Abstract
Distinct ecotypes of the snail Littorina saxatilis, each linked to a specific shore microhabitat, form a mosaic-like pattern with narrow hybrid zones in between, over which gene flow is 10–30% of within-ecotype gene flow. Multi-locus comparisons cluster populations by geographic affinity independent of ecotype, while loci under selection group populations by ecotype. The repeated occurrence of partially reproductively isolated ecotypes and the conflicting patterns in neutral and selected genes can either be explained by separation in allopatry followed by secondary overlap and extensive introgression that homogenizes neutral differences evolved under allopatry, or by repeated evolution in parapatry, or in sympatry, with the same ecotypes appearing in each local site. Data from Spain, the UK and Sweden give stronger support for a non-allopatric model of ecotype formation than for an allopatric model. Several different non-allopatric mechanisms can, however, explain the repeated evolution of the ecotypes: (i) parallel evolution by new mutations in different populations; (ii) evolution from standing genetic variation; and (iii) evolution in concert with rapid spread of new positive mutations among populations inhabiting similar environments. These models make different predictions that can be tested using comprehensive phylogenetic information combined with candidate loci sequencing.
Keywords: Littorina saxatilis, ecotype evolution, parallel evolution, selective sweep, evolution in concert, incipient speciation
1. Introduction
Reproductive barriers that evolve repeatedly in independent populations offer a replicated experiment for testing of speciation mechanisms. Stochastic forces alone cannot explain the repeated patterns of evolution, and the primary conclusion is that natural selection is involved (Schluter & Nagel 1995; Johannesson 2001; Coyne & Orr 2004; Schluter et al. 2004). Furthermore, reproductive barriers between host races or ecotypes function in the presence of gene flow, and studies of these types of barriers are hence more informative to understand initial steps of speciation than studies of barriers between already separated species, as the latter barriers may have accumulated after the completion of speciation (Coyne & Orr 2004; Via & West 2008; Schluter 2009). But, of course, one must be aware that not all partial reproductive barriers evolve to completion (Nosil et al. 2009).
The evolution of partially reproductively isolated ecotypes in the seashore periwinkle Littorina saxatilis has been suggested as an example of parallel evolution of reproductive isolation/incipient speciation (Rolán-Alvarez et al. 2004; Panova et al. 2006; Quesada et al. 2007; Schluter 2009) showing circumstantial evidence for strong ecological separation being the main factor behind the evolution of reproductive barriers. Nevertheless, it has been suggested that these barriers may, at least in part, be due to genetic differences accumulated during an earlier period of allopatric separation (Grahame et al. 2006; Butlin et al. 2008). Indeed, other earlier presumed cases of sympatric speciation have recently been shown to involve components of allopatric origin, such as the Mexican inversion in Rhagoletis that contributed to the production of a host-shift (Feder et al. 2003).
Ecotype formation in L. saxatilis provides an excellent model system for studies of both ecological mechanisms of divergence and origin of genetic variation for ecological adaptation (Johannesson 2009). Firstly, microhabitat-specific ecotypes have evolved in a variety of different rocky shore areas over the complete range of species, providing a test of general mechanisms under a variety of different circumstances. Secondly, ecotype formation, including the evolution of reproductive isolation, can be studied at different scales. At a local scale, ecotypes are distributed in such a way that pairs of contrasting ecotypes are present on islands in an archipelago, or on different parts of the same shore. At a regional scale, similar ecotypes occur at geographical distances of at least 200–400 km. Finally, at distances of 1000–3000 km, ecotypes living under similar selective regimes share basic phenotypic characteristics such as size, shell thickness and behaviour (table 1). This gives ample opportunities to study phenotypic similarities and divergences at different phylogeographic levels and at different levels of contemporary gene flow. Thus far, comprehensive studies of this system have been undertaken in Spain, the UK and Sweden. Here, we compare results obtained in the three systems, and synthesize the current knowledge on the mechanisms of ecotype formation, in particular the evolution of reproductive isolation. We suggest four mechanisms that alone or in various combinations may explain the repeated evolution of ecotypes in L. saxatilis, and we outline the genomic signatures that will be predicted from each of these mechanisms.
Table 1.
Summary of data gathered from ecotype pairs of Littorina saxatilis in Spain, the UK and Sweden.
| Spain |
UK |
Sweden |
references | ||||
|---|---|---|---|---|---|---|---|
| ecotype | RB | SU | M | H | S | E | |
| metapopulation structure | coastal and large islands | coastal | small islands | ||||
| age of establishment | <40 000 yr BP | <10 000 yr BP | <10 000 yr BP | Quesada et al. (2007) | |||
| morphological phenotype | large, thick-shelled, narrow aperture, ridged | small, thin-shelled, large aperture, smooth | large, thick-shelled, narrow aperture, smooth | small, thin-shelled, large aperture, smooth | large, thick-shelled, narrow aperture, smooth | small, thin-shelled, large aperture, smooth | Johannesson & Johannesson (1996); Hollander et al. (2006); Conde-Padin et al. (2009) |
| behavioural phenotype | warya | bolda | wary | bold | Johannesson & Johannesson (1996) | ||
| habitat | upper intertidal | lower intertidal | mean intertidal boulders | upper intertidal vertical cliffs | intertidal cliffs | intertidal boulders | Janson (1982); Johannesson et al. (1983); Grahame et al. (2006) |
| inferred main selection | crabs | waves | crabs | waves | crabs | waves | Johannesson (1986) |
| hybrid zone extension | sympatricb | sympatricb (low density) | parapatric | ||||
| hybrid fitness | hybrids as fit as parental forms in hybrid zone | inconclusive, possibly postzygotic barrier | bounded hybrid superiority | Janson (1983); Hull et al. (1996); Rolán-Alvarez et al. (1997); Johannesson et al. (2000); Cruz & Garcia (2001) | |||
| evidence of reproductive barrier | assortative mating in microsympatric sites in the field | assortative mating in laboratory experiments | assortative mating in laboratory experiments | Johannesson et al. (1995); Pickles & Grahame (1999); Hollander et al. (2005); Johannesson et al. (2008) | |||
| neutral gene flow over hybrid zone compared with within-ecotypec | 8.8% (allozymes) | 29.2% (AFLP) | 12.7% (microsatellites) | Rolán-Alvarez et al. (1996); Panova et al. (2006); Grahame et al. (2006); Galindo et al. (2009) | |||
| 19.4% (AFLP) | |||||||
| 7.5% (microsatellites) | |||||||
aPersonal observation.
bFollowing the definition of Futuyama & Mayer (1980). All individuals are capable of crossing the hybrid zone.
cNm over hybrid zone compared with Nm over same distance within populations of pure ecotype.
2. On terminology
Recently, Arendt & Reznick (2007) made the case that the standard use of the two terms ‘parallel evolution’ and ‘convergent evolution’ for independent evolution of similar traits in closely and distantly related taxa, respectively, is misleading, as the same gene can sometimes be found to cause the same phenotypic effect in distantly related species, while at the same time, different genes may give the same effect in populations of the same species. Furthermore, Butlin et al. (2008) emphasize the importance of distinguishing between parallel ecological divergence of ecotypes and independent parallel origin of alleles. The latter is just one possible mechanism explaining parallel evolution of ecotypes, host races, etc., but this distinction is mostly not made in the literature (Schluter & Nagel 1995; Johannesson 2001; Schluter et al. 2004; Colosimo et al. 2005; Arendt & Reznick 2007; Schluter 2009). The key feature of parallel evolution is the repeated evolution of similar phenotypes in independent situations. Independence is critical to be able to conclude that parallel evolution is a result of selection alone. Indeed, there are alternative processes that can lead to repeated evolution of phenotypes in which the genetic variation that selection is acting on has a common origin, rather than several independent origins. If this is so, evolution may follow a path that is partly dictated by the available genetic variation, and the repeated evolution of similar phenotypes is partly related to connections between populations. To stress the importance of independence, here we use the term ‘repeated evolution of phenotypes’ when the genetic backgrounds of traits are unknown or not independent, and we use ‘parallel evolution’ when similar phenotypic traits in different populations have evolved de novo from independent new mutations with similar phenotypic effects, or from repeated substitutions of the same nucleotide (Wood et al. 2005).
We make the distinction between allopatry and non-allopatry (parapatry and sympatry) and consider these terms equivalent to ‘in the absence of gene flow’ or ‘in the presence of gene flow’, respectively. Following Bolnick & Fitzpatrick (2007) (see also discussions in Mallet et al. 2009 and Fitzpatrick et al. 2009), we consider the spatial context of speciation to be important, because it reflects the level of connectivity/independence of populations although we do not expect to find complete sympatry nor complete allopatry in nature (Butlin et al. 2008; Fitzpatrick et al. 2008). As underlined by Rieseberg & Burke (2001), levels of migration that are much smaller than what is needed to prevent divergence of populations by drift (i.e. Nm ≪ 1) will be crucial in the process of transferring positively selected alleles among populations of a species (or even among species). Unfortunately, migration rates at these low levels are extremely hard to measure (Strasburg & Rieseberg 2008).
3. The biology of littorina saxatilis
The rough periwinkle, L. saxatilis, is widely distributed with dense continuous populations (100–1000 individuals per square metre) along wave-impacted rocky shores, and in lagoons and salt-marshes of the northern Atlantic, from Portugal to Novaya Zemlya and Svalbard, and from North Carolina to Greenland (Reid 1996). A few, probably introduced, populations are present elsewhere (Italy, South Africa, California). The species forms distinct ecotypes in different microhabitats and gene flow between adjacent ecotypes is strongly (although not completely) impeded with the formation of micro-scale hybrid zones (Johannesson et al. 1993, 1995a; Rolán-Alvarez et al. 1999; Grahame et al. 2006; Panova et al. 2006). A most important trait is the lack of migratory (pelagic) larvae resulting in restricted dispersal among populations. Snails may either crawl along the shore in the range of 2–10 m per generation (Janson 1983; Erlandsson et al. 1998) or occasionally drift attached to rafting pieces of macroalgae or frozen up in ice floes or similar.
Migration rates through rafting are hard to assess. However, after a toxic algal bloom that wiped out all snails on small islands (at distances of 0.1–1 km from larger islands), the rate of island re-colonization was 1.5 per cent per generation (Johannesson & Johannesson 1995). As a successful recolonization requires only one adult female (see below), migration between islands in the archipelago can be estimated to be in the region of 0.03 migrants (assuming male and female rafting to be equally frequent) per generation, that is, Nm ≪ 1. Obviously, migration over long geographical distances (greater than 100–1000 km) is likely to be much less frequent. Notably, founding a new population will require no more than a single mated female as she has the capacity to release a few hundred offspring that will remain in the area throughout life. (Although not all offspring survive until the adult stage, observations after the algal bloom indicated that populations of re-colonized islands expanded exponentially and returned to normal densities in a few generations (Johannesson & Johannesson 1995).) Hence, one female being successfully rafted to a new site is probably enough to establish a new population in that site, and this may explain this species being established in extremely remote places like the small and isolated island of Rockall (Johannesson 1988). In addition, females of L. saxatilis are strongly promiscuous and usually carry sperm from eight to ten males (Mäkinen et al. 2007). This together with a rapid population growth prevents a serious loss of genetic variation even if a new site is colonized by only a single female (Nei et al. 1975; Chakraborty & Nei 1977; Janson 1987a).
4. Ecotypes of littorina saxatilis
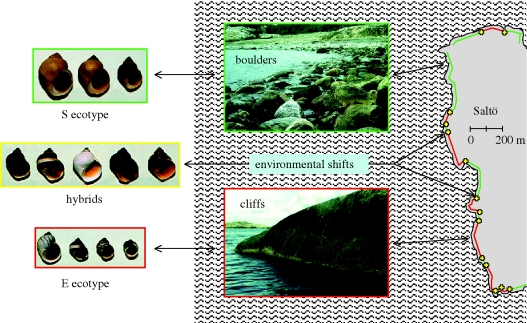
A number of different ecotypes of L. saxatilis have been described on the basis of morphological, ecological and genetic relationships. The best investigated ones are the S and E ecotypes (from Sweden), the H and M (from the UK) and the RB and SU (from Spain), however, additional ecotypes include the mudflat ecotype tenebrosa, the extremely small neglecta living in empty barnacle shells and the subarctic groenlandica (figure 1). Although earlier studies resulted in descriptions of more than 20 different species, based on the genetic and morphological analyses all these are currently considered ecotypes of L. saxatilis (for an extensive review, see Reid 1996), however, adjacent ecotypes are separated by relatively strong reproductive barriers (table 1). In principle, a distinct ecotype of L. saxatilis is expected whenever the species occupies a new microhabitat. This 1 : 1 relationship between phenotype of L. saxatilis populations and the microenvironment they inhabit suggests that phenotypic evolution is strongly linked to ecological factors. Indeed, a microhabitat covering only a few metres of shore embedded in another habitat (e.g. a few boulders in a crevice surrounded by smooth cliffs) will host a population of snails with a phenotype matching the local boulder habitat.
Figure 1.
Six ecotypes of Littorina saxatilis illustrating the extensive variation in shell size and form that is present within this species. All the individuals are adults of representative size and shape. Upper row from left to right: ‘neglecta’—an ecotype living in the empty shells of dead barnacles in the UK and France, ‘S’—a crab-resistant ecotype found on boulder shores along the Swedish west coast, ‘E’—a wave-resistant ecotype confined to granite cliffs on the Swedish west coast. Lower row from left to right: ‘groenlandica’—a large ecotype found in subarctic areas along with ecotypes of smaller size, ‘SU’—a wave-resistant Spanish ecotype and ‘RB’—a crab-resistant Spanish ecotype.
In Sweden, Spain and the UK contrasting ecotypes are present in what can simply be denoted as either crab-rich or wave-swept intertidal habitats. Although the particular habitats are found in somewhat different intertidal contexts, the impact of crab crushing and wave-surge select for either a snail with a large and thick shell, a relatively small aperture and wary behaviour, or a snail with a small and thin shell, a relatively large aperture and bold behaviour (table 1). An important piece of information is that differences between ecotypes in morphology and behaviour are largely inherited although a minor part of the difference is because of phenotypic plasticity (e.g. Janson 1982; Johannesson & Johannesson 1996; Carballo et al. 2001; Hollander et al. 2006; Conde-Padín et al. 2007, 2009). In the UK and Spain, both ecotypes are present on the same shores but at different tidal heights. In the mid-shore, narrow zones of contact or overlap, depending on the shore, are formed and here hybrids are also present (Johannesson et al. 1993; Hull et al. 1996). In Sweden, the ecotypes are confined to different shores of contrasting microhabitats (boulder or cliffs) and in microhabitat shifts, ecotypes overlap and hybrids are produced. The distribution of different microhabitats, and in particular, the contrast of crab selection (selecting for large size, thick shell and a snail that quickly retracts into the shell) and wave-surf (selecting for small size to minimize drag forces, large aperture to increase foot size and grip, and a snail that rapidly emerges from the shell to secure the grip to the substratum) provides the basis of the existence of parallel phenotypes, in the first place within each region (e.g. Sweden, Spain and the UK) but, superficially at least, also among regions (table 1).
5. Evidences of partial reproductive isolation
Estimates of gene flow outside and across hybrid zones using genetic markers show that gene flow over hybrid zones is only 10–30%, or sometimes even less, of gene flow over similar distances within continuous populations of the same ecotype (Rolán-Alvarez et al. 1996; Grahame et al. 2006; Panova et al. 2006; Galindo et al. 2009). Both extrinsic and intrinsic effects are likely to contribute to the impeded gene flow. For example, immigrant inviability is likely to be a major force preventing gene flow. That is, snails crossing habitat borders (either by creeping or by rafting) reduce their chance of survival to about 10–20% of their earlier value (Janson 1983; Rolán-Alvarez et al. 1997). In addition, parental ecotype snails disperse non-randomly at least in the Spanish hybrid zone, contributing to the gene flow barrier (Erlandsson et al. 1999; Cruz et al. 2004). The presence of a postzygotic component of isolation has been suggested from observations from a hybrid zone in the UK, where five hybrid females out of seven sampled had strongly elevated levels of aborting embryos (Hull et al. 1996). However, the abortion rate varies extensively among females both within and among local populations of any ecotype (Janson 1985) and hence there are alternative explanations for this observation that cannot be rejected, such as simply an effect of a small sample size, risk of cross-mating with a sibling species (L. arcana), risk of sperm depletion owing to very few mates being available (the UK hybrid zones were reported to be of low density), or presence of some local disease. In contrast, comprehensive data of abortive embryos in samples from both Sweden and Spain showed no indication of hybrid individuals producing higher numbers of abnormal embryos than other females (Janson 1985; Johannesson et al. 2000). Hybrid male and female fecundity (another indicator of possible intrinsic postzygotic isolation) was similar to that of pure ecotypes in one study (Johannesson et al. 2000), while slightly less in another study (Cruz & Garcia 2001). Moreover, survival rates of hybrids in the hybrid zone have been shown to be higher, or as high as that of both parental ecotypes, and higher than outside the zone, in both Spain and Sweden (Janson 1983; Rolán-Alvarez et al. 1997), while for the UK the evidence is less conclusive (table 1).
In both field and laboratory experiments, assortative mating of ecotypes is very strong at a local scale as well as across regional scales, with barriers of around 80 per cent isolation (Johannesson et al. 1995a; Hull et al. 1996; Rolán-Alvarez et al. 1999; Pickles & Grahame 1999; Cruz et al. 2004; Hollander et al. 2005; Johannesson et al. 2008; Conde-Padín et al. 2008). In the laboratory, assortative mating is observed at various stages of pair-formation and copulation. The first step is when males actively search for a female to mate and use the mucous trails of other snails to encounter a female (Erlandsson et al. 1999). A male that encounters trails of females of two different ecotypes, more frequently follows trails of females of his own ecotype (Johannesson et al. 2008). The next step of mating is when the male mounts the female and inserts the penis under the female shell. Males of one ecotype that have the possibility of mating with females of two different ecotypes copulate with females of their own ecotype for longer than the other females (Hollander et al. 2005), and from studies of males mating parasitized females, other males or juveniles we know that short matings (less than 5 min) are likely to represent failed copulation attempts with no transfer of sperm, while a full-length mating is usually around 20–60 min (Saur 1990). Furthermore, laboratory experiments show that males mate longer with females of the same ecotype from a population 150 km away than with females of a different ecotype from an adjacent shore, despite the former being genetically much more distantly related to the males than the latter (Hollander et al. (2005), and see Janson (1987b) for isolation-by-distance effects in allozymes over geographical distances of 0–300 km).
Size is likely to be a major factor explaining the assortative mating between ecotypes. In the field, sizes of mates in a pair are positively correlated (Johannesson et al. 1995a; Hull 1998; Conde-Padín et al. 2008), indicating a general trend for size-assortative mating. In fact, the observed degree of assortative mating across seven geographically distant populations was apparently predicted using information on their size population variability (Conde-Padín et al. 2008). As snails of crab-rich environments (ecotypes RB, M and S, see table 1) are roughly twice the size of snails from wave-exposed environments (SU, H and E, see table 1), size differences between ecotypes are likely to explain a major part of their assortative mating. Indeed, if size differences are removed (by experiments in the laboratory choosing extremely large and small individuals of ecotypes with different average sizes) mating barriers largely disappear (Hollander et al. 2005; Johannesson et al. 2008). Snails of different ecotypes have different growth rates, and these are largely inherited, but also extremely variable within populations (Janson 1982; Johannesson et al. 1997; Conde-Padín et al. 2007). This suggests that there is a large amount of additive genetic variation for selection to operate on. Indeed, quantitative population differentiation estimates (QST) for different shell shape and size traits between sympatric ecotypes were significantly higher than molecular FST estimates (table 2 from Conde-Padín et al. 2007), suggesting that divergent natural selection is mainly responsible for the inflated QST estimates (Merilä & Crnokrak 2001). Most probably, crab predation favours rapid growth and large adult size (Kemppainen et al. 2005), while wave surf selects for slow growth and small adult size; a small size reduces drag forces that can be excessive on rocky shores (Denny 2000) and increases the availability of protective cracks (Raffaelli & Hughes 1978). Hence, size could be a ‘magic trait’ (Gavrilets 2004) with pleiotropic effects on ecotype fitness and mate choice. Size differences are under strong directional selection with survival rates reduced to 10–20% of original values when snails are transplanted between contrasting environments (Janson 1983; Rolán-Alvarez et al. 1997), and as size is the major component of the phenotypic differences between ecotypes of the same area (Sundberg 1988), selection is likely to act directly on size. The exact mechanism of the size-based assortative mating remains unclear, but with respect to the males' tracking of females' mucous trails, the width of the trail will reflect the size of the female that laid the trail, and males should be able to probe the trail width. Both models of speciation (Gavrilets 2004) and experimental trials (Rice & Hostert 1993) have earlier shown that a magic trait facilitates the evolution of a reproductive barrier in the face of gene flow, and may be the most likely way new species are formed in non-allopatric systems (Bolnick & Fitzpatrick 2007). Size is the dominant component of the difference between the two Swedish ecotypes (S and E, Sundberg 1988) and is also likely to be the major difference between ecotypes in the UK and Spain (table 1).
Table 2.
Alternative mechanisms and their predicted genomic signatures for repeated evolution of ecotypes of Littorina saxatilis in a postglacial area (e.g. Sweden).
| phylogenetic patternsa |
population differentiation |
||||
|---|---|---|---|---|---|
| model | mechanism | non-candidate locib | candidate locic, neutral variationd | candidate loci, functional variatione | candidate versus non-candidate loci |
| (1) allopatry | ancestralf origin of ecotype-specific alleles. Double colonization | haplotypes cluster by ecotype, or, following extensive introgression, by geographical relatedness | haplotypes cluster by ecotype. Large variation within each ecotype indicating old lineages | same non-synonymous substitutions, correlated with ecotype, across geographical areas | differentiation among geographically distant populations may be smaller in candidate loci because drift is opposed by selection |
| (2A) non-allopatry (parallel)—parallel evolution due to same substitutions | in situ evolution of ecotypes due to repeated independent non-synonymous substitutions of the same nucleotide | haplotypes cluster by geographical relatedness (if different lineages colonized different areas), or haplotype frequencies vary across geographical areas due to drift (as lineage sorting progresses, the haplotype will eventually cluster by geographical area) | haplotypes cluster by geographical relatedness (if different lineages colonized different areas), or haplotype frequencies vary across geographical areas due to drift (as lineage sorting progresses, the haplotype will eventually cluster by geographical area). Sites closely linked to functional substitutions affected by independent local selective sweeps | same non-synonymous substitutions, correlated with ecotype, across geographical areas. These substitutions appear against different genetic background (i.e. sequence of a particular allele, that acquired mutation) in different geographical areas | differentiation among geographically distant populations smaller at candidate than at non-candidate loci |
| (2B) non-allopatry (parallel)—parallel evolution due to different substitutions | in situ evolution of ecotypes due to repeated independent non-synonymous substitutions at different loci influencing the same traits | as 2A | as 2A | unique non-synonymous substitutions, correlated with ecotype in each geographical area. These substitutions appear in different loci in different geographical areas | differentiation among geographically distant populations greater at candidate than at non-candidate loci |
| (3) non-allopatry (repeated) standing genetic variation | alleles that are positively selected in one ecotype are present in low frequencies as neutral or slightly deleterious alleles in other ecotypes including the ancestral lineage that invaded the area | as 2A | as 1 | as 1 | as 1 |
| (4) non-allopatry (repeated)—evolution in concert | recent mutations favoured by selection in one habitat spread relatively rapidly and successively improve local adaptation of the different ecotypes | as 2A | large haplotype variation in one ecotype. Very low genetic variation owing to recent selective sweep of a derived allele in the other ecotype | same non-synonymous substitutions, correlated with ecotype, across geographical areas, at least in one ecotype. These substitutions appear against the same genetic background (i.e. sequence of a particular allele, that acquired mutation) in different geographical areas for sites in close genomic proximity, but different backgrounds for unlinked sites | differentiation among geographically distant populations much smaller for candidate loci in one ecotype (may vary among loci) but not in the other ecotype |
aPredominant patterns, stochastic variation among loci expected.
bNuclear genes, not affected by ecological selection and mtDNA.
cNon-synonymous substitutions of coding regions (adaptive mutations of non-coding regions are not considered).
dIntrons, untranslated regions and synonymous substitions in coding sequences of candidate genes.
eNon-synonymous substitutions in the coding sequence of candidate genes, possibly also mutations in regulatory regions, coupled with differential expression.
fPre-dating colonization.
To investigate the mechanisms of the repeated evolution of reproductive barriers, we need to unveil the evolution of ecotype differences in size as well as in any other traits contributing to the reproductive isolation. We might also be interested in the more general issue of how similar ecotypes evolve repeatedly in different sites, independent of the fact that they also evolve reproductive barriers. A key issue for both these questions is what mechanisms have been involved in creating the ecotype differences we observe at present. Have they all evolved in the presence of gene flow between incipient ecotypes? Or, have both allopatric and parapatric or even sympatric elements been involved in ecotype evolution? Existing genetic data provide some input to this, as do the results of a recent model (Sadedin et al. 2009), and we review these below. However, a major step forward will require linking phenotypic differentiation to patterns of evolution at the molecular level (Colosimo et al. 2005; Rogers & Bernatchez 2005; Seehausen et al. 2008), and so we here propose specific hypotheses that may explain repeated ecotype differentiation in L. saxatilis, and from each of these we suggest testable predictions that will be useful to design the next generation of studies.
6. Mechanisms of ecotype formation
Using the two Swedish ecotypes (E and S) as an example, here we propose different mechanisms for their evolution, illustrating the various possible alternative mechanisms that need to be considered. The E and S ecotypes are confined to the Swedish west coast and adjacent areas of southern Norway, and they are present on wave-exposed boulder (S) and cliff (E) shores of islands and mainland shores (figure 2). The coast is a postglacial environment that became available for marine species 10 000 years ago, and since then postglacial uplift of land has continuously formed new islands. The establishment of L. saxatilis populations in this area has taken place in two steps; the first step being colonization of the first available habitat in the area, while the second step (still ongoing) is the colonization of new emerging islands.
Figure 2.
The distribution of Littorina saxatilis S and E ecotypes and hybrid forms along a rocky shore of a Swedish island. This island is rather typical for the Swedish west coast in that its shore consists of a mosaic of boulders and cliffs, and the distribution of the two ecotypes strictly follows the microhabitat distribution with hybrid zones where the habitat shifts.
A key question is where and when did the genetic variation originate that shapes the E and S ecotypes, including the differences that maintain their reproductive barrier. Here, we discuss four mechanisms that alone or in various combinations are likely to have contributed to the formation of the two ecotypes and the reproductive barrier:
(a). Allopatry
The essential formation of the two ecotypes predates the colonization of Sweden and they were introduced by double colonization from elsewhere (with minor later modifications). Upon colonizing Sweden they established secondary contact resulting in local introgression. Although the original evolution of the separate ecotypes may, or may not, have been in allopatry, the Swedish establishment was essentially an allopatric process.
(b). Parallel evolution
A single Swedish colonization followed by non-allopatric separation in local islands where genetic differences between E and S evolved repeatedly by parallel selection pressures acting on mutations of local origin (parallel evolution). We can think of either identical substitutions occurring repeatedly and independently in different populations, or unique mutations occurring in each location, but these having similar effects on the phenotype (Wood et al. 2005; Arendt & Reznick 2007).
(c). Standing genetic variation
Non-allopatric separation as in (b) but selection acting on ancestral alleles present in low frequencies in the first founder group that invaded the Swedish archipelago, or introduced later by long-distance migrating snails. Specific alleles would rapidly increase in frequency whenever populations expand into new microhabitats where these are favoured (Colosimo et al. 2005; Barrett & Schluter 2007). This variation could originally have evolved either in allopatry or in non-allopatry, but the formation of E and S is based on pre-existing variation.
(d). Evolution in concert
Non-allopatric separation as in (b) and (c) but the variation shaping E and S has a recent origin, essentially originating in the evolving Swedish populations by the accumulation of positively selected mutations in each habitat that spread rapidly by selective sweeps, and successively promoted the formation of more extreme ecotypes (Rieseberg & Burke 2001; Morjan & Rieseberg 2004).
From each of these mechanisms one may outline the patterns that will be predicted in neutral as well as adaptive parts of the genome (table 2). A limitation with earlier attempts based only on multi-loci comparisons of neutral markers (see below) is that extensive secondary introgression can erode ancestral differences formed during earlier periods of allopatry. However, differences in neutral sequences linked to adaptive loci will be maintained in the presence of hybridization, as selection impedes introgression of these sequences. We therefore expect variation in these neutral sequences to distinguish between ancestral origin and double invasion, on the one hand, and recent and repeated origins of ecotypes, on the other (table 2—1 versus 2 and 4). However, if ecotypes are formed from standing genetic variation, linked neutral sequences will diverge in much the same way as under a double invasion (Barrett & Schluter 2007). Although in this case, there will still be different phylogenetic patterns in non-candidate loci (species trees) separating the two models of evolution, unless extensive introgression has removed allopatric differences under model 1 (table 2—1 versus 3). One additional expectation is that differences between ecotypes in variation linked to adaptive loci would extend much further (genomically) in the allopatric case than it would in the standing variation case. This is because recombination erodes the linkage disequilibrium more effectively within a population than between populations. Recently, it has been suggested that genomic hitchhiking around key QTLs may be an important effect in the early divergence of species (Smadja et al. 2008; Via & West 2008).
It is important that the various models described in table 2 must be considered under specified scales of time or space (or both). For example, the independent substitution of the same nucleotide in separate sub-areas along the Swedish coast may be considered as an example of parallel evolution (model 2) within a larger area (Sweden), while at a scale of local islands, among which the new mutations spread, this will be an example of evolution in concert (model 4). Furthermore, it is important to stress that gene and population trees will not coincide for all loci in any of the models.
7. Allopatry versus non-allopatry—existing data
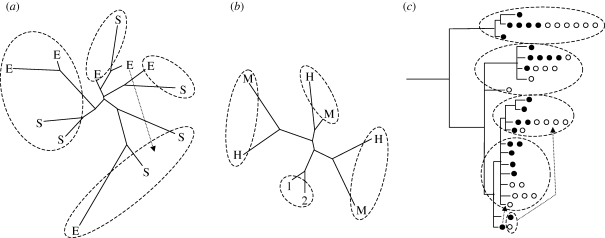
Given the limitations of multi-locus techniques discussed above, here we summarize the conclusions from the existing data. A major question that has been addressed in several earlier studies is whether the formation of the reproductively isolated ecotypes in L. saxatilis is largely a result of allopatric separation followed by secondary overlap and hybridization, or if most of the genetic variation that separates ecotypes (neutral as well as adaptive) has a non-allopatric origin (model 1 versus 2–4). A number of studies have used genetic markers (allozymes, microsatellites, AFLP) to assess the differentiation within- and between-ecotypes within a geographical area (UK: Wilding et al. 2001; Grahame et al. 2006, Spain: Johannesson et al. 1993; Rolán-Alvarez et al. 2004, Sweden: Johannesson & Tatarenkov 1997; Panova et al. 2006; Mäkinen et al. 2008). These analyses show notably similar patterns; multi-locus comparisons based on allozymes, microsatellites or AFLP variation cluster samples by geographical relatedness rather than by ecotypes (e.g. figure 3a,b). Furthermore, in Spain, the same mtDNA haplotypes are shared between ecotypes within the same region, while haplotypes of different regions are widely separated in the phylogeny (Quesada et al. 2007; figure 3c). In addition, candidate AFLP loci showed significantly larger geographic differentiation than non-candidate loci, which is in agreement with our expectations from parallel evolution of reproductive isolation (table 2).
Figure 3.
Genetic clustering of contrasting ecotypes of Littorina saxatilis from Sweden, the UK and Spain based either (a,b) on multi-locus estimates of genetic distances (microsatellites and AFLP) or (c) inferred from the phylogeny of mtDNA. Ecotypes are denoted with letters according to the terminology used in the different countries where S, M and RB are crab-tolerant phenotypes and E, H and SU are wave-tolerant (see table 1 for details). Samples from the same geographical sites (sampled within 50–100 m distance) are encircled. Open circles, RB; closed circles, SU.
To fit these data into a general allopatric model, where ecotypes evolve only when physically isolated from each other is complicated, and requires the complete removal of all differences accumulated during earlier allopatric phases. Indeed, this requires extensive secondary introgression between local ecotypes in combination with periods of demographic fluctuations. Notably, extensive secondary hybridization between allopatrically isolated lineages of bivalve species (Mytilus edulis/trossulus/galloprovincialis, and two subspecies of Macoma balthica) has not removed differences in allozymes, nuclear and mitochondrial genes accumulated during the earlier allopatric phases (Riginos & Cunningham 2005, 2007; Nikula et al. 2008).
Existing data hence support a non-allopatric model of separation, but the data are superficial and inconclusive for individual loci, some of which may be extremely important in the reproductive isolation of ecotypes. Therefore, there is a need for a more comprehensive phylogenetic analysis based on both selected and neutral variation of nuclear genes, not least because a mixed model including both allopatric and non-allopatric elements might be expected.
8. A scenario of ecotype formation
To illustrate a largely non-allopatric path of ecotype formation, let us consider the formation of E and S ecotypes in Sweden. The snails that colonized Sweden probably rafted here from the UK or France (more adjacent North Sea coasts are dominated by sandy beaches and suitable habitats are sparse), and in the light of the biology of the species and the estimated Nm = 0.03 between islands at 0.1–1 km distance (see above), an educated guess is that Nm for long-distance colonization should be at least one order of magnitude smaller. As described above, colonization is likely to be initiated by a single mated female and a new population will expand rapidly to population sizes of 10 000 snails or more (as shown by empirical observations, Johannesson & Johannesson (1995), and by modelling, Sadedin et al. (2009)). Following the initial colonization, the species probably quite rapidly colonized additional areas along the coast, a process that would possibly have been completed over some 100 years ago, assuming a step-wise pattern and Nm ∼ 0.01 between adjacent sites. Assuming that long-distance migration is much less likely than migration between nearby islands along the Swedish coast, it seems likely that after the establishment of the first island population, other islands were colonized by females from this island, and so forth. Indeed, colonization of new islands that continued to emerge out of the sea is still ongoing. The progress of this second step is likely to start with the appearance of an uplifted island in the form of a wave-swept cliff (an E habitat). A rafted E ecotype female from a nearby larger island is the most likely founder of this little island, and in a few generations, gives rise to a population of several thousands of snails (such as has been observed following repopulation events of small islands after a toxic algal bloom, Johannesson & Johannesson 1995). After the top of the island has emerged a few metres above mean water, the first boulder habitat becomes available. The only way to explain the common origin of all snails within an island in neutral markers described earlier is that the boulder habitat is colonized by members of the already established E ecotype population. This population will be restricted by either the mutation rate (parallel evolution), the available genetic variation already present (standing genetic variation) or genes introduced to the population by rafting snails from a boulder habitat already established in the area (evolution in concert). In all of these models, the neutral variation on the island will derive from the first colonization event, and even if S alleles are introduced by single migrating individuals thereafter, their effect on the pool of neutral alleles will be minor (as these events are likely to be few in number when compared with the number of snails of the established population). Hence, all three parapatric models can explain the observations referred to above, that contrasting ecotypes of the same island share a pool of neutral genes.
9. A role for parallel evolution?
An individual-based model parameterized with data from the Swedish E–S ecotype system shows that, within an island, one ecotype can readily form from the other under parapatric conditions over less than 1000 generations, under the assumption that the mutation rate is 10−5 and 2–16 independently evolving loci affect ecotype formation (Sadedin et al. 2009). Few loci with large effects rather than many loci with small effects, moderate selection pressures and the presence of intermediate zones provides the most favourable conditions for the rapid formation of ecotypes. Overall, the model supports parallel evolution of ecotypes by independent local mutations, whenever migration contributing genetic variation from other sources is low (Nm < 0.001).
Nevertheless, for adaptive alleles, extremely low levels of gene flow may occasionally introduce alleles that are selectively favoured and become important to the new population. Field densities and dispersal distances suggest Ne ∼ 10 000 in most populations of L. saxatilis and thus loci with positive mutation rates of 10−7 or higher may possibly evolve by parallel evolution if Nm < 0.001, which seems likely to be the case over large geographical distances (among countries), while less likely at a scale separating islands within an archipelago (or close localities along a coastline). At a local geographical scale, parallel evolution seems less likely unless mutation rates are high. Notably, the evolutionary history of individual alleles can only be reconstructed from gene trees linked to phylogeographic information and not from allele frequency information alone.
10. Prospects for the future
Notably, it is likely that the formation of E and S includes elements of two or more of these mechanisms, that is, some genes (possibly of allopatric origin) may be ancestral and present as standing genetic variation in the founder group, while others evolved more recently by repeated substitutions of the same nucleotide in independent sites, or through the rapid spread of new and strongly favoured alleles. Simply for this reason—that we have to consider the phylogenetic history and the function of individual genes or regions of genes, one at a time—we need to approach this problem using ‘omics-techniques’. Today, genomics, transcriptomics, proteomics and even metabolomics offer new approaches in non-model organisms that did not exist a few years ago (Ellegren & Sheldon 2008). Genome- and transcriptome-wide sequencing generate large amounts of data that can be used to develop panels of neutral markers or search for selected loci. The latter can be identified, among other approaches, from associations between ecotype and single variable nucleotides (SNPs), by looking for genome regions with decreased diversity, probably produced by selective sweeps, or by comparing silent and non-silent substitution ratios in candidate genes. For a single candidate gene, it is also possible to relate sequence variation between alleles to differences in their functional properties (Andersen et al. 2009). Using the microarray technique, we can compare expression levels of many genes in contrasting habitats or ecotypes, to estimate divergence at the transcriptomic level and at the same time identify more loci involved in ecotype differences (Ranz & Machado 2006; Shiu & Borevitz 2008). Finally, combining genomics with methodologies for quantitative traits may be the most successful approach (Stinchcombe & Hoekstra 2008).
Our suggestion that size is a crucial factor involved in the reproductive isolation of local ecotypes both in Sweden and Spain (and probably also in the UK) needs to be linked to knowledge on the genetic background of size. If we can identify dominant size genes and analyse their phylogenetic history on a background of the neutral phylogeny and phylogeography, we may finally be able to answer the question of how the reproductive isolation between ecotypes of L. saxatilis evolved, and hence cast new light on an intriguing example of repeated evolution of reproductive barriers.
Acknowledgements
This work was supported by a Linnaeus grant from the Swedish Research Councils (VR and Formas, to K.J. and C.A.), a grant from the MEC (CGL2008-00135/BOS) (to E.R.A.) and an EU Transfer of knowledge grant (to R.K.B.).
We are grateful to Juan Galindo, John Grahame, Johan Hollander, Humberto Quesada and Erik Svensson, and not least to one anonymous reviewer, for discussions and suggestions, and to Hans Ellegren and Staffan Ulfstrand for organizing the Speciation Conference at Kristineberg, Sweden.
Footnotes
One contribution of 11 to a Theme Issue ‘Genomics of speciation’.
References
- Andersen O., Wetten O. F., De Rosa M. C., Andre C., Alinovi C. C., Colafranceschi M., Brix O., Colosimo A.2009Haemoglobin polymorphisms affect the oxygen-binding properties in Atlantic cod populations. Proc. R. Soc. B 276, 833–841 (doi:10.1098/rspb.2008.1529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt J., Reznick D.2007Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation? Trends Ecol. Evol. 23, 26–32 (doi:10.1016/j.tree.2007.09.011) [DOI] [PubMed] [Google Scholar]
- Barrett R. D. H., Schluter D.2007Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 (doi:10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- Bolnick D. I., Fitzpatrick B. M.2007Sympatric speciation: models and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 38, 459–487 (doi:10.1146/annurev.ecolsys.38.091206.095804) [Google Scholar]
- Butlin R. K., Galindo J., Grahame J.2008Sympatric, parapatric or allopatric: the most important way to classify speciation? Phil. Trans. R. Soc. B 363, 2997–3007 (doi:10.1098/rstb.2008.0076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo M., García C., Rolán-Alvarez E.2001Heritability of shell traits in wild Littorina saxatilis populations: results across a hybrid zone. J. Shell Res. 20, 415–422 [Google Scholar]
- Chakraborty R., Nei M.1977Bottleneck effects on average heterozygosity and genetic distance with the step-wise mutation model. Evolution 31, 347–356 (doi:10.2307/2407757) [DOI] [PubMed] [Google Scholar]
- Colosimo P. F., et al. 2005Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science 307, 1928–1933 (doi:10.1126/science.1107239) [DOI] [PubMed] [Google Scholar]
- Conde-Padín P., Carvajal-Rodríguez A., Carballo M., Caballero A., Rolán-Alvarez E.2007Genetic variation for shell traits in a direct-developing marine snail involved in a putative sympatric ecological speciation process. Evol. Ecol. 21, 635–650 (doi:10.1007/s10682-006-9142-8) [Google Scholar]
- Conde-Padín P., Cruz R., Hollander J., Rolán-Alvarez E.2008Revealing the mechanism of sexual isolation in a case of sympatric and parallel ecological divergence. Biol. J. Linn. Soc. 94, 513–526 (doi:10.1111/j.1095-8312.2008.00998.x) [Google Scholar]
- Conde-Padín P., Caballero A., Rolán-Alvarez E.2009Relative role of genetic determination and plastic response during ontogeny for shell-shape traits subjected to diversifying selection. Evolution 63, 1356–1363 (doi:10.1111/j.1558-5646.2009.00636.x) [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation Sunderland, MA: Sinauer Associates [Google Scholar]
- Cruz R., Garcia C.2001Disruptive selection on female reproductive characters in a hybrid zone of Littorina saxatilis. Evol. Ecol. 15, 167–182 (doi:10.1023/A:1014878523629) [Google Scholar]
- Cruz R., Carballo M., Conde-Padín P., Rolán-Alvarez E.2004Testing alternative models for sexual isolation in natural populations of Littorina saxatilis: indirect support for by-product ecological speciation? J. Evol. Biol. 17, 288–293 (doi:10.1111/j.1420-9101.2003.00689.x) [DOI] [PubMed] [Google Scholar]
- Denny M. W. 2000Are there mechanical limits to size in wave-swept organisms? J. Exp. Biol. 202, 3463–3467 [DOI] [PubMed] [Google Scholar]
- Ellegren H., Sheldon B. C.2008Genetic basis of fitness differences in natural populations. Nature 452, 169–175 (doi:10.1038/nature06737) [DOI] [PubMed] [Google Scholar]
- Erlandsson J., Rolán-Alvarez E., Johannesson K.1998Migratory differences between ecotypes of the snail Littorina saxatilis on Galician rocky shores. Evol. Ecol. 12, 913–924 (doi:10.1023/A:1006559904596) [Google Scholar]
- Erlandsson J., Kostylev V., Rolán-Alvarez E.1999Mate search and aggregation behaviour in the Galician hybrid zone of Littorina saxatilis. J. Evol. Biol. 12, 891–896 (doi:10.1046/j.1420-9101.1999.00087.x) [Google Scholar]
- Feder J. L., Roethele F. B., Filchak K., Niedbalski J., Romero-Severson J.2003Evidence for inversion polymorphism related to sympatric host race formation in the apple maggot fly Rhagoletis pomonella. Genetics 163, 939–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick B. M., Fordyce J. A., Gavrilets S.2008What, if anything, is sympatric speciation? J. Evol. Biol. 21, 1452–1459 (doi:10.1111/j.1420-9101.2008.01611.x) [DOI] [PubMed] [Google Scholar]
- Fitzpatrick B. M., Fordyce J. A., Gavrilets S.2009Patterns, processes and geographic modes of speciation. J. Evol. Biol. 22, 2342–2347 (doi:10.1111/j.1420-9101.2009.01833.x) [DOI] [PubMed] [Google Scholar]
- Futuyama D. J., Mayer G. C.1980Non-allopatric speciation in mammals. Syst. Zool. 29, 254–271 [Google Scholar]
- Galindo J., Morán P., Rolán-Alvarez E.2009Comparing geographical genetic differentiation between candidate and noncandidate loci for adaptation strengthens support for parallel ecological divergence in the marine snail Littorina saxatilis. Mol. Ecol. 18, 919–930 (doi:10.1111/j.1365-294X.2008.04076.x) [DOI] [PubMed] [Google Scholar]
- Gavrilets S.2004Fitness landscapes and the origin of species Princeton, NJ: Princeton University Press [Google Scholar]
- Grahame J., Wilding C. S., Butlin R. K.2006Adaptation to a steep environmental gradient and an associated barrier to gene exchange in Littorina saxatilis. Evolution 60, 268–278 [PubMed] [Google Scholar]
- Hollander J., Lindegarth M., Johannesson K.2005Local adaptation but not geographic separation promotes assortative mating in a snail—support for ecological speciation. Anim. Behav. 70, 1209–1219 (doi:10.1016/j.anbehav.2005.03.014) [Google Scholar]
- Hollander J., Collyer M. L., Adams D. C., Johannesson K.2006Phenotypic plasticity in two marine snails: constraints superseding life-history. J. Evol. Biol. 19, 1861–1872 (doi:10.1111/j.1420-9101.2006.01171.x) [DOI] [PubMed] [Google Scholar]
- Hull S. L.1998Assortative mating between two morphs of Littorina saxatilis on a shore in Yorkshire. Hydrobiologia 378, 79–88 (doi:10.1023/A:1003237521419) [Google Scholar]
- Hull S. L., Grahame J., Mill P. J.1996Morphological divergence and evidence for reproductive isolation in Littorina saxatilis (Olivi) in northeast England. J. Moll. Stud. 62, 89–99 (doi:10.1093/mollus/62.1.89) [Google Scholar]
- Janson K.1982Genetic and environmental effects on the growth rate of Littorina saxatilis Olivi. Mar. Biol. 69, 73–78 (doi:10.1007/BF00396963) [Google Scholar]
- Janson K.1983Selection and migration in two distinct phenotypes of Littorina saxatilis in Sweden. Oecologia 59, 58–61 (doi:10.1007/BF00388072) [DOI] [PubMed] [Google Scholar]
- Janson K.1985Variation in the occurrence of abnormal embryos in females of the intertidal gastropod Littorina saxatilis Olivi. J. Moll. Stud. 51, 64–68 [Google Scholar]
- Janson K.1987aGenetic drift in small and recently founded populations of the marine snail Littorina saxatilis. Heredity 58, 31–37 (doi:10.1038/hdy.1987.5) [Google Scholar]
- Janson K.1987bAllozyme and shell variation in two marine snails (Littorina, Prosobranchia) with different dispersal abilities. Biol. J. Linn. Soc. 30, 245–256 (doi:10.1111/j.1095-8312.1987.tb00299.x) [Google Scholar]
- Johannesson K.1988The paradox of Rockall: why is a brooding gastropod (Littorina saxatilis) more widespread than one having a planktonic larval dispersal stage (L. littorea)? Mar. Biol. 99, 507–513 (doi:10.1007/BF00392558) [Google Scholar]
- Johannesson K.2001Parallel speciation: a key to sympatric divergence. Trends Ecol. Evol. 16, 148–153 (doi:10.1016/S0169-5347(00)02078-4) [DOI] [PubMed] [Google Scholar]
- Johannesson K.2009Inverting the null-hypothesis of speciation: a marine snail perspective. Evol. Ecol. 23, 5–16 (doi:10.1007/s10682-007-9225-1) [Google Scholar]
- Johannesson K., Johannesson B.1995Dispersal and population expansion in a direct developing marine snail (Littorina saxatilis) following a severe population bottleneck. Hydrobiologia 309, 173–180 (doi:10.1007/BF00014485) [Google Scholar]
- Johannesson B., Johannesson K.1996Population differences in behaviour and morphology in Littorina saxatilis: Phenotypic plasticity or genetic differentiation? J. Zool. 240, 475–493 (doi:10.1111/j.1469-7998.1996.tb05299.x) [Google Scholar]
- Johannesson K., Tatarenkov A.1997Allozyme variation in a snail (Littorina saxatilis)—deconfounding the effects of microhabitat and gene flow. Evolution 51, 402–409 (doi:10.2307/2411112) [DOI] [PubMed] [Google Scholar]
- Johannesson K., Johannesson B., Rolán-Alvarez E.1993Morphological differentiation and genetic cohesiveness over a microenvironmental gradient in the marine snail Littorina saxatilis. Evolution 47, 1770–1787 (doi:10.2307/2410220) [DOI] [PubMed] [Google Scholar]
- Johannesson K., Rolán-Alvarez E., Ekendahl A.1995aIncipient reproductive isolation between two sympatric morphs of the intertidal snail Littorina saxatilis. Evolution 49, 1180–1190 (doi:10.2307/2410443) [DOI] [PubMed] [Google Scholar]
- Johannesson K., Rolán-Alvarez E., Erlandsson J.1997Growth rate differences between upper and lower shore ecotypes of the marine snail Littorina saxatilis (Olivi) (Gastropoda). Biol. J. Linn. Soc. 61, 267–279 [Google Scholar]
- Johannesson K., Larsson A., Cruz R., Garcia C., Rolán-Alvarez E.2000Hybrid fitness seems not to be an explanation for the partial reproductive isolation between ecotypes of Galician Littorina saxatilis. J. Moll. Stud. 66, 149–156 (doi:10.1093/mollus/66.2.149) [Google Scholar]
- Johannesson K., Havenhand J. N., Jonsson P. R., Lindegarth M., Sundin A., Hollander J.2008Male discrimination of female mucous trails permits assortative mating in a marine snail species. Evolution 62, 3178–3184 (doi:10.1111/j.1558-5646.2008.00510.x) [DOI] [PubMed] [Google Scholar]
- Kemppainen P., Nes S., Ceder C., Johannesson K.2005Refuge function of marine algae complicates selection in an intertidal snail. Oecologia 143, 402–411 (doi:10.1007/s00442-004-1819-5) [DOI] [PubMed] [Google Scholar]
- Mäkinen T., Panova M., Andre C.2007High levels of multiple paternity in Littorina saxatilis: hedging the bets? J. Hered. 98, 705–711 (doi:10.1093/jhered/esm097) [DOI] [PubMed] [Google Scholar]
- Mäkinen T., Panova M., Johannesson K., Tatarenkov A., Appelqvist C., André C.2008Genetic differentiation on multiple spatial scales in an ecotype-forming marine snail with limited dispersal: Littorina saxatilis. Biol. J. Linn. Soc. 94, 31–40 (doi:10.1111/j.1095-8312.2008.00960.x) [Google Scholar]
- Mallet J., Meyer A., Nosil P., Feder J. L.2009Space, sympatry and speciation. J. Evol. Biol. 22, 2332–2341 (doi:10.1111/j.1420-9101.2009.01816.x) [DOI] [PubMed] [Google Scholar]
- Merilä J., Crnokrak P.2001Comparison of genetic differentiation at marker loci and quantitative traits. J. Evol. Biol. 14, 892–903 (doi:10.1046/j.1420-9101.2001.00348.x) [Google Scholar]
- Morjan C. L., Rieseberg L. H.2004How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles. Mol. Ecol. 13, 1341–1356 (doi:10.1111/j.1365-294X.2004.02164.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Maruyama T., Chakraborty R.1975The bottleneck effect and genetic variability in populations. Evolution 29, 1–10 (doi:10.2307/2407137) [DOI] [PubMed] [Google Scholar]
- Nikula R., Strelkov P., Väinölä R.2008A broad transition zone between an inner Baltic hybrid swarm and a pure North Sea subspecies of Macoma balthica (Mollusca, Bivalvia). Mol. Ecol. 17, 1506–1522 (doi:10.1111/j.1365-294X.2007.03688.x) [DOI] [PubMed] [Google Scholar]
- Nosil P., Harmon L. J., Seehausen O.2009Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 24, 145–156 (doi:10.1016/j.tree.2008.10.011) [DOI] [PubMed] [Google Scholar]
- Panova M., Hollander J., Johannesson K.2006Site-specific genetic divergence in parallel hybrid zones suggests non-allopatric evolution of reproductive barriers. Mol. Ecol. 15, 4021–4031 (doi:10.1111/j.1365-294X.2006.03067.x) [DOI] [PubMed] [Google Scholar]
- Pickles A., Grahame J.1999Mate choice in divergent morphs of Littorina saxatilis (Olivi); speciation in action? Anim. Behav. 58, 181–184 (doi:10.1006/anbe.1999.1115) [DOI] [PubMed] [Google Scholar]
- Quesada H., Posada D., Caballero A., Morán P., Rolán-Alvarez E.2007Phylogenetic evidence for multiple sympatric ecological diversification in a marine snail. Evolution 61, 1600–1612 (doi:10.1111/j.1558-5646.2007.00135.x) [DOI] [PubMed] [Google Scholar]
- Raffaelli D. G., Hughes R. N.1978The effects of crevice size and availability on populations of Littorina rudis and Littorina neritoides. J. Anim. Ecol. 47, 71–83 (doi:10.2307/3923) [Google Scholar]
- Ranz J. M., Machado C. A.2006Uncovering evolutionary patterns of gene expression using microarrays. Trends Ecol. Evol. 21, 29–37 (doi:10.1016/j.tree.2005.09.002) [DOI] [PubMed] [Google Scholar]
- Reid D.1996Systematics and evolution of Littorina London, UK: Ray Society [Google Scholar]
- Rice W. R., Hostert E. E.1993Laboratory experiments on speciation—what have we learned in 40 years. Evolution 47, 1637–1653 (doi:10.2307/2410209) [DOI] [PubMed] [Google Scholar]
- Rieseberg L. H., Burke J. M.2001The biological reality of species: gene flow, selection, and collective evolution. Taxon 50, 47–67 (doi:10.2307/1224511) [Google Scholar]
- Riginos C., Cunningham C. W.2005Local adaptation and species segregation in two mussel (Mytilus edulis × Mytilus trossulus) hybrid zones. Mol. Ecol. 14, 381–400 (doi:10.1111/j.1365-294X.2004.02379.x) [DOI] [PubMed] [Google Scholar]
- Riginos C., Cunningham C. W.2007Hybridization in postglacial marine habitats. Mol. Ecol. 16, 3971–3972 (doi:10.1111/j.1365-294X.2007.03505.x) [DOI] [PubMed] [Google Scholar]
- Rogers S. M., Bernatchez L.2005Integrating QTL mapping and genome scans towards the characterization of candidate loci under parallel selection in the lake whitefish (Coregonus clupeaformis). Mol. Ecol. 14, 351–361 (doi:10.1111/j.1365-294X.2004.02396.x) [DOI] [PubMed] [Google Scholar]
- Rolán-Alvarez E., Rolán E., Johannesson K.1996Differentiation in radular and embryonic characters, and further comments on gene flow, between two sympatric morphs of Littorina saxatilis (Olivi). Ophelia 45, 1–15 [Google Scholar]
- Rolán-Alvarez E., Johannesson K., Erlandsson J.1997The maintenance of a cline in the marine snail Littorina saxatilis: the role of home site advantage and hybrid fitness. Evolution 51, 1838–1847 (doi:10.2307/2411006) [DOI] [PubMed] [Google Scholar]
- Rolán-Alvarez E., Erlandsson J., Johannesson K., Cruz R.1999Mechanisms of incomplete prezygotic reproductive isolation in an intertidal snail; testing behavioural models in wild populations. J. Evol. Biol. 12, 879–890 (doi:10.1046/j.1420-9101.1999.00086.x) [Google Scholar]
- Rolán-Alvarez E., Carballo M., Galindo J., Moran P., Fernández B., Caballero A., Cruz R., Boulding E. G., Johannesson K.2004Nonallopatric and parallel origin of local reproductive barriers between two snail ecotypes. Mol. Ecol. 13, 3415–3424 (doi:10.1111/j.1365-294X.2004.02330.x) [DOI] [PubMed] [Google Scholar]
- Sadedin S., Hollander J., Panova M., Johannesson K., Gavrilets S.2009Case studies and mathematical models of ecological speciation 3. Ecotype formation in a Swedish snail. Mol. Ecol. 18, 4006–4023 (doi:10.1111/j.1365-294X.2009.04320.x) [DOI] [PubMed] [Google Scholar]
- Saur M.1990Mate discrimination in Littorina littorea (L.) and L. saxatilis (Olivi) (Mollusca: Prosobranchia). Hydrobiologia 193, 261–270 (doi:10.1007/BF00028082) [Google Scholar]
- Shiu S. H., Borevitz J. O.2008The next generation of microarray research: applications in evolutionary and ecological genomics. Heredity 100, 141–149 (doi:10.1038/sj.hdy.6800916) [DOI] [PubMed] [Google Scholar]
- Schluter D., Nagel L. M.1995Parallel speciation by natural selection. Am. Nat. 146, 292–301 (doi:10.1086/285799) [Google Scholar]
- Schluter D.2009Evidence for ecological speciation and its alternative. Science 323, 737–741 (doi:10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- Schluter D., Clifford E. A., Nemethy M., McKinnon J. S.2004Parallel evolution and inheritance of quantitative traits. Am. Nat. 163, 809–822 (doi:10.1086/383621) [DOI] [PubMed] [Google Scholar]
- Seehausen O., et al. 2008Speciation through sensory drive in cichlid fish. Nature 455, 620–627 (doi:10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- Smadja C., Galindo J., Butlin R. K.2008Hitching a lift on the road to speciation. Mol. Ecol. 17, 4177–4180 (doi:10.1111/j.1365-294X.2008.03917.x) [DOI] [PubMed] [Google Scholar]
- Stinchcombe J. R., Hoekstra H. E.2008Combining population genomics and quantitative genetics: finding the genes underlying ecologically important traits. Heredity 100, 158–170 (doi:10.1038/sj.hdy.6800937) [DOI] [PubMed] [Google Scholar]
- Strasburg J. L., Rieseberg L. H.2008Molecular demographic history of the annual sunflowers Helianthus annuus and H. petiolaris—large effective population sizes and rates of long-term gene flow. Evolution 62, 1936–1950 (doi:10.1111/j.1558-5646.2008.00415.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg P.1988Microgeographic variation in shell characters of Littorina saxatilis Olivi—a question mainly of size. Biol. J. Linn. Soc. 35, 169–184 (doi:10.1111/j.1095-8312.1988.tb00464.x) [Google Scholar]
- Via S., West J.2008The genetic mosaic suggests a new role for hitchhiking in ecological speciation. Mol. Ecol. 17, 4334–4345 (doi:10.1111/j.1365-294X.2008.03921.x) [DOI] [PubMed] [Google Scholar]
- Wilding C. S., Butlin R. K., Grahame J.2001Differential gene exchange between parapatric morphs of Littorina saxatilis detected using AFLP markers. J. Evol. Biol. 14, 611–619 (doi:10.1046/j.1420-9101.2001.00304.x) [Google Scholar]
- Wood T. E., Burke J. M., Rieseberg L. H.2005Parallel genotypic adaptation: when evolution repeats itself. Genetica 123, 157–170 (doi:10.1007/s10709-003-2738-9) [DOI] [PMC free article] [PubMed] [Google Scholar]