Abstract
Box jellyfish (Cubomedusae) possess a unique visual system comprising 24 eyes of four morphological types. Moreover, box jellyfish display several visually guided behaviours, including obstacle avoidance and light-shaft attractance. It is largely unknown what kind of visual information box jellyfish use for carrying out these behaviours. Brightness contrast is almost certainly involved, but it is also possible that box jellyfish extract colour information from their surroundings. The possible presence of colour vision in box jellyfish has previously been investigated using behavioural, electrophysiological and immunohistochemical methods. However, the results from these studies are to some degree conflicting and inconclusive. Here, we present results from an investigation into the visual system of the box jellyfish Chiropsella bronzie, using microspectrophotometry and immunohistochemistry. Our results strongly indicate that only one type of visual pigment is present in the upper and lower lens eyes with a peak absorbance of approximately 510 nm. Additionally, the visual pigment appears to undergo bleaching, similar to that of vertebrate visual pigments.
Keywords: Cubomedusae, Chiropsella bronzie, microspectrophotometry, vision, eyes, visual pigment
1. Introduction
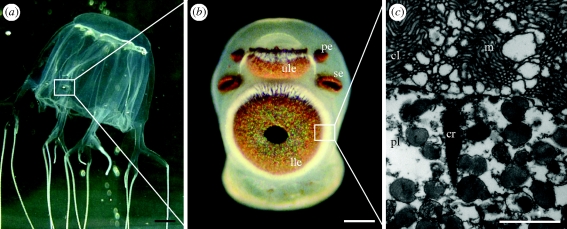
Unlike most other jellyfish, box jellyfish (cubomedusae) are highly visually orientated. They are agile swimmers and use visual information to avoid obstacles (Garm et al. 2007b), orient themselves towards light shafts (Buskey 2003) and optimize feeding time when in light shafts (Garm & Bielecki 2008). They have an elaborate visual system, which consists of four sensory structures called rhopalia, each holding six eyes of four morphological types: a pair of pit eyes, a pair of slit eyes, a small upper lens eye and a larger lower lens eye (Nilsson et al. 2005; Garm et al. 2008; figure 1b).
Figure 1.
The visual system of box jellyfish. (a) A Chiropsella bronzie medusa. Each medusa has four sensory clubs known as rhopalia. (b) Each rhopalium possesses six eyes; two pit eyes (pe), two slit eyes (se), one upper lens eye (ule) and one lower lens eye (lle). (c) The retina of the eyes consist of three layers; a ciliary layer (cl), a pigment layer (pl) and a neural layer. All photoreceptors are ciliary, demonstrated here by the presence of a ciliary rootlet (cr), and the cilium carries in microvilli (m), which appear to have no strict organization. Scale bars, (a) 1 cm, (b) 100 µm and (c) 1 µm.
Box jellyfish have ciliary photoreceptors (figure 1c) in contrast to most invertebrates, which employ rhabdomeric photoreceptors (Arendt & Wittbrodt 2001). Ciliary and rhabdomeric photoreceptors differ in their structure, developmental origin, transduction pathway, electrophysiological response polarity and mechanism of photopigment regeneration (Arendt & Wittbrodt 2001; Pepe 2001). In light of their basal position in animal phylogeny, identifying biochemical mechanisms and components in the photoreceptors of box jellyfish may help understand the path of photoreceptor evolution (Lamb 2009).
The spectral sensitivity of a visual system is determined primarily by the spectral absorbance properties of the visual pigment(s) within the retinal photoreceptors. The typical visual pigment is a protein (opsin) with a bound chromophore, and both the type of chromophore and the amino acid sequence of the opsin influence the absorption spectrum of the pigment (Dartnall 1953; Dartnall & Lythgoe 1965; Yokoyama 2000). Two or more different visual pigments, present in separate photoreceptors, can be used for colour vision, given the presence of a neural circuitry comparing the signals from spectrally different receptors. It has been proposed that colour vision may have evolved in shallow water to eliminate luminance (brightness) noise in the form of flickering caused by surface ripple (Maximov 2000). Colour vision can eliminate luminance noise because spectral composition is relatively insensitive to fluctuations in intensity. Box jellyfish are often found in shallow water and therefore would be exposed to such constant flickering light.
The question of colour vision in box jellyfish has been addressed several times. One immunocytochemical study suggested the presence of several opsin types in the lens eyes of the species Carybdea marsupialis (Martin 2004). However, other immunocytochemical, molecular and electrophysiological studies have indicated the presence of only a single opsin (Coates et al. 2006; Garm et al. 2007a; Ekström et al. 2008; Koyanagi et al. 2008; Kozmik et al. 2008).
In this study, we have used microspectrophotometry (MSP) and immunocytochemistry to examine the two types of lens eyes of the Australian box jellyfish Chiropsella bronzie (Gershwin 2006) (figure 1a). The results indicate that a single visual pigment type is present in the lens eyes. Moreover, the visual pigment present in the photoreceptors of the lens eyes appears to bleach in a way similar to that of vertebrate visual pigments.
2. Material and methods
(a). Animals and sampling
Adult specimens of C. bronzie were hand-collected off sandy beaches, close to Port Douglas, Queensland, Australia. For immunocytochemical examinations, whole rhopalia were fixed in 4 per cent paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.2), and stored in cacodylate buffer at 4–8°C until use. For MSP examination, animals were maintained in a 10 000 l tank of fresh sea water and illuminated by standard fluorescent tubes. They were flown to Brisbane 1–2 days after capture, where they were kept in a 200 l tank with artificial sea water illuminated by True Light (Phillips, Australia) and ultraviolet (UV)-rich Blacklight (Phillips) fluorescent tubes. The medusae were fed fresh prawns daily and used within 2–3 days.
(b). Microspectrophotometry
Jellyfish were dark-adapted for a minimum of 30 min before their rhopalia were removed. Electrophysiology studies have shown that 30 min is sufficient for full dark adaptation of the lens eyes (Coates et al. 2006; Garm et al. 2007a). Preparations were made from either an individual eye or a whole rhopalium with the statocyst removed. Whole rhopalia preparations were generally more successful than single eye preparations. All dissections and preparations were carried out under infrared (IR) illumination with the aid of an IR-image converter. Each tissue sample was placed on a coverslip in a small amount of artificial sea water (pH approx. 7.8) and then gently squashed under a second coverslip. The edges of the top coverslip were sealed with nail varnish to prevent dehydration and movement of the tissue. Sea water was deemed an appropriate medium based on the isotonic nature of jellyfish (Mills 1984) and because electrophysiological recordings have been successfully carried out in sea water (Weber 1982; Garm et al. 2007a). When using whole rhopalium preparations, the orientation of the rhopalium was carefully monitored before and after squashing, and the upper and lower lens eyes were identified using both orientation and morphology.
The photosensitive layer of the retina of the lens eyes is composed of the ciliary outer segments of the photoreceptors, with microvilli extending from the ciliary membrane (figure 1c). These microvilli are approximately 0.05 µm in diameter (Yamasu & Yoshida 1976) and densely packed in a tangle without alignment, except for occasional parallel bundles (figure 1c). Measurements were made on these microvillar masses, which potentially originated from several, adjacent photoreceptors.
MSP recordings were performed using a computer-controlled, single-beam, wavelength-scanning microspectrophotometer, described in detail elsewhere (Hart 2004). Briefly, each scan consisted of a ‘downward’ long- to short-wavelength spectral pass from 800 to 300 nm and an ‘upward’ short-to-long wavelength spectral-pass covering the same spectrum. Corresponding wavelength data from the downward and upward spectral passes were averaged together, and the average spectrum was then converted to an absorbance spectrum at 1 nm intervals. The average size of the beam was 5 × 4 µm.
Following the initial scans of dark-adapted preparations (pre-bleach scans), the outer segments were bleached with full-spectrum ‘white’ light for 1 min. The ‘white’ light source was that from a 12 V 50 W quartz halogen bulb at the blaze angle of the microspectrophotometer. A post-bleach scan was then performed and a difference spectrum was obtained by subtracting the post-bleach spectrum from the pre-bleach spectrum. Baseline spectra were made from adjacent tissue-free areas of the preparation. On a number of occasions, 400 nm light was shone on the microvilli, post-bleach, to investigate possible reconversion. Absorbance spectra were also measured from the upper lens-eye lens, which was dissected out from the rhopalium. Unfortunately, a lower lens-eye lens could not be obtained from the available animals.
(c). Analysis of visual pigment absorbance spectra
Visual pigment absorbance spectra were analysed as described elsewhere (Hart 1998, 2004). Briefly, absorbance spectra were fitted with a variable-point running average and normalized. The wavelength of maximum absorbance (λmax) was estimated by fitting a least-square regression line to the data between 70 and 30 per cent of the normalized long-wavelength absorbance, following the methods of MacNichol (1986) and Govardovskii et al. (2000). For comparison, a rhodopsin (vitamin A1-based) visual pigment template (Govardovskii et al. 2000) of the appropriate λmax was plotted with the measured spectra.
(d). Immunofluorescence and confocal microscopy
Excised rhopalia were cryoprotected overnight in 0.1 M phosphate buffer containing 25 per cent sucrose and then transferred to the embedding medium (Richard-Allan Scientific, Neg-50, frozen section medium). Rhopalia were then quickly frozen in the embedding medium at −60°C, serially sectioned with a Microm HM560 (Microm, Walldorf, Germany) cryostat (section thickness 14 µm) and thaw-mounted on chrome-alum gelatin-coated microscope slides. Following rinses in phosphate-buffered saline (PBS) containing 10 per cent normal goat serum, 1 per cent bovine serum albumin (BSA) and 0.25 per cent Triton X-100, the slides were incubated overnight at room temperature with primary antibody. In an initial screening, antibodies against the five different zebrafish retinal opsins (Vihtelic et al. 1999) were tested at different dilutions using the protocol below. Of these, only the antibody raised against the UV opsin labelled photoreceptors in C. bronzie.
Optimal immunolabelling was obtained by incubation at room temperature for 20 h with rabbit anti-zebrafish cone UV opsin (1 : 500–1 : 1000), diluted in PBS (pH 7.2) containing 1 per cent BSA and 0.25 per cent Triton X-100. Immunoreactivity was visualized by immunofluorescence (goat-anti-rabbit IgG-Alexa Fluor 546, diluted 1 : 600).
Immunolabelled cryostat sections were examined using a Zeiss LSM 510 META confocal laser scanning microscope. High-resolution scans were obtained with a ×63/1.4 NA DIC plan-apochromat oil-immersion objective, using a 561 nm DPPS diode laser as excitation light. Images were captured as 1024 × 1024 pixel frames (pixel size: 0.14–0.20 µm; sampling interval: 0.46 µm). Overview scans were performed using a ×20/0.50 NA Epiplan-Neofluar objective (1024 × 1024 pixel frame; pixel size: 0.62 µm; sampling interval: 2.4 µm). All specimens were scanned with a parallel Nomarski differential image contrast channel.
3. Results
(a). Microspectrophotometry
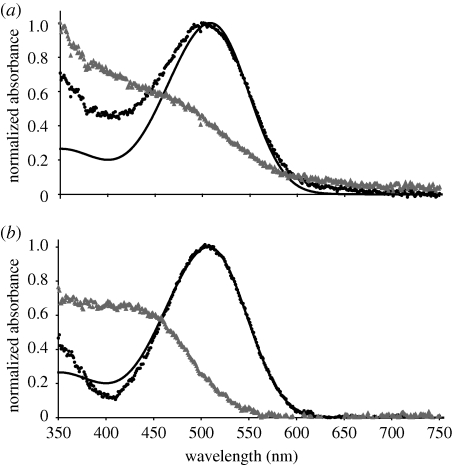
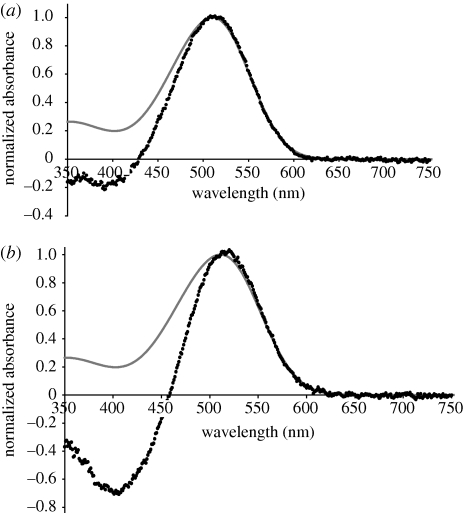
In both eye-types, the obtained spectra indicated the presence of a single visual pigment only. MSP analysis showed little variation in λmax within or between eye types. The λmax of the mean pre-bleach spectrum for the upper lens eye and the lower lens eye was 508 (n = 7) and 507 nm (n = 7), respectively (figure 2), while the λmax calculated for the mean difference spectrum was 510 (n = 7) and 512 nm (n = 5) for the upper and lower lens eyes, respectively (figure 3).
Figure 2.
Normalized mean pre-bleach (black data points) and post-bleach (grey data points) absorbance spectra of microvillar regions of the lower and upper lens eyes of C. bronzie. Values of λmax for pre-bleach spectra were calculated using the method of MacNichol (1986) and the corresponding A1-rhodopsin template of Govardovskii et al. (2000) (black solid line) is plotted for comparison. (a) The average pre-bleach spectrum of the upper lens eye has a λmax at 508 nm while (b) the mean pre-bleach lower lens eye spectrum has a λmax at 507 nm. In both eye types, the visual pigment bleaches when exposed to white light (grey data points).
Figure 3.
Normalized mean difference spectra (black data points) with a fitted A1-Govardovskii rhodopsin template (grey line). The calculated λmax of the (a) upper lens eye and (b) the lower lens eye are 510 and 512 nm, respectively.
The post-bleach spectra revealed a photoproduct that strongly absorbed wavelengths below approximately 460 nm (figure 2). This photoproduct accounts for the slightly higher λmax values of the mean difference spectra compared with the pre-bleach spectra (figure 3). Efforts were made to bleach the accumulated photoproduct, but no photoconversion was observed. However, as the MSP measurements were performed on a mass of microvilli, possibly originating from multiple photoreceptors, photoconversion analysis is confounded by the possibility of diffusion of visual pigments in their active form. Bleaching with various durations and wavelengths was also tested, and revealed just different degrees of bleaching, but no shift in peak absorbance (data not shown). Hence, all indications of the present study point towards a vertebrate-like visual pigment in box jellyfish.
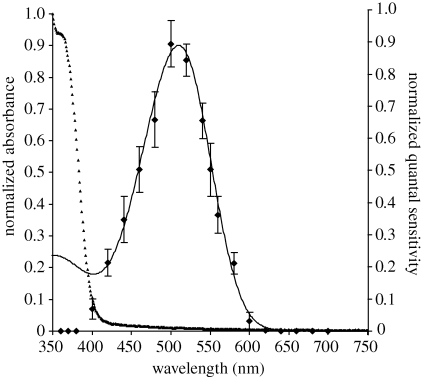
The mean pre-bleach spectrum of the upper lens eye is slightly wider than the fitted rhodopsin template at shorter wavelengths, and thus indicates the presence of a stable photoproduct (figure 2a). By contrast, the mean pre-bleach spectrum of the lower lens eye did not appear to contain a significant amount of photoproduct, and matched the fitted rhodopsin template closely (figure 2b). A relative increase in photoproduct, present after bleaching in both eyes, is demonstrated by the difference spectra in figure 3. The relative increase in absorbance at shorter wavelengths is larger for the lower lens eye than for the upper lens eye (figure 3). The different amounts of pre-bleach photoproducts may reflect differences in the state of dark adaptation of the two eyes prior to measurement. The lens of the upper lens eye was found to strongly absorb wavelengths shorter than 400 nm (figure 4), thus explaining the absence of a β-peak in the spectral sensitivity curves in the previous electrophysiological measurements (Garm et al. 2007a).
Figure 4.
The lens of the upper lens eye strongly absorbs light at shorter wavelengths (small triangles). Previous spectral sensitivity measurements obtained by extracellular electrophysiological recordings lacked a β-peak (Garm et al. 2007a). The absorbance properties of the lens fully accounts for this absence. Here, the spectral sensitivity measurements have been converted to absorptance (diamonds with s.e.m. bars) using a path length of 35 µm (Coates et al. 2006) and a typical invertebrate specific absorbance value of 0.0067 µm−1 (Warrant & Nilsson 1998). Solid line corresponds to a λmax, 510 nm, A1-Govardovskii rhodopsin template.
(b). Immunohistochemistry
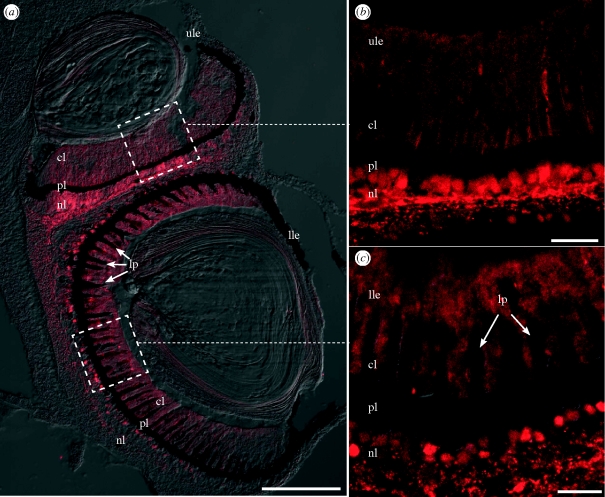
Antibodies against five different zebrafish opsins (Vihtelic et al. 1999) were reacted against cryosections of jellyfish eyes, but only the antibody against zebrafish UV opsin-labelled any of the photoreceptors (figure 5). Only the photoreceptors of the upper and lower lens eyes were immunoreactive, leaving the photoreceptors of the pit and slit eyes unlabelled. The labelling patterns of the photoreceptors in the two lens eyes were similar. Both outer segments and cell bodies were immunoreactive. The rather homogeneous labelling of the outer segments in the entire retinas indicates that all receptor cells are immunoreactive for zebrafish UV opsin, although the cell bodies were heterogeneously labelled. Abundant immunoreactive structures with the appearance of neuronal processes were observed in the neuropil layer surrounding the lens eyes. Because immunoreactive cell bodies were not observed outside the retinas of the lens eyes, the labelled neuronal processes were interpreted as photoreceptor basal processes.
Figure 5.
The photoreceptors of both the upper (ule) and lower (lle) lens eyes of C. bronzie stained with zebrafish UV opsin antibody. Sagittal sections of the lens eyes show the three layers of the retina; ciliary layer (cl), pigment layer (pl) and neural layer (nl). In addition to photoreceptors, the lower lens eye contains long-pigmented cells (lp). Labelling occurred in the cell bodies in the neural layer and the outer segments in the ciliary layer in both lens eyes. (a) Sagittal section of both lens eyes at low magnification. (b) Higher magnification of the upper lens eye retina. (c) Higher magnification of the lower lens eye retina. Scale bars, (a) 100 µm and (b,c) 20 µm.
4. Discussion
The MSP and immunohistochemical data collected in this study suggest that each of the lens eyes in the box jellyfish species C. bronzie possesses only one visual pigment. Furthermore, the similarity of the calculated λmax values between the two lens eyes and the similar immunostaining pattern throughout their ciliary layers indicate that the upper and lower lens eyes use the same opsin protein.
The techniques used here allow the entire retina to be investigated, overcoming the limitation of previous electrophysiological studies where only the lateral parts of the retina could be accessed (Coates et al. 2006; Garm et al. 2007a). Our finding of λmax values of the upper and lower lens eyes, 508 and 507 nm, respectively, are similar to those previously obtained from C. bronzie by electrophysiological measurements (Garm et al. 2007a), which were 510 and 497 nm for the upper and lower lens eye, respectively.
The MSP measurements of the lens of the upper lens eye show that it acts as a UV filter, absorbing wavelengths below 400 nm. This explains the absence of a β-peak (Govardovskii et al. 2000) in the spectral sensitivity curves from the lens eye obtained by electrophysiological measurements (Garm et al. 2007a). Further, this suggests that the lens protects the retina from UV damage, and potentially improves visual contrast by absorbing scattered short-wavelength light (Lythogoe 1979).
The post-bleach spectra obtained in this study indicate that the visual pigment in the upper and lower lens eye in C. bronzie undergo a bleaching similar to that of vertebrate visual pigments. In vertebrate visual pigments, photoisomerization of the chromophore leads to the hydrolysis of the Schiff-base bond between opsin and chromophore (Pepe 2001). In invertebrates, photoisomerization of the chromophore does not break the bond between opsin and chromophore, but instead results in a stable metarhodopsin in which the chromophore can be photoconverted to the 11-cis isoform, thus restoring the original visual pigment (Brown & Brown 1958; Hubbard & St George 1958). Recently, Koyanagi et al. (2008) expressed the opsin of the box jellyfish species Carybdea rastonii in HEK293S cells. Irradiation of the reconstituted visual pigment yielded a photostable photoproduct with a 455 nm peak and thereby also demonstrated that the visual pigments of box jellyfish are not bi-stable photopigments as those found in invertebrate rhabdomeric receptors. Differences in the shape of the post-bleach absorbance spectra of Koyanagi et al. (2008) and those of our study are most probably owing to differences in the intracellular environment between HEK293S cells and box jellyfish photoreceptors.
The presence of considerable amounts of photoproducts in upper lens-eye pre-bleach spectra indicates that the preparation was not fully dark-adapted. However, both lens eyes were subject to the same treatment and the lower lens eye did not show significant levels of photoproduct in the pre-bleach spectrum. Hence, rates of visual pigment regeneration may differ between the two lens eyes. One morphological difference between the lens eyes is the presence of long-pigmented cells in the lower lens eye (O'Connor et al. 2009, figure 5a). It is possible that the long-pigmented cells take part in photopigment regeneration, resulting in a proportionally larger amount of regeneration components available in the lower lens eye than in the upper lens eye. This said, mechanisms of photopigment regeneration in box jellyfish are unknown. Alternatively, the lower lens eye may have been less bleached at the beginning of dark adaptation owing to shielding by the long-pigmented cells.
As discussed previously, the photopigment expressed by box jellyfish appears to bleach like a vertebrate visual pigment. Vertebrate visual pigments regenerate via enzymatic pathways involving an isomerohydrolase (Lamb & Pugh 2004) or photoisomerases belonging to the opsin protein family (Chen et al. 2001; Terakita 2005). To date, screening for opsins in box jellyfish has not indicated the presence of any photoisomerase-like opsins (Koyanagi et al. 2008; Kozmik et al. 2008). Hence, the mechanisms of photopigment regeneration in box jellyfish remain elusive. The recently sequenced box jellyfish opsins belong to the newly created opsin clade, ‘cnidops’, which appears to be a sister group to the rhabdomeric + Go opsin clade (Plachetzki et al. 2007; Koyanagi et al. 2008). Indeed, box jellyfish opsins may use yet unknown mechanisms of photoactivation/photoregeneration, which can potentially help elucidate the evolution of the photocycle of visual pigments.
The two box jellyfish opsins sequenced hitherto are expressed in the retina of the upper and lower lens eyes, but not in the slit or pit eyes (Koyanagi et al. 2008; Kozmik et al. 2008). These studies, combined with the immunohistochemical investigation presented here, suggest that the lesser eyes employ a different opsin or a light-sensitive molecule when compared with the lens eyes. Unfortunately, we were unable to identify outer segments from the pit or slit eyes on which to perform MSP. Therefore, the possibility that multiple visual pigment types are present in the box jellyfish visual system still remains; however, MSP does not give any indication that the lens eyes are based on more than a single visual pigment.
Acknowledgements
All experiments conducted in this study comply with the ‘Principles of animal care’, publication no. 86-23, revised 1985 of the National Institute of Health and with the current laws for animal care in Australia and Sweden.
The authors wish to thank Dr Thomas Vihtelic for generously providing antibodies against zebrafish UV opsin, Eva Landgren and Carina Rasmussen for expert technical assistance and Jamie Seymour, Matthew Gordon and Teresa Carrette for support and assistance in the field. The authors would like to acknowledge financial support: grants from the Swedish Research Council and Swedish Foundation for Strategic Research (BioX) to D.-E.N., an Australian Research Council Discovery Project Grant (DP0558681) and ARC QEII Fellowship to N.S.H. and an ARC grant to N.J.M. and a grant (no. 272-07-0163) from the Danish Research Council (FNU) to A.G.
References
- Arendt D., Wittbrodt J.2001Reconstructing the eyes of Urbilateria. Phil. Trans. R. Soc. Lond. B 356, 1545–1563 (doi:10.1098/rstb.2001.0971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. K., Brown P. S.1958Visual pigments of octopus and cuttlefish. Nature 182, 1288–1290 (doi:10.1038/1821288a0) [DOI] [PubMed] [Google Scholar]
- Buskey E. J.2003Behavioral adaptations of the cubozoan medusa Tripedalia cystophora for feeding on copepod (Dioithona oculata) swarms. Mar. Biol. 142, 225–232 (doi:10.1007/s00227-002-0938-y) [Google Scholar]
- Chen P., et al. 2001A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat. Genet. 28, 256–260 (doi:10.1038/90089) [DOI] [PubMed] [Google Scholar]
- Coates M. M., Garm A., Theobald J. C., Thompson S. H., Nilsson D.-E.2006The spectral sensitivity of the lens eyes of a box jellyfish, Tripedalia cystophora (Conant). J. Exp. Biol. 209, 3758–3765 (doi:10.1242/jeb.02431) [DOI] [PubMed] [Google Scholar]
- Dartnall H. J. A.1953The interpretation of spectral sensitivity curves. Br. Med. Bull. 9, 24–30 [DOI] [PubMed] [Google Scholar]
- Dartnall H. J. A., Lythgoe J. N.1965The spectral clustering of visual pigments. Vis. Res. 5, 81–100 (doi:10.1016/0042-6989(65)90057-X) [DOI] [PubMed] [Google Scholar]
- Ekström P., Garm A., Palsson J., Vihtelic T. S., Nilsson D.-E.2008Immunohistochemical evidence for multiple photosystems in box jellyfish. Cell Tissue Res. 333, 115–124 (doi:10.1007/s00441-008-0614-8) [DOI] [PubMed] [Google Scholar]
- Garm A., Bielecki J.2008Swim pacemakers in box jellyfish are modulated by the visual input. J. Comp. Physiol. A 194, 641–651 (doi:10.1007/s00359-008-0336-0) [DOI] [PubMed] [Google Scholar]
- Garm A., Coates M. M., Gad R., Seymour J., Nilsson D.-E.2007aThe lens eyes of the box jellyfish Tripedalia cystophora and Chiropsalmus sp. are slow and color-blind. J. Comp. Physiol. A 193, 547–557 (doi:10.1007/s00359-007-0211-4) [DOI] [PubMed] [Google Scholar]
- Garm A., O'Connor M., Parkefelt L., Nilsson D.-E.2007bVisually guided obstacle avoidance in the box jellyfish Tripedalia cystophora and Chiropsella bronzie. J. Exp. Biol. 210, 3616–3623 (doi:10.1242/jeb.004044) [DOI] [PubMed] [Google Scholar]
- Garm A., Andersson F., Nilsson D.-E.2008Unique structure and optics of the lesser eyes of the box jellyfish Tripedalia cystophora. Vis. Res. 48, 1061–1073 (doi:10.1016/j.visres.2008.01.019) [DOI] [PubMed] [Google Scholar]
- Gershwin A.2006Comments on Chiropsalmus (Cnidaria: Cubozoa: Chirodropida): a preliminary revision of the Chiropsalmidae, with descriptions of two new genera and two new species. Zootaxa 1231, 1–42 [Google Scholar]
- Govardovskii V. I., Fyhrquist N., Reuter T., Kuzmin D. G., Donner K.2000In search of the visual pigment template. Vis. Neurosci. 17, 509–528 (doi:10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- Hart N. S.1998Avian photoreceptors Bristol, UK: University of Bristol [Google Scholar]
- Hart N. S.2004Microspectrophotometry of visual pigments and oil droplets in a marine bird, the wedge-tailed shearwater Puffinus pacificus: topographic variations in photoreceptor spectral characteristics. J. Exp. Biol. 207, 1229–1240 (doi:10.1242/jeb.00857) [DOI] [PubMed] [Google Scholar]
- Hubbard R., St George R. C.1958The rhodopsin system of the squid. J. Gen. Physiol. 41, 501–528 (doi:10.1085/jgp.41.3.501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M., Takano K., Tsukamoto H., Ohtsu K., Tokunaga F., Terakita A.2008Jellyfish vision starts with cAMP signaling mediated by opsin-Gs cascade. Proc. Natl Acad. Sci. USA 105, 15 576–15 580 (doi:10.1073/pnas.0806215105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z., et al. 2008Assembly of the cnidarian camera-type eye from vertebrate-like components. Proc. Natl Acad. Sci. USA 105, 8989–8993 (doi:10.1073/pnas.0800388105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D.2009Evolution of vertebrate retinal photoreception. Phil. Trans. R. Soc. B 364, 2911–2924 (doi:10.1098/rstb.2009.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr2004Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 23, 307–380 (doi:10.1016/j.preteyeres.2004.03.001) [DOI] [PubMed] [Google Scholar]
- Lythogoe J. N.1979The ecology of vision Oxford, UK: Clarendon Press [Google Scholar]
- MacNichol E. F., Jr1986A unifying presentation of photopigment spectra. Vis. Res. 26, 1543–1556 [DOI] [PubMed] [Google Scholar]
- Martin V. J.2004Photoreceptors of cubozoan jellyfish. Hydrobiologia 530/531, 135–144 (doi:10.1007/s10750-004-2674-4) [Google Scholar]
- Maximov V. V.2000Environmental factors which may have led to the appearance of colour vision. Phil. Trans. R. Soc. Lond. B 355, 1239–1242 (doi:10.1098/rstb.2000.0675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C. E.1984Density is altered in Hydromedusae and Ctenophores in response to changes in salinity. Biol. Bull. 166, 206–215 (doi:10.2307/1541442) [Google Scholar]
- Nilsson D.-E., Gilsén L., Coates M., Skogh C., Garm A.2005Advanced optics in a jellyfish eye. Nature 435, 201–205 (doi:10.1038/nature03484) [DOI] [PubMed] [Google Scholar]
- O'Connor M., Garm A., Nilsson E.2009Structure and optics of the eyes of the box jellyfish Chiropsella bronzie. J. Comp. Physiol. A 195, 557–568 [DOI] [PubMed] [Google Scholar]
- Pepe I. M.2001Recent advances in our understanding of rhodopsin and phototransduction. Prog. Retin. Eye Res. 20, 733–759 (doi:10.1016/S1350-9462(01)00013-1) [DOI] [PubMed] [Google Scholar]
- Plachetzki D. C., Degnan B. M., Oakley T. H.2007The origins of novel protein interactions during animal opsin evolution. PLoS ONE 2, e1054 (doi:10.1371/journal.pone.0001054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakita A.2005The opsins. Genome Biol. 6, 213 (doi:10.1186/gb-2005-6-3-213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic T. S., Doro C. J., Hyde D. R.1999Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis. Neurosci. 16, 571–585 [DOI] [PubMed] [Google Scholar]
- Warrant E., Nilsson E.1998Absorption of white light in photoreceptors. Vision Res. 38, 195–207 (doi:10.1016/S0042-6989(97)00151-X) [DOI] [PubMed] [Google Scholar]
- Weber C.1982Electrical activity in response to light of the ocellus of the hydromedusan Sarsia Tubulosa. Biol. Bull. 162, 413–422 [Google Scholar]
- Yamasu T., Yoshida M.1976Fine structure of complex ocelli of a cubomedusan, Tamoya-Bursaria Haeckel. Cell Tissue Res. 170, 325–339 (doi:10.1007/BF00219415) [DOI] [PubMed] [Google Scholar]
- Yokoyama S.2000Molecular evolution of vertebrate visual pigments. Prog Retin. Eye Res. 19, 385–419 (doi:10.1016/S1350-9462(00)00002-1) [DOI] [PubMed] [Google Scholar]