Abstract
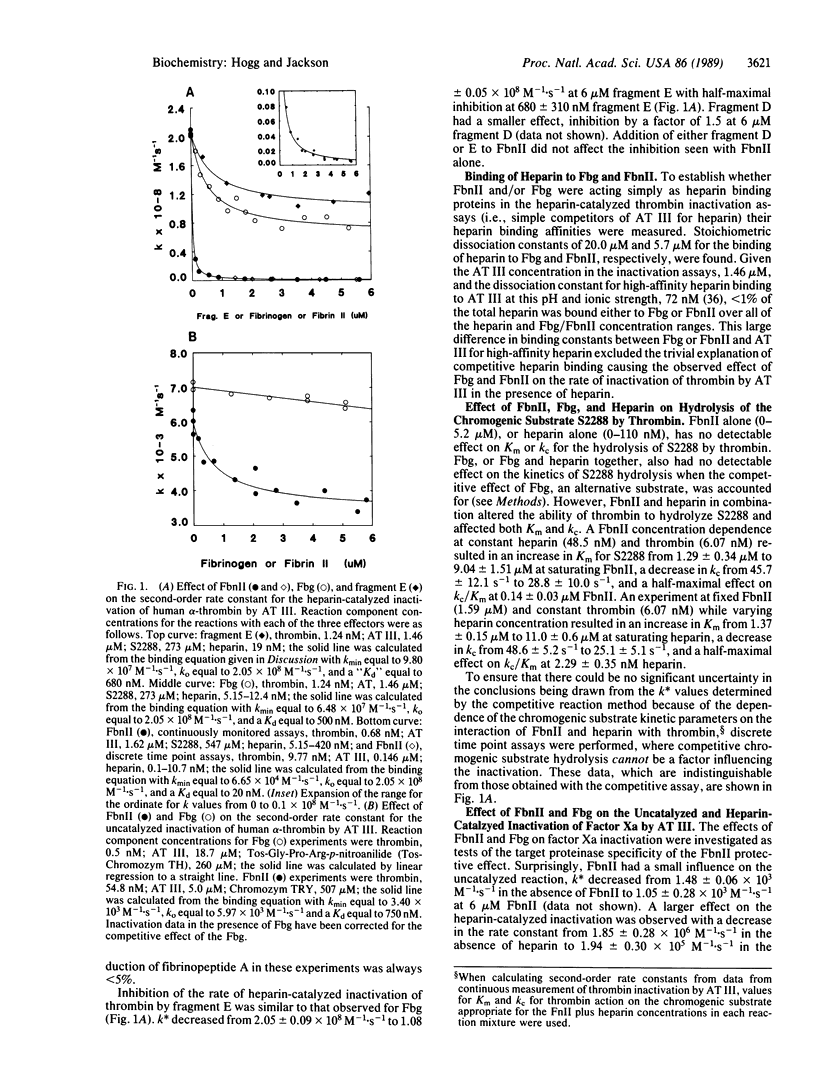
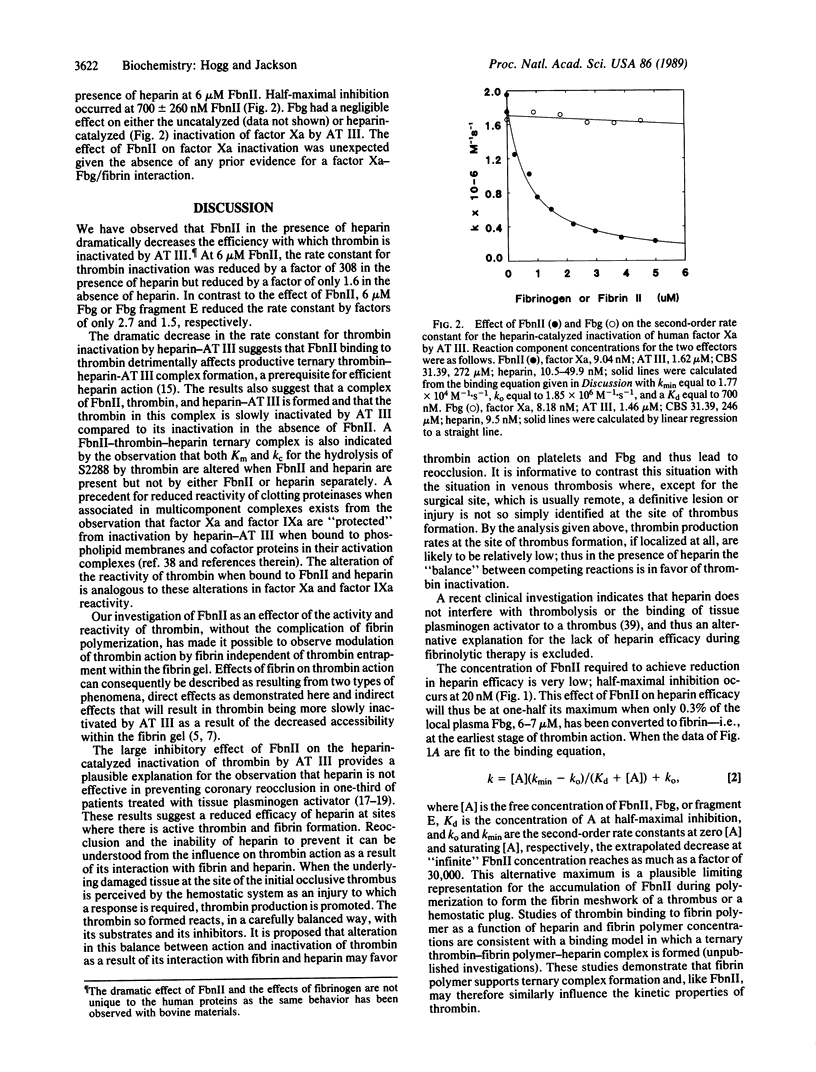
Fibrin II monomer has a dramatic inhibitory effect on the rate of heparin-catalyzed inactivation of human alpha-thrombin by antithrombin III. At 6 microM fibrin II monomer, equivalent to the concentration of fibrinogen in plasma, the second-order rate constant was reduced by a factor of 308--from 2.05 x 10(8) M-1.s-1 to 6.65 x 10(5) M-1.s-1. Fibrin II monomer minimally affected the uncatalyzed rate of thrombin inactivation showing a reduction in the second-order rate constant by a factor of only 1.6. Fibrinogen and the product of plasmin degradation of fibrinogen, fragment E, at 6 microM concentrations also decreased the second-order rate constant for heparin-catalyzed thrombin inactivation, but by factors of only 2.7 and 1.9, respectively. On the basis of these observations it is proposed that protection of thrombin from inactivation by heparin-antithrombin III by fibrin II monomer can explain the limited efficacy of heparin in preventing coronary reocclusion in patients treated with tissue plasminogen activator and other fibrinolytic agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berliner L. J., Sugawara Y., Fenton J. W., 2nd Human alpha-thrombin binding to nonpolymerized fibrin-Sepharose: evidence for an anionic binding region. Biochemistry. 1985 Nov 19;24(24):7005–7009. doi: 10.1021/bi00345a038. [DOI] [PubMed] [Google Scholar]
- Björk I., Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982 Oct 29;48(3):161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G., Morrison J. F. The analysis of progress curves for enzyme-catalysed reactions by non-linear regression. Biochim Biophys Acta. 1977 Apr 12;481(2):297–312. doi: 10.1016/0005-2744(77)90264-9. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin specificity. Ann N Y Acad Sci. 1981;370:468–495. doi: 10.1111/j.1749-6632.1981.tb29757.x. [DOI] [PubMed] [Google Scholar]
- Francis C. W., Markham R. E., Jr, Barlow G. H., Florack T. M., Dobrzynski D. M., Marder V. J. Thrombin activity of fibrin thrombi and soluble plasmic derivatives. J Lab Clin Med. 1983 Aug;102(2):220–230. [PubMed] [Google Scholar]
- Fry E. T., Sobel B. E. Lack of interference by heparin with thrombolysis or binding of tissue-type plasminogen activator to thrombi. Blood. 1988 May;71(5):1347–1352. [PubMed] [Google Scholar]
- Gold H. K., Leinbach R. C., Garabedian H. D., Yasuda T., Johns J. A., Grossbard E. B., Palacios I., Collen D. Acute coronary reocclusion after thrombolysis with recombinant human tissue-type plasminogen activator: prevention by a maintenance infusion. Circulation. 1986 Feb;73(2):347–352. doi: 10.1161/01.cir.73.2.347. [DOI] [PubMed] [Google Scholar]
- Hogg P. J., Winzor D. J. Quantitative affinity chromatography: further developments in the analysis of experimental results from column chromatography and partition equilibrium studies. Arch Biochem Biophys. 1984 Oct;234(1):55–60. doi: 10.1016/0003-9861(84)90323-0. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Jesty J. The kinetics of inhibition of alpha-thrombin in human plasma. J Biol Chem. 1986 Aug 5;261(22):10313–10318. [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kaminski M., McDonagh J. Inhibited thrombins. Interactions with fibrinogen and fibrin. Biochem J. 1987 Mar 15;242(3):881–887. doi: 10.1042/bj2420881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M., McDonagh J. Studies on the mechanism of thrombin. Interaction with fibrin. J Biol Chem. 1983 Sep 10;258(17):10530–10535. [PubMed] [Google Scholar]
- Latallo Z. S., Jackson C. M. Reaction of thrombins with human antithrombin III: II. Dependence of rate of inhibition on molecular form and origin of thrombin. Thromb Res. 1986 Sep 1;43(5):523–537. doi: 10.1016/0049-3848(86)90072-1. [DOI] [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Studies on synthetic peptides that bind to fibrinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences. Biochemistry. 1980 Mar 4;19(5):1013–1019. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- Lewis S. D., Janus T. J., Lorand L., Shafer J. A. Regulation of formation of factor XIIIa by its fibrin substrates. Biochemistry. 1985 Nov 19;24(24):6772–6777. doi: 10.1021/bi00345a007. [DOI] [PubMed] [Google Scholar]
- Lewis S. D., Shields P. P., Shafer J. A. Characterization of the kinetic pathway for liberation of fibrinopeptides during assembly of fibrin. J Biol Chem. 1985 Aug 25;260(18):10192–10199. [PubMed] [Google Scholar]
- Liu C. Y., Nossel H. L., Kaplan K. L. The binding of thrombin by fibrin. J Biol Chem. 1979 Oct 25;254(20):10421–10425. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. High molecular weight derivatives of human fibrinogen produced by plasmin. I. Physicochemical and immunological characterization. J Biol Chem. 1969 Apr 25;244(8):2111–2119. [PubMed] [Google Scholar]
- Nichol L. W., Ward L. D., Winzor D. J. Multivalency of the partitioning species in quantitative affinity chromatography. Evaluation of the site-binding constant for the aldolase-phosphate interaction from studies with cellulose phosphate as the affinity matrix. Biochemistry. 1981 Aug 18;20(17):4856–4860. doi: 10.1021/bi00520a008. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Nyström C., Björk I. The size and shape of human and bovine antithrombin III. Eur J Biochem. 1977 Aug 15;78(1):195–203. doi: 10.1111/j.1432-1033.1977.tb11730.x. [DOI] [PubMed] [Google Scholar]
- Olson S. T., Srinivasan K. R., Björk I., Shore J. D. Binding of high affinity heparin to antithrombin III. Stopped flow kinetic studies of the binding interaction. J Biol Chem. 1981 Nov 10;256(21):11073–11079. [PubMed] [Google Scholar]
- Olson S. T. Transient kinetics of heparin-catalyzed protease inactivation by antithrombin III. Linkage of protease-inhibitor-heparin interactions in the reaction with thrombin. J Biol Chem. 1988 Feb 5;263(4):1698–1708. [PubMed] [Google Scholar]
- Rosenberg R. D. Chemistry of the hemostatic mechanism and its relationship to the action of heparin. Fed Proc. 1977 Jan;36(1):10–18. [PubMed] [Google Scholar]
- Rupp C., Sievi R., Furlan M. Fractionation of plasmic fibrinogen digest on lysine-agarose. Isolation of two fragments D, fragment E and simultaneous removal of plasmin. Thromb Res. 1982 Jul 1;27(1):117–121. doi: 10.1016/0049-3848(82)90285-7. [DOI] [PubMed] [Google Scholar]
- Sache E., Maillard M., Bertrand H., Maman M., Kunz M., Choay J., Fareed J., Messmore H. Studies on a highly active anticoagulant fraction of high molecular weight isolated from porcine sodium heparin. Thromb Res. 1982 Mar 15;25(6):443–458. doi: 10.1016/0049-3848(82)90086-x. [DOI] [PubMed] [Google Scholar]
- Seegers W. H., Nieft M., Loomis E. C. NOTE ON THE ADSORPTION OF THROMBIN ON FIBRIN. Science. 1945 May 18;101(2629):520–521. doi: 10.1126/science.101.2629.520. [DOI] [PubMed] [Google Scholar]
- Thaler E., Schmer G. A simple two-step isolation procedure for human and bovine antithrombin II/III (heparin cofactor): a comparison of two methods. Br J Haematol. 1975 Oct;31(2):233–243. doi: 10.1111/j.1365-2141.1975.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Tian W. X., Tsou C. L. Determination of the rate constant of enzyme modification by measuring the substrate reaction in the presence of the modifier. Biochemistry. 1982 Mar 2;21(5):1028–1032. doi: 10.1021/bi00534a031. [DOI] [PubMed] [Google Scholar]
- Vali Z., Scheraga H. A. Localization of the binding site on fibrin for the secondary binding site of thrombin. Biochemistry. 1988 Mar 22;27(6):1956–1963. doi: 10.1021/bi00406a023. [DOI] [PubMed] [Google Scholar]
- Verstraete M., Arnold A. E., Brower R. W., Collen D., de Bono D. P., De Zwaan C., Erbel R., Hillis W. S., Lennane R. J., Lubsen J. Acute coronary thrombolysis with recombinant human tissue-type plasminogen activator: initial patency and influence of maintained infusion on reocclusion rate. Am J Cardiol. 1987 Aug 1;60(4):231–237. doi: 10.1016/0002-9149(87)90219-0. [DOI] [PubMed] [Google Scholar]
- Wilner G. D., Danitz M. P., Mudd M. S., Hsieh K. H., Fenton J. W., 2nd Selective immobilization of alpha-thrombin by surface-bound fibrin. J Lab Clin Med. 1981 Mar;97(3):403–411. [PubMed] [Google Scholar]



