
2007 Kunmingella douvillei (Mansuy); Williams et al., figs 2.4–6
2007 Kunmingella douvillei (Mansuy 1912); Vannier, fig. 5i.
2008 Kunmingella douvillei (Mansuy 1912); Siveter, pl. 1, figs 4, 6
2008 Kunmingella douvillei (Mansuy 1912); Williams et al., text-fig. 2.3
Diagnosis. Kunmingella with shield lacking a posteroventral spine.
Syntypes. Shields and valves on a slab of mudstone (Mansuy 1912, p. 22, pl. 1, fig. 8). Kebaocun section, Yiliang County, Yunnan Province; Heilinpu Formation, Lower Cambrian. The repository for this material is unknown.
Material. Countless thousands of specimens are known from numerous localities in Sichuan and especially Yunnan provinces, southern China (see Hou et al. (2002) and Zhang (2007) for details), but individuals with preserved soft-part anatomy are extremely rare. Previously figured specimens with soft parts are detailed in Hou et al. (1996, 2002, 2004), Shu et al. (1999), Gabbott et al. (2004), Williams et al. (2007, 2008) and Siveter (2008). The 32 newly collected specimens documented here, many together with their counterparts, all bear soft-part anatomy: YKLP10988–94, 10996–11001, 11003–18 and (formerly RCCBYU) 10196 and 10258–59. Shields are 1.25–6 mm long (a greater range than that recorded by Hou et al. (2002): 1.76–5.24 mm); those over 3.5 mm long are regarded as adults (see §3b).
In the following description, we refer to only those specimens that most clearly exhibit a particular feature.
(a). Adults
The morphology of the shield is given in the diagnosis of the genus. The assumed division between the head and the trunk is indicated by the disposition of appendages: trunk appendages point backwards, head appendages forward (e.g. figure 1d,f,g). The body has 10 limb-bearing segments, five each in the head and the trunk, each bearing a single pair of appendages, the limb bases of which are not preserved (figure 1g,o). The adult body has a maximum width of about 1.5 mm and narrows posteriorly from the sixth segment. Segments 2–8 are typically 0.5 mm long axially (figure 1g). The posterior-most segments, including the tiny triangular-shaped tail-piece, form a cone-shaped termination to the trunk that protrudes beyond the posterior margin of the shield (figure 1g).
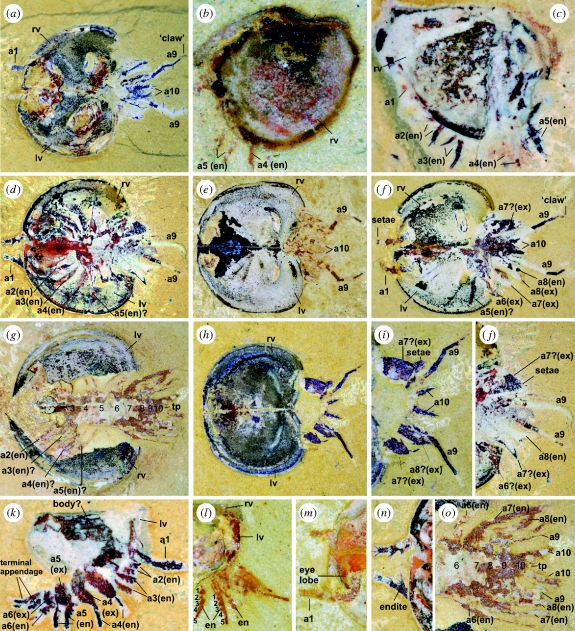
Figure 1.
Bradoriida with soft-part anatomy preserved, Yu'anshan Member, Heilinpu Formation, Lower Cambrian, Haikou, Kunming area and Chengjiang area, Yunnan Province, South China; (a,c–f,h–k,n) from Ercaicun, Haikou, Kunming area; (b,l,m) from Mafeng, Haikou, Kunming area; (g,o), from Xiaolantian, Chengjiang area. (a,d–o) Kunmingella douvillei: (a) YKLP (formerly RCCBYU) 10259, fully open shield, dorsal view, ×6.25; (d,j,n) YKLP 10994, (d) fully open shield, dorsal view, ×6.6, (j) posterior part, dorsal view, ×8, (n) anterior part, dorsal view, ×11; (e) YKLP10993 fully open shield, dorsal view, ×6; (f) YKLP10998, fully open shield, dorsal view, ×7.4; (g,o) YKLP10988b, (g) fully open shield, dorsal view (1–10: body segmentation), ×5.5, (o) posterior part, dorsal view, ×10; (h,i) YKLP (formerly RCCBYU) 10258, (h) fully open shield, dorsal view, ×6; (i) posterior part, dorsal view, ×10; (k) YKLP11001a, juvenile, right lateral view, with left valve behind soft parts, ×24; (l) YKLP11000, juvenile, lateral view of posterior part of excavated closed shield (1–5: podomeres of endopod), ×13; (m) YKLP10990a, fully open shield, dorsal view of anterior part, ×10. (b,c) Kunyangella cheni: (b) YKLP 11003, right lateral view, ×18.5; (c) YKLP 11002, left lateral view, with right valve behind soft parts, ×21.5. Abbreviations: a1–a5, head appendages; a6–a10, trunk appendages; en, endopod; ex, exopod; rv, right valve; lv, left valve; td, terminal limb-bearing division; tp, tail piece; 1–5, podomeres of the endopod.
A pair of lateral eyes occurs just in front of the insertion point of the first appendage (antenna) at the site of the anterodorsal node of the shield (figure 1a,m). The first, uniramous appendage is about 2.5 mm long, tapers distally and consists of five podomeres, of which typically the distal three protrude beyond the shield anteriorly (figure 1d,m). There are five to six short, stiff setae on the outer and distal edges of the distal-most podomere (figure 1d,n). Podomeres 2–4, at least, each have an endite distally on their inner margins, with the endite of at least the third and fourth podomeres bearing splays of setae.
Appendages 2–8 are biramous, each having a large leaf-shaped exopod (most evident on the trunk in figure 1d,f,h–j and on the head in YKLP 10990a) and an endopod consisting of five podomeres (e.g. figure 1l; also unfigured specimen YKLP 10196 and Shu et al. 1999, figs 1a–c). The four post-antennal cephalic appendages can each be traced back to a discrete body segment (figure 1g); each endopod bears a single elongate endite on the inner margin of each podomere (Shu et al. 1999), but it cannot be determined whether any of the exopods are fringed by marginal setae (as is the case with the exopods of the trunk appendages; see below). Each endopod of trunk appendages 6–8 has a single endite on (possibly the inner margin of) each podomere (figure 1l and YKLP10196; Shu et al. 1999) and each exopod bears stiff and distally progressively longer setae on its distal outer and inner margins (figure 1d,j).
The ninth and tenth pairs of appendages are uniramous (figure 1a,e,f,l,o). The ninth appendage is narrow, elongate, about 2 mm long excluding the short terminal ‘claw-like’ structure and posteriorly it projects well beyond the shield (figure 1a,f,h,i). It comprises at least five podomeres, each about 0.5–0.75 mm long, with setae originating from the inner surface of each podomere; adjacent podomeres are geniculate. The tenth appendage is about 1 mm long and consists of at least three podomeres each bearing short, stiff setae, at least six of which occur on the distal-most podomere (figure 1a,h,i).
(b). Ontogeny
Based on isolated shields and valves, Km. douvillei has five ontogenetic stages (Zhang 2007). Growth stages 1 and 2 each have a tiny (ca 200–230 µm diameter), univalved, dome-like shield; later pre-adult growth stages each have a small bivalved shield. The anterior and posterior lobes of the shield become well developed first in growth stage 4. On these criteria, YKLP11001a (figure 1k), with soft-part anatomy, is considered to be growth stage 4. In the new material presented here, all specimens with 10 appendages (collected from various horizons) are considered to be adults, and these range from 3.5 to 6 mm long (figure 1a,d–h). YKLP11001a (figure 1k) differs principally from adults by having seven appendages and a trunk that does not appear to extend beyond the posterior margin of the shield. Its first appendage is uniramous, at least 0.55 mm long, and comprises five podomeres; no setae are determinable, but this may be a factor of preservation. Appendages 2–6 each have an endopod composed of at least four podomeres. An exopod is not discernible on the second and third appendages; appendages 4–6 each have a leaf-shaped exopod. Appendage 7, seen as a single, posteriorly projecting ramus, consists of at least three elongate podomeres.
Family: Comptalutidae Öpik, 1968
Genus: Kunyangella Huo, 1965
2002 Kunyangella Huo, 1965; Hou et al., p. 387 (q.v. for full synonymy).
2007 Kunyangella Huo, 1965; Zhang, p. 128
Diagnosis. Comptalutids with the penultimate growth stage having three head appendages, including two post-antennal limbs each with a narrow, elongate endopod bearing at least two stout setae terminally; the endopod of each trunk appendage is broader, and each podomere has an endite with setae. Shield with mid-dorsal node, but no other lobation. A weak latero-admarginal ridge occurs between cardinal corners, demarcated from the lateral valve surface by a furrow.
Species: Kunyangella cheni Huo, 1965
2002 Kunyangella cheni Huo, 1965; Hou et al., p. 387, figs 20d–k, 21a (q.v. for full synonymy).
2007 Kunyangella cheni; Zhang, p. 129
Diagnosis. Kunyangella with shield bearing a bulbous, elongate to arcuate mid-dorsal node.
Holotype. External mould of a (juvenile?) left valve, Northwest University, Xi'an, China, no. 0006; Huo, 1965, pl. 1. fig. 6. Jinning County, Yunnan Province; Heilinpu Formation, Lower Cambrian.
Material. Known from a few hundred specimens from several localities in Yunnan Province (Hou et al. 2002). Herein, we document the first (two) specimens with soft-part anatomy preserved, both from Haikou: YKLP11002–3. Both shields are about 2 mm long and, bearing in mind that specimens from Yunnan Province are up to 2.38 mm long (Hou et al. 2002), each probably represents a penultimate growth stage.
(c). Soft-part anatomy
There are remnants of a mass of soft tissue within the shield of YKLP 11002 (figure 1c), but no more detail of the body is preserved. YKLP 11003 (figure 1b) shows red coloration mid-posteriorly that might represent the remnants of soft tissues protruding beyond the body. However, the posteroventral position of the trunk appendages may indicate that much of the body was accommodated within the shield. An anterodorsal node (‘eye lobe’) is absent, and there is no indication of the presence of an eye.
YKLP 11002 preserves remnants of five appendage pairs, three anteriorly that project forwards on the presumed head and two behind that project posteroventrally on the presumed trunk. Given that YKLP 11002 probably represents a penultimate growth stage, it is considered that adults would have four pairs of appendages in the head (for ontogenetic patterns of segmentation in Crustacea, e.g. Waloszek & Maas 2005).
The presumed first pair of appendages (occurring in a similar position and with similar morphology to the distal end of the first appendage of Kunmingella) are preserved in the lateral view as two short, stout podomeres protruding from the shield mid-anteriorly (these very delicate podomeres were visible when originally observed, but subsequently they mostly flaked off from the shale matrix and therefore are not now seen clearly in figure 1c). More posteriorly, there are narrow, presumed endopods of two appendage pairs extending beyond the shield (a2, a3), each consisting of two podomeres with elongate, almost claw-like pairs of setae terminally. Remnants of two trunk appendages (a4, a5)—presumed endopods—are broader than appendages 1–3 and have stout setae terminally. The fifth appendage shows at least three podomeres, each having an endite with setae. YKLP 11003 preserves remnants of two pairs of trunk appendages (presumed a4, a5) protruding posteroventrally from a closed shield.
4. The anatomy and lifestyle of kunmingella douvillei
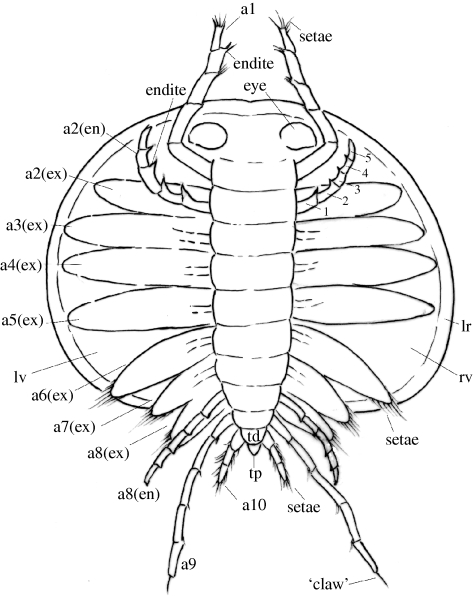
The considerably increased dataset of soft-part anatomy for Km. douvillei allows a revised reconstruction of the animal (figure 2). The occurrence of five limb-bearing segments in the head and five in the trunk (figure 1g,o) and the disposition of the associated appendages confirm the cephalic-trunk boundary suggested by Hou et al. (1996) and Shu et al. (1999, p. 287). However, Shu et al. (1999) identified only eight appendage pairs. The new material shows for the first time, to our knowledge, that the first appendage is long (2.5 mm) and that its insertion point is just behind that of the eye (figure 1m) and in a more posterior position than envisaged by Shu et al. (1999). Our material confirms the observation of the latter authors that the biramous post-antennal appendages each have an endopod comprising five podomeres, with the inner margin of each podomere bearing an endite (‘lobate expansions’ of Shu et al. 1999). Each exopod of these biramous appendages is leaf-like (YKLP11005) and, at least for appendages 6–8, is fringed by marginal setae. The new material shows that the posterior morphology of Km. douvillei comprises uniramous ninth and tenth appendages (equal to biramous eighth appendage and furca of Shu et al. 1999, fig. 6e), and that the last three trunk segments, together with the associated appendages, extend beyond the posterior margin of the shield (figure 1g,o; cf. Shu et al. 1999, p. 287). Our new material also shows that three appendages (and presumably three segments) are added between growth stage 4 (sensu Zhang 2007) and the adult stage (see above).
Figure 2.
Reconstruction of Km. douvillei (Mansuy 1912), based on the new material here and, in part, on evidence from Hou et al. (1996) and Shu et al. (1999). Interpretation of the morphology of the exopods and endopods of the head appendages is based on specimen YKLP 10990a and Shu et al. (1999), respectively. For clarity, some of the appendages have been omitted from the reconstruction. The reconstruction shows the dorsal aspect of the animal with the shield stripped away; in appendage 2, the endopod, which lies below the exopod from the dorsal aspect, is emphasized to show the five podomeres. Information on the limb bases of all appendages is lacking. Abbreviations as for figure 1, except lr, latero-admarginal ridge.
Kunmingella douvillei possibly crawled on and swam near the substrate, with its dorsal shield held widely open in life (Shu et al. 1999; Hou et al. 2004). Vannier (2007, fig. 3) suggested that the species may have formed a component of the Early Cambrian meiofauna, living at the sediment–water interface, with a lifestyle and ecological niche similar to some Recent ostracods. The eyes and possibly the long first appendage would have acted as sensory devices. The leaf-shaped exopods may have aided swimming. The trailing ninth and short tenth appendages may have functioned to stabilize the animal during locomotion. An approximate morphological analogue of the ninth appendage is the antenniform cercus in the Burgess Shale trilobite Olenoides serratus (Whittington 1975). Remains of Km. douvillei shields occur in considerable numbers in elliptical aggregates interpreted as coprolites, indicating possible predation by anomalocaridids and/or unknown epibenthic predators (Vannier & Chen 2005).
5. Phylogenetic position of bradoriida
Bradoriida encompasses a wide range of bivalved arthropod morphologies that traditionally were regarded as ostracod or ostracod-like crustaceans (e.g. Sylvester-Bradley 1961). Previous studies based on less complete soft-part evidence concluded that Kunmingella was perhaps a stem-lineage crustacean (Hou et al. 1996; Shu et al. 1999). The new material described here allows testing of the systematic relationship of two bradoriid families.
Following the scheme of Waloszek and co-authors (e.g. Walossek & Müller 1998; Walossek 1999, 2003; Maas et al. 2004; Waloszek et al. 2005, 2007; Stein et al. 2005; Zhang et al. 2007), the series of homogeneous, unspecialized post-antennal biramous limbs with setose leaf-shaped exopods in Km. douvillei represent the plesiomorphic condition as in the ground plan of Euarthropoda (e.g. Waloszek 2003). Even though the proximal parts of the appendages are not preserved, the generalized, homogeneous nature of the limb rami suggests that the limb bases are also unspecialized. The endopods of Kunmingella each consist of five podomeres: possession of fewer than seven podomeres is characteristic of members of Crustacea (Waloszek 2003; Waloszek et al. 2007; Zhang et al. 2007; figures 1i,j and 2), while possession of five head appendages indicates a level on the crustacean stem line equivalent to that of Henningsmoenicaris or Oelandocaris (Stein et al. 2005, 2008) or more derived Labrophora (Zhang et al. 2007, fig. 3). In contrast to the morphology of Kunmingella, the adult growth stage of Ky. cheni is here considered to have only four head appendages. According to the scheme of Waloszek and others, possession of just four head segments, rather than five, would place Kunyangella in a more basal position than Kunmingella, and comptalulitids and kunmingellids would form a paraphyletic group. Alternatively, if Kunyangella does not belong to Crustacea, then similarities between comptalutids and kunmingellids, such as those of the shield, would represent convergence and the Bradoriida would be polyphyletic.
However, these scenarios also require consideration in terms of global parsimony. To this end, we have coded Kunmingella and Kunyangella for inclusion in the arthropod morphological database presented and analysed by Wills et al. (1998): although this analysis is in need of revision, it represents the most comprehensive database of fossil arthropod characters. To ensure that we are considering the range of previous hypotheses for bradoriid relationships, we have also included Klausmuelleria and Vestrogrothia to represent the Phosphatocopina. Heuristic search methods (see the electronic supplementary material) recovered a monophyletic Bradoriida as the sister taxon to crown-group crustaceans. The placement of the Bradoriida on the crustacean stem is supported by their possession of a mobile appendage on the terminal division of the trunk and five head segments (depending upon optimization criteria; see the electronic supplementary material), and by their lack of crown-group characters such as specialized second and third appendages. Conversely, Phosphatocopina are recovered as crown-group crustaceans on the basis of their placement relative to Remipedia (see the electronic supplementary material). The marrellomorphs are placed as sister group to the clade (Bradoriida + Eucrustacea), at the base of the crustacean stem. Alternative interpretations of arthropod phylogeny (e.g. using molecular data) can be applied using back-bone constraint trees. When the pancrustacean hypothesis of Regier et al. (2005) is taken into account, the same placement of Bradoriida is recovered.
Acknowledgements
This study was supported by Royal Society International Joint Projects (2004/R2-CH and 2008/R4) awarded to David J. Siveter and X.H.; the National Natural Foundation of China (40730211); Program 973 of China (2006CB806400); and the Department of Science and Technology of Yunnan Province (2005D0002Z). We thank Matt Wills (University of Bath) for providing the arthropod data matrices used in the phylogenetic analyses and Tom Harvey (University of Leicester) for discussion on arthropod relationships. We are very grateful for the constructive comments of the two anonymous referees.
References
- Gabbott S. E., Hou X.-G., Norry M., Siveter D. J.2004Preservation of early Cambrian animals of the Chengjiang biota. Geology 32, 901–904 (doi:10.1130/G20640.1) [Google Scholar]
- Hinz-Schallreuter I.1993Ostracodes from the Middle Cambrian of Australia. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, no. 188, pp. 305–326 Stuttgart, Germany: E. Schweizerbart'sche Verlagsbuchhandlung. [Google Scholar]
- Hou X.-G., Siveter D. J., Williams M., Walossek D., Bergström J.1996Appendages of the arthropod Kunmingella from the Early Cambrian of China: its bearing on the systematic position of the Bradoriida and the fossil record of the Ostracoda. Phil. Trans. R. Soc. Lond. B B351, 1131–1145 (doi:10.1098/rstb.1996.0098) [Google Scholar]
- Hou X.-G., Siveter D. J., Williams M., Feng X.-H.2002A monograph of the Bradoriid arthropods from the Lower Cambrian of SW China. Trans. R. Soc. Edin. Earth Sci. 92, 347–409 [Google Scholar]
- Hou X.-G., Aldridge R. J., Bergström J., Siveter D. J., Siveter D. J., Feng X.-H.2004The Cambrian fossils of Chengjiang, China: the flowering of early animal life, pp. 233 Oxford, UK: Blackwell Science Ltd [Google Scholar]
- Huo S.-C.1956Brief notes on Lower Cambrian Archaeostraca from Shensi and Yunnan. Acta Palaeontol. Sin. 4, 425–445 [Google Scholar]
- Huo S.-C.1965Additional notes on Lower Cambrian Archaeostraca from Shensi and Yunnan. Acta Palaeontol. Sin. 13, 291–307 [Google Scholar]
- Huo S.-C., Shu D.1985Cambrian Bradoriida of south China, pp. 251 Xian, China: Northwest University Press; (In Chinese, English summary) [Google Scholar]
- Jones P. J., Laurie J. R.2007Bradoriida and Phosphatocopida (Arthropoda) from the Arthur Creek Formation (Middle Cambrian), Georgina Basin, central Australia. Assoc. Aust. Palaeontol. Mem. 35, 205–223 [Google Scholar]
- Jones P. J., McKenzie K. G.1980Queensland Middle Cambrian Bradoriida (Crustacea): new taxa, palaeobiogeography and biological affinities. Alcheringa 4, 203–225 (doi:10.1080/03115518008618932) [Google Scholar]
- Maas A., Waloszek D., Müller K. J.2003Morphology, ontogeny and phylogeny of the Phosphatocopina (Crustacea) from the Upper Cambrian ‘Orsten’ of Sweden. Fossil. Strata 49, 1–238 [Google Scholar]
- Maas A., Waloszek D., Chen J.-Y., Braun A., Wang X.-Q., Huang D.-Y.2004Phylogeny and life habits of early arthropods: predation in the Early Cambrian sea. Prog. Nat. Sci. 14, 158–166 (doi:10.1080/10020070412331343301) [Google Scholar]
- Mansuy H.1912Pt. 2, Paléontologie. In Mémoires du service géologique de 1'Indochine, vol. 1 (eds Deprat J., Mansuy H.), pp. 146 Etude géologique du Yun-Nan oriental, Hanoi, Vietnam: Imprimerie d'Extreme Orient [Google Scholar]
- Matthew G. F.1899Preliminary notice of the Etcheminian Fauna of Cape Breton. Bull. Nat. Hist. Soc. New Brunswick 4, 198–208 [Google Scholar]
- Müller K. J.1979Phosphatocopine ostracodes with preserved appendages from the Upper Cambrian of Sweden. Lethaia 12, 1–27 (doi:10.1111/j.1502-3931.1979.tb01234.x) [Google Scholar]
- Öpik A. A.1968Ordian (Cambrian) Crustacea Bradoriida of Australia. Bull. Bur. Miner. Resour. Geol. Geophys. 103, 1–46 [Google Scholar]
- Raymond P. E.1935Leonchoila and other Mid-Cambrian Arthropoda. Bull. Mus. Comp. Zool. Harvard Univ. 76, 205–230 [Google Scholar]
- Regier J. C., Shultz J. W., Kambic R. E.2005Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc. R. Soc. B 272, 395–401 (doi:10.1098/rspb.2004.2917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M. J., Vannier J. M. C., Williams M.2007Early Ordovician ostracods from Argentina: their bearing on the origin of the binodicope and palaeocope clades. J. Paleontol. 81, 1384–1395 (doi:10.1666/05-134.1) [Google Scholar]
- Shu D., Vannier J., Luo H.-L., Chen L.-Z., Zhang X.-L., Hu S.-X.1999Anatomy and lifestyle of Kunmingella (Arthropoda, Bradoriida) from the Chengjiang fossil Lagerstätte (Lower Cambrian, southwest China). Lethaia 32, 279–298 [Google Scholar]
- Siveter D. J.2008Ostracods in the Palaeozoic? Palaeobiodivers. Palaeoenviron. (Senckenbergiana Lethaea) 88, 1–9 (doi:10.1007/BF03043973) [Google Scholar]
- Siveter D. J., Williams M.1997Cambrian bradoriid and phosphatocopid arthropods of North America. Spec. Pap. Palaeontol. 57, 1–69 [Google Scholar]
- Siveter D. J., Williams M., Peel J. S., Siveter D. J.1996Bradoriids (Arthropoda) from the Early Cambrian of North Greenland. Trans. R. Soc. Edin. Earth Sci. 116, 113–121 [Google Scholar]
- Siveter D. J., Williams M., Waloszek D.2001A three-dimensionally preserved phosphatocopid arthropod with appendages from the Early Cambrian. Science 293, 479–481 (doi:10.1126/science.1061697) [DOI] [PubMed] [Google Scholar]
- Siveter D. J., Waloszek D., Williams M.2003aAn Early Cambrian phosphatocopid crustacean with three-dimensionally preserved soft parts from Shropshire, England. Spec. Pap. Palaeontol. 70, 9–30 [Google Scholar]
- Siveter D. J., Sutton M., Briggs D. E. G., Siveter D. J.2003bAn ostracode crustacean with soft parts from the Lower Silurian. Science 302, 1749–1751 (doi:10.1126/science.1091376) [DOI] [PubMed] [Google Scholar]
- Siveter D. J., Siveter D. J., Sutton M. D., Briggs D. E. G.2007Brood care in a Silurian ostracod. Proc. R. Soc. B 274, 465–469 (doi:10.1098/rspb.2006.3756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M., Waloszek D., Maas A.2005Oelandocaris oelandica and the stem lineage of Crustacea. In Crustacea and Arthropod relationships (eds Koenemann S., Jenner R. A.), pp. 55–71 London, UK: Taylor & Francis [Google Scholar]
- Stein M., Waloszek D., Maas A., Haug J. T., Müller K. J.2008The stem crustacean Oelandocaris oelandica re-visited. Palaeontol. Pol. 53, 461–484 [Google Scholar]
- Sylvester-Bradley P. C.1961Archaeocopida. In Treatise on invertebrate Paleontology Part Q, Arthropoda 3, Crustacea, Ostracoda (eds Moore R. C., Pitrat C. W.), pp. Q100–Q103 Boulder, CO: Geological Society of America and University of Kansas Press [Google Scholar]
- Vannier J.2007Early Cambrian origin of complex marine ecosystems. In Deep time perspectives on climate change (eds Williams M., Haywood A. M., Gregory F. J., Schmidt D. N.), pp. 81–100 The Micropalaeontological Society Special Publication Bath, VA: Geological Society Publishing House [Google Scholar]
- Vannier J., Chen J.-Y.2005Early Cambrian food chain: new evidence from fossil aggregates in the Maotianshan shale biota, SW China. Palaios 20, 3–26 (doi:10.2110/palo.2003.p03-40) [Google Scholar]
- Walossek D.1993The Upper Cambrian Rehbachiella and the phylogeny of Branchiopoda and Crustacea. Fossil. Strata 32, 1–202 [Google Scholar]
- Walossek D.1999On the Cambrian diversity of Crustacea. In Crustaceans and the biodiversity crisis (eds Schram F. R., von Vaupel Klein J. C.), pp. 3–27 Leiden, The Netherlands: Brill [Google Scholar]
- Waloszek D.2003Cambrian ‘Orsten’-type preserved arthropods and the phylogeny of Crustacea. In The new panorama of animal evolution (eds Legakis A., Sfenthourakis S., Polymeni R., Thessalou-Legakis M.), pp. 69–87 Sofia, Bulgaria: Pensoft Publishers [Google Scholar]
- Waloszek D., Maas A.2005The evolutionary history of crustacean segmentation: a fossil-based perspective. Evol. Dev. 7, 515–527 (doi:10.1111/j.1525-142X.2005.05056.x) [DOI] [PubMed] [Google Scholar]
- Walossek D., Müller K. J.1998Early arthropod phylogeny based on fossil and Recent taxa. In Arthropod fossils phylogeny (ed. Edgecombe G. D.), pp. 185–231 New York, NY: Columbia University Press [Google Scholar]
- Waloszek D., Chen J.-Y., Maas A., Wang X.-Q.2005Early Cambrian arthropods: new insights into arthropod head and structural evolution. Arthropod Struct. Dev. 34, 189–205 (doi:10.1016/j.asd.2005.01.005) [Google Scholar]
- Waloszek D., Maas A., Chen J.-Y., Stein M.2007Evolution of cephalic feeding structures and the phylogeny of Arthropoda. Palaeogeogr. Palaeoclimatol. Palaeoecol. 254, 273–287 (doi:10.1016/j.palaeo.2007.03.027) [Google Scholar]
- Whittington H. B.1975Trilobites with appendages from the Middle Cambrian, Burgess Shale, British Columbia. Fossil. Strata 4, 97–136 [Google Scholar]
- Williams M., Siveter D. J., Popov L. E., Vannier J. M. C.2007Biogeography and affinities of the bradoriid arthropods: cosmopolitan microbenthos of the Cambrian seas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 248, 202–232 (doi:10.1016/j.palaeo.2006.12.004) [Google Scholar]
- Williams M., Siveter D. J., Salas M. J., Vannier J., Popov L. E., Ghobadi Pour M.2008The earliest ostracods: the geological evidence. Palaeobiodivers. Palaeoenviron. (Senckenbergiana Lethaea) 88, 11–21 (doi:10.1007/BF03043974) [Google Scholar]
- Wills M. A., Briggs D. E. G., Fortey R. A., Wilkinson M., Sneath P. H. A.1998An arthropod phylogeny based on fossil and recent taxa. In Arthropod fossils and phylogeny (ed. Edgecombe G. D.), pp. 33–105 New York, NY: Columbia University Press [Google Scholar]
- Zhang X.-G.2007Phosphatized bradoriids (Arthropoda) from the Cambrian of China. Palaeontogr. Abteilung A 281, 93–173 [Google Scholar]
- Zhang X.-G., Siveter D. J., Waloszek D., Maas A.2007An epipodite-bearing crown-group crustacean from the Lower Cambrian. Nature 449, 595–598 (doi:10.1038/nature06138) [DOI] [PubMed] [Google Scholar]