Abstract
Yeasts are ubiquitous in terrestrial and aquatic microbiota, yet their ecological functionality remains relatively unexplored in comparison with other micro-organisms. This paper formulates and tests the novel hypothesis that heat produced by the sugar catabolism of yeast populations inhabiting floral nectar can increase the temperature of floral nectar and, more generally, modify the within-flower thermal microenvironment. Two field experiments were designed to test this hypothesis for the winter-blooming herb Helleborus foetidus (Ranunculaceae). In experiment 1, the effect of yeasts on the within-flower thermal environment was tested by excluding them from flowers, while in experiment 2 the test involved artificial inoculation of virgin flowers with yeasts. Nectary temperature (Tnect), within-flower air temperature (Tflow) and external air temperature (Tair) were measured on experimental and control flowers in both experiments. Experimental exclusion of yeasts from the nectaries significantly reduced, and experimental addition of yeasts significantly increased, the temperature excess of nectaries (ΔTnect = Tnect − Tair) and the air space inside flowers in relation to the air just outside the flowers. In non-experimental flowers exposed to natural pollinator visitation, ΔTnect was linearly related to log yeast cell density in nectar, and reached +6°C in nectaries with the densest yeast populations. The warming effect of nectar-dwelling yeasts documented in this study suggests novel ecological mechanisms potentially linking nectarivorous microbes with winter-blooming plants and their insect pollinators.
Keywords: floral microbiology, floral microclimate, Helleborus foetidus, Metschnikowia reukaufii, nectar yeasts, yeast communities
1. Introduction
Micro-organisms play pivotal functions in ecosystems, such as promoting nutrient cycling and mediating important interactions between the biotic and abiotic components of the enviroment (Paul 2007; Peay et al. 2008). Unicellular fungi, usually known as yeasts, are ubiquitous in terrestrial and aquatic habitats, including such extreme environments as deep-sea hydrothermal vents, polar soils or extreme acidic continental waters (Gadanho & Sampaio 2005; Gadanho et al. 2006; Connell et al. 2008). Yeasts form part of hyperdiverse microbiota associated with plant surfaces and soil, but in comparison with other components of these microbial communities, their biodiversity and ecological functionality remains relatively unexplored (Lachance 2006). This contrasts with the vast amount of effort devoted to elucidating the ecology of yeasts of economic importance in highly artificial environments, notably baker's yeast (Saccharomyces cerevisiae) (Goddard 2007; Replansky et al. 2008). Increasing evidence is showing, however, that the yeast component of microbial communities in natural habitats plays significant ecological functions, including litter decomposition, favouring root growth and plant performance, enhancing plant–mycorrhiza interactions, and establishing mutualistic links with plants and invertebrates (Lachance et al. 2001; Sampaio et al. 2007; Boby et al. 2008; Cloete et al. 2009; Rodrigues et al. 2009). Particularly intriguing from an evolutionary ecological viewpoint are recent findings showing that some yeasts exploit mutualistic interactions, leading to complex symbioses where phyla from three kingdoms are involved. For example, in the tripartite mutualism between fungus-growing ants, their fungal cultivars and antibiotic-producing bacteria, black yeasts intimately associated with the ants compromise their antibiotic defenses by consuming the ants' mutualistic bacteria (Little & Currie 2008). In the flowers of some bumble-bee-pollinated plants, dense populations of specialized nectarivorous yeasts deplete the sugar content of nectar, which reduces the reward available to the plants' mutualistic pollinators (Herrera et al. 2008; de Vega et al. 2009).
Ascertaining the effect of yeasts on the other participants in these mutualistic systems may prove elusive, partly because the consequences of such interactions may be subject to subtle context-dependent shifts (Thompson 1988), but also because intricate combinations of direct and indirect cascading effects are to be expected whose elucidation may require complex experiments that will not always be feasible under natural conditions (Boby et al. 2008; Little & Currie 2008; Wiens et al. 2008). As a first step towards that goal, however, proximate consequences of the activity of yeasts that could potentially impinge upon the dynamics of mutualistic interactions should be identified. In the case of nectar-dwelling yeasts, depletion of the nectar's energy content through metabolic degradation of sugar is one of such consequences (Herrera et al. 2008; de Vega et al. 2009), but some connected effects may also be envisaged. Both fermentative and fermentative–oxidative metabolic activity of yeasts produce significant amounts of heat, particularly during exponential growth and when exposed to high carbon : nitrogen ratios (Gustafsson 1991; Cooney et al. 1996; Lamprecht 2003), as ordinarily found in floral nectar (Nicolson et al. 2007). We therefore hypothesized that, in natural conditions, the extensive sugar catabolism of quickly growing nectar yeast populations could increase the temperature of nectar and, more generally, modify the within-flower thermal microenvironment. The hypothesized floral warming effect of nectar yeasts would be most ecologically relevant in environments where low ambient temperature often limits pollinator visitation, pollen germination, fertilization success and fruit development (Corbett et al. 1992; Jakobsen & Martens 1994; Herrera 1995; Kudo 1995; Krannitz 1996; McKee & Richards 1998). We report in this paper an experimental field study testing and verifying the novel hypothesis of thermal modification of the floral microenvironment by nectar yeasts, using as study subject the bumble-bee-pollinated Helleborus foetidus (Ranunculaceae), a winter-flowering plant whose nectar harbours dense yeast populations (Brysch-Herzberg 2004; Herrera et al. 2008). Although our goal here is only to demonstrate experimentally for the first time an hitherto unrecognized biological phenomenon rather than evaluating its ecological consequences, yeast-induced floral warming can under some circumstances have significant implications for plants and pollinators. As reviewed in §4, in cool environments floral warming can benefit both the plants (e.g. by faster growth of pollen tubes) and the pollinators (by providing a heat reward), and yeasts can become important floral warming agents for plants living in shady forest understories, which are unable to use direct sunshine to warm their flowers.
2. Material and methods
Helleborus foetidus is a perennial herb widely distributed in western and southwestern Europe. Plants produce one or a few inflorescences in early–mid winter, each bearing 20–75 flowers that open gradually over the following 1.5–2.5 months. Individual flowers last for one to three weeks, and are mostly pollinated by bumble-bees. The large globular perianth (length = 15–17 mm, width = 13–15 mm) consists exclusively of five large, overlapping green sepals, as the petals have become modified into nectaries. Each flower generally contains five nectaries shaped like flattened horns and hidden deeply inside the perianth. These form a distinct ring between the stamens and the sepals and, if unvisited, each nectary may contain up to 5 µl of sucrose-dominated nectar. Further details on the autoecology, floral biology and pollination biology of H. foetidus can be found in Vesprini et al. (1999), Herrera et al. (2001, 2006, 2008) and Canto et al. (2008).
This study was carried out during March–April 2009 at two H. foetidus populations in mountain habitats of the Sierra de Cazorla, Jaén province, southeastern Spain (‘Las Navillas’ and ‘Puerto Llano’ hereafter, 1220 m and 1810 m a. s. l., respectively). The two sites were 14 km apart, and at both localities H. foetidus plants were growing in the understory of mature Pinus nigra forests. Experiments were made at each site during the local flowering peak. Snowfalls were unusually frequent and abundant at the study region during the 2009 winter–early spring, which resulted in a generalized delay of the flowering season of H. foetidus in comparison with average years. Weather was cool and overcast on most study dates at both sites, with mean ambient temperature (±s.e.) being 7.3 ± 0.2°C (interquartile range = 5.9–8.6°C) and 10.0 ± 0.2°C (interquartile range = 6.1–14.2°C) in Las Navillas and Puerto Llano, respectively. In Puerto Llano, there were daily frosts and occasional snowfalls until late April, when this study had already begun and H. foetidus plants were at peak flowering there. Despite adverse weather, during study dates, bumble-bees were seen regularly visiting H. foetidus flowers at the two study sites.
Two complementary manipulative experiments were designed to test the hypothesized effect of nectar yeasts on the within-flower thermal environment. In experiment 1, the effect was tested by experimentally excluding yeasts from flowers, while in experiment 2 the test involved adding yeasts to flowers. In experiment 1, we compared flowers exposed to natural bumble-bee visitation (control) with virgin flowers from which visitors had been excluded with mesh bags (treatment). As yeast inocula are brought to H. foetidus nectar by foraging bumble-bees (Canto et al. 2008), the nectaries of naturally visited inflorescences should harbour yeasts frequently while those of unvisited inflorescences should not. The experiment followed a randomized block design. Pairs of neighbouring inflorescences (blocks) from different plants were selected at each study locality (n = 7 and 10 pairs in Las Navillas and Puerto Llano, respectively). In each pair, one inflorescence was bagged (treatment) after removing any open flower, which ensured that only virgin flowers would be present thereafter, and the other inflorescence (control) was left exposed to natural pollinator visitation. The assumption that exposed flowers harboured yeasts while virgin flowers did not was verified by microscopical examination of single-nectary nectar samples. At each locality, nectar samples from exposed, naturally visited flowers from plants in the neighbourhood of experimental pairs were examined microscopically (two nectaries from each of two flowers per plant, n = 9 and 10 plants, Las Navillas and Puerto Llano, respectively), and yeast cell density determined following the methods described by Herrera et al. (2009). Single-nectary nectar samples from virgin flowers within pollinator exclosures were also examined microscopically (n = 10 and 30, Las Navillas and Puerto Llano, respectively).
In experiment 2, the thermal environment within virgin flowers that opened inside pollinator exclosures (control) was compared with that of similarly virgin flowers whose nectaries had been experimentally inoculated with yeasts (treatment) and remained subsequently within exclosures for the whole experiment. Experiment 2 was performed only at the Puerto Llano site, according to a randomized block design. Newly open virgin flowers within each of 10 bagged inflorescences (blocks) were randomly assigned to either control or treatment groups. Every nectary of all flowers in the treatment group (n = 214 nectaries in 43 flowers) was inoculated with yeasts by injecting into the nectar 1 µl of a liquid culture of Metschnikowia reukaufii, the dominant yeast in H. foetidus nectar at the study region (Herrera et al. 2010). The culture had an estimated density of 5.15 × 104 cells mm−3 and was started with a single M. reukaufii strain isolated from the glossa of a bumble-bee captured while foraging on H. foetidus flowers at Las Navillas (Herrera et al. 2010). Efficacy of the inoculation treatment for establishing yeast populations in the nectaries of experimental flowers was verified at the end of the experiment by microscopically estimating cell density in nectar samples from n = 155 inoculated nectaries.
In both experiments, the following variables were measured on individual flowers using ultra fast (time constant = 0.005 s), fine (tip diameter = 0.22 mm) type T thermocouples (model IT-23, Physitemp Instruments, Clifton, NJ) connected to a digital thermometer: nectary temperature, Tnect, was measured on a single nectary per flower by dipping the thermocouple tip into the nectar; within-flower air temperature, Tflow, was measured halfway between nectary aperture and flower opening; and external air temperature, Tair, was measured approximately 10 mm away from the flower opening (figure 1a). The order of Tnect, Tflow and Tair measurements on individual flowers was altered systematically to prevent spurious patterns. In experiment 1, temperature measurements were delayed 6 days after bagging of experimental inflorescences to allow for the opening of new, virgin flowers within exclosures. In experiment 2, measurements were delayed 3 days after nectary inoculation to allow for growth of yeast populations. To avoid the effects of direct solar radiation on the few sunny days during the study period, temperatures were always measured when experimental plants had been in the shade for at least 1 h before measurement.
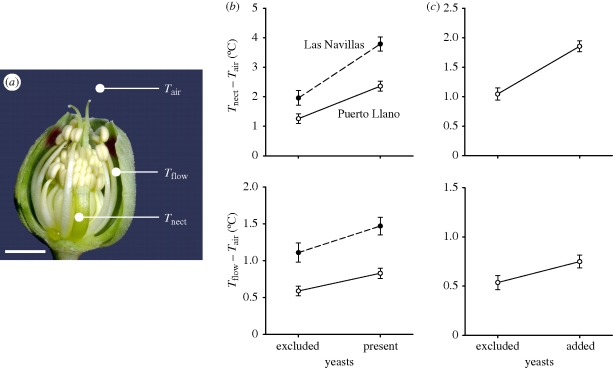
Figure 1.
(a) Flower of H. foetidus with the perianth partly removed, showing the three temperature measurement points (Tnect, Tflow and Tair). One translucent nectary almost full of nectar is visible in the foreground. Scale bar, 5 mm. (b,c) Model-adjusted, least-squares means (±1 s.e.) of temperature excess of the nectary (ΔTnect = Tnect − Tair) and the air inside the flower (ΔTflow = Tflow − Tair) in experiments 1 and 2, respectively. For experiment 1, means are shown separately for the two study sites. See table 1 for statistical significance of yeast, site and yeast × site effects.
In addition to experiments 1 and 2, the relationship between naturally occurring yeast populations and the within-flower thermal microenvironment was further investigated by examining the relationship between ΔTnect and yeast cell density across individual nectaries. Seven H. foetidus inflorescences exposed to natural pollinator visitation were collected from a site near Las Navillas, taken indoors, and kept overnight in the dark at room temperature (range 17–21°C) in water-filled plastic vases. Next morning, Tair and Tnect were measured for individual nectaries (n = 46) as detailed above for field measurements. Yeast cell density was then estimated for the same nectaries by microscopical examination within 5–15 min of temperature measurements.
The temperature excess of the nectar (ΔTnect = Tnect − Tair) and the air within the flower (ΔTflow = Tflow − Tair) in relation to the air just outside the flower were used as response variables in statistical analyses of experiments 1 and 2. Significance of treatment effects on ΔTnect and ΔTflow were tested by fitting mixed model analysis of variance (ANOVAs) to the data using SAS procedure MIXED, with treatment as fixed effect, and blocks and flowers within blocks as random effects. In experiment 1, site (Las Navillas and Puerto Llano) and site × treatment effects were also included as fixed effects in the model. The LSMEANS statement was used to obtain model-adjusted least-squares means and standard errors of response variables for treatment levels. All means will be reported ±1 s.e.
3. Results
On the study dates, nectar from H. foetidus flowers exposed to natural pollinator visitation almost invariably contained yeasts at both study sites, often at very high densities. Yeasts were present in 100 per cent and 92.5 per cent of single-nectary nectar samples from Las Navillas (n = 36) and Puerto Llano (n = 40), respectively. Judging by cell morphology, M. reukaufii was the only yeast species present in all samples examined microscopically. This was corroborated by sequencing the D1/D2 domain of the 26S subunit ribosomal DNA for a sample of yeast isolates from H. foetidus nectar samples from both study sites, following the methods of Herrera et al. (2010). In Las Navillas, mean yeast density was 3.29 × 104 ± 4.16 × 103 cells mm−3 (range = 3.57 × 102–9.46 × 104 cells mm−3, n = 36). In Puerto Llano, mean density for yeast-containing samples was 4.82 × 104 ± 7.90 × 103 cells mm−3 (range = 5.87 × 102–1.94 × 105 cells mm−3, n = 37). In experiment 2, virgin flowers artificially inoculated with yeasts had a mean cell density of 8.02 × 104 ± 6.73 × 103 cells mm−3 (range = 1.75 × 103–5.38 × 105 cells mm−3, n = 155) by the end of the experiment. None of the n = 40 nectar samples from virgin flowers within exclosures examined microscopically contained yeasts.
The interior of H. foetidus flowers was significantly warmer on average than the air just outside flowers. In experiment 1, mean thermal excess of nectar (ΔTnect = 1.6 ± 0.15 and 3.1 ± 0.15°C, treatment and control flowers, respectively) and air (ΔTflow = 0.8 ± 0.07 and 1.2 ± 0.07°C, treatment and control flowers, respectively) were significantly greater than zero (p < 0.0001 in all cases). Corresponding figures for experiment 2 were 1.0 ± 0.10 and 1.9 ± 0.09°C for ΔTnect, and 0.5 ± 0.07 and 0.7 ± 0.07°C for ΔTflow, all of which were also significantly greater than zero (p < 0.0001). Flowers with and without yeasts differed in their thermal excesses, as denoted by the highly significant yeast effect on ΔTnect and ΔTflow in the two experiments (table 1). Irrespective of whether yeast populations were natural (experiment 1) or established artificially (experiment 2), both ΔTnect and ΔTflow were greater in flowers whose nectaries contained yeasts than in those that did not (figure 1) or, in other words, flowers with yeasts had significantly warmer interiors than flowers without yeasts. The estimated warming effect of yeasts on Tnect was +1.5 ± 0.14 and +0.8 ± 0.12°C, and on Tflow +0.3 ± 0.09 and +0.2 ± 0.07°C, in experiments 1 and 2, respectively. In experiment 1, there was a significant site × yeast interaction effect on ΔTnect (table 1), denoting that the thermal effect of yeasts differed between sites. The difference between sites involved only the magnitude of the effect, which was greater in Las Navillas (+1.8°C) than in Puerto Llano (+1.1°C) (figure 1b).
Table 1.
Summary of mixed model ANOVAs testing for the effects of nectar yeasts on the temperature excess of nectar (ΔTnect) and air inside the flower (ΔTflow), in relation to the temperature of the air immediately outside the floral perianth (figure 1a). In experiment 1, the ‘yeasts’ effect refers to the comparison of flowers exposed to pollinator visitation (control) with flowers from which yeasts had been excluded (treatment). In experiment 2, yeasts effect refers to the comparison between virgin flowers that opened inside pollinator exclosures (control) and virgin flowers whose nectaries had been inoculated with yeasts (treatment). The site effect in experiment 1 refers to differences between study sites (Las Navillas and Puerto Llano). Experiment 2 was performed only at Puerto Llano.
| dependent variable |
||||||
|---|---|---|---|---|---|---|
|
ΔTnect = Tnect − Tair |
ΔTflow = Tflow − Tair |
|||||
| effect | d.f. | F | p | d.f. | F | p |
| experiment 1 | ||||||
| yeasts | 1,229 | 112.21 | <0.0001 | 1,229 | 10.02 | 0.0018 |
| site | 1,15 | 16.00 | 0.0012 | 1,15 | 27.72 | <0.0001 |
| yeasts × site | 1,229 | 7.04 | 0.0085 | 1,229 | 0.42 | 0.52 |
| experiment 2 | ||||||
| yeasts | 1,132 | 42.52 | <0.0001 | 1,132 | 8.17 | 0.0049 |
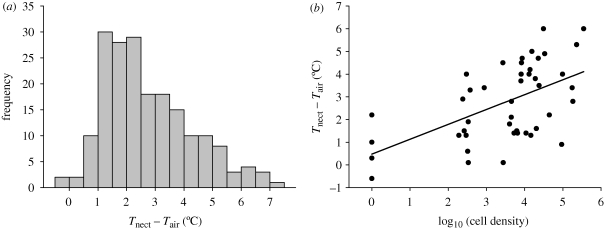
Model-adjusted means referred to in the preceding paragraph reflect central trends, but temperature excesses varied widely, as illustrated by the frequency distribution of ΔTnect for non-experimental flowers in experiment 1 (i.e. flowers exposed to natural pollinator visits and presumably containing yeasts) (figure 2a). Variation among nectaries in temperature excess was partly explained by differences in yeast density, as shown by the significant direct relationship between ΔTnect and cell density (log-transformed) in nectaries of field-collected inflorescences kept indoors in a thermally stable ambient (figure 2b). Variation in log cell density accounted for 35 per cent of variance in ΔTnect, and the slope of the regression line indicates that increasing cell density by a factor of 10 led to a predicted increase of 0.66°C in ΔTnect (figure 2b). Yeast densities approximately 1.50 × 105 cells mm−3 were associated with ΔTnect of up to 5–6°C. The relationship between ΔTnect and log cell density remained statistically significant after omitting from computations the nectar samples without yeasts (y = −0.151 + 0.810x; r2 = 0.21, F1,38 = 10.3, p = 0.0027), which further strengthens the conclusion that yeast presence was causatively linked to nectary temperature excess.
Figure 2.
(a) Frequency distribution of nectary temperature excess (ΔTnect = Tnect − Tair) in control H. foetidus flowers from experiment 1 (i.e. flowers exposed to natural pollinator visitation and presumably containing yeasts). (b) Relationship between nectary temperature excess and yeast cell density in the same nectaries of field-collected, non-experimental H. foetidus inflorescences exposed to pollinator visitation. Line is the least-squares-adjusted regression (y = 0.475 + 0.655x; r2 = 0.35, F1,44 = 24.0, p < 0.0001).
4. Discussion
Results of this study support our hypothesis that metabolic heat produced by nectarivorous yeasts alters the within-flower thermal environment of winter-blooming H. foetidus. Flowers with yeast-containing nectaries had warmer interiors than flowers with ‘clean’ nectar, and the magnitude of warming depended on the density of yeast populations in nectar. Experimental exclusion of yeasts from the nectaries significantly reduced (experiment 1), and experimental addition of yeasts to virgin flowers significantly increased (experiment 2), the temperature excess of the nectaries and of the air inside flowers in relation to the surrounding air. Furthermore, nectary temperature excess was directly related to yeast population density in nectar. One unexpected result was the finding that flowers without yeasts also exhibited statistically significant, albeit quantitatively minor, thermal excess in the nectaries and air within flowers. This warming possibly reflects the respiratory activity of stamens or carpels, as thermogenic activity by the thin-walled, holocrine nectaries themselves seems unlikely (Vesprini et al. 1999, 2008; J. Vesprini 2009, personal communication), but this interpretation is speculative until confirmed by experimentation.
Thermal excess of floral structures with respect to the immediately surrounding air has been documented for many species from a variety of plant families and ecological scenarios, including tropical habitats, Arctic and alpine environments, and the winter season of temperate habitats. These previous investigations have shown that floral warming either is the outcome of active, respiration-based endothermy by floral structures themselves (Seymour & Schultze-Motel 1997; Lamprecht et al. 2002), or arises from passive absorption of incident solar irradiance, often enhanced by floral heliotropism (sun-tracking), flower colour, or perianth architecture (Stanton & Galen 1989; Herrera 1995; Kudo 1995; Totland 1996; McKee & Richards 1998; Orueta 2002; Sapir et al. 2006). The present study has documented a third, novel mechanism whereby flowers can raise their temperatures over that of the ambient, namely by harbouring dense populations of heat-dissipating yeasts in their nectaries. Details on the sequence and types of sugar metabolism by M. reukaufii, the dominant yeast in H. foetidus nectar (Herrera et al. 2010), are not known, but the species is able to metabolize sucrose (Barnett et al. 2000), the dominant sugar in H. foetidus nectar (Canto et al. 2008). Under natural field conditions, the dense populations of M. reukaufii metabolize the sugar of nectar down to almost complete exhaustion (Herrera et al. 2008). We therefore suggest that the thermal effects associated with the presence of M. reukaufii in H. foetidus nectar is the outcome of intense fermentative–oxidative sugar metabolism, as found for other yeasts in artificial environments during the exponential growth phase, particularly when it takes place under extreme C : N imbalance (Gustafsson 1991; Cooney et al. 1996; Goddard 2007), as typically found in floral nectar.
As revealed by experiment 1, the mean thermal effect of yeasts on nectary temperature was +1.8 and +1.1°C in Las Navillas and Puerto Llano, respectively. About one-fifth of individual nectaries in exposed flowers had thermal excesses ≥4°C, and excesses of 5–7°C were not exceptional (figure 2). These figures are appreciably lower than those exhibited by flowers of endothermic species at peaks of heat production (Patiño et al. 2000; Lamprecht et al. 2002), but comparable with thermal excesses reported for passively heating flowers under direct solar radiation (Kjellberg et al. 1982; Stanton & Galen 1989; Corbett et al. 1992; Herrera 1995; Kudo 1995; Krannitz 1996; Totland 1996; Orueta 2002; Sapir et al. 2006). At the study region, H. foetidus mainly occupies the understory of montane evergreen forests, where plants are infrequently exposed to direct sunlight and the possibilities of floral warming by direct solar radiation are limited (Sánchez-Lafuente et al. 2005). The quantitative similarity between the thermal effect of yeasts on H. foetidus flowers and that of direct solar radiation on flowers from open habitats suggests the intriguing possibility that in cool, shady forest environments nectarivorous yeasts could act as a replacement of the floral warming role played by direct solar radiation. Consistent with this hypothesis are the observations that (i) the flowers of Primula vulgaris, another winter-blooming plant of shady forest understory in the study region, harbour dense populations of M. reukaufii in nectar and are warmer than the surrounding air (C. M. Herrera 2009, unpublished data); and (ii) M. reukaufii strains isolated from nectar of winter flowers are able to multiply profusely at ambient temperature near to freezing point (M. I. Pozo 2008, unpublished data).
Although we have not directly evaluated the ecological consequences of yeast-induced floral warming of H. foetidus flowers in this study, considerable circumstantial evidence suggests that some consequences are to be expected. Irrespective of whether it is produced by active endothermy or passive solar heating, previous studies have shown that floral warming can enhance plant reproduction through mechanisms including increased pollinator visitation, pollen germination, pollen tube growth, fertilization success, fruit development and seed size (Corbett et al. 1992; Jakobsen & Martens 1994; Herrera 1995; Kudo 1995; Krannitz 1996; Seymour & Schultze-Motel 1997; McKee & Richards 1998; Seymour et al. 2003). If, as it seems likely, the warming of H. foetidus flowers’ interior by yeasts raises the temperature of the gynoecium, then yeast warming could have beneficial effects on the maternal component of reproductive success, as found in other species. Floral warming could also increase reproductive success via enhanced pollinator visitation. Warmth alone can act as a metabolic reward for pollinators (Herrera 1995; Seymour et al. 2003; Dyer et al. 2006; Sapir et al. 2006), and this effect should be most likely under the cool conditions characteristic of the flowering season of H. foetidus. Small ectothermic pollinators of H. foetidus (e.g. Andrena) should benefit most from the broadened activity window provided by warmer flower interiors, as documented by Herrera (1995) for small bees foraging on passively warmed flowers of the early-blooming daffodil Narcissus longispathus, but enhanced pollinator visitation would not be necessarily restricted to them. The endothermic bumble-bee Bombus terrestris, one of the main pollinators of H. foetidus (Herrera et al. 2001), prefers to land on warmer flowers when given a choice between artificial flowers that differ slightly in temperature but are otherwise similar in nectar rewards (Dyer et al. 2006). In addition, B. terrestris foragers keep the head, thorax and most of the abdomen inside the perianth of H. foetidus flowers while foraging for nectar, hence they can also benefit not only from the warmer nectar but also from the warmer air in flower interiors. As pollinator visitation to H. foetidus flowers is infrequent and seed production may sometimes be pollen-limited (Herrera et al. 2001; Herrera 2002), any mechanism enhancing pollinator visitation such as floral warming might ultimately translate into improved fecundity. Foraging preferences of bumble-bees for warmer flowers, however, may vanish if higher floral temperature is associated with lower sugar reward (Whitney et al. 2008). In this study, the thermal excess of individual nectaries was directly related to yeast cell density, and the latter correlates inversely with per cent sugar in nectar as shown by Herrera et al. (2008). Warmer nectaries will thus be predictably associated with lower sugar concentration in nectar, and vice versa, as heating is just a byproduct of the metabolic degradation of sugars. This can pose a dilemma to bumble-bees foraging on H. foetidus flowers, which would have to base their choices on either warmth reward or sugar reward. This dilemma would mostly apply to choices among nectaries of the same flower, rather than among flowers in the same plant, as it is at the former level where most variance in yeast cell density and nectar sugar composition occur in H. foetidus populations (Herrera et al. 2006; Pozo et al. 2009). In any case, plausible scenarios may be envisaged where bumble-bees could shift from warmth-based choices (e.g. at low ambient temperatures near their lower thermal limit) to sugar-based ones (e.g. under non-limiting thermal environments). Further studies are needed to elucidate the effects of floral warming by yeasts on pollinator foraging and, ultimately, on pollen import and export of individual flowers.
As noted in §1, ascertaining the net effect of yeasts when they participate as third partners in mutualisms may prove elusive. Because they degrade floral nectar by metabolizing nectar sugar, a reward produced by plants to attract pollinators, nectarivorous yeasts should in principle be seen as parasitic exploiters of plant–pollinator mutualisms (Herrera et al. 2008). This interpretation, however, assumes that neither plants nor pollinators obtain a benefit from the presence of yeasts in nectar, an assumption that may sometimes be unwarranted. For example, the abundant alcohol accumulating in the nectar of a tropical palm as a consequence of yeast metabolism may ultimately enhance the attractiveness of inflorescences to alcohol-seeking mammalian pollinators (Wiens et al. 2008). Floral warming by yeasts documented in this study provides another example whereby yeasts in nectar could under some circumstances benefit plants, pollinators or both, as discussed above. Subtle trade-offs are to be expected between the advantages and disadvantages derived to mutualists from nectar contamination by yeasts, the ecological and evolutionary implications of which will only be elucidated by more observational and experimental work.
Acknowledgements
We are grateful to Carlos A. Herrera, André Lachance, Clara de Vega and José Vesprini for discussion and helpful comments on this manuscript, and Azucena Canto for providing the yeast culture for experiment 2. Permission to work in the Sierra de Cazorla was granted by Consejería de Medio Ambiente, Junta de Andalucía. Work supported by grants CGL2006-01355 and EXPLORA CGL2007-28866-E/BOS to C.M.H., and FPI Predoctoral Fellowship BES-2007-17142 to M.I.P., from Ministerio de Educación y Ciencia.
References
- Barnett J. A., Payne R. W., Yarrow D.2000Yeasts: characteristics and identification, 3rd edn Cambridge, UK: Cambridge University Press [Google Scholar]
- Boby V. U., Balakrishna A. N., Bagyaraj D. J.2008Interaction between Glomus mosseae and soil yeasts on growth and nutrition of cowpea. Microbiol. Res. 163, 693–700 (doi:10.1016/j.micres.2006.10.004) [DOI] [PubMed] [Google Scholar]
- Brysch-Herzberg M.2004Ecology of yeasts in plant–bumblebee mutualism in Central Europe. FEMS Microbiol. Ecol. 50, 87–100 (doi:10.1016/j.femsec.2004.06.003) [DOI] [PubMed] [Google Scholar]
- Canto A., Herrera C. M., Medrano M., Pérez R., García I. M.2008Pollinator foraging modifies nectar sugar composition in Helleborus foetidus L. (Ranunculaceae): an experimental test. Am. J. Bot. 95, 315–320 (doi:10.3732/ajb.95.3.315) [DOI] [PubMed] [Google Scholar]
- Cloete K. J., Valentine A. J., Stander M. A., Blomerus L. M., Botha A.2009Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a sclerophyllous medicinal shrub, Agathosma betulina (Berg.) Pillans. Microb. Ecol. 57, 624–632 (doi:10.1007/s00248-008-9457-9) [DOI] [PubMed] [Google Scholar]
- Connell L., Redman R., Craig S., Scorzetti G., Iszard M., Rodriguez R.2008Diversity of soil yeasts isolated from South Victoria Land, Antarctica. Microb. Ecol. 56, 448–459 (doi:10.1007/s00248-008-9363-1) [DOI] [PubMed] [Google Scholar]
- Cooney M. J., Marison I. W., van Gulik W. M., von Stockar U.1996Calorimetric and stoichiometric analysis of growth of Kluyveromyces fragilis in continuous culture: nitrogen limitation imposed upon carbon-limited growth. Appl. Microbiol. Biotechnol. 44, 643–653 (doi:10.1007/BF00172498) [Google Scholar]
- Corbett A. L., Krannitz P. G., Aarssen L. W.1992The influence of petals on reproductive success in the Arctic poppy (Papaver radicatum). Can. J. Bot. 70, 200–204 (doi:10.1139/b92-027) [Google Scholar]
- de Vega C., Herrera C. M., Johnson S. D.2009Yeasts in floral nectar of some South African plants: quantification and associations with pollinator type. S. Afr. J. Bot. 75, 798–806 (doi:10.1016/j.sajb.2009.07.016) [Google Scholar]
- Dyer A. G., Whitney H. M., Arnold S. E. J., Glover B. J., Chittka L.2006Bees associate warmth with floral colour. Nature 442, 525–525 (doi:10.1038/442525a) [DOI] [PubMed] [Google Scholar]
- Gadanho M., Sampaio J. P.2005Occurrence and diversity of yeasts in the mid-Atlantic ridge hydrothermal fields near the Azores Archipelago. Microb. Ecol. 50, 408–417 (doi:10.1007/s00248-005-0195-y) [DOI] [PubMed] [Google Scholar]
- Gadanho M., Libkind D., Sampaio J. P.2006Yeast diversity in the extreme acidic environments of the Iberian Pyrite Belt. Microb. Ecol. 52, 552–563 (doi:10.1007/s00248-006-9027-y) [DOI] [PubMed] [Google Scholar]
- Goddard M. R.2007Quantifying the complexities of Saccharomyces cerevisiae's ecosystem engineering via fermentation. Ecology 89, 2077–2082 (doi:10.1890/07-2060.1) [DOI] [PubMed] [Google Scholar]
- Gustafsson L.1991Microbiological calorimetry. Thermochim. Acta 193, 145–171 (doi:10.1016/0040-6031(91)80181-H) [Google Scholar]
- Herrera C. M.1995Floral biology, microclimate, and pollination by ectothermic bees in an early-blooming herb. Ecology 76, 218–228 (doi:10.2307/1940644) [Google Scholar]
- Herrera C. M.2002Censusing natural microgametophyte populations: variable spatial mosaics and extreme fine-graininess in winter-flowering Helleborus foetidus (Ranunculaceae). Am. J. Bot. 89, 1570–1578 (doi:10.3732/ajb.89.10.1570) [DOI] [PubMed] [Google Scholar]
- Herrera C. M., Sánchez-Lafuente A. M., Medrano M., Guitián J., Cerdá X., Rey P. J.2001Geographical variation in autonomous self-pollination levels unrelated to pollinator service in Helleborus foetidus (Ranunculaceae). Am. J. Bot. 88, 1025–1032 (doi:10.2307/2657084) [PubMed] [Google Scholar]
- Herrera C. M., Pérez R., Alonso C.2006Extreme intraplant variation in nectar sugar composition in an insect-pollinated perennial herb. Am. J. Bot. 93, 575–581 (doi:10.3732/ajb.93.4.575) [DOI] [PubMed] [Google Scholar]
- Herrera C. M., García I. M., Pérez R.2008Invisible floral larcenies: microbial communities degrade floral nectar of bumblebee-pollinated plants. Ecology 89, 2369–2376 (doi:10.1890/08-0241.1) [DOI] [PubMed] [Google Scholar]
- Herrera C. M., de Vega C., Canto A., Pozo M. I.2009Yeasts in floral nectar: a quantitative survey. Ann. Bot. 103, 1415–1423 (doi:10.1093/aob/mcp026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C. M., Canto A., Pozo M. I., Bazaga P.2010Inhospitable sweetness: nectar filtering of pollinator-borne inocula leads to impoverished, phylogenetically clustered yeast communities. Proc. R. Soc. B 277, 747–754 (doi:10.1098/rspb.2009.1485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen H. B., Martens H.1994Influence of temperature and ageing of ovules and pollen on reproductive success in Trifolium repens L. Ann. Bot. 74, 493–501 (doi:10.1006/anbo.1994.1146) [Google Scholar]
- Kjellberg B., Karlsson S., Kerstensson I.1982Effects of heliotropic movements of flowers of Dryas octopetala L. on gynoecium temperature and seed development. Oecologia 54, 10–13 (doi:10.1007/BF00541101) [DOI] [PubMed] [Google Scholar]
- Krannitz P. G.1996Reproductive ecology of Dryas integrifolia in the high Arctic semi-desert. Can. J. Bot. 74, 1451–1460 (doi:10.1139/b96-175) [Google Scholar]
- Kudo G.1995Ecological significance of flower heliotropism in the spring ephemeral Adonis ramosa (Ranunculaceae). Oikos 72, 14–20 (doi:10.2307/3546032) [Google Scholar]
- Lachance M. A.2006Yeast biodiversity: how many and how much? In Biodiversity and ecophysiology of yeasts (eds Rosa C., Péter G.), pp. 1–9 Berlin, Germany: Springer [Google Scholar]
- Lachance M. A., Starmer W. T., Rosa C. A., Bowles J. M., Barker J. S. F., Janzen D. H.2001Biogeography of the yeasts of ephemeral flowers and their insects. FEMS Yeast Res. 1, 1–8 [DOI] [PubMed] [Google Scholar]
- Lamprecht I.2003Calorimetry and thermodynamics of living systems. Thermochim. Acta 405, 1–13 (doi:10.1016/S0040-6031(03)00123-0) [Google Scholar]
- Lamprecht I., Schmolz E., Blanco L., Romero C. M.2002Flower ovens: thermal investigations on heat producing plants. Thermochim. Acta 391, 107–118 (doi:10.1016/S0040-6031(02)00168-5) [Google Scholar]
- Little A. E. F., Currie C. R.2008Black yeast symbionts compromise the efficiency of antibiotic defenses in fungus-growing ants. Ecology 89, 1216–1222 (doi:10.1890/07-0815.1) [DOI] [PubMed] [Google Scholar]
- McKee J., Richards A. J.1998Effect of flower structure and flower colour on intrafloral warming and pollen germination and pollen-tube growth in winter flowering Crocus L. (Iridaceae). Bot. J. Linn. Soc. 128, 369–384 [Google Scholar]
- Nicolson S. W., Nepi M., Pacini E.(eds)2007Nectaries and nectar Dordrecht, The Netherlands: Springer [Google Scholar]
- Orueta D.2002Thermal relationships between Calendula arvensis inflorescences and Usia aurata bombyliid flies. Ecology 83, 3073–3085 [Google Scholar]
- Patiño S., Grace J., Bänziger H.2000Endothermy by flowers of Rhizanthes lowii (Rafflesiaceae). Oecologia 124, 149–155 (doi:10.1007/s004420050001) [DOI] [PubMed] [Google Scholar]
- Paul E. A.(ed.)2007Soil microbiology and biochemistry, 3rd edn.Oxford, UK: Elsevier [Google Scholar]
- Peay K. G., Kennedy P. G., Bruns T. D.2008Fungal community ecology: a hybrid beast with a molecular master. BioScience 58, 799–810 (doi:10.1641/B580907) [Google Scholar]
- Pozo M. I., de Vega C., Canto A., Herrera C. M.2009Presence of yeasts in floral nectar is consistent with the hypothesis of microbial-mediated signaling in plant–pollinator interactions. Plant Signal. Behav. 4, 1102–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Replansky T., Koufopanou V., Greig D., Bell G.2008Saccharomyces sensu stricto as a model system for evolution and ecology. Trends Ecol. Evol. 23, 494–501 (doi:10.1016/j.tree.2008.05.005) [DOI] [PubMed] [Google Scholar]
- Rodrigues A., Cable R. N., Mueller U. G., Bacci M., Pagnocca F. C.2009Antagonistic interactions between garden yeasts and microfungal garden pathogens of leaf-cutting ants. Antonie Van Leeuwenhoek 96, 331–342 (doi:10.1007/s10482-009-9350-7) [DOI] [PubMed] [Google Scholar]
- Sampaio A., Sampaio J. P., Leao C.2007Dynamics of yeast populations recovered from decaying leaves in a nonpolluted stream: a 2-year study on the effects of leaf litter type and decomposition time. FEMS Yeast Res. 7, 595–603 (doi:10.1111/j.1567-1364.2007.00218.x) [DOI] [PubMed] [Google Scholar]
- Sánchez-Lafuente A. M., Guitián J., Medrano M., Herrera C. M., Rey P. J., Cerdá X.2005Plant traits, environmental factors, and pollinator visitation in winter-flowering Helleborus foetidus L. (Ranunculaceae). Ann. Bot. 96, 845–852 (doi:10.1093/aob/mci236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir Y., Shmida A., Ne'eman G.2006Morning floral heat as a reward to the pollinators of the Oncocyclus irises. Oecologia 147, 53–59 (doi:10.1007/s00442-005-0246-6) [DOI] [PubMed] [Google Scholar]
- Seymour R. S., Schultze-Motel P.1997Heat-producing flowers. Endeavour 21, 125–129 (doi:10.1016/S0160-9327(97)80222-0) [Google Scholar]
- Seymour R. S., White C. R., Gibernan M.2003Heat reward for insect pollinators. Nature 426, 243–244 (doi:10.1038/426243a) [DOI] [PubMed] [Google Scholar]
- Stanton M. L., Galen C.1989Consequences of flower heliotropism for reproduction in an alpine buttercup (Ranunculus adoneus). Oecologia 78, 477–485 (doi:10.1007/BF00378737) [DOI] [PubMed] [Google Scholar]
- Thompson J. N.1988Variation in interspecific interactions. Ann. Rev. Ecol. Syst. 19, 65–87 (doi:10.1146/annurev.es.19.110188.000433) [Google Scholar]
- Totland Ø.1996Flower heliotropism in an alpine population of Ranunculus acris (Ranunculaceae): effects on flower temperature, insect visitation, and seed production. Am. J. Bot. 83, 452–458 (doi:10.2307/2446214) [Google Scholar]
- Vesprini J. L., Nepi M., Pacini E.1999Nectary structure, nectar secretion patterns and nectar composition in two Helleborus species. Plant Biol. 1, 560–568 (doi:10.1111/j.1438-8677.1999.tb00784.x) [Google Scholar]
- Vesprini J. L., Nepi M., Ciampolini F., Pacini E.2008Holocrine secretion and cytoplasmic content of Helleborus foetidus L. (Ranunculaceae) nectar. Plant Biol. 10, 268–271 (doi:10.1111/j.1438-8677.2007.00023.x) [DOI] [PubMed] [Google Scholar]
- Whitney H. M., Dyer A., Chittka L., Rands S. A., Glover B. J.2008The interaction of temperature and sucrose concentration on foraging preferences in bumblebees. Naturwissenschaften 95, 845–850 (doi:10.1007/s00114-008-0393-9) [DOI] [PubMed] [Google Scholar]
- Wiens F., Zitzmann A., Lachance M. A., Yegles M., Pragst F., Wurst F. M., von Holst D., Guan S. L., Spanagel R.2008Chronic intake of fermented floral nectar by wild treeshrews. Proc. Natl Acad. Sci. USA 105, 10 426–10 431 (doi:10.1073/pnas.0801628105) [DOI] [PMC free article] [PubMed] [Google Scholar]