Abstract
The polychromatic and trophically polymorphic Midas cichlid fish species complex (Amphilophus cf. citrinellus) is an excellent model system for studying the mechanisms of speciation and patterns of phenotypic diversification in allopatry and in sympatry. Here, we first review research to date on the species complex and the geological history of its habitat. We analyse body shape variation from all currently described species in the complex, sampled from six crater lakes (maximally 1.2–23.9 kyr old) and both great lakes in Nicaragua. We find that Midas cichlid populations in each lake have their own characteristic body shape. In lakes with multiple sympatric species of Midas cichlid, each species has a distinct body shape. Across the species complex, most body shape change relates to body depth, head, snout and mouth shape and caudal peduncle length. There is independent parallel evolution of an elongate limnetic species in at least two crater lakes. Mitochondrial genetic diversity is higher in crater lakes with multiple species. Midas cichlid species richness increases with the size and age of the crater lakes, though no such relationship exists for the other syntopic fishes. We suggest that crater lake Midas cichlids follow the predicted pattern of an adaptive radiation, with early divergence of each crater lake colonization, followed by intralacustrine diversification and speciation by ecological adaptation and sexual selection.
Keywords: geometric morphometrics, mitochondrial DNA genetic diversity, ecomorphology, limnology, Mesoamerica, adaptive radiation
1. Introduction
A major challenge in speciation research is that environmental, biological, ecological and geographical conditions change over time and can obscure past evolutionary processes and events. Arguably, species complexes and incipient species are among the best ways to understand speciation, as species or populations in the complex might be at different stages of divergence and thereby offer a chronological perspective of change. Furthermore, since the divergence may be recent or ongoing, the current biotic and abiotic conditions are likely to be similar as those that are or were involved in the initial differentiation.
The species complex of Midas cichlid (Amphilophus cf. citrinellus (Günther)) fishes is an ideal model system for studying speciation in nature because of its biology and geographical setting (table 1). The complex is distributed across the great lakes and crater lakes of Nicaragua (figure 1) and, at lower densities, some of the larger nearby rivers (Barlow 1976; Smith & Bermingham 2005). The Midas cichlid species complex is highly variable within and across species, including a pronounced polychromatism and trophic polymorphism. Much of the early research on the species complex focused on behavioural aspects of mate choice and competition (e.g. Barlow 1973, 1986, 1998; Baylis 1976a,b; McKaye & Barlow 1976). That line of inquiry was followed by an interest in trophic ecology, especially feeding apparatus variation and phenotypic plasticity (Meyer 1989, 1990a,b). More recently and using newly available molecular tools, questions about phylogeography, population history and speciation have been addressed in this species complex (Wilson et al. 2000; Barluenga & Meyer 2004; Barluenga et al. 2006; Bunje et al. 2007; Elmer et al. 2009, 2010). Several species in this complex arose very recently and some through sympatric speciation (Wilson et al. 2000; Barluenga et al. 2006; Elmer et al. 2009). Each crater lake in Nicaragua is likely to harbour a different set of endemic species, which makes these lakes an excellent system to study the relative speed and strength by which geographical isolation and natural and sexual selection promote phenotypic diversification and speciation.
Table 1.
Natural history and biological details about the Nicaraguan crater lakes and great lakes that are home to the Midas cichlid species complex.
| lake | natural history | maximum agea (years before present) | lake surface area (km2) | Midas cichlid speciesb | number of other fish speciesc |
|---|---|---|---|---|---|
| crater lakes | |||||
| Apoyeque | The name ‘Apoyeque’ means ‘salty water’ in the Náhuatl language; it is so-named because of its high mineral content, as is characteristic of Nicaragua's crater lakes (Barlow et al. 1976). Recent research indicates there are two ecological morphs of Midas cichlid in the lake (K. R. Elmer, T. K. Lehtononen & A. Meyer 2010, unpublished data) and some authors have called these different species (Waid et al. 1999). | 1900d | 2.50e | A. citrinellus | 2 |
| Apoyo | This is the largest and oldest crater lake and is the most Midas cichlid species-rich. A. zaliosus (Barluenga et al. 2006), and likely other endemic species, arose by sympatric speciation. Apoyo is under pressure from lakeside development and introduced or cultivated exotic fishes (McCrary et al. 2007). | 23 890f | 21.10g | A. astorquii, A. chancho, A. flaveolus, A. zaliosus | 5h |
| Asososca León | Little is known about this small lake's volcanic history or age. The lake is home to a large population of introduced African tilapia. | 4500i | 0.81j | A. citrinellus | 3 |
| Asososca Managua | This crater lake is the water source for the city of Managua. It may be the youngest crater lake. There are ancient paintings on the rocks, indicating that it was historically a sacred site. | 1245k | 0.74l | A. citrinellus | 3 |
| Masaya | This lake is where Midas cichlid expert George Barlow collected most of his laboratory stocks. The lake originated ca 6000 years ago in the San Antonio eruption. Approximately 2120 and 1800 years ago there were major eruptions at one end of the lake: fish may or may not have survived. | 6000m | 8.38n | A. citrinellus | 9 |
| Monte Galán | This is probably a young lake, formed by recent volcanic activity of nearby Momotombo (Altamiro 1982). | ? | 0.79o | A. citrinellus | 8 |
| Tiscapa | Located in the city of Managua, this tiny lake is surrounded by a tourist nature park. The lake is contaminated from channelling activity (INETER 2009b) and local pollution. | ? | 0.13p | A. citrinellus | 2 |
| Xiloá | This lake is located just above great lake Managua and beside crater lake Apoyeque. Lake Managua water level has historically periodically risen (e.g. 9 m high in last eruption), resulting in exchange between Xiloá and Managua (Cowan et al. 2002). | 6100q | 3.75r | A. amarillo, A. sagittae, A. xiloaensis | 14 |
| great lakes | |||||
| Managua (or Xolotlán) | The lake drains intermittently to Lake Nicaragua by the river Tipitapa. | Early Pleistocenes | 1053t | A. citrinellus, A. labiatus | 27u |
| Nicaragua (or Cocibolca) | This is the largest lake in the Western Hemisphere south of the North America Great Lakes and north of Lake Titicaca. Is connected to the Caribbean Sea by the San Juan river. | Early Pleistocenev | 8143w | A. citrinellus, A. labiatus | 45x |
aAge of last major eruption, which created the caldera. Crater lake age must be less than this estimate.
bAs formally described to date.
cFrom Waid et al. (1999) and personal observation unless otherwise indicated.
hStauffer et al. (2008), including two introduced species Gobiomorus dormitor (Tate Bedarf et al. 2001) and Oreochromis niloticus (McCrary et al. 2007).
uVilla (1982) in McCrary et al. (2006) lists 26, plus now there is introduced tilapia (McKaye et al. 1995).
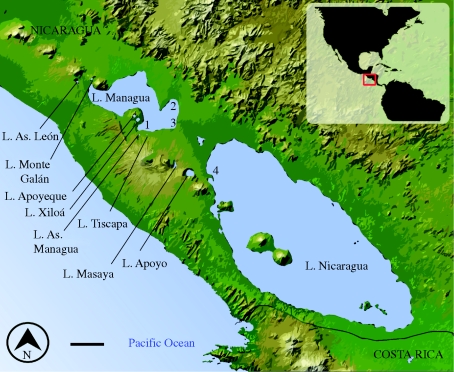
Figure 1.
The crater lakes of Nicaragua lie in the craters of dormant volcanoes along the fault lines that run along the western coast. Lakes discussed in the text are noted. 1. Miraflores, 2. San Antonio, 3. Tipitapa, 4. Las Isletas, As. = Asososca. Scale bar = 20 km.
Here we summarize the state of research on this system, with a focus on the geography, ecology and genetics of speciation. Further, we present a distribution-wide analysis of body shape variation and genetic diversity in the Midas cichlid species complex that is based on both new morphometric data and mitochondrial DNA (mtDNA) sequence.
(a). Geographical setting
Western Nicaragua is underlain by one of the world's most volcanically active areas, known as the Central American Volcanic Arc. After a volcano erupts and subsequently cools the resultant cone-shaped crater fills with ground- and rainwater to become a crater lake (also known as a caldera lake or maar). Nicaraguan crater lakes are characteristically oligotrophic, with a high proportion of dissolved solids and no in- or out-flow (Barlow et al. 1976). After some time, these crater lakes are colonized by aquatic organisms, including cichlid fishes. At least eight Nicaraguan crater lakes have been successfully colonized by Midas cichlids (figure 1, table 1). It is not known for each case how or when crater lake colonizations happen; it is generally thought to be from natural phenomena like hurricanes or piscivorous birds, though some lakes may have been purposely colonized by humans (Villa 1976a). In general, crater lakes have an impoverished fauna and a lot of ecological niche space to be filled (Schluter 2000a). Because of their geographical isolation and known geological history, crater lakes are excellent natural systems for studying the evolution of species in both allopatry and sympatry.
Nicaragua is subject not only to volcanic but also tectonic activity, which has resulted in the two largest and oldest lakes in Central America: great lakes Managua and Nicaragua. These date to the Early Pleistocene (Kutterolf et al. 2007) and together cover approximately 9000 km2 (Cole 1976). Lake Managua, to the north, lies at 7 or 8 m higher elevation than Lake Nicaragua. Connectivity between these two lakes has varied depending on lake fluctuations (Swain 1966). River Tipitapa rarely but periodically connects Lake Managua to Nicaragua when water levels are high (Cole 1976). The great lakes are exceptionally shallow (mean depth around 8.6 m for Lake Managua and 12.4 m for Lake Nicaragua) and have gently sloping basins, allowing wind to stir the sediments (Cole 1976). This and a high prevalence of phytoplankton (Barlow 1976) make the water of the great lakes very turbid. Thus, this habitat is very different from the clear, deep waters of the crater lakes.
(b). Midas cichlid diversity
The A. citrinellus species complex is part of the most genus-rich clade of neotropical cichlids: the tribe Heroini (Říčan et al. 2008), which display a wide diversity of morphological, ecological and behavioural adaptations. Some of their morphologies are phylogenetically discordant because of homoplasy and convergent evolution (Říčan et al. 2008), as has been found for cichlids in general (Meyer 1993; Stiassny & Meyer 1999).
Based on evidence from geology, biogeography and population genetics, Nicaragua's great lakes are thought to contain the ancestral population of Midas cichlids from which all crater lake populations originate (Barlow 1976; Barluenga & Meyer 2004; Barluenga et al. 2006). Midas cichlids within a crater lake are genetically more similar to each other than to those studied in any other lake (Barluenga et al. 2006; Bunje et al. 2007; Elmer et al. 2009).
Currently, there are nine species described in the Midas species complex: Amphilophus amarillo Stauffer and McKaye (Lake Xiloá endemic), Amphilophus astorquii Stauffer, McCrary and Black (Lake Apoyo endemic), Amphilophus chancho Stauffer, McCrary and Black (Lake Apoyo endemic), A. citrinellus (Günther), Amphilophus flaveolus Stauffer, McCrary and Black (Lake Apoyo endemic), Amphilophus labiatus (Günther), Amphilophus sagittae Stauffer and McKaye (Lake Xiloá endemic), Amphilophus xiloaensis Stauffer and McKaye (Lake Xiloá endemic) and Amphilophus zaliosus Barlow (Lake Apoyo endemic) (table 1). Rapidly evolving genetic markers can discern many of these species (Barluenga & Meyer 2004; Barluenga et al. 2006; Elmer et al. 2009), though not all species have been suitably investigated. We expect that more species from the crater lakes will be described in the future and that the taxonomic validity of the original species A. ‘citrinellus’ will need to be revisited. Therefore, we refer to Midas cichlids found in multiple crater lakes as A. cf. citrinellus.
Midas cichlids are polymorphic for trophically relevant attributes such as body shape, pharyngeal jaw apparatus and hypertrophied lips. Limnetic species A. sagittae and A. zaliosus are elongate ecotypes with papilliform pharyngeal jaws, while benthic species such as A. astorquii, A. chancho and A. flaveolus in Apoyo and A. amarillo and A. xiloaensis in Xiloá are more high bodied and tend to have molariform pharyngeal jaws (Barlow & Munsey 1976; Vivas & McKaye 2001; Klingenberg et al. 2003; Parsons et al. 2003; Barluenga et al. 2006). Amphilophus labiatus has a slightly more elongate body, more pointed snout, lateral compression and, most obviously, dramatically hypertrophied lips compared with A. citrinellus (Barlow & Munsey 1976; Klingenberg et al. 2003). A similarly thick-lipped ecotype is found at moderate frequencies (approx. 20%) in Lake Apoyeque, where it occupies an ecological niche distinct from the more abundant thin-lipped morph (K. R. Elmer, T. K. Lehtonen & A. Meyer 2010, unpublished data), and much more rarely in lakes Masaya and Xiloá (Barlow 1976; McKaye et al. 2002).
Some species in the Midas cichlid species complex are polychromatic: the common colour morph is greyish with spotted, striped and barred patterns (referred to as the ‘normal’ or ‘dark’ morph) while the ‘gold’ morph is uniformly orange, yellow or even white (Barlow 1976). All fish begin life dark and some later lose their melanophores and become gold (or more rarely white, lacking both dark melanocytes and yellow xanthophores) permanently (Barlow 1976; Dickman et al. 1988). Though there is an environmental (e.g. diet) component to the brightness of the gold morph (Webber et al. 1973), amelanism is under genetic control (Henning et al. in press). Gold morphs are found in moderate to low frequencies (e.g. less than 20% in A. xiloaensis, less than 7% in A. sagittae) in crater lakes Xiloá, Masaya, Asososca León and Asososca Managua (Barlow 1976; Elmer et al. 2009; K. R. Elmer, T. K. Lehtonen & A. Meyer 2007, personal observation) and in great lakes Managua and Nicaragua (8–10%; Webber et al. 1973). Historically, the melanic morph has been called normal (or sometimes ‘grey’) because it is the most common colour morph, is drab like most freshwater fishes and is similar to generalized cichlids in the genus (Webber et al. 1973; Barlow 1976). Unfortunately, normal is a loaded semantic descriptor that is uninformative for readers not familiar with Midas cichlids. It also implies that the gold phenotype is ‘abnormal’ or mutant, which is not true because the system is a natural (probably stable) colour polymorphism. Consequently, we prefer the more neutral and inclusive term dark to describe the common melanic morph.
Strong assortative mating within the colour morphs has been shown in the field (McKaye 1980, 1986; Elmer et al. 2009) and laboratory (e.g. Barlow et al. 1977; Barlow 1986). In Lake Xiloá, this assortative mating may be resulting in incipient speciation, where gold and dark morphs are significantly genetically differentiated at neutral markers (Elmer et al. 2009). In contrast, gold and dark morphs in the great lakes show little or no genetic differentiation (Barluenga & Meyer 2004).
(c). Midas cichlid mating behaviour
In comparison to African cichlids, which are remarkably adapted to diverse and extremely tight ecological niches and show highly specialized sexual strategies (Salzburger & Meyer 2004; Salzburger 2009), Midas cichlids were thought to be rather unspecialized (Barlow 1976; Baylis 1976b). Though the sexes are generally isomorphic (i.e. show little or no difference in overall body shape), males tend to be larger, have longer dorsal and pectoral fins and bigger nuchal humps. Females typically choose to pair with males that are larger than themselves and aggressive (Barlow 1992), though the exact dynamics of mate choice and partner stability are complicated. Males and females pair at the beginning of the breeding season, acquire and defend a territory while raising and guarding their broods (Barlow 1986, 1992), though the extent of biparental care may vary across pairs and species (T. K. Lehtonen 2007, unpublished data). Mating is assortative by ecological species (Baylis 1976a; Vivas & McKaye 2001; Elmer et al. 2009), though this has not been researched in detail or for all species. Competition for breeding sites and predation on fry is intense (McKaye 1977; McKaye & McKaye 1977), which may exert strong selective pressure for suitable good mate communication and coordination within pairs (Baylis 1976b).
(d). Sympatric speciation
Because crater lakes are isolated environments, this biological system has become a model for sympatric speciation in nature (Baylis 1976a; Barluenga & Meyer 2004; Barluenga et al. 2006; Gavrilets et al. 2007; Luz-Burgoa et al. 2007; Elmer et al. 2009). In all cases where multiple Midas cichlid species have so far been recognized within a crater lake, they have different ecologies and morphologies, strongly suggestive of the effect of disruptive, and then divergent, natural selection to exploit different intralacustrine niches (Baylis 1976a; Stauffer & McKaye 2002; Barluenga et al. 2006; Stauffer et al. 2008) (sensu Schluter 2000b). Breeding of each species commonly occurs within a metre or two from sister taxa, which argues for strong pre-zygotic reproductive isolation mechanisms (Barlow & Munsey 1976; Stauffer et al. 2008; Elmer et al. 2009; T. K. Lehtonen 2005–2008, personal observation). Even when speciation is incomplete or undetermined, there can be intralacustrine ecological differentiation (e.g. Lake Apoyeque). Ecological differentiation can occur much faster than even rapidly evolving neutral molecular markers can track; thus the absence of genetic differentiation between ecotypes does not refute ecological speciation (Thibert-Plante & Hendry 2009).
Incipient sympatric speciation by sexual selection based on colour (either gold or dark) has been proposed in this group, first inferred from observational field studies (McKaye 1980; Meyer 1989, 1990a) and later by genetic inference (Wilson et al. 2000; Elmer et al. 2009). Theoretical verbal and mathematical models suggest such speciation is possible (Turner & Burrows 1995; Higashi et al. 1999; Takimoto et al. 2000; Kirkpatrick & Ravigné 2002), but the mode of gold inheritance (a dominant allele) (Barlow 1983; Henning et al. in press), effect of brood adoptions on sexual imprinting (Barlow 1992), frequency of mixed colour morph matings (Barlow 1992; Elmer et al. 2009) and possibly late gold colour change after sexual maturity is reached (Barlow 1998) might all be factors that could inhibit speciation based on colour.
(e). Revealing morphological and genetic variation
The research field of geometric morphometrics allows hypothesis testing by describing and quantifying differences in shape between biological groups (Rohlf & Marcus 1993; Adams et al. 2004; Zelditch et al. 2004) because multivariate shape variation is statistically comparable. Founded on landmarks that describe overall body shape, and subsequent Procrustes superimposition that minimizes inter-individual variation, modern analyses of shape can discern much more subtle differences between groups than could traditional morphometrics (Rohlf & Marcus 1993; Parsons et al. 2003; Adams et al. 2004). The methods have been very successful in discerning biological groups of fishes, including cichlids, and relating the body shape differentiation to ecology or phylogeny (Ruber & Adams 2001; Klingenberg et al. 2003; Trapani 2003; Zelditch et al. 2004; Clabaut et al. 2007).
In the present study, we use geometric morphometrics to characterize body shape variation within and between eight lakes and all currently described species of the Midas cichlid species complex in Nicaragua. We further test whether abiotic environmental characteristics of each crater lake are correlated with genetic diversity and species richness in the Midas cichlid species complex. We take advantage of the crater lakes as natural replicate evolutionary experiments to infer some general patterns of the Midas cichlid adaptive radiation across time and space.
2. Material and methods
(a). Sample collection
Samples were collected in 2001, 2003, 2005 and 2007 from great lakes Nicaragua (one location: Las Isletas) and Managua (three locations: Miraflores, San Antonio and Tipitapa) and from six crater lakes (north to south): Asososca León, Apoyeque, Xiloá, Asososca Managua, Masaya and Apoyo (figure 1). Fish were collected using gill nets. Sample sizes for lakes Nicaragua and Managua were augmented by purchasing specimens from local fishermen.
In the field, fish were placed on their right side on a flat surface and photographed from directly above using a tripod and a Nikon Coolpix 995 or S4 digital camera. A scale ruler was included in each photograph. Tissue samples (fin clip and muscle) were collected from each specimen and stored in pure ethanol. Fish heads were taken as vouchers and stored in 70 per cent ethanol. Species were initially taxonomically classified on site. Later the specimen photographs also served in assigning each specimen to a species independently by more than one author. The currently available taxonomy was used.
A total of 1334 specimens from all nine currently recognized species of the Midas cichlid species complex were included in this study (table 2; electronic supplementary material, appendix table S1). This sampling represents the entire known crater lake and great lake distribution, except for Monte Galán and Tiscapa, for which our sample sizes were not sufficient for geometric morphometric analyses. For this study, we limited ourselves to the investigation of external phenotypic differences only between lakes and species. Intraspecific polymorphisms (e.g. in colour, jaw type or lip shape) have not been investigated here and will be the subject of future study. Males and females are combined because other research has indicated no significant body shape differences between the sexes (K. R. Elmer 2010, unpublished data). Previous morphometric studies on this species complex also combined sexes (e.g. Klingenberg et al. 2003; Parsons et al. 2003; Barluenga et al. 2006). Sampling locations within lakes have been pooled, because in none of the previous studies on crater lakes has any intralacustrine genetic structure been observed (Barluenga et al. 2006; Elmer et al. 2009). Juvenile fish (total body length approx. less than 10 cm) were excluded because fish of this size cannot be reliably assigned to species.
Table 2.
Specimens by lake and species used in the geometric morphometrics study. Total sample size is 1334.
| lake | species | N per species |
|---|---|---|
| crater lakes | ||
| Apoyo | A. astorquii | 114 |
| A. chancho | 22 | |
| A. flaveolus | 13 | |
| A. zaliosus | 81 | |
| Apoyeque | A. cf. citrinellus | 132 |
| Asososca León | A. cf. citrinellus | 162 |
| Asososca Managua | A. cf. citrinellus | 111 |
| Masaya | A. cf. citrinellus | 135 |
| Xiloá | A. amarillo | 50 |
| A. sagittae | 41 | |
| A. xiloaensis | 68 | |
| great lakes | ||
| Managua | A. citrinellus | 122 |
| A. labiatus | 17 | |
| Nicaragua | A. citrinellus | 138 |
| A. labiatus | 128 | |
(b). Genetic diversity analyses
(i). DNA sequencing
Genomic DNA was extracted from tissues using a standard high-salt protocol (Bruford et al. 1998). The mtDNA control region was amplified with primers LProF (Meyer et al. 1994) and 12S5R (GGC GGA TAC TTG CAT GT) using standard PCR conditions. PCR products were cleaned using a FastAP Thermosensitive Alkaline Phophatase dephosphorylation protocol and cycle sequenced in the forward and reverse directions by BigDye Terminator Cycle Sequencing Ready Reaction using standard conditions and the same primers as in the PCR. After cleaning the single-stranded product with Zymo ZR-96 DNA Sequencing Clean-up Kit, samples were resuspended in water and electrophoresed in an ABI3130xl DNA sequencer (Applied Biosystems). Forward and reverse contigs were assembled in Sequencher v. 4.2.2. Additional sequences were obtained from GenBank.
(ii). Genetic diversity analyses
Sequences were aligned in MacClade v. 4 (Maddison & Maddison 2003) and trimmed to a common length that resulted in no terminal gaps (733 bp). The distribution of haplotypes was calculated in DnasP v. 5 (Librado & Rozas 2009) (20 sites with alignment gaps were not considered). Haplotype or allelic richness (rarefied to a sample size of 63 to avoid any bias induced by unequal sample sizes per crater lake) and gene diversity were calculated in Contrib v. 1.02 (Petit et al. 1998) for each crater lake.
(c). Geometric morphometrics
(i). Data acquisition
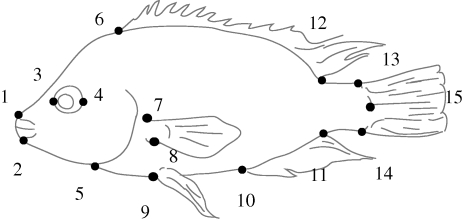
Fifteen landmarks that describe body shape were digitized in TpsDig 2.12 (Rohlf 2001) by a single investigator (H.K.) from the photograph of each specimen (figure 2). Two additional landmarks representing 10 cm on the size scale were used to determine the standard length of each fish, calculated in Past 1.89 (Hammer et al. 2001).
Figure 2.
Location of 15 homologous landmarks used for geometric morphometrics to describe body shape.
Specimens were grouped in such a way as to test three main questions:
—Morphological variation across all crater and great lakes. To investigate the overall morphometric variation in the entire Midas cichlid species complex across lakes, all specimens within each lake were pooled (n = 1334 across eight lakes; table 2) and comparisons drawn across lakes.
—Morphological variation within lakes with multiple species. To assess the variation among different Midas species within particular lakes, we created pooled and separate datasets for crater lakes Apoyo (four species) and Xiloá (three species) and for great lakes Managua (two species) and Nicaragua (two species) (table 2). Morphometric analyses were the same for all datasets.
—Diversity within crater lakes and correlations with lake characteristics. To test correlations between biological diversity in crater lakes and crater lake abiotic characteristics such as size and age of lakes, specimens in each crater lake were pooled for calculating partial disparity (PD) and mtDNA diversity.
(ii). Body shape analysis
Geometric morphometric methodologies largely follow Klingenberg et al. (2003). Analyses were performed in Morphoj 1.00k (Klingenberg 2008). The first step was a generalized least squares Procrustes superimposition (Dryden & Mardia 1998), in which the configuration of 15 landmarks for each specimen was scaled to unit centroid size, translated to a common position and rotated to minimize Procrustes distances between all landmark configurations (Dryden & Mardia 1998; Rohlf 1999; Zelditch et al. 2004). Therefore, the Procrustes distances become relative measures of shape differences between biological groups. The reliability of the Procrustes distance in distinguishing groups was assessed by permutation tests (1000 iterations) and p-values were corrected for non-independence (α/k − 1) (Rice 1989).
We applied a size correction to our data to account for any allometric effects associated with growth, i.e. any dependence of body shape on absolute body size (Loy et al. 1996; Reis et al. 1998; Klingenberg 2003). We divided the dataset into subgroups according to the comparison we were performing (e.g. by lake, or species within a lake). A multivariate regression of shape (dependent variable: Procrustes coordinates) on size (independent variable: centroid size) (Monteiro 1998) was performed for each subgroup using a permutation test against the null hypothesis of independence (10 000 iterations). The regression residuals were then used for all geometric morphometric analyses.
The thin plate spline technique (Dryden & Mardia 1998) was used to visualize shape changes in particular body regions. This method displays the average body shape for a set of individuals of interest compared with an ‘average’ (or ‘consensus’) body shape of all fish included in the analysis. The two body shapes (i.e. configurations of 15 landmarks) are superimposed onto a grid. The differences distort the grid in those body regions that differ the most between the average body shape of the groups being compared.
To assess the total amount of variation in body shape between groups, we used canonical variates analysis (CVA; when comparing multiple groups) and discriminant function analysis (DFA; when comparing pairs of groups). These are commonly used ordination analyses to capture the multi-dimensional variation that is inherent in body shapes (Mardia et al. 1979; Albrecht 1980; Klingenberg et al. 2003; Zelditch et al. 2004). These multivariate analyses reduce the amount of variation within groups and express the variation among groups in n dimensions, where n is the number of groups minus one. Multi-dimensional plots were used to describe the morphospace (i.e. the abstract space where each point represents a particular individual) and to distinguish between groups simultaneously. Each axis successively describes the greatest proportion of variation in body shape. Principal components analyses (PCAs) were also conducted. This ordination method is hypothesis-free in that it assumes no a priori groupings in assessing variation but can obscure true and biologically relevant variation between groups.
To quantify the contribution a group makes to the overall morphological variation across the crater lakes, we estimated PD for each group (i.e. lake) compared with the grand mean as:
where Di is the Procrustes distance of the ith group from the grand mean and N is the total number of groups (Zelditch et al. 2004, p. 302).
3. Results
(a). Morphological variation across all lakes
Mean standard length (±standard deviation) of all specimens pooled was 13.1 ± 2.9 cm (n = 1334). Centroid size accounted for significant proportions of shape variation across lakes (minimum of 1.99% in Lake Apoyeque up to 8.47% in Lake Nicaragua, p < 0.01) and for all groups pooled (2.01%, p < 0.0001) (table 3). Therefore, body shape is correlated to centroid size and an allometry correction was applied for all analyses (as described in §2; table 3).
Table 3.
Regression of body shape (Procrustes coordinates) on centroid size indicates notable allometry (i.e. non-independence of shape and size). The percentage of variation for which allometry accounts is given in per cent predicted. p-Values indicate the significance of the regression relationship. As. = Asososca. Analyses are grouped into (a) all eight lakes, (b) each great lake, (c) Lake Apoyo and (d) Lake Xiloá.
| (a) | Apoyo | Apoyeque | As. León | As. Managua | Managua | Masaya | Nicaragua | Xiloá | pooled |
| % predicted | 2.86 | 1.99 | 4.72 | 2.84 | 6.58 | 2.60 | 8.47 | 2.66 | 2.01 |
| p-value | <0.0001 | 0.0028 | <0.0001 | 0.0056 | <0.0001 | 0.0009 | <0.0001 | 0.0019 | <0.0001 |
| (b) | Managua | Nicaragua | |||||||
| A. citrinellus | A. labiatus | pooled | A. citrinellus | A. labiatus | pooled | ||||
| % predicted | 2.08 | 5.65 | 1.55 | 4.64 | 5.43 | 4.56 | |||
| p-value | 0.0138 | 0.4888 | 0.1146 | <0.0001 | <0.0001 | <0.0001 | |||
| (c) | Apoyo | ||||||||
| A. astorquii | A. chancho | A. flaveolus | A. zaliosus | pooled | |||||
| % predicted | 3.11 | 7.57 | 4.35 | 5.00 | 1.01 | ||||
| p-value | 0.0004 | 0.1333 | 0.8369 | 0.0003 | 0.0031 | ||||
| (d) | Xiloá | ||||||||
| A. amarillo | A. sagittae | A. xiloaensis | pooled | ||||||
| % predicted | 5.88 | 8.58 | 10.68 | 1.77 | |||||
| p-value | 0.0067 | <0.0001 | <0.0001 | 0.0029 |
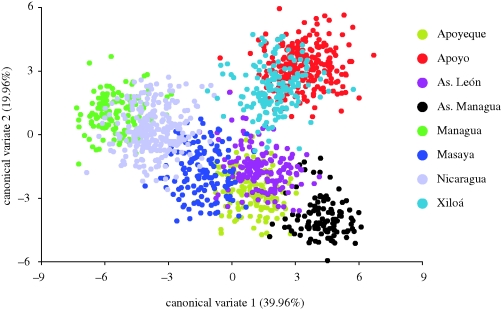
Each crater lake and great lake occupies a unique field of morphospace variation. Specimens from any given lake only slightly or moderately overlap with specimens from any other lake (figure 3). The distinctiveness of each lake is less pronounced but is still evident in a plot of the first two PCs, which makes no a priori grouping (electronic supplementary material, appendix figure S1). In the PCA and CVA, lakes Apoyeque, Masaya and Asososca León contain specimens with the most central body shapes in the analyses, i.e. that deviate least from the average Midas cichlid body shape. Specimens from great lakes Managua and Nicaragua form an overlapping cluster, indicating that the body shapes in those lakes are quite similar to each other but different from those in the crater lakes. Individuals in lakes Apoyo and Xiloá cluster together along CVs 1 and 2 and are set apart from the other lakes. Fish in Asososca Managua are the most distinct. Pronounced shape changes associated with CV1 are: lip/snout thickness (landmark (LM) 1 and 2), body height (LM 6) and width of the mid-body region (LM 5, 7–9), a shift of the anterior insertion of the anal fin (LM 10) and differences in the length of the caudal peduncle region (LM 11–15) (figure 4).
Figure 3.
Plot of all specimens derived from a CVA. Each lake is shown in a different colour. Each dot represents the multivariate morphospace of a different specimen in CV1 and CV2. As. = Asososca.
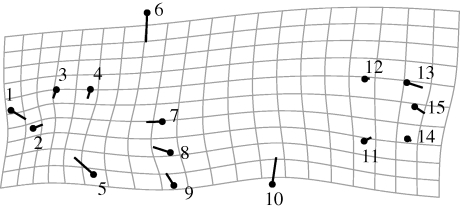
Figure 4.
Shape change associated with CV1 across the species complex from the analysis of all eight lakes combined. The circle represents an average consensus body shape for all Midas cichlids. The bar terminus represents the shift in body shape that is associated with the first CV. The distortion in the grid shows the local shape change associated with the difference between the consensus shape and the shape change described in CV1. Thus, the primary change in body shape across all Midas cichlids relates to body elongation and mid-body height. Scale factor = 10.
Procrustes distances are a metric to describe the difference in body shape between two groups. In a comparison of Procrustes distances between all lakes, Midas cichlid body shape always differs significantly (p < 0.001) (table 4). Average body shapes are the least different between crater lake Masaya and great lake Nicaragua (Procrustes distance 0.0385) and the most different between crater lake Asososca Managua and great lake Managua (Procrustes distance 0.1234).
Table 4.
Pairwise Procrustes distances between all lakes. All comparisons were statistically significant (p < 0.001).
| Apoyeque | Apoyo | Asososca León | Asososca Managua | Managua | Masaya | Nicaragua | |
|---|---|---|---|---|---|---|---|
| Apoyo | 0.0647 | ||||||
| Asososca León | 0.0490 | 0.0613 | |||||
| Asososca Managua | 0.0811 | 0.0793 | 0.0758 | ||||
| Managua | 0.0867 | 0.1140 | 0.0863 | 0.1234 | |||
| Masaya | 0.0512 | 0.0790 | 0.0555 | 0.0828 | 0.0511 | ||
| Nicaragua | 0.0596 | 0.0874 | 0.0661 | 0.0905 | 0.0613 | 0.0385 | |
| Xiloá | 0.0465 | 0.0460 | 0.0440 | 0.0809 | 0.0839 | 0.0576 | 0.0652 |
(b). Morphological variation within lakes
(i). Great lakes Managua and Nicaragua
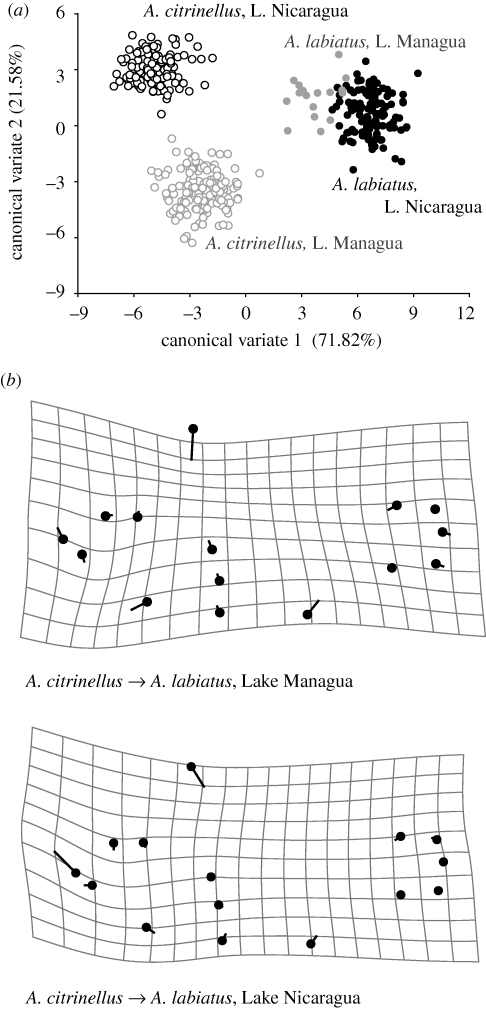
Amphilophus citrinellus and A. labiatus are considered to be two species that inhabit both great lakes (Astorqui 1971). However, the body shape of A. citrinellus is different in Lake Managua from its conspecifics in Lake Nicaragua (figure 5a, table 5). The body shape of A. labiatus is also different in each of the two great lakes (figure 5a, table 5).
Figure 5.
(a) There is a complete morphospace difference between A. citrinellus from each of the two lakes, which is primarily described by CV2. Conversely, most of the variation between the two A. labiatus populations and between species is described along CV1. All interspecific and population comparisons are significantly different in body shape (p < 0.001) Each dot represents the multivariate morphospace of an individual specimen. (b) The shape changes between the average (i.e. consensus) shape of A. citrinellus (black dot) and A. labiatus (line terminus) in great lakes Managua and Nicaragua. In both lakes, both species are completely differentiated by a DFA. No scale factor applied.
Table 5.
Pairwise Procrustes distances between A. citrinellus and A. labiatus from both great lakes. All comparisons were statistically significant (p < 0.001).
| Managua A. citrinellus | Managua A. labiatus | Nicaragua A. citrinellus | |
|---|---|---|---|
| Managua A. labiatus | 0.0878 | ||
| Nicaragua A. citrinellus | 0.0623 | 0.0703 | |
| Nicaragua A. labiatus | 0.1007 | 0.0649 | 0.0797 |
Amphilophus citrinellus and A. labiatus in Lake Managua are completely morphologically distinguished by the DFA, i.e. there is no morphospace overlap between these two species. This pronounced differentiation is primarily due to a greater body depth of A. citrinellus compared with A. labiatus (LM 6). Shape differences in gill opercula–body intersection (LM 5), placement of the anal fin (LM 10) and caudal peduncle length (LM 14 and 15) are also important (figure 5b).
DFA also completely discriminates A. citrinellus from A. labiatus in Lake Nicaragua based on body shape. As in Lake Managua, A. labiatus has a more shallow body (LM 6), elongate head and voluminous lip/snout region (LM 1) relative to A. citrinellus (figure 5b).
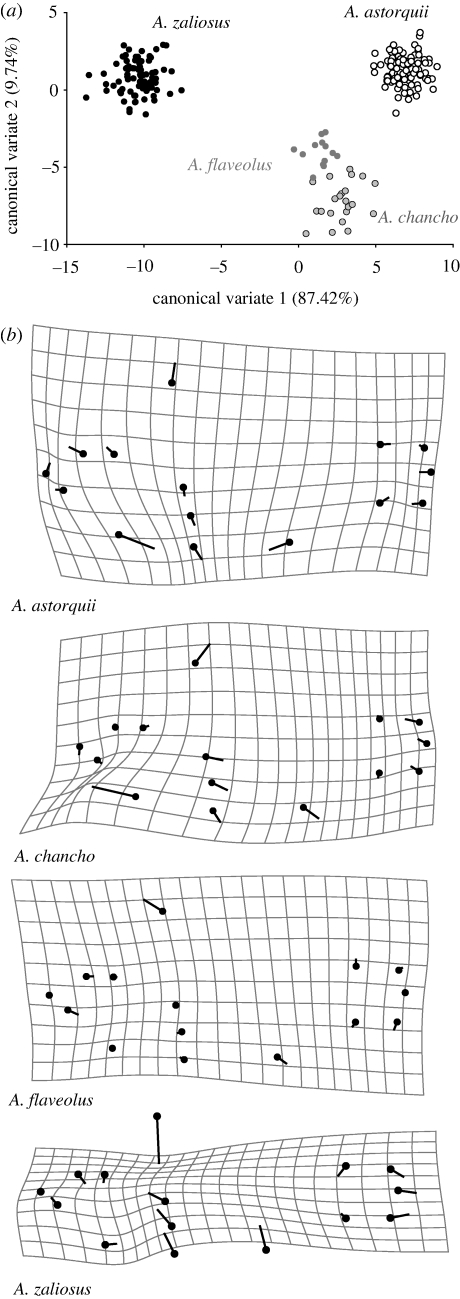
(ii). Crater lake Apoyo: four species
There is a significant difference in body shape among all four species of Midas cichlids in Apoyo (figure 6, table 6). The fields of morphospace variation for A. astorquii and A. zaliosus are each tightly clustered, divergent from each other, and almost completely attributable to the body shape variation described in CV1. Amphilophus chancho and A. flaveolus are both high-bodied benthic species that show very little difference in morphospace between them and, in contrast to A. astorquii and A. zaliosus, are only separated along CV2. The shape change associated with CV1 across all specimens is primarily a broadening of the mid-body region (LM 5–9) and an elongation of the caudal peduncle (LM 10–15) (data not shown).
Figure 6.
(a) The first two axes of the CVA can distinguish all four species within crater Lake Apoyo. Each dot represents the multivariate morphospace of an individual specimen. (b) Body shape variation for each of the four species in Apoyo compared with a consensus body shape. The line terminus indicates the average local shape change in the species of interest, compared with a consensus average shape for all four species of Midas cichlid that are endemic to Lake Apoyo (black dot). Scale factor = 7.5.
Table 6.
Pairwise Procrustes distances between all four species in crater lake Apoyo. All comparisons were statistically significant (p < 0.001).
| A. astorquii | A. chancho | A. flaveolus | |
|---|---|---|---|
| A. chancho | 0.0991 | ||
| A. flaveolus | 0.0682 | 0.0736 | |
| A. zaliosus | 0.1151 | 0.1342 | 0.0961 |
Each species in Apoyo clearly has a different average body shape (figure 6b). Amphilophus astorquii is a relatively compact fish with a robust mid-body (LM 3–10), a short caudal peduncle (LM 11–15) and eyes shifted rostrally (LM 3 and 4). Amphilophus chancho has a shorter lower jaw (LM 5), a larger head and a deep body (LM 6–10) and a short caudal peduncle (LM 13–15). Amphilophus flaveolus is characterized by a more bulky and steep forehead region (LM 6) and is the most similar to the consensus shape. Amphilophus zaliosus is the most morphologically distinct species: its body is very narrow (LM 6–10), lower jaw very long (LM 5) and caudal peduncle narrow and elongate (LM 11–15).
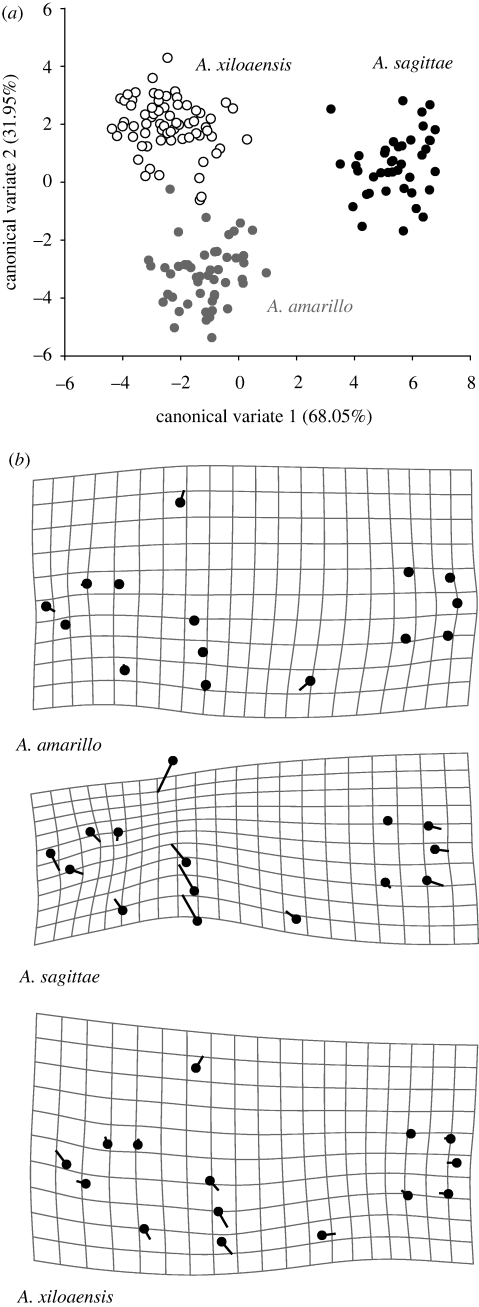
(iii). Crater lake Xiloá: three species
There are significant body shape differences between all three Xiloá Midas cichlid species (figure 7, table 7). Amphilophus sagittae has the most distinct morphology, evidenced by high interspecific Procrustes distances and differentiation along CV1. Amphilophus amarillo and A. xiloaensis are primarily differentiated along CV2. CV1 encodes a shape change in head size, mid-body depth (LM 6–10) and caudal peduncle length (LM 11–15) (data not shown). Amphilophus amarillo is the most similar to the average Xiloá Midas cichlid shape, except for being slightly more high bodied (LM 6) and having the anal fin shifted ventro-rostrally (figure 7b). Amphilophus sagittae has a relatively small head and eye (LM 1–5), is elongated through the mid-body and caudal peduncle (LM 5, 6, 9–10, 13–15) and differs most from consensus. Amphilophus xiloaensis is slightly more deep bodied (LM 6–9) with a shorter caudal peduncle (LM 13–15) and a longer lower jaw (LM 2 and 5).
Figure 7.
(a) The first two axes of the CVA can distinguish all three species within crater Lake Xiloá. Each dot represents the multivariate morphospace of an individual specimen. (b) Body shape variation for each of the three described species of Xiloá compared with the consensus body shape of all three species of the lake. The line terminus indicates the average local shape change in the species of interest, compared with a consensus average shape (black dot). Scale factor = 7.5.
Table 7.
Pairwise Procrustes distances between all three species in crater lake Xiloá. All interspecies comparisons were statistically significant (p < 0.001).
| A. amarillo | A. sagittae | |
|---|---|---|
| A. sagittae | 0.0768 | |
| A. xiloaensis | 0.0475 | 0.1069 |
(iv). Parallelism across crater lakes Apoyo and Xiloá
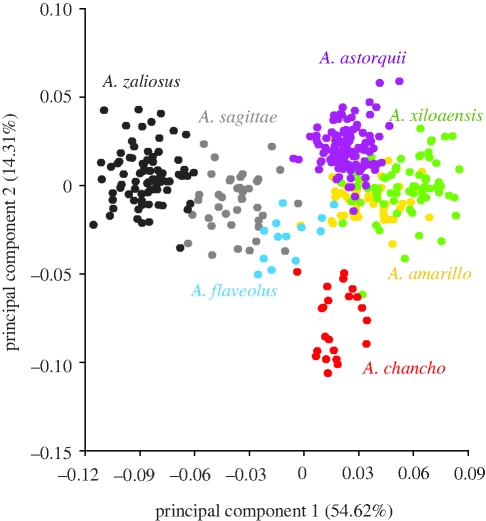
Midas cichlids in lakes Apoyo and Xiloá share similar morphospaces (figure 3). Species in different lakes but with similar ecologies showed equivalent shifts in body shape (e.g. lateral compression, elongated candal peduncle) (figures 6b,7b). This is especially clear in the limnetic species A. sagittae and A. zaliosus. A PCA to compare the morphospace variation between all endemic species of Apoyo and Xiloá demonstrates that these elongate limnetics have their morphospace explained best by the same directions on the PC, with the older species A. zaliosus showing the more extreme variation (figure 8). This suggests that similar ecological forces through divergent selection caused the parallel evolution of a limnetic species in both crater lakes Apoyo and Xiloá.
Figure 8.
The morphospaces of individual specimens in the limnetic species, A. zaliosus (endemic to Lake Apoyo) and A. sagittae (endemic to Lake Xiloá) are explained by the same direction of principal components (primarily along PC1). Benthic species are better distinguished along PC2.
(c). Biological diversity within crater lakes
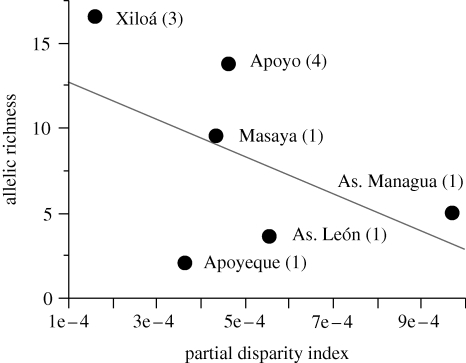
One hundred and forty-four new mtDNA sequences (GenBank accession numbers GU016624–GU016703, GU016998–GU017061) were combined with previously available sequences (electronic supplementary material, appendix table S2) for a total dataset of 929 sequences from all crater lakes. These collapsed to 73 different haplotypes for the entire Midas cichlid species complex. Lake Xiloá had the highest rarefied allelic richness (16.48) and Apoyeque the lowest (1.90) (figure 9; electronic supplementary material, appendix table S3). This pattern was generally similar to that observed for gene diversity, which is a measure that is not corrected for sample size differences (electronic supplementary material, appendix table S3).
Figure 9.
There is a negative trend between PD, which describes the amount of body shape variation within each crater lake, and rarefied allelic richness. A line of best fit is drawn in grey. Each crater lake is noted, with the number in parentheses indicating the number of currently described Midas cichlid species in the lake.
We were interested in lake and faunal features that may suggest why some crater lakes house multiple species while most other crater lakes apparently contain only a single species of Midas cichlid. Because only six crater lakes could be included in this study, this small sample size renders rigorous statistical analyses difficult. Nonetheless, some relevant patterns emerge.
Crater lakes with one species (A. cf. citrinellus) have significantly lower allelic richness than lakes with multiple Midas cichlid species (mean ± s.d.: 5.02 ± 3.24 versus 15.14 ± 1.90; t-test assuming equal variances: t = 3.94, d.f. = 4, two-tailed p = 0.017) (calculated in JMP v. 7; SAS 2008) (figure 9). PD is higher in lakes with one species (5.81 × 10−4 ± 2.73 × 10−4) than it is in lakes with multiple species (3.11 × 10−4 ± 2.17 × 10−4), though the relationship is not significant (t-test assuming equal variances: t = −1.197, d.f. = 4, two-tailed p = 0.297) (figure 9). Allelic richness tends to decrease weakly with increasing PD (F1,4 = 1.374, p = 0.31) (figure 9).
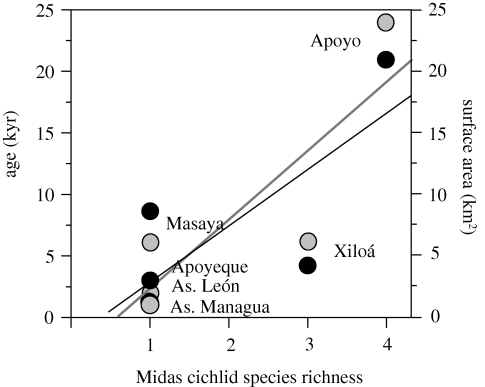
Midas cichlid species richness increases significantly with crater lake age (F1,4 = 10.59, p = 0.031) and size (F1,4 = 5.586, p = 0.077) (figure 10). This effect is leveraged by Apoyo, which is the most species-rich, oldest and largest of the crater lakes. Xiloá has apparently relatively high species richness for a mid-aged lake age of small size. The same relationship between species richness and crater lake size and age does not hold when comparing syntopic fish species other than Midas cichlids, including (crater lake age: F1,4 = 0.035, p = 0.861; crater lake size: F1,4 = 0.045, p = 0.842) or excluding (crater lake age: F1,4 = 0.014, p = 0.911; crater lake size: F1,4 = 0.003, p = 0.960) alien species (table 1). Any undescribed species, in the Midas complex or otherwise, would affect the inferences drawn here, as we will discuss below.
Figure 10.
Midas cichlid species richness increases significantly with crater lake age (in thousands of years; grey dots and grey line of best fit) and surface area (in km2; black dots and black line of best fit). Some crater lakes will increase in species richness (along x-axis) if more species are formally described in the future. The same relationship does not hold for other fish species in the crater lakes.
4. Discussion
(a). Each lake has a unique Midas cichlid morphology
Morphometric analysis of the entire Midas cichlid species complex across six crater lakes and both great lakes revealed that Midas cichlids from each lake have their own characteristic body shapes (figure 3). Each lake is significantly different from all other lakes, whether crater or great lake (table 4). This includes lakes that supposedly have the same species composition, e.g. A. cf. citrinellus in crater lakes or A. labiatus and A. citrinellus in great lakes.
Populations in crater lakes Asososca Managua and Apoyo are the most divergent in body shape (figure 3, table 4). Specimen morphospaces from some crater lakes overlap, particularly from lakes Asososca León and Apoyeque, and Xiloá and Apoyo, and from the great lakes. This suggests that similar body shapes independently evolved in species of Midas cichlids that inhabit those pairs of lakes. The morphospaces of specimens from all other lakes, however, are almost completely non-overlapping.
The greatest apportioning of body shape change across the complex occurs in the head and mouth shape, head size, body depth and length of the caudal peduncle (figure 4). Interestingly, these are also body shape characteristics that have been found in other cichlid studies to relate to trophic niche and ecological selection (e.g. Rüber & Adams 2001; Hulsey & García de León 2005; Barluenga et al. 2006; Clabaut et al. 2007; Konow et al. 2008). Thus, though we have not addressed trophic differences here, our findings in light of previous research on eco-morphology in the species complex (Barlow & Munsey 1976; Meyer 1989, 1990a,b; Vivas & McKaye 2001; Barluenga et al. 2006) suggest a strong role for ecological selection and adaptation in the diversification of Midas cichlid body shapes.
The body shapes of Midas cichlids from the great lakes are distinct from those inhabiting the crater lakes. Such a pronounced body shape variation between great lakes and crater lakes is not unexpected because the clear, deep waters of crater lakes are quite different from the shallow and turbid environment in the great lakes of Nicaragua. Nonetheless, some crater lakes are very young, especially Asososca Managua (1.2 kyr ago) and Apoyeque (1.8 kyr ago) (table 1). Therefore, the change in body shape that comes with colonization of a new crater lake must occur quite quickly. Such morphological changes are expected as a first macrohabitat shift, or colonization of a new environment, during the formation of an adaptive radiation (Schluter 2000a; Gavrilets & Losos 2009). Drift or random sampling of the ancestral population may also be important (Mayr 1954). The role of local adaptation and ecological character displacement very likely interact strongly in crater lakes with multiple ecological species, e.g. minimally Apoyo, Xiloá and nominally also Apoyeque (K. R. Elmer, T. K. Lehtonen & A. Meyer 2010, unpublished data). An analysis of body shape with ecological niche and inferred adaptation (sensu Schluter 1993) across lakes would be important in order to clarify whether similar or different selection regimes act in each of these crater lakes.
(b). Variation in crater lakes with one described species
The crater lakes with A. cf. citrinellus—Apoyeque, Asososca León, Asososca Managua and Masaya—may hold several species that are as of yet undiscovered or formally described. For example, morphs with hypertrophied lips occur to different extents in lakes Apoyeque and Masaya (Waid et al. 1999). In Asososca Managua, which has the highest PD, we collected a relatively elongate A. cf. citrinellus at low frequencies (K. R. Elmer, H. Kusche & A. Meyer 2009, unpublished data), and future research may identify it as an incipient limnetic ecotype or species. Asososca Managua has a large water volume, the smallest littoral zone of all crater lakes (Waid et al. 1999) and clear water: thus, it is feasible that rapid speciation to exploit the limnetic niche might occur (sensu Barluenga et al. 2006).
Lake Masaya is maximally 6000 years old. Given the examples of other crater lakes of the region with similar age, Lake Masaya might be expected to harbour multiple species, though they have not yet been described. However, the lake has experienced frequent volcanic activity (Kutterolf et al. 2007), even as recently as 1800 years ago. It is unknown to what extent these eruptions affect a crater lake's fauna, but they could have repeatedly eliminated or decimated the entire fish population. Additionally, historical documents indicate that there were no large fish, such as Midas cichlids, in Masaya 450 years ago (Villa 1976a), which raises the interesting possibility that the Midas population in Lake Masaya is only a few centuries old.
(c). Patterns of diversity in Nicaraguan great lakes
In both Nicaraguan great lakes there is significant body shape differentiation between the species A. citrinellus and A. labiatus (figure 5, table 5). Though the taxonomy has been far from clear (Astorqui 1971; Villa 1976b), these species are recognized as having distinct morphologies (Astorqui 1971; Klingenberg et al. 2003), though little or no difference in meristic characters has been documented (Barlow & Munsey 1976). The body shape of A. labiatus is rather elongated, and it has a more pointed snout relative to that of A. citrinellus (figure 5b). Additionally, A. labiatus has large, hypertrophied lips, which gave it its name.
Intriguingly, both A. citrinellus and A. labiatus have body shapes that are significantly different from their conspecifics in the neighbouring great lake (figure 5a, table 5). Differences among populations of A. citrinellus and A. labiatus between the two great lakes may indicate phenotypic divergence by drift promoted by long periods of geographical isolation. Amphilophus citrinellus in lakes Managua and Nicaragua are significantly genetically differentiated at nuclear microsatellites and mtDNA (Barluenga & Meyer 2004), suggesting a strong role for geographical isolation. Alternatively, the significant intraspecific, interpopulation body shape differences may represent local adaptation to different lake conditions. Abiotic environmental factors such as turbidity, depth, slope and temperature are very similar for both great lakes (Cole 1976), but the lakes differ dramatically in water chemistry (Cole 1976). Lake Managua is much more concentrated in dissolved solids, probably because it has no major out-flow (Cole 1976). Our results for A. labiatus in Managua and A. citrinellus in Nicaragua should be treated with some caution, as the former has a relatively small sample size (table 2) and the latter relies on samples drawn only from the west–northwestern area of the lake. Additional studies to discern the ecological causes of the intraspecific variation between the populations in lakes Managua and Nicaragua are underway, as these populations represent the source of biological variation that colonized the isolated crater lakes.
(d). Midas cichlid diversity and the question of species
An overall assessment of the Midas cichlid species complex was due, given that in recent years six new species have been described (Stauffer & McKaye 2002; Stauffer et al. 2008). Prior to those species descriptions, Klingenberg et al. (2003) analysed body shape differences among the species that were at that time properly taxonomically described: A. citrinellus, A. labiatus and A. zaliosus. The authors found that some species had body shapes more similar to the syntopic populations of their sister taxa than to allotopic populations of their apparent conspecifics. Our current findings indicate that the earlier lack of interspecific resolution in the study of Klingenberg et al. (2003) was due to an A. citrinellus sample that included specimens from different lakes. Thus, this ‘lumping’ of species would have exaggerated perceived ‘intraspecific’ variation of A. citrinellus. Future study will probably identify that we too have lumped species that might await taxonomic description. Certainly, more ecological and behavioural research is needed, but it would seem clear already that some of the crater lakes contain as yet undescribed species.
Whether populations of Midas cichlids in each lake should be considered different species—given the significant morphological variation and distinctiveness we have shown here—depends on one's species concept (see Mallet 1995; Sites & Marshall 2003; Wiens 2004). Allopatric populations of Midas cichlids in different crater lakes cannot come into physical contact or hybridize in nature, so they are isolated genetic units on independent evolutionary trajectories. However, reproductive isolation is not complete and Midas cichlids from different species and lakes will breed in captivity if no homospecifics are offered (Baylis 1976b) and there is no evidence for F1 hybrid inviability (to date only tested between A. ‘citrinellus’ × A. zaliosus, Baylis (1976a)). Thus, we suggest that populations in each crater lake are diverging because of geographical isolation, drift and, ultimately, local adaptation (Templeton 1980). Conversely, diversification within lakes probably occurs under sympatric conditions, depending on specific lake environments and disruptive ecological selection (discussed below). Thus, paradoxically, the question of crater lake Midas cichlid species is less clear in allopatry than in sympatry.
(e). Crater lake characteristics and species richness
Crater lakes harbouring multiple described endemic Midas cichlid species tend to have populations with higher genetic diversity and lower PD than populations in lakes of only A. cf. citrinellus (figure 9). Species-rich lakes may have higher genetic diversity because of intralacustrine population genetic structuring. Perhaps for similar reasons, temperate lakes with benthic and limnetic stickleback ecotypes are also more haplotype-rich than lakes with a generalist ecotype (Taylor & McPhail 1999). Alternatively, allelic richness may simply increase linearly with time when crater lakes are isolated and/or with lake size (size is correlated with genetic diversity in stickleback lacustrine populations; Caldera & Bolnick 2008). Reduced PD suggests a reduction in interindividual body shape variation, perhaps with increasing specialization.
Midas cichlid species-rich crater lakes are older and larger than crater lakes with one species (figure 10). Waid et al. (1999) also found Nicaraguan crater lake size and age to be important in the number of fish species therein. There is no relationship between crater lake age or size and the number of other fish species therein. This contrast argues for a rapid speciation rate in Midas cichlids compared with the other syntopic crater lake cichlids such as Neetroplus nematopus or Parachromis managuensis.
The population in Lake Xiloá has an exceptionally high haplotype diversity relative to the lake's young age. Xiloá has at least once in the past been merged with Lake Managua (Villa 1976c). An historical influx of A. citrinellus and A. labiatus into the Xiloá Midas cichlid population could have increased diversity within the crater lake by contributing new alleles or promoting character displacement into benthic and limnetic ecotypes (Grant 1972; Schluter 2000b), a pattern that is common in freshwater fishes (Robinson & Wilson 1994). Finally, Lake Xiloá has a heterogeneous bottom profile, with steep slopes to the north and shallow, sandy substrate to the south, which effectively increases the number of potential habitats (Lim et al. 1976) and may promote intralacustrine diversification and speciation. The combination of ecological and genetic factors promoting speciation in isolated populations may together further increase species richness (Emerson & Kolm 2005).
Some Nicaraguan lakes are better studied than others. Arguably, we know of multiple species in Apoyo and Xiloá because they are the cleanest, most accessible and therefore best studied of Nicaragua's crater lakes. Finding and describing new species in other crater lakes could change these perceived relationships.
(f). Intralacustrine diversity in crater lakes Apoyo and Xiloá
In both crater lakes that house multiple described species of Midas cichlid, we find that all species can be statistically significantly distinguished in body shape from their sympatric sister taxa. Body shape differentiation among species in Lake Apoyo is higher than it is in Lake Xiloá (tables 6 and 7).
In Lake Apoyo, Nicaragua's largest and oldest crater lake, there is pronounced body shape variation among the four endemic cichlid species (figure 6, table 6). Amphilophus zaliosus, the species with the illustrative common name ‘arrow cichlid’, is the most morphologically distinct. It is characterized by a narrow, elongate shape, reflecting adaptation to the limnetic niche (Barlow & Munsey 1976; Barluenga et al. 2006). Amphilophus astorquii, a benthic, colony-breeding species, has a short mid-body and is also quite distinct from the other Apoyo species; it is particularly separated from A. zaliosus along CV1. Amphilophus chancho, also a benthic species, has a bulky mid-body and a short lower jaw, which might reflect adaptation to prey types (Meyer 1989, 1990b) different from the other benthic species. The most basal shape in Lake Apoyo can be found in A. flaveolus. Larger sample sizes of A. chancho and A. flaveolus would give us greater power to describe their differences, which are subtle but morphometrically and meristically consistent and significant (present study; Stauffer et al. 2008). Species in Apoyo are endemic, reproduce assortatively in close geographical proximity (Baylis 1976a; T. K. Lehtonen 2008, unpublished data) and are ecologically distinct sister taxa (Barluenga et al. 2006; Stauffer et al. 2008). Thus, the system has been argued as a strong case for sympatric speciation by disruptive ecological selection and associated divergence in assortative mating (Baylis 1976a; Barluenga et al. 2006; Gavrilets et al. 2007; Stauffer et al. 2008). However, to date, this has only been researched in A. zaliosus (Barlow & Munsey 1976; Baylis 1976a; Barluenga et al. 2006).
Lake Xiloá is the only other crater lake that currently has several described endemic Midas cichlid species. All species are significantly different from each other in body shape (table 7, figure 7). As in Apoyo, Xiloá's limnetic species, A. sagittae, is the most distinct in its elongate body shape and small head (figure 7b). It was also named for its ‘arrow’ body shape (Stauffer & McKaye 2002), like A. zaliosus, and feeds on a predatory diet (Vivas & McKaye 2001). Amphilophus amarillo closely approximates the consensus shape and is only distinguished from A. xiloaensis along CV2. Amphilophus amarillo and A. xiloaensis are both high bodied putatively benthic species that feed on a diet richer in snails (Vivas & McKaye 2001).
There are ecological differences between all three species in the pharyngeal jaw apparatus, choice of breeding substrates (Vivas & McKaye 2001) and nest site depth (Elmer et al. 2009). Xiloá Midas cichlids are a distinct genetic cluster compared with neighbouring crater and great lakes (Bunje et al. 2007; K. R. Elmer, A. Kautt & A. Meyer 2010, unpublished data). Given the sister taxon relationships, ecological distinctiveness, assortative mating, novel environment and complete endemism, there is a strong argument for sympatric speciation of all the extant species in crater lake Xiloá.
(g). Adaptive radiation and parallel evolution
In Apoyo and Xiloá, we identify a pattern of morphological and genetic variation that agrees with expectations of intralacustrine divergence into available ecological niches, resulting in eco-morphological adaptation and subsequent sympatric speciation. Parallel evolution of a limnetic species in multiple crater lakes has been hypothesized (McKaye et al. 2002; Stauffer & McKaye 2002; Barluenga et al. 2006; Stauffer et al. 2008) but not previously demonstrated.
Here, we show the independent parallel body shape evolution of an elongate limnetic ecotype in crater lakes Apoyo and Xiloá (figures 6b,7b). Amphilophus sagittae is limnetic (Vivas & McKaye 2001) and ecologically equivalent to A. zaliosus (Stauffer & McKaye 2002), which is also limnetic (Barlow & Munsey 1976; Barluenga et al. 2006). The original species description of A. zaliosus noted that ‘fish similar in appearance’ were living in Xiloá (Barlow & Munsey 1976), but until now no formal morphological comparison of similarity and parallelism had been pursued. As first suggested generally by Barlow & Munsey (1976), the morphospace of species is generally equivalent in lakes Apoyo and Xiloá (figure 3). Morphological variation is described in the same direction of shape change into benthic and limnetic forms (figure 8). Body shapes of A. zaliosus and A. sagittae are generally more similar to each other than they are to other species in their native lake. Both species have slender, elongate bodies and longer caudal peduncles compared with their syntopic, less morphologically differentiated species (figures 6,7).
In Lake Apoyo, A. zaliosus originated sympatrically from an ‘A. citrinellus’ ancestor within the last 10 000 years (Barluenga et al. 2006; Elmer et al. 2010). Amphilophus sagittae is almost certainly younger, since Lake Xiloá itself is only 6100 years old. The less dramatic morphological differentiation between A. sagittae and its syntopic Midas cichlids compared with the distinctiveness of A. zaliosus relative to its syntopics might be due to the comparatively recent speciation. Rapid evolution of a limnetic morph, even under crater lake sympatric conditions, is in agreement with theory and model predictions (Gavrilets et al. 2007).
Ecomorphological parallelism across independent lakes is a prediction of ecological speciation and strong evidence for adaptation to local environment, rather than variation owing to drift (Schluter 1996). Such divergence can occur quickly (Hendry et al. 2007), especially if adaptation or speciation is based on standing genetic variation, i.e. alleles that are present in the ancestral population, rather than novel mutations (reviewed in Barrett & Schluter 2007). The limnetic ecological niche associated with the deep open water found in crater lakes is a novel habitat for Midas cichlids compared with the shallow ancestral habitat of the great lakes. Thus, disruptive selection on eco-morphology and concomitant assortative mating is thought to drive speciation in the crater lakes (Barlow & Munsey 1976; Baylis 1976a; Barluenga et al. 2006). In the temperate zone, competition and predation are thought to be the main drivers of diversification rather than lake physical characteristics (Robinson & Wilson 1994; Vamosi 2003), though some physical and chemical attributes may be important as well (Vamosi 2003; Landry et al. 2007; Gow et al. 2008).
Ecological speciation by disruptive selection is thought to be difficult to accomplish (Felsenstein 1981; Kirkpatrick & Ravigné 2002; Bürger et al. 2006; Nosil et al. 2009). It requires that multiple factors occur in concert: strong trade-offs in efficiencies to maximize fitness (Levins 1962); spatial and/or ecological conditions that maintain polymorphism and frequency-dependent selection (e.g. Udovic 1980; Otto et al. 2008); the evolution of reproductive isolation between ecologically dissimilar types (Maynard Smith 1966; Rice & Salt 1990; Kirkpatrick & Ravigné 2002); and a mechanism for ecologically relevant mate choice (Higashi et al. 1999; Takimoto et al. 2000). Conversely, under the forces of natural selection, parallel evolution may be rather likely (Orr 2005) and disruptive selection rather common (Martin & Pfennig 2009). Correspondingly, a number of taxa do show parallel evolution of eco-morphological traits (reviewed in Schluter 2000a) and cichlid fishes in particular include convincing cases of parallel evolution (Meyer 1993; Galis & Metz 1998; Stiassny & Meyer 1999; Turner 2008; Salzburger 2009). The only known limnetic neotropical cichlid species are A. zaliosus (Barlow & Munsey 1976) and A. sagittae (Stauffer & McKaye 2002), so ecological speciation into this niche may be rare. However, our findings suggest that intralacustrine diversification can occur very quickly when environmental conditions are suitable.
5. Conclusion
Understanding the environmental and biological conditions that result in an acceleration or stasis of speciation is important if we are to test rigorously the current theories of ecological speciation and adaptive radiation (Schluter 2000a; Seehausen 2006; Nosil et al. 2009). Our findings suggest that Midas cichlids in Nicaraguan crater lakes follow an expected pattern of adaptive radiation: divergence in macrohabitat (lake colonization), then microhabitat (benthics and limnetics within lakes) and associated divergence in adaptive traits and assortative mating (Gavrilets & Losos 2009). Albeit the Midas cichlid species complex does not match the species richness of, for example, the African cichlids (Kocher 2004), we consider the Midas cichlid species complex across crater lakes to be an incipient adaptive radiation (sensu Schluter 2000a). This groundwork should allow for predictive hypothesis testing in future research. It also supports the notion that the crater lakes in Nicaragua represent repeated ‘natural experiments’ (Wilson et al. 2000; Barluenga et al. 2006).
Because all Nicaraguan crater lakes are unique with respect to colonization history and environmental factors, it may be that different histories influence the rate of speciation in various lakes (e.g. age and isolation in Apoyo, flux in Xiloá). We are restricted in replicates of these natural experiments to the number of crater lakes that exist. Nonetheless, many natural model systems with sympatric ecotypes of freshwater fishes in young, isolated and simplified lakes have a similar number of replicate environments, e.g. five lakes with sympatric benthic and limnetic stickleback ecotypes in British Columbia (Gow et al. 2008), six St John River lakes with dwarf and normal lake whitefish ecotypes (Landry et al. 2007), three Cameroonian crater lakes (Schliewen et al. 1994; Schliewen & Klee 2004). These systems have offered unparalleled insights into adaptive radiation and speciation in fishes.
Myriad aspects of the Nicaraguan Midas cichlid species complex call for further ecological and evolutionary investigation. For example, quantifying ecological and genetic diversification that occurs shortly after colonizing a new lake; testing the extent of reproductive isolation within ecotypes across lakes (i.e. parallel speciation sensu Schluter & Nagel 1995); assessing the adaptive roles of morphological and colour polymorphisms. Because of parallel evolution across lakes and the emerging genome resources (whole genome sequence: International Cichlid Genome Consortium 2006), the Midas cichlids also offer an exciting avenue for inferring genomic and transcriptomic bases to phenotypic variation (Elmer et al. 2010) and the role of standing genetic variation versus novel mutation in parallel evolution and natural selection (Schluter & Conte 2009; Via 2009). The success of other freshwater fishes with divergent sympatric ecotypes offers a roadmap for further experimental and conceptual challenges (e.g. Peichel et al. 2001; Kingsley et al. 2004; Rogers & Bernatchez 2005; Jeukens et al. 2009; Schluter 2009) that can be tackled with the diverse Midas cichlid species complex.
Acknowledgements
We thank M. Barluenga and W. Salzburger for some specimen photographs. Many thanks to W. Challenger and A. Cardini for statistical advice and A. Freundt and S. Kutterolf for help sourcing geological information. This research was supported by an Alexander von Humboldt and Natural Sciences and Engineering Research Council fellowship to K.R.E., Alexander von Humboldt and Academy of Finland fellowships to T.K.L. and grants of the DFG and the University of Konstanz to A.M. A.M. thanks the Institute for Advanced Study in Berlin for a fellowship that aided in the preparation of this manuscript and H. Ellegren for the invitation to the Wren-Foundation Kristineberg workshop on speciation and for permitting us to contribute to this special issue.
Footnotes
One contribution of 11 to a Theme Issue ‘Genomics of speciation’.
References
- Adams D. C., Rohlf F. J., Slice D. E.2004Geometric morphometrics: ten years of progress following the ‘revolution’. Ital. J. Zool. 71, 5–16 (doi:10.1080/11250000409356545) [Google Scholar]
- Albrecht G. H.1980Multivariate analysis and the study of form, with special reference to canonical variate analysis 1. Integr. Comp. Biol. 20, 679–693 (doi:10.1093/icb/20.4.679) [Google Scholar]
- Altamiro G.1982Volcan Mombotombo Managua, Nicaragua: Instituto Nicaragüense de Estudios Territoriales; See http://www.ineter.gob.ni/geofisica/vol/momotombo/doc/altamirano1982.html [Google Scholar]
- Astorqui I.1971Peces de la cuenca de los grandes lagos de Nicaragua. Rev. Biol. Trop. 19, 7–57 [Google Scholar]
- Barlow G. W.1973Competition between color morphs of the polychromatic Midas cichlid Cichlasoma citrinellum. Science 179, 806–807 (doi:10.1126/science.179.4075.806) [DOI] [PubMed] [Google Scholar]
- Barlow G. W.1976The Midas cichlid in Nicaragua. In Investigations of the ichthyology of Nicaraguan lakes (ed. Thorson T. B.), pp. 333–358 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Barlow G. W.1983The benefits of being gold: behavioral consequences of polychromatism in the Midas cichlid, Cichlasoma citrinellum. Environ. Biol. Fish. 8, 235–247 (doi:10.1007/BF00001089) [Google Scholar]
- Barlow G. W.1986Mate choice in the monogamous and polychromatic Midas cichlid, Cichlasoma citrinellum. J. Fish Biol. 29, 123–133 (doi:10.1111/j.1095-8649.1986.tb05004.x) [Google Scholar]
- Barlow G. W.1992Is mating different in monogamous species? The Midas cichlid fish as a case study. Am. Zool. 32, 91–99 [Google Scholar]
- Barlow G. W.1998Sexual-selection models for exaggerated traits are useful but constraining. Am. Zool. 38, 59–69 [Google Scholar]
- Barlow G. W., Munsey J. W.1976The red devil–Midas-arrow cichlid species complex in Nicaragua. In Investigations of the ichthyology of Nicaraguan lakes (ed. Thorson T. B.), pp. 359–369 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Barlow G. W., Baylis J. R., Roberts D.1976Chemical analyses of some crater lakes in relation to adjacent Lake Nicaragua. In Investigations of the ichthyology of Nicaraguan lakes (ed. Thorson T. B.), pp. 17–20 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Barlow G. W., Rogers W., Cappeto R. V.1977Incompatibility and assortative mating in the Midas cichlid. Behav. Ecol. Sociobiol. 2, 49–59 (doi:10.1007/BF00299288) [Google Scholar]
- Barluenga M., Meyer A.2004The Midas cichlid species complex: incipient sympatric speciation in Nicaraguan cichlid fishes? Mol. Ecol. 13, 2061–2076 (doi:10.1111/j.1365-294X.2004.02211.x) [DOI] [PubMed] [Google Scholar]
- Barluenga M., Stölting K. N., Salzburger W., Muschick M., Meyer A.2006Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439, 719–723 (doi:10.1038/nature04325) [DOI] [PubMed] [Google Scholar]
- Barrett R. D. H., Schluter D.2007Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 (doi:10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- Baylis J. R.1976aA quantitative study of long-term courtship: I. Ethological isolation between sympatric populations of the Midas cichlid, Cichlasoma citrinellum, and the arrow cichlid. Behaviour 59, 59–69 (doi:10.1163/156853976X00460) [Google Scholar]
- Baylis J. R.1976bA quantitative study of long-term courtship: II. A comparative study of the dynamics of courtship in two New World cichlid fishes. Behaviour 59, 117–140 (doi:10.1163/156853976X00343) [Google Scholar]
- Bruford M. W., Hanotte O., Brookfield J. F. Y., Burke T.1998Multi-locus and single-locus DNA fingerprinting. In Molecular genetic analysis of populations (ed. Hoezel A. R.), pp. 283–336 New York, NY: Oxford University Press [Google Scholar]
- Bunje P. M. E., Barluenga M., Meyer A.2007Sampling genetic diversity in the sympatrically and allopatrically speciating Midas cichlid species complex over a 16 year time series. BMC Evol. Biol. 7, 25 (doi:10.1186/1471-2148-7-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürger R., Schneider K. A., Willensdorfer M.2006The conditions for speciation through intraspecific competition. Evolution 60, 2185–2206 [PubMed] [Google Scholar]
- Caldera E. J., Bolnick D. I.2008Effects of colonization history and landscape structure on genetic variation within and among three-spine stickleback (Gasterosteus aculeatus) populations in a singe watershed. Evol. Ecol. Res. 10, 575–598 [Google Scholar]
- Clabaut C., Bunje P. M. E., Salzburger W., Meyer A.2007Geometric morphometric analyses provide evidence for the adaptive character of the Tanganyikan cichlid fish radiations. Evolution 61, 560–578 (doi:10.1111/j.1558-5646.2007.00045.x) [DOI] [PubMed] [Google Scholar]
- Cole G. A.1976Limnology of the Great Lakes of Nicaragua. In Investigations of the ichthyology of Nicaraguan lakes (ed. Thorson T. B.), pp. 9–15 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Cowan H., Prentice C., Pantosti D., de Martini P., Strauch W.& Workshop Participants 2002Late Holocene earthquakes on the Aeropuerto Fault, Managua, Nicaragua. Bull. Seismol. Soc. Am. 92, 1694–1707 (doi:10.1785/0120010100) [Google Scholar]
- Dickman M. C., Schliwa M., Barlow G. W.1988Melanophore death and disappearance produces color metamorphosis in the polychromatic Midas cichlid (Cichlasoma citrinellum). Cell Tissue Res. 253, 9–14 (doi:10.1007/BF00221733) [DOI] [PubMed] [Google Scholar]
- Dryden I. L., Mardia K. V.1998Statistical shape analysis. Chichester, UK: Wiley [Google Scholar]
- Elmer K. R., Lehtonen T., Meyer A.2009Color assortative mating contributes to sympatric divergence of Neotropical crater lake cichlid fish. Evolution 63, 2750–2757 (doi:10.1111/j.1558-5646.2009.00736.x) [DOI] [PubMed] [Google Scholar]
- Elmer K. R., Fan S., Gunter H. M., Jones J. C., Boekhoff S., Kuraku S., Meyer A.2010Rapid evolution and selection inferred from the transcriptomes of sympatric crater lake cichlid fishes. Mol. Ecol. 19Suppl.1), 197–211 [DOI] [PubMed] [Google Scholar]
- Emerson B. C., Kolm N.2005Species diversity can drive speciation. Nature 434, 1015–1017 (doi:10.1038/nature03450) [DOI] [PubMed] [Google Scholar]
- Felsenstein J.1981Skepticism towards Santa Rosalia, or why are there so few kinds of animals? Evolution 35, 124–138 (doi:10.2307/2407946) [DOI] [PubMed] [Google Scholar]
- Galis F., Metz J. A. J.1998Why are there so many cichlid species? Trends Ecol. Evol. 13, 1–2 (doi:10.1016/S0169-5347(97)01239-1) [DOI] [PubMed] [Google Scholar]
- Gavrilets S., Losos J. B.2009Adaptive radiation: contrasting theory with data. Science 323, 732–738 (doi:10.1126/science.1157966) [DOI] [PubMed] [Google Scholar]
- Gavrilets S., Vose A., Barluenga M., Salzburger W., Meyer A.2007Case studies and mathematical models of ecological speciation. 1. Cichlids in a crater lake. Mol. Ecol. 16, 2893–2909 (doi:10.1111/j.1365-294X.2007.03305.x) [DOI] [PubMed] [Google Scholar]
- Gow J. L., Rogers S. M., Jackson M., Schluter D.2008Ecological predictions lead to the discovery of a benthic–limnetic sympatric species pair of three-spine stickleback in Little Quarry Lake, British Columbia. Can. J. Zool. 86, 564–571 (doi:10.1139/Z08-032) [Google Scholar]
- Grant P. R.1972Convergent and divergent character displacement. Biol. J. Linn. Soc. 4, 39–68 (doi:10.1111/j.1095-8312.1972.tb00690.x) [Google Scholar]
- Hammer Ø., Harper D. A. T., Ryan P. D.2001Past: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 41, 1–9 [Google Scholar]
- Hendry A. P., Nosil P., Rieseberg L. H.2007The speed of ecological speciation. Funct. Ecol. 21, 455–464 (doi:10.1111/j.1365-2435.2007.01240.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning F., Renz A., Fukamachi S., Meyer A.In press Genetic comparative genomic expression analyses of the Mc1r locus in the polychromatic Midas cichlid fish (Teleostei: Cichlidae Amphilophus sp.) species group. J. Mol. Evol. [DOI] [PubMed] [Google Scholar]
- Higashi M., Takimoto G., Yamamura N.1999Sympatric speciation by sexual selection. Nature 402, 523–526 (doi:10.1038/990087) [DOI] [PubMed] [Google Scholar]
- Hulsey C. D., García de León F. J.2005Cichlid jaw mechanics: linking morphology to feeding specialization. Funct. Ecol. 19, 487–494 (doi:10.1111/j.1365-2435.2005.00987.x) [Google Scholar]
- INETER 2008aLaguna Apoyeque. Managua, Nicaragua: Instituto Nicaragüense de Estudios Territoriales; See http://www.ineter.gob.ni/Direcciones/Recursos%20Hidricos/HIDROGRAFIA%20WEB/Lagunas/Apoyeque.htm (Accessed 2 February 2009) [Google Scholar]
- INETER 2008bLaguna Asososca Managua. Managua, Nicaragua: Instituto Nicaragüense de Estudios Territoriales; See http://www.ineter.gob.ni/Direcciones/Recursos%20Hidricos/HIDROGRAFIA%20WEB/Lagunas/Asososca.htm (Accessed 2 February 2009) [Google Scholar]
- INETER 2009aLagos y Lagunas de Nicaragua. Managua, Nicaragua: Instituto Nicaragüense de Estudios Territoriales; See http://www.ineter.gob.ni/Direcciones/Recursos%20Hidricos/dirgral/LAGOS.htm (Accessed 15 July 2009) [Google Scholar]
- INETER 2009bLaguna de Apoyo. Managua, Nicaragua: Instituto Nicaragüense de Estudios Territoriales.See http://www.ineter.gob.ni/Direcciones/Recursos%20Hidricos/HIDROGRAFIA%20WEB/Lagunas/Apoyo.htm (Accessed 5 April 2009) [Google Scholar]
- INETER 2009cLaguna de Tiscapa. Managua, Nicaragua: Instituto Nicaragüense de Estudios Territoriales; See http://www.ineter.gob.ni/Direcciones/Recursos%20Hidricos/HIDROGRAFIA%20WEB/Lagunas/Tiscapa.htm (Accessed 5 April 2009) [Google Scholar]
- INETER 2009dLaguna de Xiloá. Managua, Nicaragua: Instituto Nicaragüense de Estudios Territoriales; See http://www.ineter.gob.ni/Direcciones/RecursosHidricos/HIDROGRAFIAWEB/Lagunas (Accessed 12 November 2008) [Google Scholar]
- International Cichlid Genome Consortium 2006Genetic basis of vertebrate diversity: the cichlid fish model, 1–24 See http://www.genome.gov/Pages/Research/Sequencing/SeqProposals/CichlidGenomeSeq.pdf (28 March 2006)
- Jeukens J., Bittner D., Knudsen R., Bernatchez L.2009Candidate genes and adaptive radiation: insights from transcriptional adaptation to the limnetic niche among coregonine fishes (Coregonus spp., Salmonidae). Mol. Biol. Evol. 26, 155–166 (doi:10.1093/molbev/msn235) [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., et al. 2004New genomic tools for molecular studies of evolutionary change in threespine sticklebacks. Behaviour 141, 1331–1344 (doi:10.1163/1568539042948150) [Google Scholar]
- Kirkpatrick M., Ravigné V.2002Speciation by natural and sexual selection: models and experiments. Am. Nat. 159, S22–S35 (doi:10.1086/338370) [DOI] [PubMed] [Google Scholar]
- Klingenberg C. P.2003Quantitative genetics of geometric shape: heritability and the pitfalls of the univariate approach. Evolution 57, 191–195 [DOI] [PubMed] [Google Scholar]
- Klingenberg C. P.2008MorphoJ Faculty of Life Sciences, University of Manchester, UK: See http://www.flywings.org.uk/MorphoJ_page.htm [Google Scholar]
- Klingenberg C. P., Barluenga M., Meyer A.2003Body shape variation in cichlid fishes of the Amphilophus citrinellus species complex. Biol. J. Linn. Soc. 80, 397–408 (doi:10.1046/j.1095-8312.2003.00246.x) [Google Scholar]
- Kocher T. D.2004Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5, 288–298 (doi:10.1038/nrg1316) [DOI] [PubMed] [Google Scholar]
- Konow N., Bellwood D. R., Wainwright P. C., Kerr A. M.2008Evolution of novel jaw joints promote trophic diversity in coral reef fishes. Biol. J. Linn. Soc. 93, 545–555 (doi:10.1111/j.1095-8312.2007.00893.x) [Google Scholar]
- Kutterolf K., Freundt A., Pérez W., Wehrmann H., Schmincke U.2007Late Pleistocene to Holocene temporal succession and magnitudes of highly-explosive volcanic eruptions in west-central Nicaragua. J. Volcanol. Geotherm. Res. 163, 55–82 (doi:10.1016/j.jvolgeores.2007.02.006) [Google Scholar]
- Landry L., Vincent W. F., Bernatchez L.2007Parallel evolution of lake whitefish dwarf ecotypes in association with limnological features of their adaptive landscape. J. Evol. Biol. 20, 971–984 (doi:10.1111/j.1420-9101.2007.01304.x) [DOI] [PubMed] [Google Scholar]
- Levins R.1962Theory of fitness in a heterogeneous environment. I. The fitness set and adaptive function. Am. Nat. 96, 361–373 (doi:10.1086/282245) [Google Scholar]
- Librado P., Rozas J.2009DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (doi:10.1093/bioinformatics/btp187) [DOI] [PubMed] [Google Scholar]
- Lim T. M., McKaye K. R., Weiland D. J.1976An investigation into the use of artificial habitats as a mean of increasing the fisheries productivity of the great leaks complex of Nicaragua. In Investigations of the ichthyofauna of Nicaraguan lakes (ed. Thorson T. B.), pp. 311–319 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Loy A., Cataudella S., Corti M.1996Shape changes during the growth of the sea bass, Dicentrarchus labrax (Teleostea: Perciformes), in relation to different rearing conditions. Adv. Morphometrics 284, 399–414 [Google Scholar]
- Luz-Burgoa K., de Oliveira S. M., Sá Martins J. S.2007. Computer simulations on the sympatric speciation modes for the Midas cichlid species complex. See Nature Preceedingshttp://hdl.handle.net/10101/npre.2007.798.1
- Maddison D. R., Maddison W. P.2003MacClade 4: analysis of phylogeny and character evolution. Version 4.07. Sunderland, MA: Sinauer Associates [Google Scholar]
- Mallet J.1995A species definition for the modern synthesis. Trends Ecol. Evol. 10, 294–299 (doi:10.1016/0169-5347(95)90031-4) [DOI] [PubMed] [Google Scholar]
- Mardia K. V., Kent J. T., Bibby J. M.1979Multivariate analysis. New York, NY: Academic Press [Google Scholar]
- Martin R. A., Pfennig D. W.2009Disruptive selection in natural populations: the roles of ecological specialization and resource competition. Am. Nat. 174, 268–281(doi:10.1086/600090) [DOI] [PubMed] [Google Scholar]
- Martínez C. S.1976Relative abundance and distribution of the mojarra (Cichlasoma citrinellum) in Lake Nicaragua. In Investigations of the ichthyology of Nicaraguan lakes (ed. Thorson T. B.), pp. 371–374 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Maynard Smith J.1966Sympatric speciation. Am. Nat. 100, 637–650 (doi:10.1086/282457) [Google Scholar]
- Mayr E.1954Change of genetic environment and evolution. In Evolution as a process (eds Huxley J., Hardy A. C., Ford E. B.), pp. 157–180 New York, NY: Collier Books [Google Scholar]
- McCrary J. K., Castro M., McKaye K. R.2006Mercury in fish from two Nicaraguan lakes: a recommendation for increased monitoring of fish for international commerce. Environ. Pollut. 141, 513–518 (doi:10.1016/j.envpol.2005.08.062) [DOI] [PubMed] [Google Scholar]
- McCrary J. K., Murphy B. R., Stauffer J. R., Jr, Hendrix S. S.2007Tilapia (Teleostei: Cichlidae) status in Nicaraguan natural waters. Environ. Biol. Fish. 78, 107–114 (doi:10.1007/s10641-006-9080-x) [Google Scholar]
- McKaye K. R.1977Competition for breeding sites between the cichlid fishes of Lake Jiloa, Nicaragua. Ecology 58, 291–302 (doi:10.2307/1935604) [Google Scholar]
- McKaye K. R.1980Seasonality in habitat selection by the gold color morph of Cichlasoma citrinellum and its relevance to sympatric speciation in the family Cichlidae. Environ. Biol. Fish. 5, 75–78 (doi:10.1007/BF00000953) [Google Scholar]
- McKaye K. R.1986Mate choice and size assortative mating by the cichlid fishes of Lake Jiloa, Nicaragua. J. Fish Biol. 29, 135–150 (doi:10.1111/j.1095-8649.1986.tb05005.x) [Google Scholar]
- McKaye K. R., Barlow G. W.1976Competition between color morphs of the Midas cichlid, Cichlasoma citrinellum, in Lake Jiloá, Nicaragua. In Investigations of the ichthyofauna of Nicaraguan lakes (ed. Thorson T. B.), pp. 467–475 Lincoln, NE: University of Nebraska Press [Google Scholar]
- McKaye K. R., McKaye N. M.1977Communal care and kidnapping of young by parental cichlids. Evolution 31, 674–681 (doi:10.2307/2407533) [DOI] [PubMed] [Google Scholar]
- McKaye K. R., Ryan J. D., Stauffer J. R., Jr, Lopez Perez L. J., Vega G. I., van den Berghe E.1995African tilapia in Lake Nicaragua. BioScience 45, 406–411 (doi:10.2307/1312721) [Google Scholar]
- McKaye K. R., et al. 2002Behavioral, morphological and genetic evidence of divergence of the Midas cichlid species complex in two Nicaraguan crater lakes pp. 19–47 Managua, Nicaragua: Dirección de Investigación de la Universidad Centroamericana [Google Scholar]
- Meyer A.1989Costs and benefits of morphological specialization: feeding performance in the trophically polymorphic Neotropical cichlid fish Cichlasoma citrinellum. Oecologia 80, 431–436 (doi:10.1007/BF00379047) [DOI] [PubMed] [Google Scholar]
- Meyer A.1990aEcological and evolutionary consequences of the trophic polymorphism in Cichlasoma citrinellum (Pisces: Cichlidae). Biol. J. Linn. Soc. 39, 279–299 (doi:10.1111/j.1095-8312.1990.tb00517.x) [Google Scholar]
- Meyer A.1990bMorphometrics and allometry in the trophically polymorphic cichlid fish, Cichlasoma citrinellum: alternative adaptations and ontogenetic changes in shape. J. Zool. 221, 237–260 (doi:10.1111/j.1469-7998.1990.tb03994.x) [Google Scholar]
- Meyer A.1993Phylogenetic relationships and evolutionary processes in East African cichlid fishes. Trends Ecol. Evol. 8, 279–285 (doi:10.1016/0169-5347(93)90255-N) [DOI] [PubMed] [Google Scholar]
- Meyer A., Morrissey J. M., Schartl M.1994Recurrent origin of a sexually selected trait in Xiphophorus fishes inferred from a molecular phylogeny. Nature 368, 539–542 (doi:10.1038/368539a0) [DOI] [PubMed] [Google Scholar]
- Monteiro L. A.1998Multivariate regression models and geometric morphometrics: the search for causal factors in the analysis of shape. Syst. Biol. 48, 192–199 (doi:10.1080/106351599260526) [DOI] [PubMed] [Google Scholar]
- Nosil P., Harmon L. J., Seehausen O.2009Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 24, 145–156 (doi:10.1016/j.tree.2008.10.011) [DOI] [PubMed] [Google Scholar]
- Orr H. A.2005The probability of parallel evolution. Evolution 59, 216–220 [PubMed] [Google Scholar]
- Otto S. P., Servedio M. R., Nuismer S. L.2008Frequency-dependent selection and the evolution of assortative mating. Genetics 179, 2091–2112 (doi:10.1534/genetics.107.084418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo N., Avellán D. R., Macías J. L., Scolamacchia T., Rodríguez D.2008The ∼1245 yr BP Asososca maar: new advances on recent volcanic stratigraphy of Managua (Nicaragua) and hazard implications. J. Volcanol. Geotherm. Res. 176, 493–512 (doi:10.1016/j.jvolgeores.2008.04.020) [Google Scholar]
- Parsons K. J., Robinson B. W., Hrbek T.2003Getting into shape: an empirical comparison of traditional truss-based morphometric methods with a newer geometric method applied to New World cichlids. Environ. Biol. Fish. 67, 417–431 (doi:10.1023/A:1025895317253) [Google Scholar]
- Peichel C. L., Nereng K. S., Ohgi K. A., Cole B. L. E., Colosimo P. F., Bueride C. A., Schluter D., Kingsley D. M.2001The genetic architecture of divergence between threespine stickleback species. Nature 414, 901–905 (doi:10.1038/414901a) [DOI] [PubMed] [Google Scholar]
- Petit R. J., El Mousadik A., Pons O.1998Identifying populations for conservation on the basis of genetic markers. Conserv. Biol. 12, 844–855 (doi:10.1046/j.1523-1739.1998.96489.x) [Google Scholar]
- Reis R. E., Zelditch M. L., Fink W. L.1998Ontogenetic allometry of body shape in the Neotropical catfish Callichthys (Teleostei: Siluriformes). Copeia 1998, 177–182 (doi:10.2307/1447715) [Google Scholar]
- Říčan O., Zardoya R., Doadrio I.2008Phylogenetic relationships of Middle American cichlids (Cichlidae, Heroini) based on combined evidence from nuclear genes, mtDNA, and morphology. Mol. Phylogenet. Evol. 49, 941–957 (doi:10.1016/j.ympev.2008.07.022) [DOI] [PubMed] [Google Scholar]
- Rice W.1989Analyzing tables of statistical tests. Evolution 43, 223–225 (doi:10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- Rice W., Salt G. W.1990The evolution of reproductive isolation as a correlated character under sympatric conditions: experimental evidence. Evolution 44, 1140–1152 (doi:10.2307/2409278) [DOI] [PubMed] [Google Scholar]
- Robinson B. W., Wilson D. S.1994Character release and displacement in fishes: a neglected literature. Am. Nat. 144, 596 (doi:10.1086/285696) [Google Scholar]
- Rogers S. M., Bernatchez L.2005Integrating QTL mapping and genome scans towards the characterization of candidate loci under parallel selection in lake whitefish (Coregonus clupeaformis). Mol. Ecol. 14, 351–361 (doi:10.1111/j.1365-294X.2004.02396.x) [DOI] [PubMed] [Google Scholar]
- Rohlf F. J.1999Shape statistics: Procrustes superimpositions and tangent spaces. J. Classif. 16, 197–223 (doi:10.1007/s003579900054) [Google Scholar]
- Rohlf F. J. TPSDig2: a program for landmark development and analysis. 2001. See http://life.bio.sunysb.edu/morph/ [Google Scholar]
- Rohlf F. J., Marcus L. F.1993A revolution in morphometrics. Trends Ecol. Evol. 8, 129–132 [DOI] [PubMed] [Google Scholar]
- Rüber L., Adams D. C.2001Evolutionary convergence of body shape and trophic morphology in cichlids from Lake Tanganyika. J. Evol. Biol. 14, 325 (doi:10.1046/j.1420-9101.2001.00269.x) [Google Scholar]
- Salzburger W.2009The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Mol. Ecol. 18, 169–185 (doi:10.1111/j.1365-294X.2008.03981.x) [DOI] [PubMed] [Google Scholar]
- Salzburger W., Meyer A.2004The species flocks of East African cichlid fishes: recent advances in molecular genetics and population genetics. Naturwissenschaften 91, 277–290 [DOI] [PubMed] [Google Scholar]
- SAS 2008JMP statistical discovery version 7 Cary, NC: SAS Institute Inc [Google Scholar]
- Schliewen U. K., Klee B.2004Reticulate sympatric speciation in Cameroonian crater lake cichlids. Front. Zool. 1, e5 (doi:10.1186/1742-9994-1-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliewen U. K., Tautz D., Paabo S.1994Sympatric speciation suggested by monophyly of crater lake cichlids. Nature 368, 629–632 (doi:10.1038/368629a0) [DOI] [PubMed] [Google Scholar]
- Schluter D.1993Adaptive radiation in sticklebacks: size, shape, and habitat use efficiency. Ecology 74, 699–709 (doi:10.2307/1940797) [Google Scholar]
- Schluter D.1996Ecological speciation in postglacial fishes. Phil. Trans. R. Soc. Lond. B 351, 807–814 (doi:10.1098/rstb.1996.0075) [Google Scholar]
- Schluter D.2000aThe ecology of adaptive radiations. New York, NY: Oxford University Press Inc [Google Scholar]
- Schluter D.2000bEcological character displacement in adaptive radiation. Am. Nat. 156, S4–S16 (doi:10.1086/303412) [Google Scholar]
- Schluter D.2009Evidence for ecological speciation and its alternative. Science 323, 737–741 (doi:10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- Schluter D., Conte G. L.2009Genetics and ecological speciation. Proc. Natl Acad. Sci. USA 106(Suppl. 1), 9995–9962 (doi:10.1073/pnas.0901264106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D., Nagel L.1995Parallel speciation by natural selection. Am. Nat. 146, 292–301 (doi:10.1086/285799) [Google Scholar]
- Seehausen O.2006African cichlid fish: a model system in adaptive radiation research. Proc. R. Soc. B 273, 1987–1998 (doi:10.1098/rspb.2006.3539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert L., Simkin T.2002Momotombo. Volcanoes of the world: an illustrated catalog of Holocene volcanoes and their eruptions. Global Volcanism Program Digital Information Series, GVP-3 Washington, DC: Smithsonian Institution; See http://www.volcano.si.edu/world/volcano.cfm?vnum=1404-09= (Accessed 10 July 2009) [Google Scholar]
- Sites J. W., Marshall J. C.2003Delimiting species: a Renaissance issue in systematic biology. Trends Ecol. Evol. 18, 462–470 (doi:10.1016/S0169-5347(03)00184-8) [Google Scholar]
- Smith S. A., Bermingham E.2005The biogeography of lower Mesoamerican freshwater fishes. J. Biogeogr. 32, 1835–1854 (doi:10.1111/j.1365-2699.2005.01317.x) [Google Scholar]
- Stauffer J. R., Jr, McKaye K. R.2002Descriptions of three new species of cichlid fishes (Teleostei: Cichlidae) from Lake Xiloá, Nicaragua, pp. 1–18 Managua, Nicaragua: Dirección de Investigación de la Universidad Centroamericana [Google Scholar]
- Stauffer J. R., Jr, McCrary J. K., Black K. E.2008Three new species of cichlid fish (Teleostei: Cichlidae) in Lake Apoyo, Nicaragua. P. Biol. Soc. Wash. 121, 117–129 (doi:10.2988/06-37.1) [Google Scholar]
- Stiassny M. L. J., Meyer A.1999Cichlids of the rift lakes. Sci. Am. 280, 64–69 (doi:10.1038/scientificamerican0299-64) [Google Scholar]
- Swain F. M.1966Bottom sediments of Lake Nicaragua and Lake Managua, western Nicaragua. J. Sed. Petrol. 36, 522–540 [Google Scholar]
- Takimoto G., Higashi M., Yamamura N.2000A deterministic genetic model for sympatric speciation by sexual selection. Evolution 54, 1870–1881 [DOI] [PubMed] [Google Scholar]
- Tate Bedarf A., McKaye K. R., Van Den Berghe E., Perez L. J. L., Secor D. H.2001Initial six-year expansion of an introduced piscivorous fish in a tropical Central American lake. Biol. Invas. 3, 391–404 (doi:10.1023/A:1015806700705) [Google Scholar]
- Taylor E. B., McPhail J. D.1999History of an adaptive radiation in species pairs of threespine sticklebacks (Gasterosteus): insights from mitochondrial DNA. Biol. J. Linn. Soc. 66, 271–2914 (doi:10.1111/j.1095-8312.1999.tb01891.x) [Google Scholar]
- Templeton A. R.1980The theory of speciation via the founder principle. Genetics 94, 1011–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibert-Plante X., Hendry A. P.2009Five questions on ecological speciation addressed with individual-based simulations. J. Evol. Biol. 88, 109–123 (doi:10.1111/j.1420-9101.2008.01627.x) [DOI] [PubMed] [Google Scholar]
- Trapani J.2003Geometric morphometric analysis of body-form variability in Cichlasoma minckleyi, the Cuatro Cienegas cichlid. Environ. Biol. Fish. 68, 357–369 (doi:10.1023/B:EBFI.0000005763.96260.2a) [Google Scholar]
- Turner G. F.2008Adaptive radiation of cichlid fish. Curr. Biol. 17, R827–R831 (doi:10.1016/j.cub.2007.07.026) [DOI] [PubMed] [Google Scholar]
- Turner G. F., Burrows M. T.1995A model of sympatric speciation by sexual selection. Proc. R. Soc. Lond. B 260, 287–292 (doi:10.1098/rspb.1995.0093) [Google Scholar]
- Udovic D.1980Frequency-dependent selection, disruptive selection, and the evolution of reproductive isolation. Am. Nat. 116, 621–641 (doi:10.1086/283654) [Google Scholar]
- Vamosi S. M.2003Presence of other fish species affects speciation in threespine sticklebacks. Evol. Ecol. Res. 5, 717–730 [Google Scholar]
- Via S.2009Natural selection in action during speciation. Proc. Natl Acad. Sci. USA 106(Suppl. 1), 9939–9946 (doi:10.1073/pnas.0901397106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa J.1976aIchthyology of the lakes of Nicaragua: historical perspective. In Investigations of the ichthyology of Nicaraguan lakes (ed. Thorson T. B.), pp. 101–113 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Villa J.1976bSystematic status of the cichlid fishes Cichlasoma dorsatum, C. granadense and C. nigritum Meek. In Investigations of the ichthyology of Nicaraguan lakes (ed. Thorson T. B.), pp. 101–113 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Villa J.1976cUna teoría sobre el origen de los peces de Xiloà. In Investigations of the ichthyology of Nicaraguan lakes (ed. Thorson T. B.), pp. 177–189 Lincoln, NE: University of Nebraska Press [Google Scholar]
- Vivas R., McKaye K. R.2001Habitat selection, feeding ecology, and fry survivorship in the Amphilophus citrinellus species complex in Lake Xiloá. J. Aquacult. Aq. Sci. IX, 32–48 [Google Scholar]
- Waid R. M., Raesly R. L., McKaye K. R., McCrary J. K.1999Zoogeografía íctica de lagunas cratéricas de Nicaragua. Encuentro 51, 65–80 [Google Scholar]
- Webber R., Barlow G. W., Brush A. H.1973Pigments of a color polymorphism in a cichlid fish. Comp. Biochem. Physiol. 44B, 1127–1135 [DOI] [PubMed] [Google Scholar]
- Wiens J. J.2004What is speciation and how should we study it? Am. Nat. 163, 914–923 (doi:10.1086/386552) [DOI] [PubMed] [Google Scholar]
- Wilson A. B., Noack-Kunnmann K., Meyer A.2000Incipient speciation in sympatric Nicaraguan crater lake cichlid fishes: sexual selection versus ecological diversification. Proc. R. Soc. Lond. B 267, 2133–2141 (doi:10.1098/rspb.2000.1260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelditch M., Swiderski D., Sheets D., Fink W.2004Geometric morphometrics for biologists. New York, NY: Elsevier [Google Scholar]