Abstract
Parasite infections often induce a reduction in host immune response either because of a direct manipulation of the immune system by the parasite or because of energy depletion. Although infection-induced immunodepression can favour the establishment of the parasite within the host, a too severe immunodepression may increase the risk of infection with opportunistic pathogens, stopping the period over which the parasite can be transmitted to other hosts. Here, we explore how the risk of contracting opportunistic diseases affects the survival of the amphipod Gammarus pulex infected by the acanthocephalan Pomphorhynchus laevis. Previous work with this system has shown that upon infection, G. pulex has a substantially reduced immune response. Non-infected and P. laevis-infected hosts were maintained either in control or in micro-organism-enriched water, so as to vary the risk of encountering opportunistic pathogens. As predicted, we found that host mortality was exacerbated when infected gammarids were maintained in micro-organism-enriched water compared with clean, control water; whereas for non-infected gammarids, living in micro-organism-enriched water only moderately increased the risk of mortality. These results show that the virulence of parasites that reduce the host immune response is an environmentally sensitive trait that depends on the concomitant risk for the host of contracting opportunistic diseases. This extra source of host mortality probably represents a cost for P. laevis, and we tentatively predict that the optimal level of parasite exploitation should depend on environmental conditions.
Keywords: disease ecology, Pomphorhynchus laevis, immunodepression, opportunistic pathogens, virulence
1. Introduction
The immune system is with little doubt one of the main selective forces driving parasite evolution. Upon entering the host, parasites are exposed to the effectors of the immune system, which may result in the clearance of the infection. In response to the threat imposed by the immune system, parasites have evolved a variety of strategies aimed at manipulating host immunity (Damian 1997; Schmid-Hempel 2008). These strategies of immune evasion are supposed to favour the establishment, growth and reproduction of the parasite within the host. Immunodepression is probably one of the most widespread mechanisms of immune evasion. Many parasites interfere with the host immune response by downregulating immune effectors. In spite of the adaptive nature of immune evasion, the study of immunomodulatory strategies has been largely overlooked by evolutionary biologists (Schmid-Hempel 2008, 2009). In particular, how immune evasion strategies might affect the evolution of parasite virulence has been largely ignored until recently. Using a verbal model, Frank & Schmid-Hempel (2008) have suggested that immune evasion should promote the evolution of virulence because immune evasion favours the establishment of the pathogen within the host (a benefit arising early in the infectious process), whereas any cost of immune evasion, in terms of host mortality, would occur late during the infectious period. Since early benefits are supposed to outweigh late costs, immune evasion should promote parasite virulence. This theoretical framework should largely apply to micro-organisms that multiply within the host and that rapidly produce transmissible stages.
Macro-parasites differ from micro-parasites in most of their life-history traits. Many parasitic helminths, for instance, have complex life cycles with one or two intermediate hosts. The transmission to the definitive host is usually ensured upon the ingestion of the intermediate host. Another major difference with respect to micro-parasites is that, in most helminths, the stages that infect the intermediate host do not reproduce and need to mature until the transmissible stage is attained. These larval stages have, therefore, to manage host survival until they mature into the transmissible stage. Helminth parasites are also masters in their ability to depress the host immune functioning (Maizels et al. 2004). Although the mechanisms of evasion and suppression of immunity have been better investigated and characterized in vertebrate hosts (Hewitson et al. 2009), helminths seem to adopt the same strategies in their mollusc and arthropod intermediate hosts (Loker 1994; Guillou et al. 2007). A trophically transmitted helminth that immunodepresses its intermediate host has to finely tune its levels of immune manipulation since a severely immunodepressed host may die before the parasite has matured into the transmissible stage (obviously a major fitness cost for the parasite). Given that these trophically transmitted parasites do not multiply within the intermediate host, the major source of mortality for immunodepressed hosts should come from opportunistic pathogens.
Theory on the evolution of parasite virulence has often assumed that high host background mortality should favour the most virulent parasite strains because a ‘prudent’ parasite that manages its host will not be rewarded. However, sources of host mortality can interact in the way that the strategy of host exploitation by the parasite can inflate the risk of background mortality. This can arise, for instance, when debilitated hosts are more vulnerable to predation or when immunodepressed hosts are more susceptible to opportunistic pathogens. Williams & Day (2001) have explored the evolutionary consequences of interactive sources of mortality on parasite virulence. Their model clearly shows that when host exploitation increases background mortality, parasites are expected to evolve towards reduced levels of virulence.
In addition to the ‘strategic’ manipulation of the host immune response by the parasite, many infectious diseases can produce an apparent immunodepression of the host simply because the parasite takes over host resources for its own maintenance and reproduction. It is now well established that the immune response is an energetically demanding function (Schmid-Hempel 2003), and therefore parasites might interfere with the amount of resources a host can allocate to the immune system, resulting in an impaired immune response. Whatever the mechanism behind the reduced immune functioning of infected hosts (parasite manipulation or energy depletion), it is important to keep in mind that the increased risk of contracting opportunistic infection can have an impact on the evolution of parasite exploitation strategies (Williams & Day 2001).
In the present study, we aim at testing the consequences of immunodepression on parasite virulence (host mortality) using a natural host–parasite association involving a freshwater amphipod Gammarus pulex and its trophically transmitted helminth parasite Pomphorhynchus laevis in two different environments. Amphipods are common intermediate hosts for acanthocephalans, in which the parasite undergoes its larval development before being transmitted via predation to the definitive hosts. Acanthocephalan-induced mortality has been studied intensively, as the manipulation of host behaviour favours the transmission of the parasite to the next host (Thomas et al. 2005). However, little is known about the acanthocephalan pathogenic effects other than the predation-induced mortality. Although there is agreement that P. laevis may affect growth and reproduction of their amphipod hosts (Bollache et al. 2002), there is less evidence that they cause direct host mortality (but see Benesh & Valtonen 2007). We recently showed that in the amphipod G. pulex, the host immune cells and the activity of the phenoloxidase (a key enzyme in the invertebrate innate immune system; Cerenius & Söderhäll 2004) are impaired following infection by P. laevis (Cornet et al. 2009a), either because of a strategic manipulation by the parasite or because of a reduction in resources available for the immune system (see also Cornet et al. 2010). Whatever the mechanism, immunodepression should be beneficial for the parasite in terms of increased persistence within the host. However, the reduced immunocompetence of parasitized amphipods makes them less resistant to opportunistic pathogens. Indeed, when injected with a bacterial suspension, live bacteria persisted longer in the haemolymph of infected hosts (Cornet et al. 2009a). Environmental conditions characterized by high risk of opportunistic infections should therefore lead to increased host mortality, and possibly to a maladaptation for the helminth.
To test the ecological consequences of parasite-induced immunodepression on virulence, we investigated the effects of exposure to opportunistic pathogens and of wounding on the survival of uninfected and P. laevis-infected gammarids. To this purpose, gammarids were maintained either in clean water (control) or in micro-organism-enriched water. Manipulating water quality is ecologically relevant since the streams where gammarids live may experience a period of increased pathogen burden (especially during hot summers). In addition, wounding also occurs at a high frequency in the field (Plaistow et al. 2003), creating a direct contact between the environment and the body cavity, and facilitating the penetration of opportunistic pathogens. Whatever the cost of parasite exploitation for the host (i.e. the differential mortality between uninfected and infected hosts in optimal conditions), we predict that immunodepressed, P. laevis-infected gammarids should have a higher mortality rate when reared in micro-organism-enriched water, compared with non-infected gammarids.
2. Material and methods
(a). Sampling of hosts and parasites
Gammarus pulex males were collected in November 2008 in a small tributary of the Suzon River at Val Suzon (northern Dijon, France). Animals were maintained in the laboratory under standard conditions (15 ± 1°C, light : dark cycle 12 : 12 h) in well-aerated tanks filled with dechlorinated UV-treated tap water and fed with elm leaves. They were acclimated for two weeks in the laboratory prior to the infestation.
Pomphorhynchus laevis parasites came from naturally parasitized chubs (Leuciscus cephalus) sampled by electrofishing in the Vouge River at Aubigny en Plaine (southern Dijon). Fish were anaesthetized, killed and dissected. Adult parasites were collected from the intestines; eggs were obtained by dissecting female worms and stored in 400 µl of water.
(b). Infection procedure
Controlled infections were made following Cornet et al. (2009a). Briefly, parasite eggs from each sampled female were examined under the microscope (200× magnification) to evaluate their number and maturity. Then, six suitable clutches were pooled and the egg suspension was set at 25 eggs per µl. Prior to the parasite exposure, gammarids were food-deprived for 24 h. Gammarids were placed, in pairs, in crystallizing dishes, and were provided with 100 eggs per individual deposited on 1 cm2 of an elm leaf, on which they were allowed to feed for 48 h. Uninfected leaves were provided to the non-infected group. Animals were maintained under standard conditions until cystacanths were detected (usually seven to eight weeks after parasite exposure). Parasitized and control, non-infected gammarids were then allocated to the different treatment groups for the survival experiment (see below).
(c). Haemolymph collection, haemocyte concentration and activities of the prophenoloxidase system
Three microlitres of haemolymph were collected into a sterile, pre-chilled glass capillary and flushed into 20 µl of cold phosphate buffer saline (Cornet et al. 2009a). Ten microlitres were immediately used for the haemocyte count, using a Neubauer counting chamber, and samples were frozen in liquid nitrogen and stored at −80°C for later phenoloxidase assays.
The activity of naturally activated phenoloxidase enzymes only (hereafter called PO activity) and the activity of the proenzymes (prophenoloxidase) in addition to that of the PO (hereafter called ProPO activity) were measured for each individual haemolymph extract using a spectrophotometric assay (Cornet et al. 2009b). The assay was performed using 5 µl of haemolymph extract added to a microplate well containing 20 µl of PBS buffer and either 140 µl of dH2O to measure PO activity only or 140 µl of chymotrypsin solution (Sigma C-7762, 0.07 mg ml−1 of dH2O) to measure ProPO activity. Then, 20 µl of l-Dopa solution (Sigma D-9628, 4 mg ml−1 of dH2O) was added and the reaction was followed in a microplate reader (Versamax, Molecular Devices) for 40 min at 490 nm. Enzyme activity was analysed using the software SOFT-MaxPro 4.0 (Molecular Devices) and measured as the slope (Vmax value) of the reaction curve.
Measures of haemocyte count and PO activity were reported for 1 µl of pure haemolymph.
(d). Survival experiment
The experiment consisted of a three-way, full factorial design. The three treatments were P. laevis infection (non-infected versus infected), water quality (control versus micro-organism-enriched water) and wounding (non-wounded versus wounded). Each group contained, at the beginning of the experiment, 30 gammarids.
Control water refers to dechlorinated tap water, oxygenated in a big tank and finally UV-treated. This step enables the elimination of any opportunistic pathogens. Micro-organism-enriched water was obtained by ageing water containing a solution of LB medium (2 ml l−1; 10 g bactotryptone, 5 g yeast extract, 10 g NaCl, 1 l of dH20, pH 7.0) and algae medium (1 ml l−1; 0.1 M KNO3, 0.007 M KH2PO4, 0.004 M MgSO4·7H2O) for 2 days (modified from Rigby & Jokela 2000). Before use, a 1 : 3 dilution of aged water with well-aerated control water was done. Gammarids were isolated in glass vials filled with 20 ml of either control or micro-organism-enriched water (Cornet et al. 2009c). The water was renewed every 2 days to provide enough oxygen to the animals since the micro-organism-enriched water might be more rapidly impoverished in dissolved oxygen.
Whatever the community of pathogens that developed in the micro-organism-enriched water, we found that 50 µl of the aged water contained at least a hundred bacterial colonies that had grown on non-selective agar–LB medium. No developing colony was observed from the spread of control water.
Gammarids were experimentally wounded by making a small hole in the cuticle (laterally on the animal's fourth dorsal segment) using a fine sterile needle, allowing a direct contact between the haemolymph and the external environment for a few hours, until the wound was naturally cicatrized. In freshwater amphipods, wounding occurs at a high frequency in the wild (Plaistow et al. 2003), being mainly the consequence of injuries when animals are drifting, but also of intraspecific interactions (e.g. male–male competition for access to females). This wounding treatment was designed to allow, for a couple of hours, a direct connection between the external environment and the body cavity in order to accentuate the penetration of bacteria into the organism.
Since parasite intensity may interfere with the level of immune defences (S. Cornet 2009, unpublished data), we only used gammarids that harboured a single P. laevis parasite. Survival was checked daily until all gammarids died. Gammarids were dissected under binocular microscope to check for the status of infection.
(e). Statistical analyses
Differences in immune defences between non-infected and P. laevis-infected gammarids were analysed using linear models on natural log-transformed data.
Survival analyses were run using Cox regressions (proportional hazards model). The best model was searched using a backward stepwise procedure and likelihood ratio (LR) significance tests for the estimation of each effect. We included in the initial model the status of P. laevis infection (non-infected versus infected), the water quality (control versus micro-organism-enriched) and the wounding (non-wounded versus wounded) as independent categorical variables, as well as the two-way and three-way interactions. Parameter estimates (±s.e.) as well as associated risk ratios are also given. Positive parameter estimates correspond to risk ratio values greater than 1. The risk ratio (or hazard ratio) is the probability of the event occurring at time t + 1, given survival to time t. It gives the ratio of hazard functions for the two groups, and an estimate greater than 1 implies that the hazard function increases as one moves from the reference, control group to the treated group.
All tests were performed using JMP 5.0 for Windows (SAS Institute) and referred to two-tailed tests with significant differences considered at the level of p ≤ 0.05.
3. Results
(a). Immune response
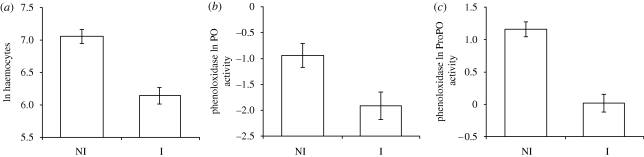
As already found in previous studies, P. laevis-infected G. pulex had an overall reduced level of immune defences compared with non-infected hosts (MANOVA: F3,43 = 18.84, p < 0.0001; figure 1). The haemocyte concentration was reduced (F1,43 = 31.38, p < 0.0001), as well as the PO (F1,43 = 7.42, p = 0.0093) and the ProPO activity (F1,43 = 39.81, p < 0.0001).
Figure 1.
Level of immune defences in G. pulex according to the status of infection by the acanthocephalan P. laevis (NI: non-infected, n = 26; I: infected, n = 19). (a) Haemocyte number, (b) PO and (c) ProPO activity per microlitre of haemolymph. Natural log-transformed data, mean ± s.e.
(b). Survival
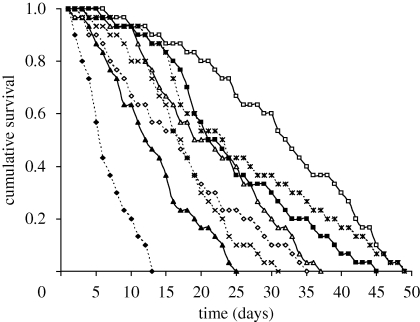
Overall, the three main factors—P. laevis infection, water quality and wounding—had a strong effect on gammarid survival (table 1, figure 2). Parasitized hosts suffered from a twofold reduction in survival (risk ratio 1.93) compared with uninfected gammarids. The micro-organism-enriched water and the wounding increased the risk of mortality by factors of 1.83 and 1.44, respectively.
Table 1.
A proportional hazard model investigating the effect of P. laevis infection, water quality and wounding on the survival of G. pulex. Parameter estimates (±s.e.) and risk ratios (with 95% CI) are given. The risk ratio refers to the increase in hazard function compared with the control group. A risk ratio value greater than 1 indicates that the mortality of the experimental group is higher than that of the control group. As an example, a risk ratio of 1.93 for the infection factor suggests that the overall hazard function for infected gammarids is increased by a factor of approximately 2.
| factors | estimate (±s.e.) | LR χ2 | d.f. | p-value | risk ratio (95% CI) |
|---|---|---|---|---|---|
| in the best modela | |||||
| infection | 0.66 (±0.07) | 77.08 | 1 | <0.0001 | 1.93 (1.66–2.24) |
| water | 0.60 (±0.07) | 65.83 | 1 | <0.0001 | 1.82 (1.57–2.11) |
| wound | 0.36 (±0.07) | 28.48 | 1 | <0.0001 | 1.44 (1.26–1.64) |
| water × wound | 0.18 (±0.06) | 7.74 | 1 | 0.0054 | 1.20 (1.06–1.37) |
| infection × water | 0.14 (±0.06) | 4.03 | 1 | 0.0447 | 1.15 (1.00–1.32) |
| not in the best model | |||||
| infection × water × wound | 0.07 (±0.06) | 0.96 | 1 | 0.3272 | |
| infection × wound | 0.06 (±0.06) | 1.05 | 1 | 0.3050 | |
aBest global model LR χ2 = 129.04, d.f.N = 5, d.f.D = 234, p < 0.0001.
Figure 2.
Survival of non-infected and P. laevis-infected G. pulex maintained in control or micro-organism-enriched water in combination with a wounding treatment (see §2 for details). Legend: status of infection (non-infected versus infected), water quality (control versus mirco-organism-enriched), wounding (non-wounded versus wounded). Open squares: non-infected, control, non-wounded. Filled squares: non-infected, micro-organism, non-wounded. Open triangles: infected, control, non-wounded. Filled triangles: infected, micro-organism, non-wounded. Asterisks: non-infected, control, wounded. Crosses: non-infected, micro-organism, wounded. Open diamonds: infected, control, wounded. Filled diamonds: infected, micro-organism, wounded.
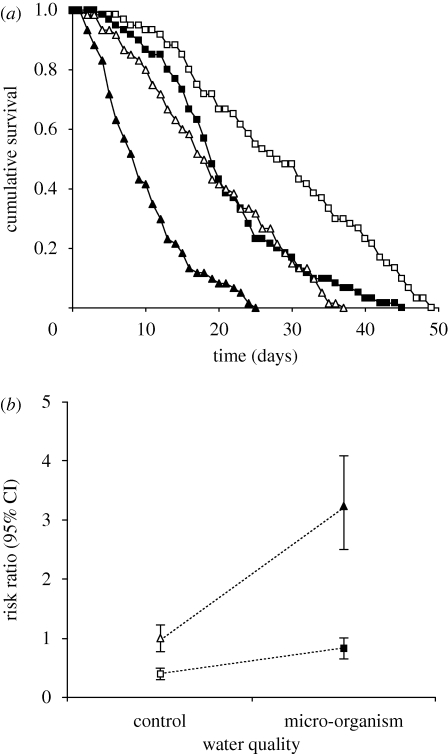
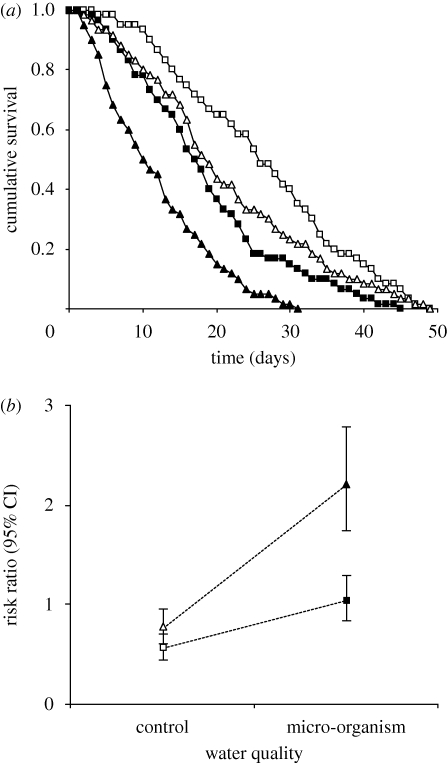
However, as expected, the survival time of P. laevis-infected gammarids was substantially lower when the hosts were exposed to micro-organism-enriched water compared with clean, control water (figure 3a,b); whereas for non-infected gammarids, living in micro-organism-enriched water only moderately increased the risk of mortality (figure 3a,b). This resulted in a statistically significant interaction between P. laevis infection and water quality (table 1). A similar interaction was found between the wounding and the water treatments (table 1). Whereas the wounding per se did not increase the risk of mortality in clean, control water (figure 4a,b), wounded gammarids suffered from a dramatic reduction in survival in micro-organism-enriched water (figure 4a,b). This confirms that the micro-organism-enriched water did contain potentially pathogenic organisms that inflicted a high cost to the gammarids when entering the haemocoel.
Figure 3.
Illustration of the significant interaction ‘status of infection × water quality’ highlighted in the survival analysis model: (a) cumulative survival and (b) risk ratio (with 95% confidence interval). Square: non-infected host. Triangle: infected host. Open symbol: control water. Filled symbol: micro-organism-enriched water.
Figure 4.
Illustration of the significant interaction ‘water quality × wounding’ highlighted in the survival analysis model: (a) cumulative survival and (b) risk ratio (with 95% confidence interval). Square: non-wounded host. Triangle: wounded host. Open symbol: control water. Fixed symbol: micro-organism-enriched water.
4. Discussion
We found that the virulence of an acanthocephalan parasite that depresses the host immune response largely depends on the environmental conditions where the host–parasite interaction takes place. As predicted, host mortality was substantially exacerbated when P. laevis-infected gammarids had to cope with other potentially pathogenic organisms in the water. This environmentally dependent parasite virulence also suggests that the optimal level of exploitation probably depends on the risk, for the host, of contracting opportunistic diseases; when this risk is high, severely immunodepressing its host might be maladaptive for the parasite.
In agreement with previous work on the same system (Cornet et al. 2009a,b), we found that the level of the PO activity and the density of immune cells (haemocytes) were dramatically impaired upon infection. Working with complex natural host–parasite systems offers the opportunity to address relevant evolutionary and ecological questions. Nevertheless, it also sets a series of limitations. For instance, one important issue is whether the observed reduction in the immune response of infected animals is the cause or the consequence of the infection. Gammarids with initially low levels of constitutive immune response might be more prone to the infection. In this case, the observed difference in the immune response between infected and non-exposed gammarids would merely reflect a differential susceptibility, instead of a parasite-induced effect. Cornet et al. (2009a) compared the level of PO activity in non-exposed, exposed but non-infected and infected hosts. Contrary to the prediction of differential susceptibility, they found that only the infected group had a lower immune activity and that non-exposed and exposed but non-infected hosts had a similar level of PO activity. Overall, these results strongly suggest that the observed variation of PO activity between non-infected and infected gammarids was not due to initial differences in susceptibility (for further details, see Cornet et al. 2009a).
Another explanation for the observed reduction in the immune response of infected hosts might stem from the parasite-induced depletion of host resources. It is now well established that the immune response is energetically costly (Schmid-Hempel 2003), and by diverting energy parasites might directly reduce the amount of host resources available for the immune system, which would result in an impaired immune response. In the absence of knowledge on the biochemical/molecular basis of parasite-induced immunodepression, this is a very plausible explanation. Nevertheless, we have recently performed an experiment for which the results suggest that the observed lower immune activity in P. laevis-infected gammarids is not the mere consequence of energy depletion. We surgically transferred parasites at the cystacanth stage from naturally infected gammarids into non-infected hosts. The level of PO in these grafted hosts was measured 3 days after the graft and compared with the PO of gammarids that were grafted with a glass bead (of similar size of the cystacanth), or with a frozen dead parasite, or left non-manipulated. The results show that the PO of gammarids grafted with a live cystacanth was significantly lower than for the other groups (Cornet 2008, unpublished data). Gammarids grafted with a glass bead or a dead parasite had similar PO levels than control, non-manipulated hosts. Interestingly, hosts grafted with a live cystacanth and naturally infected gammarids showed a similarly impaired level of PO activity. We believe that these results suggest that immunodepression is not due to resource depletion because (i) the immune response was measured 3 days after the graft, and it is unlikely that during this short period of time the parasite would have altered host body condition so dramatically; and (ii) we transferred the parasite at the cystacanth stage, when parasite growth had been arrested. It seems, therefore, unlikely that the energy requirements of cystacanths would represent so substantial a fraction of the host energy budget as to induce the observed immunodepression.
Whatever the mechanism behind the observed parasite-induced immunodepression (strategic manipulation versus energy depletion), acanthocephalans should benefit from the lower level of immune defence to persist within their intermediate host until transmission to the definitive host. However, parasites have to balance this benefit with the possible cost incurred by an immunodepressed host. The reduced immunocompetence of infected hosts might allow secondary infections by opportunistic pathogens such as bacteria or viruses present in the environment (Graham 2008) that could challenge the host and thus reinforce the deleterious effects of the parasite on host survival. This is highlighted in our study. Here, we showed that parasite infection was costly for the host since P. laevis-infected gammarids had a reduced survival under both control and micro-organism-enriched water. Water quality alteration is a common source of environmental stress for aquatic organisms (Lafferty & Kuris 1999) and has often been shown to amplify the deleterious effects of infection (Jokela et al. 2005). Changing environmental conditions, here altering water quality, had a differential effect on gammarids depending on whether they harboured a P. laevis infection. Uninfected gammarids only slightly suffered from an increased mortality in micro-organism-enriched water. Pomphorhynchus laevis-infected, immunodepressed gammarids living in a pathogen-rich environment might be rapidly overwhelmed by the pathogens that enter the body cavity, causing massive mortality.
The cost of both parasitism and immunodepression in micro-organism-enriched water was even higher when gammarids were wounded. The hole in the cuticle, present for hours before the wound heals, allows a direct contact between the general body cavity and the external environment, and probably triggers a rapid colonization of the haemocoel by bacteria and other micro-organisms. Whereas wounding had no overall significant effect on survival under control conditions, it amplified the negative effects on survival when combined with the poor-quality water. In invertebrates, haemocytes and PO are involved in coagulation and wound healing (Theopold et al. 2004). Since P. laevis-infected gammarids exhibit a lower level of immune response, they may have been less effective at healing the wound, which in turn should have facilitated the penetration of water-borne pathogens. However, contrary to this prediction, the three-way interaction between parasitism, water and wound treatments was not statistically significant (table 1).
Whatever the mechanism underlying the observed reduction in immune defences of infected hosts (direct parasite-induced suppression versus indirect effect of resource depletion), the results reported in this study fit the predictions of a theoretical model investigating the effect of interactive sources of host mortality on the evolution of parasite virulence (Williams & Day 2001). When the exploitation of the host by the parasite exacerbates the level of background mortality, virulence is predicted to go down (Williams & Day 2001). Here, indeed, we showed that infected hosts living in a pathogen-rich environment suffered exacerbated mortality risk. According to Williams & Day (2001), this interaction between infection-induced and background mortality should promote the evolution of less exploitative parasite strains. Focusing on the gammarid–acanthocephalan system, acanthocephalans would not benefit from an early death of the intermediate host. Larval parasites developing within the gammarid are infective to the definitive hosts only when they reach the cystacanth stage (Bethel & Holmes 1974). If the final host ingests the parasite before it has reached the cystacanth stage, it would fail to complete its life cycle since the parasite would not have acquired the structures to attach to the fish intestines. Host viability is therefore essential to allow the acanthocephalan larva (also true for most helminths) to be transmitted to the definitive host, meaning that the benefits of an increased immunodepression will be constrained by the decline in host survival. Highly exploitative (immunodepressive) parasite strains causing early mortality should therefore be selected against. In other words, parasites should evolve towards an optimal level of exploitation that allows them to avoid clearance by the host immune response, but also limits the risk of host death by opportunistic pathogens. In this context, the environment where the interaction takes place is likely to play a central role. One might tentatively predict that gammarid–acanthocephalan associations in environments with high pathogenic burden should evolve towards low levels of exploitation compared with environments where the risk of encountering opportunistic pathogens is low. Of course, environmental conditions can fluctuate over time, within the course of the infection. Therefore, an approach that takes into account the spatial and temporal variability is required to have quantitative predictions on the evolution of optimal exploitation in trophically transmitted parasites.
Acknowledgements
Financial support came from the Conseil Régional de Bourgogne with a FABER grant (06512AA07579-FABER 2006-178) to G.S. and a doctoral grant to S.C. We are grateful to T. Day and two anonymous referees for valuable comments on an earlier version of the manuscript. This experiment complies with the current laws of France.
References
- Benesh D. P., Valtonen E. T.2007Effects of Acanthocephalus lucii (Acanthocephala) on intermediate host survival and growth: implications for exploitation strategies. J. Parasitol. 93, 735–741 (doi:10.1645/GE-1093R.1) [DOI] [PubMed] [Google Scholar]
- Bethel W. M., Holmes J. C.1974Correlation of development of altered evasive behaviour in Gammrus lacustris (Amphipoda) harboring cystacanths of Polymorphus paradoxus (Acanthocephala) with the infectivity to the definitive host. J. Parasitol. 60, 272–274 (doi:10.2307/3278463) [PubMed] [Google Scholar]
- Bollache L., Rigaud T., Cézilly F.2002Effects of two acanthocephalan parasites on the fecundity and pairing status of female Gammarus pulex (Crustacea: Amphipoda). J. Invertebr. Pathol. 79, 102–110 (doi:10.1016/S0022-2011(02)00027-7) [DOI] [PubMed] [Google Scholar]
- Cerenius L., Söderhäll K.2004The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198, 116–126 (doi:10.1111/j.0105-2896.2004.00116.x) [DOI] [PubMed] [Google Scholar]
- Cornet S., Franceschi N., Bauer A., Rigaud T., Moret Y.2009aImmune depression induced by acanthocephalan parasites in their intermediate crustacean host: consequences for the risk of super-infection and links with host behavioural manipulation. Int. J. Parasitol. 39, 221–229 (doi:10.1016/j.ijpara.2008.06.007) [DOI] [PubMed] [Google Scholar]
- Cornet S., Franceschi N., Bollache L., Rigaud T., Sorci G.2009bVariation and covariation in infectivity, virulence and immunodepression in the host–parasite association Gammarus pulex–Pomphorhynchus laevis. Proc. R. Soc. B 276, 4229–4236 (doi:10.1098/rspb.2009.1299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet S., Biard C., Moret Y.2009cVariation in immune defence among populations of Gammarus pulex (Crustacea: Amphipoda). Oecologia 159, 257–269 (doi:10.1007/s00442-008-1211-y) [DOI] [PubMed] [Google Scholar]
- Cornet S., Sorci G., Moret Y.2010Biological invasion and parasitism: invaders do not suffer from physiological alterations of the acanthocephalan Pomphorhynchus laevis. Parasitology 137, 137–147 (doi:10.1017/S0031182009991077) [DOI] [PubMed] [Google Scholar]
- Damian R. T.1997Parasite immune evasion and exploitation: reflections and projections. Parasitology (Suppl.) 115, S169–S175 [DOI] [PubMed] [Google Scholar]
- Frank S. A., Schmid-Hempel P.2008Mechanisms of pathogenesis and the evolution of parasite virulence. J. Evol. Biol. 21, 396–404 (doi:10.1111/j.1420-9101.2007.01480.x) [DOI] [PubMed] [Google Scholar]
- Graham A. L.2008Ecological rules governing helminth microparasite coinfection. Proc. Natl Acad. Sci. USA 105, 566–570 (doi:10.1073/pnas.0707221105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou F., Roger E., Mone Y., Rognon A., Grunau C., Theron A., Mitta G., Coustau C., Gourbal B. E. F.2007Excretory–secretory proteome of larval Schistosoma mansoni and Echinostoma caproni, two parasites of Biomphalaria glabrata. Mol. Biochem. Parasitol. 155, 45–56 (doi:10.1016/j.molbiopara.2007.05.009) [DOI] [PubMed] [Google Scholar]
- Hewitson J. P., Grainger J. R., Maizels R. M.2009Helminth immunoregulation: the role of parasite-secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 167, 1–11 (doi:10.1016/j.molbiopara.2009.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela J., Taskinen J., Mutikainen P., Kopp K.2005Virulence of parasites in hosts under environmental stress: experiments with anoxia and starvation. Oikos 108, 156–164 (doi:10.1111/j.0030-1299.2005.13185.x) [Google Scholar]
- Lafferty K. D., Kuris A. M.1999How environmental stress affects the impacts of parasites. Limnol. Oceanogr. 44, 925–931 [Google Scholar]
- Loker E. S.1994On being a parasite in an invertebrate host: a short survival course. J. Parasitol. 80, 728–747 (doi:10.2307/3283252) [PubMed] [Google Scholar]
- Maizels R. M., Balic A., Gomez-Escobar N., Nair M., Taylor M. D., Allen J. E.2004Helminth parasites—masters of regulation. Immunol. Rev. 201, 89–116 (doi:10.1111/j.0105-2896.2004.00191.x) [DOI] [PubMed] [Google Scholar]
- Plaistow S. J., Outreman Y., Moret Y., Rigaud T.2003Variation in the risk of being wounded: an overlooked factor in studies of invertebrate immune function? Ecol. Lett. 6, 489–494 (doi:10.1046/j.1461-0248.2003.00455.x) [Google Scholar]
- Rigby M. C., Jokela J.2000Predator avoidance and immune defence: costs and trade-offs in snails. Proc. R. Soc. Lond. B 267, 171–176 (doi:10.1098/rspb.2000.0983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P.2003Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. Lond. B 270, 357–366 (doi:10.1098/rspb.2002.2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P.2008Parasite immune evasion: a momentous molecular war. Trends Ecol. Evol. 23, 318–326 (doi:10.1016/j.tree.2008.02.011) [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P.2009Immune defence, parasite evasion strategies and their relevance for ‘macroscopic phenomena’ such as virulence. Phil. Trans. R. Soc. B 364, 85–98 (doi:10.1098/rstb.2008.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theopold U., Schmidt O., Söderhäll K., Dushay M. S.2004Coagulation in arthropods: defence, wound closure and healing. Trends Immunol. 25, 289–294 (doi:10.1016/j.it.2004.03.004) [DOI] [PubMed] [Google Scholar]
- Thomas F., Adamo S. A., Moore J.2005Parasitic manipulation: where are we and where should we go? Behav. Proc. 68, 185–199 (doi:10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- Williams P. D., Day T.2001Interactions between sources of mortality and the evolution of parasite virulence. Proc. R. Soc. Lond. B 268, 2331–2337 (doi:10.1098/rspb.2001.1795) [DOI] [PMC free article] [PubMed] [Google Scholar]