Abstract
Many continental sister species are allopatric or parapatric, ecologically similar and long separated, of the order of millions of years. Sympatric, ecologically differentiated, species, are often even older. This raises the question of whether build-up of sympatric diversity generally follows a slow process of divergence in allopatry, initially without much ecological change. I review patterns of speciation among birds belonging to the continental Eurasian Old World leaf warblers (Phylloscopus and Seicercus). I consider speciation to be a three-stage process (range expansions, barriers to gene flow, reproductive isolation) and ask how ecological factors at each stage have contributed to speciation, both among allopatric/parapatric sister species and among those lineages that eventually led to currently sympatric species. I suggest that time is probably the critical factor that leads to reproductive isolation between sympatric species and that a strong connection between ecological divergence and reproductive isolation remains to be established. Besides reproductive isolation, ecological factors can affect range expansions (e.g. habitat tracking) and the formation of barriers (e.g. treeless areas are effective barriers for warblers). Ecological factors may often limit speciation on continents because range expansions are difficult in ‘ecologically full’ environments.
Keywords: allospecies, ecological speciation, Phylloscopus and Seicercus, speciation, time-dated phylogenies, vicariance
1. Introduction
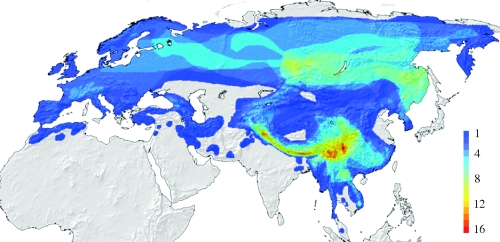
The Old World leaf warblers form a clade of small insectivorous birds, currently classified into two genera (Phylloscopus and Seicercus; Clement et al. 2006). About 60 species breed in temperate habitats throughout continental Eurasia (Clement et al. 2006), reaching their maximum diversity in the eastern Himalayas and southern China, where up to 16 species may occur along altitudinal gradients (figure 1). Diversification of these species has taken place over approximately the past 11–12 Myr (Johansson et al. 2007; figure 2).
Figure 1.
Numbers of Phylloscopus and Seicercus species in sympatry across continental Eurasia, as estimated by overlapping the range maps in Clement et al. (2006). Figure kindly constructed by B. Hawkins.
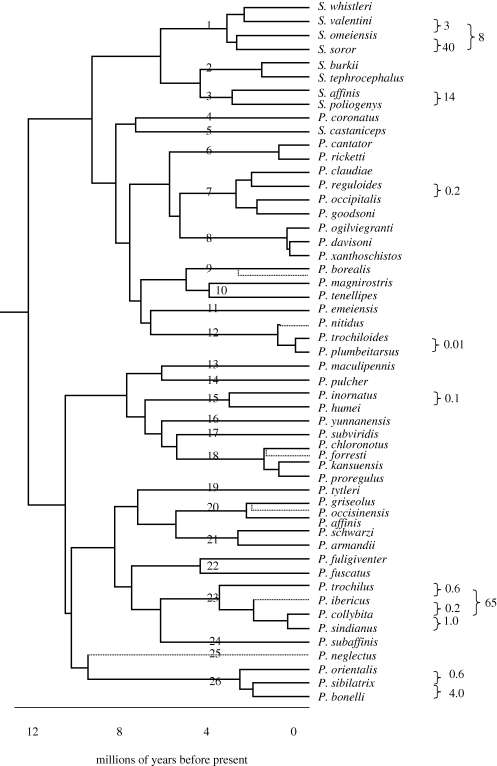
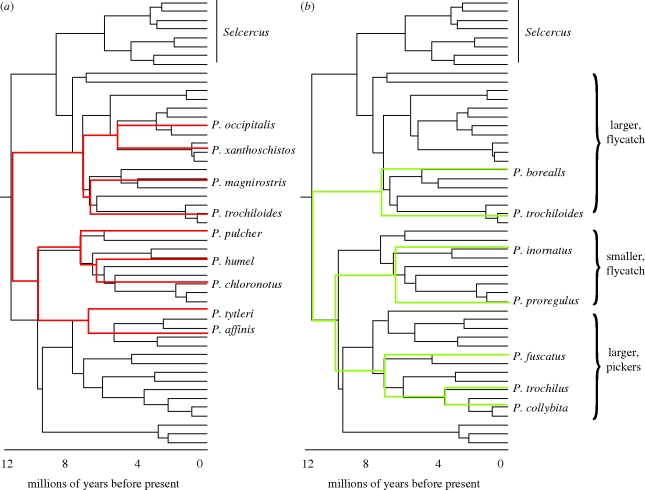
Figure 2.
Phylogenetic relationships among all species of continental Eurasian Old World leaf warblers (Seicercus and Phylloscopus) from Johansson et al. (2007), with non-continental taxa pruned. The tree was built using both mitochondrial and nuclear genes and rate-smoothed. Time scale at the bottom is based on a mitochondrial DNA rate of evolution of 2% Myr−1, as assessed by comparing the average (GTR-γ corrected) distance in mtDNA cytochrome b across the root of the tree. Numbers on the tree indicate lineages at 4 Ma, and numbers to the right of the tree the percentage of the total geographical range that is shared, for couplets of species separated by less than 4 Myr, based on ranges in Clement et al. (2006), with some corrections. Possible overlap of borealis with its sister and forresti with chloronotus is unreported, but they are considered allopatric here. If no number is indicated, the couplets are allopatric. Dashed lines indicate continental taxa not considered by Johansson et al. (2007), which have been inserted based on information from the following: unnamed sister to Phylloscopus borealis (Saitoh et al. 2006; Reeves et al. 2008), Phylloscopus nitidus (Price et al. 1997), Phylloscopus forresti (Martens et al. 2004), Phylloscopus occisinensis (Martens et al. 2008), P. ibericus (Helbig et al. 1996) and Phylloscopus neglectus (U. Olsson & P. Alström, mtDNA cytochrome b sequence provided 2009, personal communication).
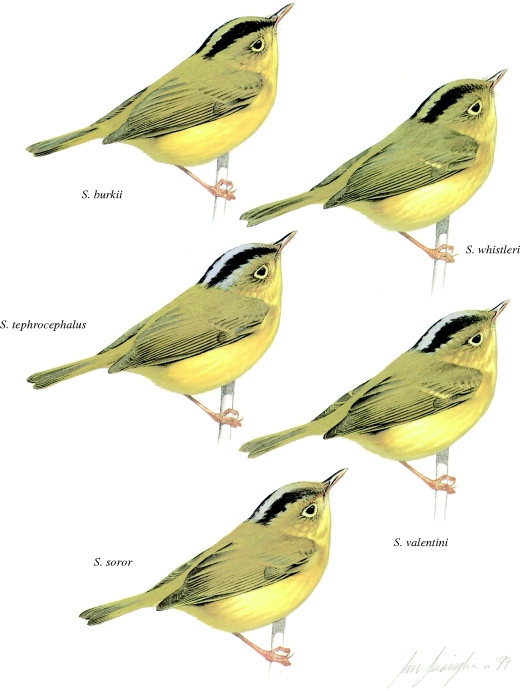
A striking feature of these warblers is their great similarity in plumage and morphology, and in some cases vocalizations, making them a classic challenge for birdwatchers. Over the past 20 years, the group has become especially well known for the number of cryptic species that have been discovered (Irwin et al. 2001a; Rheindt 2006). Sibley & Monroe (1990) recognized 36 mainland Asian species, but Clement et al. (2006), 48. Since the compilation of Clement et al. (2006), a further five species have been added (Martens et al. 2004, 2008; Olsson et al. 2005; Päckert et al. 2009), resulting in an approximately 50 per cent increase in the number of recognized continental species over the past 20 years. The additional species include not only allopatric subspecies that have been split on further study, but also sympatric taxa that were so similar as to be mistaken for conspecifics in previous work (e.g. figure 3).
Figure 3.
Five of the six recognized species in the Seicercus burkii complex. Three of the illustrated species (Seicercus soror, S. tephrocephalus, Seicercus valentini) are common along a single altitudinal gradient in Emei Shan, China, with S. soror at the lowest elevations and S. valentini at the highest elevations. Seicercus burkii (lower) and Seicercus whistleri (higher) occur together in the Himalayas. Illustration drawn by Ian Lewington to accompany the article ‘The Golden spectacled warbler: a complex of sibling species, including a previously undescribed species’, by Alström & Olsson (1999).
The great similarity in form between closely related taxa, the great diversity of species and their general abundance (Phylloscopus warblers can comprise up to 40 per cent of all birds in some localities; Price et al. 2003) has made them a suitable model for the field study of speciation on continents (Richman & Price 1992; Marchetti 1993; Helbig et al. 1996; Alström & Olsson 1999; Irwin et al. 2001a,b). Given that the external differences between the species are so small, it has been possible to infer likely intermediate stages in their evolution, and relate these to the generation of premating reproductive isolation. In this paper, I review patterns of speciation among the Old World warblers within continental Eurasia, focusing in particular on the build-up of sympatric diversity. Assuming the importance of geographical separation, speciation can be limited at any stage of a three-step process: range expansions, barrier formation and the generation of reproductive isolation (e.g. Price 2008; Phillimore & Price 2009). My main focus is on how ecological factors might limit speciation at each step in the process. In the discussion, I evaluate the findings in the light of what is known about reproductive isolation and speciation in birds (Price 2008), as well as in other groups (Coyne & Orr 2004).
2. Old world leaf warblers
I follow the taxonomy of Clement et al. (2006), but add the additional species described in Martens et al. (2004, 2008), Olsson et al. (2005) and Päckert et al. (2009). Other species are present in Indonesia, the Philippines, the Canary Islands and Africa, but these species are not considered further here. An estimate of phylogenetic relationships among the continental Eurasian species is shown in figure 2, taken from Johansson et al. (2007) but with the non-continental taxa in that study pruned from the tree. This phylogeny was built using both nuclear and mitochondrial sequences, mostly collected by Olsson et al. (2004, 2005) and rate-smoothed. The time scale is based on the average distance across the root in the mitochondrial cytochrome b gene, assuming a rate of approximately 2 per cent divergence/million years (Weir & Schluter 2008). The approximate insertion of six taxa (dashed lines) missing from Johansson et al. (2007) was determined either on the basis of cytochrome b gene sequences, or described relationships in other papers (see the legend to figure 2).
Many of the critical nodes in figure 2 discussed in this paper have strong support (Olsson et al. 2004, 2005; electronic supplementary material). The relative timings of cladogenesis depicted in figure 2 are largely consistent with a relaxed clock constructed using the cytochrome b mitochondrial sequence data in the program BEAST (Drummond & Rambaut 2007; electronic supplementary material), except that dates are in general 1–2 Myr older in the BEAST tree, and one date in particular (insertion of Phylloscopus trochilus) is 3 Myr older (confidence limits: 1–2 Myr). The mitochondrial distance from P. trochilus to other related taxa (approx. 12%) supports the older date, and it is possible that the short branch length in the rate-smoothed tree in figure 2, which was based on both mitochondrial and nuclear DNA, represents nuclear introgression (Bensch et al. 2006). Thus, the relative time for this split in figure 2 may be underestimated. I present dates based on the rate-smoothed tree in the following discussion, but it should be borne in mind that they generally come with 1–2 Myr error (see electronic supplementary material).
3. Causes of reproductive isolation
Reproductive isolation forms the foundation of the biological species concept (Mayr 1942, 1963; Coyne & Orr 2004) and is essential for persistence of species in sympatry. Before considering the process of speciation in the group, I review the mechanisms that prevent sympatric species from interbreeding.
(a). Premating isolation
Among parapatric species, only one hybrid zone is known, in the Phylloscopus collybita superspecies group (figure 4). Sympatric species rarely, if ever, hybridize, with only a few European examples of apparent hybrids between Phylloscopus bonelli/Phylloscopus sibilatrix and P. collybita/P. trochilus (McCarthy 2006). We have banded both parents and nestlings at over 500 nests in two localities in the western Himalayas, containing eight and 11 species, respectively (Price et al. 2003), as well as made many behavioural observations on hundreds of additional pairs, and never observed hybridization (Price & Jamdar 1991; unpublished observations). Thus, premating reproductive isolation among sympatric species is strong.
Figure 4.
The chiffchaff (P. collybita) superspecies complex (redrawn from Martens 1996, after Helbig et al. 1996). Phylloscopus sindianus includes lorenzii as a subspecies; P. collybita includes tristis, abietinus and caucasicus. The complex includes (a) the only known hybrid zone in the Phylloscopus, (b) a large zone of introgression between two subspecies, and (c) possibly another contact zone. In addition, P. sindianus lorenzii and P. collybita caucasicus co-occur apparently without interbreeding in the Caucuses (Martens 1982). Adapted from illustration drawn by Emiko Paul for the book ‘Speciation in Birds’ by T. Price (2008, Roberts and Company).
Subtle differences in plumage patterns may affect species recognition (Päckert et al. 2004), but we have little direct evidence that this is the case. Phylloscopus humei and Phylloscopus tytleri breed in the same location, and have very similar plumages, differing most obviously in the presence of a wing bar in P. humei (e.g. see photographs in Burke 1992). Marchetti (1993, 1998) painted out the wing bar of P. humei, and this created a likeness between the species sufficient to confuse human observers (T. Price & K. Marchetti 1989, 1990, personal observations). The painting experiment affected territory sizes of P. humei males, probably a result of interactions between conspecifics, but had no effect on conspecific mating success among those males who retained territories (Marchetti 1998). Likewise, males of P. collybita can be induced to attack a stuffed mount of the very-similar looking, but sympatric, P. trochilus, when played P. collybita song (Saether 1983).
In contrast to plumage, vocalizations are clearly an important species recognition mechanism, as demonstrated by the failure of males to respond aggressively to playback of other species' songs. Tests of female responses have only been done once in these warblers (Salomon 1989), but in other systems have been shown to correlate with male aggressiveness (Price 2008, ch. 10). We have conducted many playback experiments of songs of one species to another sympatric species and found that male responses to heterospecific song are extremely rare. This is true in experiments even between conspecifics with quite similar songs (e.g. Phylloscopus reguloides versus Phylloscopus occipitalis, Seicercus affinis versus Seicercus poliogenys; T. Price 1994–2009, unpublished observations). Thielcke et al. (1978), Saether (1983), Alström et al. (1992) and Alström & Olsson (1992, 1995, 1999) report similar findings. The main exception to a lack of conspecific response in regions where species co-occur is in the hybrid zone between Phylloscopus ibericus and P. collybita in the Pyrenees (figure 4). Here, P. ibericus males respond aggressively to P. collybita songs, even though the songs are readily distinguishable (Salomon 1989). In addition to songs, call notes often differ strikingly between species, and these may have an important role in species recognition (Martens 1982; Päckert et al. 2004), but this has not been tested.
(b). Postmating isolation
Hybrids may have reduced fitness for multiple ecological causes, but because they are not generally produced in nature we do not know. However, Irwin & Irwin (2005) note that non-interbreeding taxa of both the Phylloscopus trochiloides and Phylloscopus inornatus superspecies complexes, which meet in parapatric zones of overlap complex to the north of the Tibetan plateau, migrate in different directions. They suggest that hybrids would take intermediate flight paths, fly into Tibet and have high mortality. In central Sweden, one subspecies of P. trochilus has spread from the north and another from the south, and they meet and interbreed in central Sweden (Bensch et al. 1999, 2009). The zone of hybridization is sufficiently narrow to indicate selection against hybrids (Bensch et al. 1999), which may be a consequence of the alternative migratory routes of the two subspecies, resulting in intermediacy of the hybrids (Bensch et al. 1999, 2009).
If these migratory effects are present, they are examples of extrinsic postmating isolation between relatively young taxa that come into contact. Many allopatric taxa and most sympatric species are older. A survey of crosses across multiple passerine bird species indicates that by 3 Myr hybrids are generally showing reduced fertility, and many other intrinsic health problems, which may lower fitness greatly in nature (Price & Bouvier 2002). In the P. collybita/P. ibericus hybrid zone in the Pyrenees (separation at perhaps 2 Ma, figure 2), evidence for nuclear, but not mitochondrial, gene flow suggests female hybrids have lower fitness than male hybrids (Helbig et al. 2001). The lower fitness of female rather than male hybrids (‘Haldane's rule’) is a virtually universal finding in controlled crosses among birds (Price & Bouvier 2002; Price 2008). Explanations have always been based on intrinsic genetic incompatibilities, rather than ecology (Turelli & Orr 2000).
In the next sections, I ask how the most recent species have formed, and then consider the more distant speciation events that led to currently sympatric taxa. In each section, I consider barriers, range expansions and reproductive isolation. All geographical ranges were redrawn from Clement et al. (2006) and entered into ArcGis 9.2. I used the maps to calculate range areas, latitudinal position and area of overlap between species.
4. Divergence in allopatry
(a). Barriers
In order to evaluate factors contributing to recent speciation events, I evaluate the spatial distributions of close relatives. To use an objective comparison, I focus on sister species. Seventeen of the 21 sister pairs in figure 2 are estimated to have shared a common ancestor within the last 3 Myr or so, and hence likely to have been affected by climate change approaching, and within, the Pleistocene. Thirteen of the sisters (60%) are allopatric, two (10%) have narrowly overlapping parapatric ranges (parapatry is defined here as less than 1% of the total range of the two species overlapping, figure 2) and a further two (10%) require further study, but are either allopatric or parapatric (legend, figure 2). Only four (20%) sister pairs have range overlaps of 1 per cent or more.
There is little indication that barriers within the Himalayas have led to Pleistocene, or even earlier, speciation (Johansson et al. 2007). Much of the Himalayas, particularly to the west, was probably dry and devoid of much forest during glacial maxima (Ray & Adams 2001). Instead, three sister species pairs are separated between south Asia and further north (Johansson et al. 2007). These species often occur in fairly open habitats (i.e. close to treeline in the Himalayas), and may have retained refuges in the Himalayas, in central Asia and in eastern Siberia, which all retained steppe-like habitat during glacial maxima, and were separated by arid deserts (Ray & Adams 2001).
The majority (12) of the sister pairs are separated between the Himalayas and southern China, or within southern China (Johansson et al. 2007). Forest refuges of the kind occupied by many of these species (mixed deciduous and coniferous woodlands) were apparently present in southern China and Southeast Asia during glacial maxima, but not further north or west (Ray & Adams 2001).
(b). Range expansions
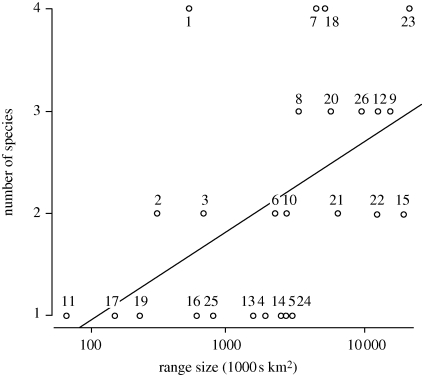
Larger ranges may be more likely to be bisected by a barrier, and hence species with large ranges are more likely to produce daughter species (Rosenzweig 1995). To assess this for the warblers, I investigated all lineages that crossed the 4 Ma timeline (figure 2), and asked whether the number of contemporary species produced by a lineage correlated with the outline of all taxa ranges combined (i.e. if there was some sympatry, the overlapping portion of the range was counted once; termed the ‘lineage range size’). The 4 Ma timeline was set because it produced a reasonable diversity of species (1–4) at the lineage tips, and because most of the component species remain allopatric/parapatric (figure 2). I repeated all the analyses described below with any area unoccupied within the lineage's range removed from the estimate of range size (see Gaston 1991): results were unchanged and are not reported.
Although there is much scatter and several recent speciation events are associated with small ranges across the mountainous regions of southern China, the number of terminal species is significantly positively correlated with lineage range size (figure 5). This could be a consequence of multiple postspeciational range changes, in that if each species independently increases its range size after speciation, the present-day lineage range size will be larger in those groups with more species. However, this seems unlikely. First, many groups still bear the signature of allopatric speciation (e.g. the P. collybita group, figure 4). Second, lineage range size is strongly correlated with the northerly position of the range (correlation between log range size and mid-point latitude, r = 0.5, p = 0.01 (after correcting for phylogeny using generalized estimating equations (Paradis & Claude 2002) and branch lengths as trimmed from figure 2 at the 4 Ma timeline, p = 0.003)). The correlation between range size and latitude is known as Rapoport's rule, and has been demonstrated previously at the species level for this group (Price et al. 1997). Price et al. (1997) argued that Rapoport's rule results because taxa occupying a similar climatic/habitat zone (high altitudes in the Himalayas, and low altitudes further north) are able to maintain large ranges, whereas species confined to lower altitudes in the south occupy climatic zones, which are not present in the north. These differences in distributions and climatic zones occupied are presumed to have been a constraint since at least the beginning of the Pleistocene. The result is that those taxa that have maintained ranges extending to the north through the last few million years are also those to have been bisected by barriers in the north.
Figure 5.
Association of the number of species in a lineage with the area covered by the lineage. Numbers by points refer to numbers on the lineages crossing the 4 Ma timeline as indicated in figure 2. Least squares trend line is drawn for illustration only. Significance was tested assuming Poisson errors with a log link, first using GLM in the statistical package R (p = 0.05), and second after correcting for phylogeny, based on the branch lengths of the truncated tree and using generalized estimating equations (Paradis & Claude 2002) implemented in APE (Paradis et al. 2004) (p = 0.01). When the mid-point of the latitudinal extent was included as a covariate, the corresponding p-values are p = 0.1 and p = 0.03, respectively.
(c). Reproductive isolation
Some allopatric taxa separated by as little as 1.5 Myr show no response to each other's song (Thielcke et al. 1978; Irwin et al. 2001a,b). Across several groups, time of separation between allospecies correlates with the magnitude of song differences between them, as measured using syntax, frequency and song length characteristics (Päckert et al. 2004, 2009). The implication is that over time, songs diverge, and song recognition diverges, leading to the accumulation of premating isolation.
Songs may diverge between populations because of ecological selection pressures. Badyaev & Leaf (1997) and Mahler & Gil (2009) found that small species sing at higher frequencies than larger ones. Some other song characteristics (emphasized frequency, repetition rate) also appear to be correlated with aspects of habitat complexity (Badyaev & Leaf 1997; Mahler & Gil 2009). However, allospecies are generally ecologically very similar (e.g. Irwin et al. 2001a,b) and a main cause of song divergence may simply be the appearance of cultural song mutations, owing to errors in the copying process (the oscine passerines or songbirds, which include these species, typically learn their songs from unrelated males during their first few months of life.).
Development of song differences between two taxa in the P. trochiloides group has been attributed to parallel selection for higher song complexity in more northerly latitudes, with the form of the complexity being arbitrary (Irwin 2000; Irwin et al. 2001b). Thielcke (1983) promoted an additional mode of cultural evolution of songs. In captivity, in the absence of tutors, young males develop unusual songs. Thielcke found that hand-raised P. collybita males from Germany sometimes sing songs that resemble those of the closely related Spanish species, P. ibericus (sonograms are in Price (2008, ch. 10)). Phylloscopus collybita males rarely respond to P. ibericus songs (Thielcke et al. 1978; Salomon 1989). These findings suggest that both song and song recognition could evolve rapidly if a population of young birds untutored by adults becomes established in an outlying area.
Postmating isolation also seems likely to develop as a result of divergence in allopatry/parapatry. For example, Irwin & Irwin (2004) note that range expansions around the Tibetan plateau in two forms in the P. trochiloides group should result in a gradual change in migratory direction, with the result that they differ strongly in migratory direction where they meet north of the plateau. In addition, it is probable that some genetic incompatibilities develop during long periods of divergence (Price & Bouvier 2002).
5. Establishment of sympatry
Under a definition of sympatry that includes species breeding within a few kilometres of each other, the number of species in sympatry varies from one to 16 (e.g. figure 1), including species that are separated along elevational gradients. Ecological differences in species-rich assemblages have been studied in the western Himalayas (Kashmir, nine species; Price 1991) and central Siberia (seven species; Forstmeier et al. 2001; figure 6). I focus on the speciation events that led to the build-up of sympatric diversity as represented in these communities. The phylogenies connecting species within both assemblages straddle the root of the whole tree (figure 6). The species cover much of the ecological and morphological diversity within the Phylloscopus (Richman 1996), but the Seicercus warblers, which are more flycatcher-like than the Phylloscopus, are not present in either community. The species in these assemblages are old. Apart from a probably underestimated date of divergence between P. collybita and P. trochilus (electronic supplementary material), the youngest species are separated by an estimated 5 Myr.
Figure 6.
Phylogenetic relationships for species in two local communities, drawn on to the tree of figure 2. (a) Western Himalayas (34° N, 75° E; Price 1991). Note that Phylloscopus xanthoschistos was not in the paper of Price (1991), because this species occurs at lower, unstudied, elevations than the other species. (b) Central Siberia (62° N, 89° E; Forstmeier et al. 2001). One species (P. trochiloides viridanus) is held in common. Ecological categories for the three main clades are consistent across the Himalayan and Siberian studies. Flycatching versus picking refers to the extent to which prey capture involves a flying movement. Within each community, both the span of average feeding methods and body sizes among species within a clade do not overlap across the three clades.
Given the likely inhospitable habitat in both locations just 18 000 years ago (Ray & Adams 2001), the assemblages in the study locations must have formed recently. However, sympatry between at least some of these species may well be ancient. The argument is as follows. The three basal lineages have led to multiple species in both assemblages (figure 6). All members derived from each lineage share a set of ecological characteristics: one lineage has led to generally small species that tend to flycatch, the second to large species that flycatch and the third to large species that tend to pick insects from the vegetation (Price 1991; Forstmeier & Kessler 2001; figure 6). Because all descendants from a lineage are similar ecologically, ancestral reconstructions imply that the three ancestral species themselves differ in body size and/or feeding method (Richman & Price 1992; Richman 1996). Similar ecological differences between species reflect differences between species in single habitats in the Himalayas at the present day (Price 1991), but such differences are not usually seen between present-day allospecies, which occupy similar ecological niches in different geographical locations (e.g. Irwin et al. 2001a,b). Thus, the inferred ancient divergence in body size and feeding method suggests sympatric interactions between ecologically divergent species.
Within each of the three clades, species in the western Himalayas are separated by elevation (e.g. coniferous versus birch versus rhododendron habitats), implying most recent divergence events in the group are along this axis (Richman & Price 1992). Ecological differences between species within the three clades are less apparent in Siberia, perhaps because the low overall population densities reduce interspecific competition (Forstmeier et al. 2001) and/or because altitudinal differentiation in habitat is not as striking.
(a). Barriers
Diversification within the group may have been initiated 11–12 Ma (figure 2; electronic supplementary material, appendix). Ancestral reconstructions of the first two splitting events indicate one barrier between tropical Southeast Asia and southern China, and the other within southern China, albeit with much uncertainty (Johansson et al. 2007). The dates of subsequent splitting events within the three clades appear remarkably similar: for the western Himalayan community, four of five splits cluster tightly around approximately 7 Ma, as do three of four for the Siberian community (figure 6) (point estimates of these dates are approx. 1.5 Myr older for the relaxed clock tree presented in electronic supplementary material, appendix). Ancestral reconstructions point to barriers forming between the Himalayas and regions to the east or north driving the majority of these population-splitting events. The implication is that there were multiple range expansions into the Himalayas prior to these splits (Johansson et al. 2007).
(b). Range expansions
The period 9–6 Ma was a time of major climate change. The south Asian monsoons began about 8–9 Ma, associated with increased seasonality of rainfall and a prolonged dry season (Zhisheng et al. 2001). The mammalian fossil record from the lower mountains of northern Pakistan indicates multiple range shifts during this time, associated with an increase in grassland species and general aridity. The major period of faunal turnover lies between 8.5 and 6 Ma (Badgley et al. 2008).
Range shifts probably applied to other taxa, including the warblers. Simultaneous dates of lineage splitting, as observed for the species under consideration here, have been interpreted in two contrasting ways. First, simultaneous splits may reflect a vicariant event, where new barriers split multiple species with congruent ranges (Zink et al. 2000). Invoked barriers include arid regions or ice sheets (Zink et al. 2000; Weir & Schluter 2004). Second, simultaneous splits may be a result of multiple successful dispersal events across a barrier, either because the barrier becomes more porous (Weir et al. 2009) or because of improved conditions for settlement beyond the barrier (Voelker 1999). In the case of dispersal across a barrier, population splitting follows on directly from the range expansion, so the range expansion can be dated. If this applies to the Old World warblers, then the simultaneous splits at approximately 7 Ma suggest that range expansions across barriers occurred at this time, perhaps as a result of climate change creating suitable habitats.
A possible two-step scenario for the build-up of sympatric diversity in the Himalayas is as follows. First, three ancestral allopatric warbler taxa, produced by barriers between the Himalayas, southern China and Southeast Asia, expanded into sympatry at about 7 Myr ago and long after their initial divergence. Sympatry was associated with differentiation into discrete ecological niches: picking, large-prey flycatching and small-prey flycatching. These range expansions themselves immediately set in the process of differentiation between the Himalayas and further east, which affected all three ancestral species. The lineages produced by this second round of allopatric differentiation spread into sympatry sometime between 7 Myr and the present day. In this case, sympatry was associated with differentiation into different elevational zones.
Siberian sympatric diversity reflects expansions of the Himalayan species, and expansions from the west.
(c). Reproductive isolation
In principle, a long period of divergence in allopatry, could lead to complete reproductive isolation when species establish sympatry. Alternatively (i) ecological interactions in sympatry could increase both premating and postmating reproductive isolation between incipient species and (ii) premating isolation could be reinforced as a result of selection to avoid cross-taxon matings. I consider each in turn.
(i). Ecological interactions
The model of speciation in allopatry inferred from current patterns implicates long time periods over which both premating and postmating isolation build among ecologically similar populations. It need not apply if speciation events in the past were associated with ecological opportunity, such as following an extinction event, or habitat alterations in response to climate change (Rosenzweig 1995). Plausibly, a range expansion into a novel habitat is rapidly followed by a second range expansion from the same source associated with exploitation of a different part of the resource spectrum. In this case, the two sympatric lineages have only had a short time to diverge, and reproductive isolation may be incomplete prior to establishment of sympatry. Ecological interactions could increase the level of both premating isolation (e.g. individuals occupy different habitats, so encounter each other less frequently, songs evolve in response to environmental selection pressures) and postmating isolation if hybrids between the taxa are inefficient in either of the parental niches. The extreme alternative to rapid formation of sympatry, associated with the generation of reproductive isolation, is the development of substantial reproductive isolation in allopatry. In this case, a major role for ecological interactions in the evolution of reproductive isolation is unlikely. It is difficult to reject this alternative, as considered further in the discussion.
(ii). Reinforcement
Premating isolation could be reinforced as a result of selection against hybrids. If hybrids have low fitness, individuals that mate with members of their own taxon have higher fitness than those that cross mate, resulting in selection leading to increased assortment (Coyne & Orr 2004, ch. 10). Indirect arguments suggest a role for this process in completing speciation. First, although song divergence in allopatry does lead to assortative mating, this is not always perfect even after a long period of divergence. In the region of overlap between P. collybita and P. ibericus (figure 4), hybrids are still produced despite song differences. Second, some sympatric non-interbreeding species differ in vocalizations and plumage in very subtle ways (Martens 1982; Alström & Olsson 1993; Päckert et al. 2004, 2009). One very similar sympatric pair is that of Seicercus omeiensis and Seicercus tephrocephalus, which in some locations in Sichuan have overlapping territories (Päckert et al. 2004). Apart from extreme similarity in plumage, the songs of these two species cover almost the same frequency range and include the same syntax structures. They differ slightly in some frequency and time parameters, and S. tephrocephalus has an introductory note separated from the rest of its song by a distinct gap, which S. omeiensis does not (Päckert et al. 2004) (however, it remains unclear whether this is a consistent across all individuals of each species; Alström & Olsson 1999). Vocal differences appear sufficient for conspecific identification, but they appear to be less than differences between pairs of allopatric taxa that continue to respond to each other's vocalizations (e.g. Thielcke et al. 1978; Alström & Olsson 1999). The implication is that sympatric taxa that are little differentiated would respond to each other's songs when they first encounter each other in sympatry, and perhaps cross-mate, were it not for selection to discriminate among subtle differences. Despite the external similarity of S. tephrocephalus and S. omeiensis, they may be separated by as much as 6 Myr (figure 2), implying hybrids have much reduced fitness (Price & Bouvier 2002) and strongly favouring individuals that mate conspecifically.
6. Discussion
The 60 or so continental species of Old World warblers diverged from a common ancestor over the past 11–12 Myr. Sympatric species are old (generally more than 3 Myr) and do not hybridize. Closely related allopatric species are also old (at least 1 Myr, often much more). Seventeen of 21 sister species are allopatric or narrowly parapatric, and none have one species range completely enclosed in the other. All indications are that species in this group follow the classic model of speciation, whereby substantial differentiation occurs in allopatry, or at least parapatry, prior to establishment of sympatry (Mayr 1942, 1963; Coyne & Orr 2004).
Long periods of divergence between allopatric taxa should result in high levels of both premating and postmating isolation, but both may be incomplete when contact is restored. Indeed, many taxa that continue to hybridize in hybrid zones are probably old. For example, J. Weir & T. Price (2009, unpublished observations) estimated the age of 41 pairs of bird taxa forming New World hybrid zones to be approximately 2 Ma at the equator and approximately 1 Ma in more temperate regions. Persistent hybridization in hybrid zones is a consequence of the parapatric arrangement of taxa. In sympatry, taxa with substantial hybridization should either collapse to one species, or form a hybrid swarm, or else premating isolation should be reinforced and hybridization reduced. Liou & Price (1994) showed that provided the two taxa occupy different ecological niches so that extinction is unlikely, strong premating isolation will inevitably be reinforced, provided postmating isolation is also strong. I suggest that the great similarity in vocalizations and behaviour of some sympatric species pairs is evidence for reinforcement of the recognition mechanism (i.e. conspecifics are under selection to discriminate what may be subtle, diagnostic, differences between taxa). This is because if allopatric taxa are compared, songs that appear more different are sometimes responded to (Thielcke et al. 1978; Irwin et al. 2001a,b).
(a). Ecology and time
In contrast to the great age of the sympatric warblers, in other groups young sympatric species, separated of the order of thousands to hundreds of thousands of years, are often found in young environments, such as postglacial or postdesiccation lakes (Schluter 1996; Seehausen 2006a), in recently formed island archipelagoes (Grant & Grant 2008), and in the Andes (Hughes & Eastwood 2006; Weir 2006). Where studied, these young species are ecologically differentiated, and the ecological differences are implicated in both sympatric coexistence and the rapid achievement of reproductive isolation. For example, body size differences and the colour of light in the environment may affect mate choice, and the intermediacy of hybrids may affect their survival (Price 2008, ch. 6). A strong linkage of reproductive isolation to divergent selection pressures has been termed ecological speciation (Schluter 2009). A multitude of recent studies on young species have left the impression that ecological speciation is a common means of generating diversity (e.g. Nosil 2008; Schluter 2009; Seehausen & Magalhaes in press).
While young sympatric species are common in young environments, they are rare in older environments. In the most comprehensive dataset, for continental New World birds, average ages of sympatric sister species vary from 3.75 Myr at the equator to 2.65 Myr in the temperate regions (J. Weir & T. Price 2009, unpublished observations). These ages seem more general. For example, the youngest species pair in the five species assemblage of Enallagma damselflies studied by McPeek (1990) in the northeast USA is estimated at 2 Myr (Turgeon et al. 2005). Up to seven species of Percina fish occur in sympatry in the southeastern USA, but only one pair is estimated to be younger than 1 Myr (Carlson et al. 2009, R. L. Carlson 2009, personal communication). Sympatric Anolis lizards on the large, old, Caribbean islands are all estimated to be millions of years separated (Losos et al. 2006).
The importance of the great age of many sympatric species is that old species are likely to be reproductively isolated from one another many times over (Coyne & Orr 1998), which makes it difficult to ascertain which mechanisms contributed to speciation and which have accumulated only afterwards (Coyne & Orr 1998, 2004, p. 69). This problem seems particularly to apply to any assessment for a role of ecological speciation: plausibly, ecological differences contributing to reproductive isolation are generated as a result of interactions in sympatry between good species, rather than during the speciation process itself (Rundell & Price 2009). For example, from his comparative study of related bird species in New Guinea, Diamond (1973) concluded that when young species come into sympatry, they often displace each other to different positions along the altitudinal gradient. The consequence of such a displacement in space must be substantial reproductive isolation between the species (most individuals of one species do not encounter individuals of the other), but this form of premating isolation has nothing to do with the origin of the species.
Ramsey et al. (2003) suggest that if species are reproductively isolated many times over, the only way to infer which reproductive isolating mechanisms contribute to speciation, rather than accumulate later, lies in the systematic investigation of reproductive isolation across related taxa at varying degrees of evolutionary divergence (see also Coyne & Orr 2004, ch. 2). If we apply this reasoning to the Old World warblers, we conclude that reproductive isolation accumulates slowly among geographically separated, ecologically similar populations, and find little demonstrable role for ecological speciation. Thus, given the large number of sympatric species, which are long separated from their closest relatives, arguments for the preponderance of ecologically divergent selection pressures in speciation (e.g. Funk et al. 2006; Schluter 2009) seem premature.
A problem with the approach advocated by Ramsey et al. (2003) is that speciation may be fundamentally different at different stages of an adaptive radiation (Rosenzweig 1995). It is possible that many currently old sympatric species were produced rapidly via ecological speciation, at a time of much ecological opportunity. They persisted in sympatry for a long time, building up multiple forms of reproductive isolation. Later in adaptive radiation, when ecological opportunity is limited because environments are full with species, sympatry becomes more difficult to establish and divergent ecological selection pressures weaker. At this late stage, speciation results from a slow process of divergence in allopatry, and persistent allospecies. The net result would be a pattern similar to that observed at the current time in the Old World warblers.
It is difficult to reject this scenario, but several arguments suggest that young ecologically produced sympatric species are often not the route to the old sympatric species currently observed in old communities. First, rapid ecological speciation seems to occur much more readily in certain groups than others (e.g. cichlid fish compared with other fish taxa in the African Great Lakes; Salzburger et al. 2005; Seehausen 2006a). In birds, seed-eating forms appear more likely to ecologically speciate than warblers. This is strikingly illustrated by ancient lineages of the arthropod-feeding warbler finches (Certhidea) on the Galápagos (up to 2 Myr), none of which are sympatric, whereas the seed-eating ground finches (Geospiza) are young and sympatric (Grant & Grant 2008; Rundell & Price 2009). Among the Old World leaf warblers, there are no young sympatric species at all. One interpretation is that warbler-like forms generally go through the slow process of divergence in allopatry, and this may apply to many other groups too. The second reason to infer a long allopatric stage for many sympatric taxa is that many taxa are indeed divided into long separated ‘allospecies’ at the present time, and if this was true in the past, these taxa seem primed to undergo adaptive diversification and move into sympatry should ecological opportunities arise; this appears to be an easier route to the sympatric accumulation of species than the de novo creation of species (Rundell & Price 2009). Finally, genetic incompatibilities may more easily develop between allopatric populations than young sympatric, ecologically separated, species, because young sympatric species continue to hybridize. For example, Grant & Grant (2010) found that gene flow between three ground finch species resident on Isla Daphne Major over the past 30 years has been between 1.5 and ×10 higher than gene flow between immigrant and resident populations of the same species. Gene flow may prevent the development of genetic incompatibilities, and sympatric species whose reproductive barriers are only enforced by the environment are subject to collapse whenever environments change (Seehausen 2006b). One can imagine a scenario where, over time, strong reproductive isolation develops between geographically isolated populations, and these species spread into sympatry, replacing any ecologically differentiated, interbreeding forms.
Mallet (2008) has suggested that there is a continuum in sympatry from ecological races and biotypes, to hybridizing species and, ultimately, to species that no longer cross. He suggests that this implies that speciation may occur, perhaps frequently, in sympatry and that the production of sympatric species is easy (whether or not a period of allopatry is involved). Mallet listed several taxa that contain sympatric ‘ecological races’, where assortative mating is strong, but not complete (cross-taxon matings greater than 1%). It is unclear how common such sympatric ecological races are. For example, bird assemblages in central Europe and in North America contain only two species complexes known or thought to be divided into such races, the crossbill (Loxia, Benkman 1993; Marquiss & Rae 2002) and redpoll (Carduelis flammea–hornemann–cabaret, Marthinsen et al. 2008). Further, the observation that many species commonly hybridize in sympatry may have little relevance. Although many species have indeed been recorded hybridizing, in true sympatry many of these are rare events and apparently represent little more than over-exuberant sexual activity, with little evolutionary consequence. Essentially non-hybridizing, often old, species seem to be the prime constituent of many ecological communities. The proposed continuum of reproductive isolation in sympatry needs more evaluation. Following Mayr (1942, 1963), I conclude that much of the action is in allopatry. Attainment of sympatry is often difficult.
(b). Barriers, range expansions and reproductive isolation as limits on speciation rate
Biogeographers have long recognized range expansions and barrier formation as important controls on speciation rate (Mayr 1947; Cracraft & Prum 1988; Zink et al. 2000). This seems to apply to the warblers reviewed here. During the past 3–4 Myr, speciation has been linked to range expansions as a result of the tracking of habitats, followed by the creation of barriers because some areas became treeless: both the cause of the range expansions and of the barriers can be considered ecological factors. Similar principles probably applied also at other times of high rates of cladogenesis, such as the multiple splits observed at about 7 Ma, a period during which seasonality and aridity increased, at least in southern China and the Himalayan region.
The Old World leaf warblers show a rate of cladogenesis that has slowed down over time (Phillimore & Price 2008, 2009), a pattern that is quite common in large clades (McPeek 2008; Phillimore & Price 2008). This slowdown towards the present has been attributed to a limit on speciation that arises because range expansions slow as environments fill up with species (Price 2008; Phillimore & Price 2009). Generally missing from previous discussions on controls on range expansions—and by implication speciation rate—has been the role that closely related species play in limiting mutual expansions into each other's range. First, if attainment of reproductive isolation among ecologically similar sister taxa takes millions of years, then the taxa will not be able to spread into each other's range for millions of years. Second, even completely reproductively isolated taxa may competitively exclude each other from one another's range. In this argument, the ultimate limit on speciation rate is not generation of reproductive isolation, which inevitably accumulates given enough time, but competitive exclusion between related taxa, preventing range expansions and further rounds of allopatric speciation. Such an ‘ecological control’ on speciation rate contrasts with so-called neutral models where ecologically identical species can diffuse into each other's range (Hubbell 2001) and the control on species diversity is set by a balance between the generation of reproductive isolation and extinction. Neutral models do not seem to fit many patterns in nature, such as the presence of regularly abutting allospecies (Rundell & Price 2009), species abundance distributions (Ricklefs 2006), changes in speciation rate through time (Phillimore & Price 2009; Rabosky 2009), the lack of a correlation between clade age and number of species (Rabosky 2009; Ricklefs 2009) and the correlation of the number of species in a clade from one region of the world to another (Ricklefs 2009).
In conclusion, results from this study emphasize time of persistence of geographically separated populations, as the key to speciation. This, and not strong divergent selection pressures, may account for much of speciation in stable, old environments, such as are observed in many places on continents. In the warblers, song divergence, which is a prime factor in premating isolation, may have both non-ecological and ecological contributions. Nothing is known about how genetic incompatibilities accumulate in this group, or in general: both non-ecological and ecological mechanisms have been proposed (Orr et al. 2004; Price 2008, ch. 16; Schluter 2009). In many slowly diverging taxa, including the warblers, ecological differences may contribute to divergence in both premating and postmating isolation. However, when environments are similar, divergent selection pressures are weak, so few new mutations will be favoured in one taxon but not the other (Price et al. in press). This places a premium on time, not ecology, if populations are to diverge to the level of species.
Note Added In Proof
Alström et al. (2010) report the discovery of yet another cryptic species within this group, Phylloscopus calciatilis, which belongs to lineage 6 in Figures 1 and 5. It is allopatric or parapatric to the two other species in this lineage and may hybridize with P. ricketti. It is inferred to be the sister species to P. cantator. Total range of the lineage is unchanged, and inclusion of the species would slightly strengthen the relevant patterns reported in this paper.
Acknowledgements
I thank Antonio Kufoy for help with GIS, Brad Hawkins for help with figure 1 and Per Alström, Darren Irwin and Jason Weir for comments. This study was supported in part by the National Science Foundation (USA).
Footnotes
One contribution of 11 to a Theme Issue ‘Genomics of speciation’.
References
- Alström P., Olsson U.1992Taxonomic status of Phylloscopus affinis and P. subaffinis. Bull. Brit. Orn. Club 112, 111–126 [Google Scholar]
- Alström P., Olsson U.1993Blyth's leaf warbler Phylloscopus reguloides found breeding in Thailand. Forktail 9, 150–152 [Google Scholar]
- Alström P., Olsson U.1995A new species of Phylloscopus warbler from Sichuan Province, China. Ibis 137, 459–468 (doi:10.1111/j.1474-919X.1995.tb03254.x) [Google Scholar]
- Alström P., Olsson U.1999The golden-spectacled warbler: a complex of sibling species, including a previously undescribed species. Ibis 141, 545–568 (doi:10.1111/j.1474-919X.1999.tb07363.x) [Google Scholar]
- Alström P., Olsson U., Colston P. R.1992A new species of Phylloscopus warbler from central China. Ibis 134, 329–334 (doi:10.1111/j.1474-919X.1992.tb08011.x) [Google Scholar]
- Alström P., Davidson P., Duckworth J. W., Eames J. C., Le T. T., Nguyen C., Olsson U., Robson C., Timmins R.2010Description of a new species of Phylloscopus warbler from Vietnam and Laos. Ibis 152, 145–168 [Google Scholar]
- Badgley C., Barry J. C., Morgan M. E., Nelson S. V., Behrensmeyer A. K., Cerling T. E., Pilbeam D.2008Ecological changes in Miocene mammalian record show impact of prolonged climatic forcing. Proc. Natl Acad. Sci. USA 105, 12 145–12 149 (doi:10.1073/pnas.0805592105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A. V., Leaf E. S.1997Habitat associations of song characteristics in Phylloscopus and Hippolais warblers. Auk 114, 40–46 [Google Scholar]
- Benkman C. W.1993Adaptation to single resources and the evolution of crossbill (Loxia) diversity. Ecol. Monogr. 63, 305–325 (doi:10.2307/2937103) [Google Scholar]
- Bensch S., Andersson T., Akesson S.1999Morphological and molecular variation across a migratory divide in willow warblers, Phylloscopus trochilus. Evolution 53, 1925–1935 (doi:10.2307/2640451) [DOI] [PubMed] [Google Scholar]
- Bensch S., Irwin D. E., Irwin J. H., Kvist L., Akesson S.2006Conflicting patterns of mitochondrial and nuclear DNA diversity in Phylloscopus warblers. Mol. Ecol. 15, 161–171 (doi:10.1111/j.1365-294X.2005.02766.x) [DOI] [PubMed] [Google Scholar]
- Bensch S., Grahn M., Muller N., Gay L., Akesson S.2009Genetic, morphological, and feather isotope variation of migratory willow warblers show gradual divergence in a ring. Mol. Ecol. 18, 3087–3096 (doi:10.1111/j.1365-294X.2009.04210.x) [DOI] [PubMed] [Google Scholar]
- Burke T.1992Molecular ecology—small, green and different. Nature 355, 775–776 (doi:10.1038/355775a0) [Google Scholar]
- Carlson R. L., Wainwright P. C., Near T. J.2009Relationship between species co-occurrence and rate of morphological change in Percina darters (Percidae: Etheostomatinae). Evolution 63, 767–778 (doi:10.1111/j.1558-5646.2008.00576.x) [DOI] [PubMed] [Google Scholar]
- Clement P., Alström P., Madge S. C.2006Species accounts of Sylviidae. In Handbook of birds of the world: Old World flycatchers to Old World warblers, vol. 11 (eds del Hoyo J., Elliott A., Christie D. A.), pp. 647–679 Barcelona, Spain: Lynx Editions [Google Scholar]
- Coyne J. A., Orr H. A.1998The evolutionary genetics of speciation. Phil. Trans. R. Soc. Lond. B 353, 287–305 (doi:10.1098/rstb.1998.0210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation Sunderland, MA: Sinauer [Google Scholar]
- Cracraft J., Prum R. O.1988Patterns and processes of diversification—speciation and historical congruence in some neotropical birds. Evolution 42, 603–620 (doi:10.2307/2409043) [DOI] [PubMed] [Google Scholar]
- Diamond J. M.1973Distributional ecology of New Guinea birds. Science 179, 759–769 (doi:10.1126/science.179.4075.759) [DOI] [PubMed] [Google Scholar]
- Drummond A. J., Rambaut A.2007BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W., Kessler A.2001Morphology and foraging behaviour of Siberian Phylloscopus warblers. J. Avian Biol. 32, 127–138 (doi:10.1034/j.1600-048X.2001.320205.x) [Google Scholar]
- Forstmeier W., Bourski O. V., Leisler B.2001Habitat choice in Phylloscopus warblers: the role of morphology, phylogeny and competition. Oecologia 128, 566–576 (doi:10.1007/s004420100678) [DOI] [PubMed] [Google Scholar]
- Funk D. J., Nosil P., Etges W. J.2006Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc. Natl Acad. Sci. USA 103, 3209–3213 (doi:10.1073/pnas.0508653103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston K. J.1991How large is a species' geographic range? Oikos 61, 434–438 (doi:10.2307/3545251) [Google Scholar]
- Grant P. R., Grant B. R.2008How and why species multiply Princeton, NJ: Princeton University Press [Google Scholar]
- Grant P. R., Grant B. R.2010Conspecific versus heterospecific gene exchange between populations of Darwin's finches. Phil. Trans. R. Soc. B 365, 1065–1076 (doi:10.1098/rstb.2009.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig A. J., Martens J., Seibold I., Henning F., Schottler B., Wink M.1996Phylogeny and species limits in the Palearctic chiffchaff Phylloscopus collybita complex: mitochondrial genetic differentiation and bioacoustic evidence. Ibis 138, 650–666 [Google Scholar]
- Helbig A. J., Salomon M., Bensch S.2001Male-biased gene flow across an avian hybrid zone: evidence from mitochondrial and microsatellite DNA. J. Evol. Biol. 14, 277–287 (doi:10.1046/j.1420-9101.2001.00273.x) [Google Scholar]
- Hubbell S. P.2001The unified theory of biodiversity and biogeography Princeton, NJ: Princeton University Press [Google Scholar]
- Hughes C., Eastwood R.2006Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl Acad. Sci. USA 103, 10 334–10 339 (doi:10.1073/pnas.0601928103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D. E.2000Song variation in an avian ring species. Evolution 54, 998–1010 [DOI] [PubMed] [Google Scholar]
- Irwin D. E., Irwin J. H.2004Siberian migratory divides: the role of seasonal migration in speciation. In Birds of two worlds (eds Greenberg R., Marra P.), pp. 27–40 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Irwin D. E., Alström P., Olsson U., Benowitz-Fredericks Z. M.2001aCryptic species in the genus Phylloscopus. Ibis 143, 233–247 (doi:10.1111/j.1474-919X.2001.tb04479.x) [Google Scholar]
- Irwin D. E., Bensch S., Price T. D.2001bSpeciation in a ring. Nature 409, 333–337 (doi:10.1038/35053059) [DOI] [PubMed] [Google Scholar]
- Johansson U. S., Alström P., Olsson U., Ericson P., Lundberg P., Price T. D.2007Build-up of the Himalayan avifauna through immigration: a biogeographical analysis of the Phylloscopus and Seicercus warblers. Evolution 61, 324–333 (doi:10.1111/j.1558-5646.2007.00024.x) [DOI] [PubMed] [Google Scholar]
- Liou L. W., Price T. D.1994Speciation by reinforcement of pre-mating isolation. Evolution 48, 1451–1459 (doi:10.2307/2410239) [DOI] [PubMed] [Google Scholar]
- Losos J. B., Glor R. E., Kolbe J. J., Nicholson K.2006Adaptation, speciation, and convergence: a hierarchical analysis of adaptive radiation in Caribbean Anolis lizards. Ann. Mo. Bot. Gard. 93, 24–33 (doi:10.3417/0026-6493(2006)93[24:ASACAH]2.0.CO;2) [Google Scholar]
- Mahler B., Gil D.2009The evolution of song in the Phylloscopus leaf warblers (Aves: Sylviidae): a tale of sexual selection, habitat adaptation, and morphological constraints. Adv. Stud. Behav. 40, 35–66 (doi:10.1016/S0065-3454(09)40002-0) [Google Scholar]
- Mallet J.2008Hybridization, ecological races and the nature of species: empirical evidence for the ease of speciation. Phil. Trans. R. Soc. B 363, 2971–2986 (doi:10.1098/rstb.2008.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti K.1993Dark habitats and bright birds illustrate the role of the environment in species divergence. Nature 362, 149–152 (doi:10.1038/362149a0) [Google Scholar]
- Marchetti K.1998The evolution of multiple male traits in the yellow-browed leaf warbler. Anim. Behav. 55, 361–376 (doi:10.1006/anbe.1997.0586) [DOI] [PubMed] [Google Scholar]
- Marquiss M., Rae R.2002Ecological differentiation in relation to bill size amongst sympatric, genetically undifferentiated crossbills Loxia spp. Ibis 144, 494–508 (doi:10.1046/j.1474-919X.2002.00041.x) [Google Scholar]
- Martens J.1982Circular distributional overlap and speciation in the chiffchaff (Phylloscopus collybita). Zeitschrift für Zoologische Systematik und Evolutionsforschung 20, 82–100 [In German.] [Google Scholar]
- Martens J.1996Vocalizations and speciation of Palearctic birds. In Ecology and evolution of acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 221–240 Ithaca, NY: Cornell University Press [Google Scholar]
- Martens J., Tietze D. T., Eck S., Veith M.2004Radiation and species limits in the Asian Pallas's warbler complex (Phylloscopus proregulus s.l.). J. Ornithol. 145, 206–222 (doi:10.1007/s10336-004-0042-9) [Google Scholar]
- Martens J., Sun Y.-H., Päckert M.2008Intraspecific differentiation of Sino-Himalayan bush-dwelling Phylloscopus leaf warblers, with description of two new taxa (P. fuscatus, P. fuligiventer, P. affinis, P. armandii, P. subaffinis). Vertebrate Zoolog. 58, 233–265 [Google Scholar]
- Marthinsen G., Wennerberg L., Lifjeld J. T.2008Low support for separate species within the redpoll complex (Carduelis flammea–hornemanni–cabaret) from analyses of mtDNA and microsatellite markers. Mol. Phylogenet. Evol. 47, 1005–1017 (doi:10.1016/j.ympev.2008.03.027) [DOI] [PubMed] [Google Scholar]
- Mayr E.1942Systematics and the origin of species from the viewpoint of a zoologist Columbia Biological Series, no. XIII New York, NY: Columbia University Press [Google Scholar]
- Mayr E.1947Ecological factors in speciation. Evolution 1, 263–288 (doi:10.2307/2405327) [Google Scholar]
- Mayr E.1963Animal species and evolution Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- McCarthy E. M.2006Handbook of avian hybrids of the world Oxford, UK: Oxford University Press [Google Scholar]
- McPeek M. A.1990Determination of species composition in the Enallagma damselfly assemblages of permanent lakes. Ecology 71, 83–98 (doi:10.2307/1940249) [Google Scholar]
- McPeek M. A.2008The ecological dynamics of clade diversification and community assembly. Am. Nat. 172, E270–E284 (doi:10.1086/593137) [DOI] [PubMed] [Google Scholar]
- Nosil P.2008Ernst Mayr and the integration of geographic and ecological factors in speciation. Biol. J. Linn. Soc. 95, 26–46 (doi:10.1111/j.1095-8312.2008.01091.x) [Google Scholar]
- Olsson U., Alström P., Sundberg P.2004Non-monophyly of the avian genus Seicercus (Aves: Sylviidae) revealed by mitochondrial DNA. Zool. Scr. 33, 501–510 (doi:10.1111/j.0300-3256.2004.00166.x) [Google Scholar]
- Olsson U., Alström P., Ericson P. G. P., Sundberg P.2005Non-monophyletic taxa and cryptic species—evidence from a molecular phylogeny of leaf-warblers (Phylloscopus, Aves). Mol. Phylogenet. Evol. 36, 261–276 (doi:10.1016/j.ympev.2005.01.012) [DOI] [PubMed] [Google Scholar]
- Orr H. A., Masly J. P., Presgraves D. C.2004Speciation genes. Curr. Opin. Genet. Dev. 14, 675–679 (doi:10.1016/j.gde.2004.08.009) [DOI] [PubMed] [Google Scholar]
- Päckert M., Martens J., Sun Y. H., Veith M.2004The radiation of the Seicercus burkii complex and its congeners (Aves: Sylviidae): molecular genetics and bioacoustics. Organ. Divers. Evol. 4, 341–364 (doi:10.1016/j.ode.2004.06.002) [Google Scholar]
- Päckert M., Blume C., Sun Y. H., Wei L., Martens J.2009Acoustic differentiation reflects mitochondrial lineages in Blyth's leaf warbler and white-tailed leaf warbler complexes (Aves: Phylloscopus reguloides, Phylloscopus davisoni). Biol. J. Linn. Soc. 96, 584–600 (doi:10.1111/j.1095-8312.2008.01159.x) [Google Scholar]
- Paradis E., Claude J.2002Analysis of comparative data using generalized estimating equations. J. Theor. Biol. 218, 175–185 (doi:10.1006/jtbi.2002.3066) [DOI] [PubMed] [Google Scholar]
- Paradis E., Claude J., Strimmer K.2004APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- Phillimore A. B., Price T. D.2008Density-dependent cladogenesis in birds. PLoS Biol. 6, 483–489 (doi:10.1371/journal.pbio.0060071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillimore A. B., Price T. D.2009Ecological influences on the temporal pattern of speciation. In Speciation and patterns of diversity (eds Butlin R., Bridle J., Schluter D.), pp. 240–256 Cambridge, UK: Cambridge University Press [Google Scholar]
- Price T.1991Morphology and ecology of breeding warblers along an altitudinal gradient in Kashmir, India. J. Anim. Ecol. 60, 643–664 (doi:10.2307/5303) [Google Scholar]
- Price T.2008Speciation in birds Boulder, CO: Roberts and Co [Google Scholar]
- Price T. D., Bouvier M. M.2002The evolution of F1 postzygotic incompatibilities in birds. Evolution 56, 2083–2089 [PubMed] [Google Scholar]
- Price T. D., Jamdar N.1991Breeding biology of eight sympatric species of Phylloscopus warblers in Kashmir. J. Bombay Nat. Hist. Soc. 88, 242–255 (doi:10.1111/j.1474-919X.1992.tb08011.x) [Google Scholar]
- Price T. D., Helbig A. J., Richman A. D.1997Evolution of breeding distributions in the Old World leaf warblers (genus Phylloscopus). Evolution 51, 552–561 (doi:10.2307/2411127) [DOI] [PubMed] [Google Scholar]
- Price T., Zee J., Jamdar K., Jamdar N.2003Bird species diversity along the Himalayas: a comparison of Himachal Pradesh with Kashmir. J. Bombay Nat. Hist. Soc. 100, 394–409 [Google Scholar]
- Price T., Phillimore A. B., Awodey M., Hudson R.In press Ecological and geographical influences on the allopatric phase of island speciation. In From field observations to mechanisms. A program in evolutionary biology (eds Grant P. R., Grant B. R.), Princeton, NJ: Princeton University Press [Google Scholar]
- Rabosky D. L.2009Ecological limits and diversification rate: alternative paradigms to explain the variation in species richness among clades and regions. Ecol. Lett. 12, 735–743 (doi:10.1111/j.1461-0248.2009.01333.x) [DOI] [PubMed] [Google Scholar]
- Ramsey J., Bradshaw H. D., Schemske D. W.2003Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57, 1520–1534 [DOI] [PubMed] [Google Scholar]
- Ray N., Adams J. M.2001A GIS-based vegetation map of the world at the last glacial maximum (25,000–15,000 BP). Internet Archaeol. 11 See http://intarch.ac.uk/journal/issue11/rayadams_toc.html [Google Scholar]
- Reeves A. B., Drovetski S. V., Fadeev I. V.2008Mitochondrial DNA data imply a stepping-stone colonization of Beringia by the Arctic warbler Phylloscopus borealis. J. Avian Biol. 39, 567–575 (doi:10.1111/j.0908-8857.2008.04421.x) [Google Scholar]
- Rheindt F. E.2006Splits galore: the revolution in Asian leaf warbler systematics. Birding ASIA 5, 25–39 [Google Scholar]
- Richman A. D.1996Ecological diversification and community structure in the Old World leaf warblers (genus Phylloscopus): a phylogenetic perspective. Evolution 50, 2461–2470 (doi:10.2307/2410713) [DOI] [PubMed] [Google Scholar]
- Richman A. D., Price T.1992Evolution of ecological differences in the Old World leaf warblers. Nature 355, 817–821 (doi:10.1038/355817a0) [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E.2006The unified neutral theory of biodiversity: do the numbers add up? Ecology 87, 1424–1431 (doi:10.1890/0012-9658(2006)87[1424:TUNTOB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E.2009Speciation, extinction, and diversity. In Speciation and patterns of diversity (eds Butlin R., Bridle J., Schluter D.), pp. 257–277 Cambridge, UK: Cambridge University Press [Google Scholar]
- Rosenzweig M. L.1995Species diversity in space and time Cambridge, UK: Cambridge University Press [Google Scholar]
- Rundell R. J., Price T. D.2009Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol. Evol. 24, 394–399 (doi:10.1016/j.tree.2009.02.007) [DOI] [PubMed] [Google Scholar]
- Saether B. E.1983Mechanism of interspecific spacing out in a territorial system of the chiffchaff Phylloscopus collybita and the willow warbler Phylloscopus trochilus. Orn. Scand. 14, 154–160 (doi:10.2307/3676020) [Google Scholar]
- Saitoh T., Nishiumi I., Alström P., Olsson U., Ueda K.2006Deep phylogeographical divergences among far eastern populations of the widespread Arctic warbler. J. Ornithol. 147, 242 [Google Scholar]
- Salomon M.1989Song as a possible reproductive isolating mechanism between 2 parapatric forms—the case of the chiffchaffs Phylloscopus collybita collybita and Phylloscopus collybita brehmii in the western Pyrenees. Behaviour 111, 270–290 (doi:10.1163/156853989X00709) [Google Scholar]
- Salzburger W., Mack T., Verheyen E., Meyer A.2005Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol. Biol. 5, 17.(doi:10.1186/1471-2148-5-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D.1996Ecological speciation in postglacial fishes. Phil. Trans. R. Soc. Lond. B 351, 807–814 (doi:10.1098/rstb.1996.0075) [Google Scholar]
- Schluter D.2009Evidence for ecological speciation and its alternative. Science 323, 737–741 (doi:10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- Seehausen O.2006aAfrican cichlid fish: a model system in adaptive radiation research. Proc. R. Soc. B 273, 1987–1998 (doi:10.1098/rspb.2006.3539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O.2006bConservation: losing biodiversity by reverse speciation. Curr. Biol 16, R334–R337 (doi:10.1016/j.cub.2006.03.080) [DOI] [PubMed] [Google Scholar]
- Seehausen O., Magalhaes I. S.In press Geographical mode and evolutionary mechanism of ecological speciation in cichlid fish. In From field observations to mechanisms: a program in evolutionary biology (eds Grant P. R., Grant B. R.). Princeton, NJ: Princeton University Press [Google Scholar]
- Sibley C. G., Monroe B. L.1990Distribution and taxonomy of birds of the world New Haven, CT: Yale University Press [Google Scholar]
- Thielcke G.1983Enstanden Dialekte des Zilpzalps Phylloscopus collybita durch Lernetzug? (Have dialects of chiffchaff Phylloscopus collybita developed by withdrawal of learning? J. Ornithol. 124, 333–368 (doi:10.1007/BF01640358) [Google Scholar]
- Thielcke G., Wüstenberg K., Becker P. H.1978Reaktionen von Zilpzalp und Fitis (Phylloscopus collybita, Ph. trochilus) auf verschiedene Gesangsformen des Zilpzalps. J. Ornithol. 119, 213–226 (doi:10.1007/BF01644590) [Google Scholar]
- Turelli M., Orr H. A.2000Dominance, epistasis and the genetics of postzygotic isolation. Genetics 154, 1663–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon J., Stoks R., Thum R. A., Brown J. M., McPeek M. A.2005Simultaneous Quaternary radiations of three damselfly clades across the Holarctic. Am. Nat. 165, E78–E107 (doi:10.1086/428682) [DOI] [PubMed] [Google Scholar]
- Voelker G.1999Molecular evolutionary relationships in the avian genus Anthus (Pipits: Motacillidae). Mol. Phylogenet. Evol. 11, 84–94 (doi:10.1006/mpev.1998.0555) [DOI] [PubMed] [Google Scholar]
- Weir J. T.2006Divergent patterns of species accumulation in lowland and highland neotropical birds. Evolution 60, 842–855 [PubMed] [Google Scholar]
- Weir J. T., Schluter D.2004Ice sheets promote speciation in boreal birds. Proc. R. Soc. Lond. B 271, 1881–1887 (doi:10.1098/rspb.2004.2803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. T., Schluter D.2008Calibrating the avian molecular clock. Mol. Ecol. 17, 2321–2328 (doi:10.1111/j.1365-294X.2008.03742.x) [DOI] [PubMed] [Google Scholar]
- Weir J. T., Bermingham E., Schluter D.2009The great American biotic interchange in birds. Proc. Natl Acad. Sci. USA 51, 21 737–21 742 (doi:10.1073/pnas.0903811106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhisheng A., Kutzbach J. E., Prell W. L., Porter S. C.2001Evolution of Asian monsoons and phased uplift of the Himalayan Tibetan plateau since Late Miocene times. Nature 411, 62–66 (doi:10.1038/35075035) [DOI] [PubMed] [Google Scholar]
- Zink R. M., Blackwell-Rago R. C., Ronquist F.2000The shifting roles of dispersal and vicariance in biogeography. Proc. R. Soc. Lond. B 267, 497–503 (doi:10.1098/rspb.2000.1028) [DOI] [PMC free article] [PubMed] [Google Scholar]