Abstract
Speciation in animals often requires that population divergence goes through three major evolutionary stages, i.e. ecological divergence, development of sexual isolation and the build-up of genetic incompatibility. There is theoretical consensus regarding favourable conditions required for speciation to reach its final and irreversible stage, but empirical tests remain rare. Here, we review recent research on processes of speciation, based on studies in hybrid zones between collared (Ficedula albicollis) and pied flycatchers (Ficedula hypoleuca). A major advantage of this study system is that questions concerning all three major sources of reproductive isolation and their interconnections can be addressed. We conclude that (i) ecological divergence is caused by divergence in life-history traits, (ii) females prefer mates of their own species based on differences in both plumage and song characteristics, (iii) male plumage characteristics have diverged but their song has converged in sympatry, (iv) there is genetic incompatibility in accordance with Haldane's rule, and (v) the Z-chromosome appears to be a hotspot for genes involved in sexual isolation and genetic incompatibility. We discuss how identification of the genes underlying the three major sources of reproductive isolation can be used to draw conclusions about links between the processes driving their evolution.
Keywords: ecological divergence, sexual isolation, genetic incompatibility, speciation, collared flycatcher, pied flycatcher
1. Introduction
Speciation lies at the heart of evolutionary biology and is the driving force behind biodiversity. Yet, many central questions remain unanswered (Coyne & Orr 2004; Dieckmann et al. 2004; Price 2008). The main reason for this is probably that the time scale needed for populations to go through all the main stages of divergence imposes severe constraints on empirical tests of many of the crucial theoretical assumptions. The aim of this paper is to advertise how this problem can be circumvented, at least partly, by using a combination of ecological, behavioural and genomic studies of natural hybrid zones. As a case study, we will review recent research on hybridizing collared (Ficedula albicollis) and pied flycatchers (Ficedula hypoleuca). Hybrid zones have long been known to offer excellent possibilities to pinpoint the traits that influence differences in niche use, sexual isolation, hybrid fitness and patterns of interspecific gene flow (e.g. Barton & Hewitt 1985; Harrison & Rand 1989; Arnold 1997). However, it has only recently been possible to start asking detailed questions such as whether genes causing ecological divergence and sexual isolation at the early stages of speciation are the same genes that eventually cause genetic incompatibility at the later stages of speciation (Qvarnström & Bailey 2009).
For sexually reproducing organisms, the development of reproductive isolation is generally viewed as a prerequisite for independent evolution of populations (Dobzhansky 1940). Consequently, studies on speciation often focus on the evolutionary basis for reproductive isolation. The development of complete reproductive isolation is in most cases a slow process, which follows a temporal pattern starting with ecological divergence and/or the evolution of traits causing sexual isolation, followed by the build-up of genetic incompatibilities (Coyne & Orr 2004; Price 2008). These three different stages of speciation partly overlap, but the relative role of extrinsic (e.g. hybrids failing to occupy or succeed in exploiting parental niches) and intrinsic (i.e. genetic incompatibilities) sources of isolation change during the process, with intrinsic sources of reproductive isolation evolving at a slower rate. What are the crucial conditions required for speciation to reach its final and irreversible stage? There has recently been a shift of scientific attention from the circumstantial conditions (such as geographical isolation) favouring speciation to the selective forces that drive evolution of the traits that are causing reproductive isolation. While empirical studies on ecology and mating patterns are needed to reveal the mechanisms underlying ecological divergence and sexual isolation, genomic studies are essential for our understanding of the build-up of genetic incompatibilities.
Natural selection is recognized as one of the major factors driving the onset of speciation. Ecological divergence may happen either when populations become subdivided by geographical barriers and are faced with different selective regimes, or because there is strong competition over resources in sympatry (Dieckmann & Doebeli 1999; Schluter 2000; Gavrilets 2004). Population divergence driven by natural selection may occur during short time scales and has the potential to lead to rapid adaptive radiations (Schluter 2000). However, there are several reasons why disruptive natural selection may not result in speciation. First, selection may not be consistent for long enough to maintain strong selection against hybrids (e.g. Seehausen et al. 2008). Second, if there is divergence in a single ecological trait, a possible alternative evolutionary outcome is stable polymorphism (Nosil et al. 2009). Third, the homogenizing effects of gene flow may prevent the build-up of distinct locally adapted genotypes (Mayr 1963; Felsenstein 1981). An important conclusion is therefore that the progressive build-up of reproductive isolation is more likely when ecological divergence leads to pre-mating isolation or when there is evolution of sexual isolation alongside ecological divergence. Sexual selection per se has been identified as a strong force driving speciation (West-Eberhard 1983; Price 1998; Panhuis et al. 2001) because closely related species often differ markedly in sexually selected traits, which gives rise to sexual isolation between them. However, theoretical models show that sexual selection is much less powerful when operating alone than when combined with either natural selection on the relevant traits and/or genetic incompatibility (reviewed by Ritchie 2007). This is because when reproductive isolation solely depends on sexual isolation, only low levels of hybridization are needed to break down the barrier.
For practical reasons, the evolution of traits (and genes) causing reproductive isolation at the different stages of divergence is generally studied using different study systems, but this hampers the ability to investigate the connections between the stages. Most of the detailed studies on the evolution of genetic incompatibility come from laboratory systems (reviewed in Coyne & Orr 2004) and support the classical theoretical models predicting that intrinsic sources of hybrid dysfunction arise through epistatic interactions between genes from different genomes (Dobzhansky 1940; Muller 1940). The role of intrinsic genetic incompatibilities in the process of speciation is debated. Because the evolution of hybrid sterility is a slow process compared with the rate of speciation in several taxa (Price & Bouvier 2002; Mendelson 2003), ecological divergence and sexual isolation may be relatively more important. In fact, some detected genetic incompatibilities between species pairs in the laboratory may have evolved long after the completion of reproductive isolation in their natural habitat (Coyne & Orr 2004). On the other hand, the role of genetic incompatibility could rather be underestimated in natural systems because effects of ‘hybrid break down’ (greater fitness reduction in later generation hybrids) and of interactions with other sources of selection against hybrids are difficult to measure (Wiley et al. 2009b). Moreover, Price (2010) argues that ecological divergence may often be redundant for speciation in birds, which instead depends on range expansions followed by the build-up of genetic incompatibilities and evolution of sexual isolation in geographically isolated populations. This argument is built on the observation that allopatric or parapatric sister species of continental Eurasian Old World leaf warblers (Phylloscopus and Seicercus) are younger but less ecologically diverged compared with sympatric species (Price 2010). An alternative interpretation is that only ecologically diverged species can persist in a long-term perspective and that many of the young allopatric sister species will vanish once they come into secondary contact. To what extent ecological differentiation evolved before or after secondary contact in the old sympatric species remains an open question. In any case, a crucial take-home message is that the relative speed by which the three different types of reproductive barriers have the potential to evolve need not correspond to their sequential role in the speciation process. It also remains unknown whether the build-up of genetic incompatibilities is directly linked with ecological divergence and the evolution of sexual isolation. One way to extract information about the transitions between the major stages of the evolution of reproductive isolation and interactions between them (for example, feedback loops, such as ‘reinforcement’) is to use study systems where all three major sources of reproductive isolation (i.e. ecological divergence, sexual isolation and genetic incompatibility) can be studied simultaneously.
In this review, we focus on two main questions regarding the transition between the three major stages of speciation. (i) To what extent are the evolutionary changes occurring at these different stages influencing each other? (ii) How can the traits causing different types of reproductive isolation remain associated through periods of gene exchange between the diverging populations when reproductive isolation partly evolves under sympatric conditions? We will focus on findings from recent studies on hybridizing Ficedula flycatchers because all three major contributions to reproductive isolation are represented in this study system. We will pinpoint possible evolutionary links between the detected sources of reproductive isolation and discuss how newly developed molecular methods can be used to test these ideas.
2. Ficedula hybrid zones
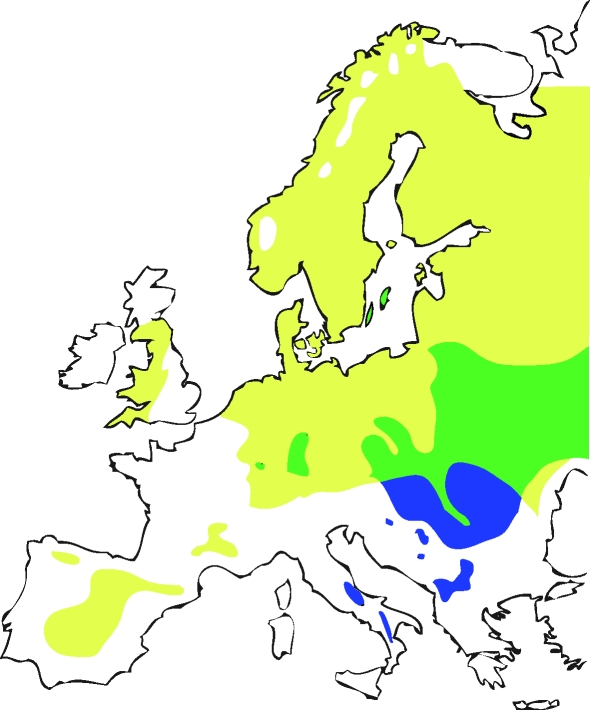
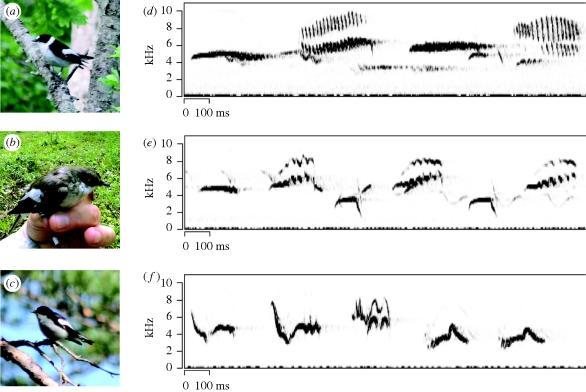
Pied and collared flycatchers are closely related bird species (divergence time probably less than 1 Ma according to nuclear DNA data; N. Backström & H. Ellegren, unpublished data) with similar breeding biology. Both species are small, short-lived, migratory passerines that winter in sub-Saharan Africa. The two species were probably periodically isolated in separate glacial refuges during the Pleistocene, and have since expanded their breeding ranges northward (Sætre et al. 2001). Today, their breeding ranges overlap in a broad hybrid zone in central and eastern Europe and in a more isolated hybrid zone on the Baltic islands of Öland and Gotland (figure 1). The Swedish hybrid zone is relatively young and arose when collared flycatchers expanded their breeding range northward where pied flycatchers were already present. Collared flycatchers have bred on Gotland for around 150 years (Lundberg & Alatalo 1992) and subsequently colonized Öland during the 1960s (Qvarnström et al. 2009). There is species-assortative mating, with occasional heterospecific pairing. Adult male collared flycatchers exhibit black and white plumage (figure 2a), with a large white forehead patch and a distinctive white collar. Male pied flycatchers exhibit a greater degree of intraspecific variation, ranging from bright black and white (figure 2c), such as that of the adult collared flycatcher male but with smaller forehead patches and no collar, to a dull female-like brown plumage (figure 2b). Females of both species are dull grey-brown and differ only slightly in the tone of their upper parts and in the amount of white at the base of their neck feathers (Svensson 1992).
Figure 1.
Breeding range distribution of collared and pied flycatchers. Yellow areas, pied flycatcher breeding range; blue areas, collared flycatcher breeding range; green areas, both species co-occur. Collared and pied flycatchers hybridize where they co-occur in central Europe and on the Baltic islands of Öland and Gotland.
Figure 2.
(a–c) Plumage divergence and (d–f) song convergence in male pied and collared flycatchers. The photographs on the left illustrate examples of variation in plumage coloration among collared and pied flycatchers on the Swedish island of Öland: (a) black and white collared flycatcher male, (b) brown pied flycatcher male, and (c) black and white pied flycatcher male. On the right, sonograms of field recordings illustrating song convergence between male collared and pied flycatchers on Öland: (d) collared flycatcher song, (e) pied flycatcher ‘mixed song’, including collared-like syllables, and (f) pure pied flycatcher song. Photo credits: (a,c) Helen Olofsson; (b) Amber Rice.
The first males arrive at the breeding grounds in northern Europe in late April and immediately start competing over natural breeding holes or nest-boxes (Alatalo et al. 1994; Pärt & Qvarnström 1997; Qvarnström 1997). Females generally arrive one week later and inspect a number of males before selecting their partner based on a number of characteristics of the male and the territory he defends (Dale & Slagsvold 1996). Females typically lay five to seven eggs, which they incubate without assistance from the males. Most clutches hatch in the beginning of June and the young are fed with insects by both parents. Monogamous pairs are most common, but polygyny (Gustafsson & Qvarnström 2006) and extra-pair matings (Sheldon & Ellegren 1999) occur. The fledglings usually leave the nest 14–15 days after hatching and are then fed by the parents for yet another week. The adult birds moult all feathers, while juveniles only moult the body feathers, before their autumn migration starts in mid-August.
The Swedish hybrid zones are the subjects of long-term study. Nest-box areas have been installed on Gotland since 1980 (Gustafsson 1989) and on Öland during 1981–1985 and since 2002 (Qvarnström et al. 2009). Detailed yearly records on breeding performance of more than a thousand breeding pairs are kept and all birds are individually marked with numbered metal rings. Birds are caught once every breeding season whereupon their morphological characters are measured and a small blood sample is taken from each individual for DNA analysis. Adult birds not recaptured in two subsequent breeding seasons are defined as dead.
3. Ecological divergence
Empirical studies on ecological speciation in animals often focus on key traits influencing foraging niches, such as beak morphology in the Galápagos finches (e.g. Schluter & Grant 1984; Price 1987) or mouth structure in African cichlids (e.g. Liem 1973). In flycatchers, there is little divergence in size (Merilä et al. 1994) or feeding techniques (Alerstam et al. 1978). Indeed, the pied and collared flycatchers bring very similar food to their nestlings, as revealed by films obtained from small infrared cameras placed inside nest-boxes (Wiley et al. 2007). Can we therefore conclude that the two Ficedula species are largely ecologically equivalent?
Detailed studies on the ecology of the two species have revealed several other differences, and some of these differences are also known to influence the relative fitness of the two species across environments. By measuring the proportion of stable isotopes in feathers grown at the wintering quarters, Veen et al. (2007) confirmed that the wintering grounds of the two species are separated. Both species are found in central Africa, but pied flycatchers have a relatively more western winter distribution (based on earlier ringing recoveries and visual sightings). However, there is no evidence suggesting that hybrids are selected against owing to an intermediate migration strategy. The isotope signature of hybrids was similar to that of pied flycatchers, and hybrids do not show any reduction in survival during migration (Veen et al. 2007). Additionally, there are clear differences in blood parasite infections between the two species. Studies based on analysis of blood smears, responses to immune challenge (Wiley et al. 2009a) and presence of parasite DNA in blood samples (Kulma submitted) show that collared flycatchers are less often infected by haemosporidians. Hybrids showed an intermediate level of response (Wiley et al. 2009a). To what extent the difference in prevalence of parasites between the two species is explained by them having separate wintering grounds, breeding microhabitat segregation or intrinsic differences in resistance to specific parasites remains unknown. Finally, there is an established link between differences in life-history traits and relative fitness of the two species across different types of environments. Collared flycatchers produce slightly smaller clutches, and their offspring grow faster when there is high food availability, but lose weight faster under poor conditions (Qvarnström et al. 2005). Nestlings of the two species also behave differently such that nestling collared flycatchers beg more intensively, especially when there is a shortage of food (Qvarnström et al. 2007). Further experiments demonstrated that the difference in nestling growth patterns between the two species influences their survival pattern. Nestling collared flycatchers are faced with a higher mortality risk in response to the seasonal decline of food availability (Qvarnström et al. 2009). Moreover, a brood size manipulation experiment showed that a reduction in clutch size (hence reduced sibling competition) resulted in increased nestling survival of late-breeding collared flycatchers, suggesting that the smaller clutch size produced by female collared flycatchers reflects an adaptive adjustment to the higher sensitivity of their nestlings to food shortage (Qvarnström et al. 2009). The most likely explanation for the divergence in life-history traits (of both nestlings and adult birds) is that pied flycatchers have been selected to breed under more harsh environmental conditions because their overall breeding distribution is more northern (figure 1).
Does the divergence in life-history traits influence reproductive isolation between the two flycatcher species? Collared flycatchers appear to be more aggressive and displace pied flycatchers from the preferred habitat where the breeding distribution of the two species overlap (Gustafsson & Pärt 1991; Sætre et al. 1999; Qvarnström et al. 2009). However, given that collared flycatchers suffer larger fitness costs under poor conditions, they are unlikely to have a competitive advantage across all environments experienced by the two species. The colonization history of the collared flycatcher on Öland is well known. Since the arrival of the first immigrants, during the 1960s, the relative proportion of pied flycatchers has quickly declined in the rich deciduous forests (Qvarnström et al. 2009). As a consequence, there is currently a measurable microhabitat segregation between the two species (Veen et al. in press). In the older hybrid zone in central Europe, there is an even more pronounced segregation such that pied flycatchers are found at higher altitudes (Sætre et al. 1999) and there is a lower level of hybridization in this hybrid zone (Sætre et al. 2003).
To summarize, although pied and collared flycatchers have very similar feeding strategies (they are both generalist insectivores), differences in life-history traits reflect different ecological adaptations. On the one hand, collared flycatchers are more successful in competition over breeding sites and their offspring have a higher growth potential under favourable environmental conditions. On the other hand, pied flycatchers are relatively more robust to harsh environments and declining food. Because pied flycatchers can escape interference competition by breeding in poorer habitats, life-history divergence appears to promote (or result from) regional coexistence of the two species. The resulting segregation in sympatry is likely to contribute to pre-zygotic isolation as a side effect. Direct evolutionary links between the divergence in life-history traits between the two species and the evolution of male display traits causing sexual isolation will be discussed in §4. Whether there are ecologically based sources of selection against hybrid nestlings (i.e. sources of post-zygotic isolation caused by the life-history divergence) and possible evolutionary links to the build-up of genetic incompatibility will be discussed in §5.
4. Sexual isolation
Hybridization provides an excellent opportunity to pinpoint the traits that are crucial for assortative mating under natural conditions (Price 2008). For example, given that divergence in male sexual display traits (e.g. plumage characteristics) between two species is important for sexual isolation, one would expect that males that are less phenotypically different from the other species should more often be involved in mixed species pairings. In addition, interactions occurring at secondary contact (such as competition and hybridization) per se may be important for the eventual completion of the speciation process in sympatry (Dobzhansky 1940; Servedio & Noor 2003).
For many birds, the quality of the breeding territory is vital for the success of the offspring. When male flycatchers arrive at the breeding grounds, they must inspect the available territories and claim a nesting site as their own. This process can lead to costly fighting between the males who have already claimed territories (residents) and the prospecting males. Field experiments have demonstrated that in allopatry, brown pied flycatcher males (figure 2b) are allowed to settle nearer to resident pied flycatcher males (Slagsvold & Sætre 1991; Sætre et al. 1993) and nearer to resident collared flycatcher males in sympatry (Alatalo et al. 1994) when compared with black male pied flycatchers (figure 2c). These brown males experience less aggression from other males than their darker counterparts (Slagsvold & Sætre 1991; Alatalo et al. 1994) and can hence avoid costly fighting when establishing breeding territories in the preferred habitat.
What are the consequences of the extensive variation in plumage colour of male pied flycatchers for speciation in sympatry? By facilitating territory establishment in the preferred deciduous habitat, which is usually dominated by the collared flycatcher, habitat isolation is decreased, and pre-zygotic isolation reduced. However, the brown plumage coloration per se may promote sexual isolation: female collared flycatchers more easily discriminate against these brown male pied flycatchers because they differ more from the jet black male collared flycatchers (Sætre et al. 1997a). Thus, the occurrence of brown coloration in male pied flycatchers has a dual effect on the rate of hybridization between the two species. First, by facilitating establishment in the preferred habitat, it increases the risk of hybridization through an increased encounter rate with heterospecific females. Second, by facilitating species recognition, it reduces the risk of hybridization. A more subtle variation in plumage coloration seen between age-classes of collared flycatchers also influences the risk of hybridization. Probably because they are more similar to male pied flycatchers, yearling male collared flycatchers experience an enhanced risk of hybridization with pied females (Wiley et al. 2005). This type of delayed plumage maturation in juvenile males is generally thought to be adaptive by preventing competition from older males (Beauchamp 2003), but aviary experiments show that female pied flycatchers, when forced to choose a heterospecific mate, prefer yearling to adult collared flycatchers (Wiley et al. 2005). Patterns of variation in plumage that may be beneficial during intraspecific territory competition in collared flycatchers hence lead to an increased risk of hybridization.
Pied and collared flycatchers exhibit pronounced spatial variation in plumage coloration across their ranges. The greater plumage divergence in sympatry (i.e. in central Europe and the Swedish Baltic islands, figure 1) compared with allopatry has been hypothesized to result from reinforcement (Alatalo et al. 1990; Sætre et al. 1993, 1997a). Reinforcement occurs when natural selection acts to limit hybridization because it has high fitness costs (Coyne & Orr 2004) and there are indeed high costs of hybridization in flycatchers (Alatalo et al. 1990; Veen et al. 2001; Svedin et al. 2008; Wiley et al. 2009b). As expected under reinforcement, female preferences have also diverged in parallel with plumage divergence in males (Sætre et al. 1997a). This sympatric divergence in plumage is more pronounced in the older central European hybrid zone than in the Swedish island hybrid zone, which is expected both under the reinforcement hypothesis and if divergence occurs through competitive interactions between heterospecific males (given that mainly brown male pied flycatchers manage to defend territories close to male collared flycatchers).
After choosing a nesting site, males have to defend their territories from other prospective males, often using complex, species-specific song (figure 2d–f) to signal that the territory is already occupied. In addition to territory defence, male song is used to attract females to nesting sites (Eriksson & Wallin 1986). In sympatry, approximately 65 per cent of male pied flycatchers sing a ‘mixed’ song including collared-like syllables (figure 2e; Gelter 1987; Alatalo et al. 1990; Haavie et al. 2004; Qvarnström et al. 2006a). This probably results from heterospecific song-learning since the frequency of ‘mixed singers’ is predicted by the relative proportion of the two species (Svedin et al. submitted). Results from playback experiments indicate that male collared flycatchers respond to the mixed song of pied flycatcher males in the same way as to conspecific song (Qvarnström et al. 2006a). Female collared flycatchers also appear to respond to male pied flycatchers singing mixed songs; female collared flycatchers only pair with heterospecifics if they sing a mixed song. These mixed singers, therefore, experience an enhanced risk of hybridization (Qvarnström et al. 2006a). A number of important consequences of this mixed singing behaviour may arise for the evolution of pre-zygotic isolation in sympatry. As we described earlier, male collared flycatchers are more aggressive and tend to be competitively dominant, sometimes displacing pied flycatchers from territories in the preferred deciduous environment. A pied flycatcher singing a mixed song, however, may more efficiently signal that the territory is occupied to prospecting male collared flycatchers, and therefore be less likely to be driven from his territory. This song convergence between pied and collared flycatchers in sympatry thereby mitigates the habitat segregation and pre-zygotic isolation. In addition, species recognition is impaired. Thus, song convergence may slow the speciation process. In the older, central European flycatcher hybrid zone, mixed singing is much less common (Haavie et al. 2004). To what extent this pattern reflects reinforcement or just stronger habitat segregation (and thereby reduced options for heterospecific song learning) is an open question.
The Ficedula flycatchers provide an example of how complex the selection patterns acting on male display traits can be. This complexity stems in part from the need to communicate different messages to males versus females. A male first has to establish a territory and then he needs to defend the territory from other males while attracting females at the same time. In the hybrid zones, males need to signal efficiently to both conspecific and heterospecific males but avoid attracting heterospecific females. Both male pied and collared flycatchers are faced with conflicting selection patterns. By having plumage traits that lower the aggressive responses of superior competitors during the establishment of territories, brown male pied flycatchers become less attractive to heterospecific females while young male collared flycatchers become more attractive to heterospecific females. However, when efficiently signalling that a territory is occupied, male pied flycatchers using the more ‘aggressive’ mixed song become more attractive to heterospecific females. The evolutionary outcome will depend on the relative strength of these conflicting selection pressures as well as the mode of inheritance. Male plumage and male song are determined in different ways, with song being a more plastic, learned signal and plumage being more fixed or heritable. Although reinforcement is nearly always studied in traits with a genetic basis, a recent model by Servedio & colleagues (2009) suggests that reinforcement may occur in learned traits as well.
There are several potential links between ecological divergence and sexual isolation in Ficedula flycatchers. First, as pointed out above, the fact that pied flycatchers are more robust against harsh environmental conditions facilitates spatial segregation in sympatry, which should promote pre-mating isolation as a side effect. Second, differences in the level of aggression during competition over territories may select for alternative male mating strategies, which may either reduce or increase sexual isolation as a side effect. Third, sexual selection may have important consequences for life-history evolution and vice versa (Andersson 1994; Svensson & Sheldon 1998; Badyaev & Qvarnström 2002; Kokko et al. 2006). Previous experiments have demonstrated a trade-off between parental effort and the size of the white forehead patch in collared flycatchers (Gustafsson et al. 1995; Qvarnström 1997). Males with relatively large forehead patches are dominant in male–male competition over nest sites (Pärt & Qvarnström 1997; Qvarnström 1997). Hence, the observed divergence in life-history traits between the two species is likely to be evolutionarily linked with changes in their sexual behaviours and/or secondary sexual traits. Future studies on coloration and song in flycatchers will provide an opportunity to more fully understand the links between ecological and social interactions, sexual isolation, reinforcement and speciation.
5. Selection against hybrids
In nature, hybrid fitness is generally determined by multiple intrinsic and extrinsic factors, with the relative importance of each factor type varying across environments and age of separation (Coyne & Orr 2004; Dieckmann et al. 2004; Price 2008). While many empirical studies have aimed to disentangle the relative roles of various isolation factors, a major next step will be to investigate to what extent the different isolation factors are evolutionarily linked to each other and in which order they occur. Genomic studies may come to play an important role in revealing such interconnections. This is because the build-up of genetic incompatibilities should generally involve complex epistatic interactions between genes, which are difficult to investigate through traditional quantitative genetic approaches.
There are several sources of reproductive isolation between pied and collared flycatchers. The divergence in life-history traits between the two parental species (see above) has consequences for their hybrids. The fitness response of nestling hybrids to environmental change is consistent with them having an intermediate growth strategy (Vallin et al. submitted). Nestling hybrids also have an intermediate begging intensity, which is likely to influence their relative fitness through yet another pathway. Nestlings with a high begging intensity are known to be more likely to be fed by their parents and to thereby gain more weight (Qvarnström et al. 2007). Female collared flycatchers have elevated levels of extra-pair conspecific young when paired to male pied flycatchers (Veen et al. 2001), and the lower begging intensity of hybrid nestlings when compared with pure-bred nestling collared flycatchers means that hybrids have a competitive disadvantage in these nests (Vallin et al. submitted). These environmentally and socially dependent sources of selection against hybrid nestlings are likely to be important because they act early in the life cycle of the hybrids.
As adults, male hybrids develop an intermediate plumage (figure 3). A detailed study on the relative fitness of F1 hybrids (where hybrid identity was established through a combination of species-specific markers and pedigree information) showed that male hybrids were less than half as fit as male collared flycatchers (Svedin et al. 2008). The reduction in fitness was mainly due to poor ability to acquire a mate and a higher incidence of extra-pair young within the nests of male hybrids. In order to disentangle the role of sexual selection from a possible effect of genetic incompatibility on the rate of being cuckolded, Svedin et al. (2008) compared male hybrids with pure-bred males expressing intermediate plumage characters. The authors' expectation was that the two groups of males should have the same reduction in fitness if selection against male hybrids was due to females finding their intermediate phenotype unattractive. Any additional reduction in the fitness of male hybrids (when compared with pure-bred males with intermediate plumage) was assumed to be caused by genetic incompatibility. In total, sexual selection against male hybrids was found to account for approximately 75 per cent of the reduction in their fitness (Svedin et al. 2008). Female F1 hybrids are totally sterile (Svedin et al. 2008); they pair and lay eggs but their eggs never hatch. Thus, Haldane's rule is followed (i.e. faster evolution of hybrid dysfunction in the heterogametic sex).
Figure 3.
A male hybrid flycatcher with a typical intermediate plumage phenotype (note the broken collar). Female hybrids are sterile (they lay eggs, which never hatch) while male hybrids only suffer partial reduction in fitness owing to a disadvantage in competition over mating. Since females constitute the heterogametic sex in birds, Haldane's rule is followed, i.e. faster evolution of hybrid dysfunction in the heterogametic sex. Photo credit Johan Träff.
The reduction in fitness remains for several backcrossed generations. When grand- and great grand-offspring from interspecific crosses were identified and used to calculate a cumulative estimate of post-zygotic isolation, hybridizing pairs were found to produce 3 per cent of the number of descendants typical of conspecific pairs (Wiley et al. 2009b). This estimate is considerably lower compared with the estimate obtained when only F1 hybrids were taken into account and illustrates that several isolation factors may interact in nature and together cause strong post-zygotic isolation. Heterospecific pairings between pied and collared flycatchers are hence highly maladaptive (i.e. strongly selected against). An interesting next step will be to investigate potential evolutionary links between the various factors that together give rise to the strong reproductive isolation between these two species. Revealing such links has the potential to shed light on the evolutionary pathways underlying the transitions between the different major stages of divergence that these two species have gone through. Since the ecological differences (i.e. adaptations to specific environments) between the two species have evolved through changes in life history, there is an interesting possibility of a direct link to the build-up of genetic incompatibility. This is because traits involved in reproduction generally are the ones that show the first signs of genetic incompatibility.
6. Genomic approaches to addressing speciation
We focus our review on two main questions. (i) To what extent are the evolutionary changes occurring in sexual isolation, ecological differentiation and genetic incompatibilities connected? (ii) How can the traits causing different types of reproductive isolation remain associated through periods of gene exchange between the diverging populations? These two questions can be rephrased from a genomic perspective. (i) What are the identity, function and interactions between genes underlying different sources of reproductive isolation? (ii) Where in the genome are these genes located (e.g. are they clustered on specific chromosomes or at chromosomal inversions)? These are topics of considerable current interest within a growing field merging evolutionary biology and genomics, i.e. the use of genomic approaches to study the genetic basis of adaptive evolution (Ellegren & Sheldon 2008).
Molecular genetic work in Ficedula flycatchers started in the early 1990s with the application of mini- and microsatellite markers for inferring paternity and relatedness (Ellegren 1992; Gelter & Tegelström 1992; Ellegren et al. 1995; Ratti et al. 1995; Sheldon & Ellegren 1998), representing one of the systems in which DNA analysis was applied to natural populations. This was also the case for the use of DNA-based methods for molecular sexing of birds, and the associated possibility to test theoretical predictions of sex allocation theory (Ellegren et al. 1996; Sheldon & Ellegren 1996; Merilä et al. 1998). Since then, DNA sequence analysis of individual genes in the pied flycatcher and the collared flycatcher has been done to address questions relating to basic aspects of mutation and selection in these genomes. Importantly, there have been some initial attempts to quantify the degree of genetic differentiation between the two species using sequence data from population samples (Sætre et al. 2001; Borge et al. 2005).
Where in the genome should we expect to find the relevant genes? Studies from natural hybrid zones suggest that reproductive isolation evolves at different rates throughout the genome and that sex chromosomes relatively quickly become incompatible between diverging populations (Tucker et al. 1992; Dod et al. 1993; Raufaste et al. 2005; Macholán et al. 2007; Teeter et al. 2008). Indeed, sex chromosomes have several characteristics that make them suitable candidates for harbouring ‘speciation genes’. They contain fast evolving genes with sex-specific fitness effects (i.e. typically involved in reproduction), their hemizygosity leads to exposure of incompatible recessive genes in hybrids and reduced recombination may shelter co-adapted gene complexes from introgression (Qvarnström & Bailey 2009). The last argument may, in particular, be true for species where females constitute the heterogametic sex (e.g. in birds and butterflies). This is because sexual isolation (pre-zygotic isolation) often depends on evolutionary changes both in male sexual display traits and in corresponding female preferences for those traits. Female heterozygosity is assumed to favour the build-up of linkage disequilibrium between the male and female component of pre-zygotic isolation (Reeve & Pfennig 2003; Kirkpatrick & Hall 2004), which in turn facilitates the critical build-up of linkage disequilibrium between genes underlying traits involved in pre- and post-zygotic isolation. In flycatchers, the Z-chromosomes have been found to be sheltered from introgression (Sætre et al. 2003) and to harbour genes underlying both pre- and post-zygotic isolation (Sæther et al. 2007). While establishing that genes causing low fitness in hybrids (see above) and male plumage characters (Sætre et al. 2003) were located on the Z-chromosome was comparatively straightforward, testing whether species recognition also was Z-linked was challenging. Sæther et al. (2007) investigated the latter question in two steps. First, they tested whether species recognition was genetically determined or socially learned by cross-fostering of young between the two species and recording the mate choice made by the females when they returned as adults the next year. In addition, the mate choice of naturally occurring extra-pair young raised by heterospecific pairs was examined for the same reason. Second, the mate choice of hybrid females was examined in order to investigate whether genetic inheritance occurred through Z-linked genes or through autosomal genes. Because hybrid females inherit their only Z-chromosome from their father, Z-linked species recognition means paternal inheritance of species recognition. F1 hybrid identity and paternal species identity were determined based on species-specific genetic markers. Indeed, hybrid females were found to more often pair with males belonging to the same species as their father than expected by chance. The alternative explanation, i.e. that this pattern was caused by mate-choice imprinting on the father's phenotype, could be ruled out by the results obtained from the swapping experiment: females raised by heterospecific males nevertheless mated to males belonging to the same species as their genetic father.
Mate-choice imprinting is when an individual's mating preference is learnt from observations of adults (reviewed by Galef & Laland 2005), and if individuals learn throughout their lives, one may argue that the pure-bred females mentioned above originally become mis-imprinted on their heterospecific step-fathers but that their preferences subsequently were reoriented back to the phenotype of conspecific males before they paired up the following year. Given that migration patterns are mainly genetically determined, the mis-imprinted females could have been reoriented by learning the phenotypes of conspecific males at the wintering ground. Yet another possibility is that mis-imprinted females became reoriented owing to lack of interest shown by hetersospecific males when they returned to breed. However, these two alternatives are not very likely scenarios: most of the time, males do not wear breeding plumage at the wintering grounds and they do not discriminate between females of the two different species (Sætre et al. 1997b). Z-linked species recognition is the most parsimonious explanation for the results. In contrast to the seemingly large genetic component of species recognition (Sæther et al. 2007), there is a high non-genetic component to variation in mate choice of female collared flycatchers choosing among conspecific males (Qvarnström et al. 2006b), and previous experiences can influence their mate choice (Qvarnström et al. 2004). This raises the question of whether the evolution of sexual isolation simply can be seen as an extension of sexual selection operating within each species. The evolution of mate choice is largely driven by costs and benefits from choosing particular partners. While direct benefits and costs normally outweigh the genetic ones when a female is choosing among conspecific males, genetic costs from heterospecific pairing can be very large (e.g. Wiley et al. 2009b). Because the relationship between the male phenotype and material benefits can vary, plasticity and/or the use of several cues may be favoured when making a choice among conspecific males. By contrast, choosing a genetically incompatible mate is always maladaptive, and the ability to restrict the mate choice to conspecifics may therefore become genetically canalized. The link between the evolution of mate choice and species recognition is an area that, in general, needs more scientific attention. At present, we can only speculate that the large genetic component of species recognition has evolved in response to high costs of hybridization and large areas of secondary contact between the two Ficedula flycatchers. To summarize, empirical studies on Ficedula flycatchers support theoretical predictions (reviewed in Qvarnström & Bailey 2009) that sex chromosomes should be hotspots for speciation genes. Patterns of genetic incompatibility follow Haldane's rule (female hybrids are sterile while males hybrids are not), the fitness reduction seen in male hybrids appears largely owing to their intermediate plumage (Svedin et al. 2008), which is a Z-linked trait (Sætre et al. 2003), and experimental studies on mate choice are in favour of Z-linked species recognition (Sæther et al. 2007).
What are the identity, function and interactions between genes underlying different sources of reproductive isolation? Identification of the more exact genomic targets for genetic incompatibilities between species is likely to require genome-wide approaches. One way of doing this is through mapping approaches. A genetic map, based on protein-coding gene markers, of the collared flycatcher genome was presented recently (Backström et al. 2006a,b, 2008) and will provide a very useful platform for identification of quantitative trait loci, for example, by marker genotyping in pedigrees of interspecific crosses of pied flycatcher and collared flycatcher. Moreover, the genetic map offers a means for linkage disequilibrium mapping in population samples (Backström et al. 2006a,b).
Needless to say, the most important current step in the application of genomics to studies of ecology and evolution is the introduction of next-generation (massive parallel) sequencing technology (Ellegren 2008; Vera et al. 2008). This will come to revolutionize molecular ecology in several ways, including the exciting possibility of retrieving the genome (or transcriptome) sequence of non-model organisms. Attempts towards flycatcher genome sequencing have recently been initiated (Künstner et al. 2010), and this may constitute the most viable approach for finding the genetic basis of flycatcher speciation. This is particularly so given that next-generation sequencing is an excellent method for obtaining data on levels of gene expression; it may very well be that hybrid incompatibilities arise owing to differences in regulatory regions of diverging lineages (Coyne & Orr 2004).
7. Conclusion
We reviewed recent research on hybrid zones between collared and pied flycatchers to illustrate how detailed studies on ecology and mating patterns can be combined with genomic approaches to test central predictions of theoretical models on speciation. The two species of Ficedula flycatchers are (i) diverged in life-history traits, which cause ecologically based selection on nestling hybrids, (ii) sexually isolated through differences in male plumage and song characteristics and corresponding female preferences, and (iii) genetically incompatible in accordance with Haldane's rule (female hybrids are sterile while male hybrids are fertile). The fact that there are reproductive isolation factors, typical for all the three major stages of speciation, opens the possibility of investigating the evolutionary links between these stages. We expect to find such links because the evolution of life-history traits (i.e. components of reproduction and survival) is generally assumed to be closely associated with the evolution of traits under sexual selection (sometimes also viewed as components of investment in reproduction). In addition, evolutionary changes in these traits may give rise to genetic incompatibilities between the two species as a side effect since traits involved in reproduction and survival both evolve fast and are sensitive to incompatibilities (hybrid intrinsic dysfunctions are manifested as sterility and lowered survival). Genomic studies on the identity, function and interaction between genes underlying the different isolation factors open novel possibilities to learn more about such links. Moreover, by pinpointing where in the genome these genes are located, physical prerequisites for the build-up and spread of reproductive isolation throughout the genome can be examined. In Ficedula flycatchers, traits causing pre- and post-zygotic isolation are linked to the Z-chromosome, which is sheltered against interspecific introgression. Speciation in Ficedula flycatchers hence appears to be, at least partly, driven by the evolution of sex-linked genes. One major future aim is to identify these genes.
Acknowledgements
We thank Richard Bailey, Trevor Price, Tomas Pärt, Nina Svedin and Chris Wiley for constructive comments on the manuscript and the Swedish Research Council (A.Q.) and the European Research Foundation (A.Q.) for financial support.
Footnotes
One contribution of 11 to a Theme Issue ‘Genomics of speciation’.
References
- Alatalo R. V., Eriksson D., Gustafsson L., Lundberg A.1990Hybridization between pied and collared flycatchers—sexual selection and speciation theory. J. Evol. Biol. 3, 375–389 (doi:10.1046/j.1420-9101.1990.3050375.x) [Google Scholar]
- Alatalo R. V., Gustafsson L., Lundberg A.1994Male coloration and species recognition in sympatric flycatchers. Proc. R. Soc. Lond. B 256, 113–118 (doi:10.1098/rspb.1994.0057) [Google Scholar]
- Alerstam T., Ebenman B., Sylvén M., Tamm S., Ulfstrand S.1978Hybridization as an agent of competition between two bird allospecies; Ficedula albicollis and F. hypoleuca on the island of Gotland in the Baltic. Oikos 31, 326–331 (doi:10.2307/3543658) [Google Scholar]
- Andersson M.1994Sexual selection Princeton, NJ: Princeton University Press [Google Scholar]
- Arnold M. L.1997Natural hybridization and evolution Oxford, UK: Oxford University Press [Google Scholar]
- Backström N., Qvarnström A., Gustafsson L., Ellegren H.2006aLevels of linkage disequilibrium in a wild bird population. Biol. Lett. 2, 435–438 (doi:10.1098/rsbl.2006.0507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backström N., Brandström M., Gustafsson L., Qvarnström A., Cheng H., Ellegren H.2006bGenetic mapping in a natural population of collared flycatchers (Ficedula albicollis): conserved synteny but gene order rearrangements on the avian Z chromosome. Genetics 174, 377–386 (doi:10.1534/genetics.106.058917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backström N., Karaiskou N., Leder E. H., Gustafsson L., Primmer C. R., Qvarnström A., Ellegren H.2008A gene-based genetic linkage map of the collared flycatcher (Ficedula albicollis) reveals extensive synteny and gene-order conservation during 100 million years of avian evolution. Genetics 179, 1479–1495 (doi:10.1534/genetics.108.088195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A. V., Qvarnström A.2002Putting sexual traits into the context of an organism: a life-history perspective in studies of sexual selection. Auk 119, 301–310 (doi:10.1642/0004-8038(2002)119[0301:PSTITC]2.0.CO;2) [Google Scholar]
- Barton N. H., Hewitt G. M.1985Analysis of hybrid zones. Ann. Rev. Ecol. Syst. 16, 113–148 (doi:10.1146/annurev.es.16.110185.000553) [Google Scholar]
- Beauchamp G.2003Delayed plumage maturation in birds in relation to social foraging and breeding competition. Evol. Ecol. Res. 5, 589–596 [Google Scholar]
- Borge T., Webster M. T., Andersson G., Sætre G. P.2005Contrasting patterns of polymorphism and divergence on the Z chromosome and autosomes in two Ficedula flycatcher species. Genetics 171, 1861–1873 (doi:10.1534/genetics.105.045120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation MA, USA: Sinauer Associates [Google Scholar]
- Dale S., Slagsvold T.1996Mate choice on multiple cues, decision rules and sampling strategies in female pied flycatchers. Behaviour 133, 903–944 (doi:10.1163/156853996X00305) [Google Scholar]
- Dieckmann U., Doebeli M.1999On the origin of species by sympatric speciation. Nature 400, 354–357 (doi:10.1038/22521) [DOI] [PubMed] [Google Scholar]
- Dieckmann U., Doebeli M., Metz J. A., Tautz D.2004Adaptive speciation. Cambridge, UK: Cambridge University Press [Google Scholar]
- Dobzhansky T.1940Speciation as a stage in evolutionary divergence. Am. Nat. 74, 312–321 (doi:10.1086/280899) [Google Scholar]
- Dod B., Jermin L. S., Boursot P., Chapman V. H., Tonnes-Nielsen J., Bonhomme F.1993Counterselection on sex chromosomes in the Mus musculus European hybrid zone. J. Evol. Biol. 6, 529–546 (doi:10.1046/j.1420-9101.1993.6040529.x) [Google Scholar]
- Ellegren H.1992Polymerase-chain-reaction (PCR) analysis of microsatellites—a new approach to studies of genetic-relationships in birds. Auk 109, 886–895 [Google Scholar]
- Ellegren H.2008Sequencing goes 454 and takes large-scale genomics into the wild. Mol. Ecol. 17, 1629–1631 (doi:10.1111/j.1365-294X.2008.03699.x) [DOI] [PubMed] [Google Scholar]
- Ellegren H., Sheldon B. C.2008Genetic basis of fitness differences in natural populations. Nature 452, 169–175 (doi:10.1038/nature06737) [DOI] [PubMed] [Google Scholar]
- Ellegren H., Lifjeld J. T., Slagsvold T., Primmer C. R.1995Handicapped males and extrapair paternity in pied flycatchers: a study using microsatellite markers. Mol. Ecol. 4, 739–744 (doi:10.1111/j.1365-294X.1995.tb00274.x) [Google Scholar]
- Ellegren H., Gustafsson L., Sheldon B. C.1996Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. Proc. Natl Acad. Sci. USA 93, 11 723–11 728 (doi:10.1073/pnas.93.21.11723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson D., Wallin L.1986Male bird song attracts females—a field experiment. Behav. Ecol. Sociobiol. 19, 297–299 (doi:10.1007/BF00300645) [Google Scholar]
- Felsenstein J.1981Skepticism towards Santa Rosalia, or why are there so few kinds of animals? Evolution 35, 124–138 (doi:10.2307/2407946) [DOI] [PubMed] [Google Scholar]
- Galef B. G., Laland K. N.2005Social learning in animals: empirical studies and theoretical models. Bioscience 55, 489–499 (doi:10.1641/0006-3568(2005)055[0489:SLIAES]2.0.CO;2) [Google Scholar]
- Gavrilets S.2004Fitness landscapes and the origin of species Princeton, NJ: Princeton University Press [Google Scholar]
- Gelter H. P.1987Song differences between the pied flycatcher Ficedula hypoleuca, the collared flycatcher Ficedula albicollis, and their hybrids. Ornis. Scand. 18, 205–215 (doi:10.2307/3676768) [Google Scholar]
- Gelter H. P., Tegelström H.1992High-frequency of extra-pair paternity in Swedish pied flycatchers revealed by allozyme electrophoresis and DNA fingerprinting. Behav. Ecol. Sociobiol. 31, 1–7 (doi:10.1007/BF00167810) [Google Scholar]
- Gustafsson L.1989Lifetime reproductive success in the collared flycatcher. In Lifetime reproductive success in birds (ed. Newton I.), pp. 75–88 London, UK: Academic Press [Google Scholar]
- Gustafsson G., Pärt T.1991Interspecific relations between collared and pied flycatchers. Proc. Int. Orn. Con. 20, 1425–1431 [Google Scholar]
- Gustafsson L., Qvarnström A.2006A test of the ‘sexy son’ hypothesis: sons of polygynous collared flycatchers do not inherit their fathers' mating status. Am. Nat. 167, 297–302 (doi:10.1086/498623) [DOI] [PubMed] [Google Scholar]
- Gustafsson L., Qvarnström A., Sheldon B. C.1995A trade-off between a life-history and a secondary sexual trait. Nature 375, 311–313 (doi:10.1038/375311a0) [Google Scholar]
- Haavie J., Borge T., Bures S., Garamszegi L. Z., Lampe H. M., Moreno J., Qvarnström A., Torok J., Sætre P.2004Flycatcher song in allopatry and sympatry—convergence, divergence and reinforcement. J. Evol. Biol. 17, 227–237 (doi:10.1111/j.1420-9101.2003.00682.x) [DOI] [PubMed] [Google Scholar]
- Harrison R. G., Rand D. M.1989Mosaic hybrid zones and the nature of species boundaries. In Speciation and its consequences (eds Otto D., Endler J. A.), pp. 111–133 Sunderland, UK: Sinauer [Google Scholar]
- Kirkpatrick M., Hall D. W.2004Male-biased mutation, sex linage, and the rate of adaptive evolution. Evolution 58, 437–440 [PubMed] [Google Scholar]
- Kokko H., Jennions M. D., Brooks R.2006Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 37, 43–66 (doi:10.1146/annurev.ecolsys.37.091305.110259) [Google Scholar]
- Kulma K., Bensch S., Qvarnström A.Submitted Malaria infections and heterospecific competition in Ficedula flycatchers. [Google Scholar]
- Künstner A., Wolf J. B. W., Backström N., Whitney O., Wilson R. K., Jarvis E. D., Warren W. C., Ellegren H.2010Comparative genomics based on massive parallel transcriptome sequencing reveals patterns of substitution and selection across 10 bird species. Mol. Ecol. 19, 266–276 (doi:10.1111/j.1365-294X.2009.04487.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem K. F.1973Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Syst. Zool. 22, 425–441 (doi:10.2307/2412950) [Google Scholar]
- Lundberg A., Alatalo R. V.1992The pied flycatcher London, UK: Poyser [Google Scholar]
- Macholán M., Munclinger P., Sugerková M., Dufková P., Bímová B., Bozíková E., Zima J., Piálek J.2007Genetic analysis of autosomal and X-linked markers across a mouse hybrid zone. Evolution 61, 746–771 (doi:10.1111/j.1558-5646.2007.00065.x) [DOI] [PubMed] [Google Scholar]
- Mayr E.1963Animal species and evolution Cambridge, UK: Belknap Press [Google Scholar]
- Mendelson T. C.2003Sexual isolation evolves faster than hybrid inviability in a diverse and sexually dimorphic genus of fish (Percidae: Etheostoma). Evolution 57, 317–327 [DOI] [PubMed] [Google Scholar]
- Merilä J., Björklund M., Gustafsson L.1994Evolution of morphological differences with moderate genetic correlations among traits as exemplified by two flycatcher species (Ficeduala; Muscicapidae). Biol. J. Linn. Soc. 52, 19–30 [Google Scholar]
- Merilä J., Sheldon B. C., Ellegren H.1998Quantitative genetics of sexual size dimorphism in the collared flycatcher, Ficedula albicollis. Evolution 52, 870–876 (doi:10.2307/2411281) [DOI] [PubMed] [Google Scholar]
- Muller H. J.1940Bearings of the Drosophila work on systematics. In The new systematics (ed. Huxley J.), pp. 185–268 Oxford, UK: Clarendon Press [Google Scholar]
- Nosil P., Harmon L. J., Seehausen O.2009Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 24, 145–156 (doi:10.1016/j.tree.2008.10.011) [DOI] [PubMed] [Google Scholar]
- Panhuis T. M., Butlin R., Zuk M., Tregenza T.2001Sexual selection and speciation. Trends Ecol. Evol. 16, 364–371 (doi:10.1016/S0169-5347(01)02160-7) [DOI] [PubMed] [Google Scholar]
- Pärt T., Qvarnström A.1997Badge size in collared flycatchers predicts outcome of male competition over territories. Anim. Behav. 54, 893–899 (doi:10.1006/anbe.1997.0514) [DOI] [PubMed] [Google Scholar]
- Price T.1987Diet variation in a population of Darwin's finches. Ecology 68, 1015–1028 (doi:10.2307/1938373) [Google Scholar]
- Price T.1998Sexual selection and natural selection in bird speciation. Phil. Trans. R. Soc. Lond. B 353, 251–260 (doi:10.1098/rstb.1998.0207) [Google Scholar]
- Price T. D.2008Speciation in birds Greenwood Village, CO: Roberts & Company [Google Scholar]
- Price T. D.2010The roles of time and ecology in the continental radiation of the Old World leaf warblers (Phylloscopus and Seicercus). Phil. Trans. R. Soc. B 365, 1749–1762 (doi:10.1098/rstb.2009.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T. D., Bouvier M. M.2002The evolution of F1 postzygotic incompatibilities in birds. Evolution 56, 2083–2089 [PubMed] [Google Scholar]
- Qvarnström A.1997Experimentally enlarged badge size increases male competition and reduces male parental care in the collared flycatcher. Proc. R. Soc. Lond. B 264, 1225–1231 (doi:10.1098/rspb.1997.0169) [Google Scholar]
- Qvarnström A., Bailey R. I.2009Speciation through evolution of sex-linked genes. Heredity 102, 2–15 [DOI] [PubMed] [Google Scholar]
- Qvarnström A., Blomgren V., Wiley C., Svedin N.2004Female collared flycatchers learn to prefer males with an artificial novel ornament. Behav. Ecol. 15, 543–548 (doi:10.1093/beheco/arh043) [Google Scholar]
- Qvarnström A., Svedin N., Wiley C., Veen T., Gustafsson L.2005Cross-fostering reveals seasonal changes in the relative fitness of two competing species of flycatchers. Biol. Lett. 1, 68–71 (doi:10.1098/rsbl.2004.0265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnström A., Haavie J., Saether S. A., Eriksson D., Pärt T.2006aSong similarity predicts hybridization in flycatchers. J. Evol. Biol. 19, 1202–1209 (doi:10.1111/j.1420-9101.2006.01140.x) [DOI] [PubMed] [Google Scholar]
- Qvarnström A., Brommer J. E., Gustafsson L.2006bTesting the genetics underlying the co-evolution of mate choice and an ornament in the wild. Nature 441, 84–86 (doi:10.1038/nature04564) [DOI] [PubMed] [Google Scholar]
- Qvarnström A., Vogel Kehlenbeck J., Wiley C., Svedin N., Sæther S. A.2007Species divergence in offspring begging intensity—difference in need or manipulation of parents? Proc. R. Soc. B 274, 1003–1008 (doi:10.1098/rspb.2006.0255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnström A., Wiley C., Svedin N., Vallin N.2009Life-history divergence facilitates regional coexistence of competing Ficedula flycatchers. Ecology 90, 1948–1957 [DOI] [PubMed] [Google Scholar]
- Ratti O., Hovi M., Lundberg A.1995Extra-pair paternity and male characteristics in the pied flycatcher. Behav. Ecol. Sociobiol. 37, 419–425 (doi:10.1007/BF00170590) [Google Scholar]
- Raufaste N., Orth A., Belkhir K., Senet D., Smadja C., Baird S. J. E., Bonhomme F., Dod B., Boursot P.2005Inferences of selection and migration in the Danish house mouse hybrid zone. Biol. J. Linn. Soc. 84, 593–616 (doi:10.1111/j.1095-8312.2005.00457.x) [Google Scholar]
- Reeve H. K., Pfennig D. W.2003Genetic biases for showy males: are some genetic systems especially conductive to sexual selection? Proc. Natl Acad. Sci. USA 100, 1089–1094 (doi:10.1073/pnas.0337427100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. G.2007Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 38, 79–102 (doi:10.1146/annurev.ecolsys.38.091206.095733) [Google Scholar]
- Sæther S. A., et al. 2007Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318, 95–97 (doi:10.1126/science.1141506) [DOI] [PubMed] [Google Scholar]
- Sætre G.-P., Kral M., Bicik V.1993Experimental evidence for interspecific female mimicry in sympatric Ficedula flycatchers. Evolution 47, 939–945 (doi:10.2307/2410197) [DOI] [PubMed] [Google Scholar]
- Sætre G.-P., Moum T., Bures S., Král M., Adamjan M., Moreno J.1997aA sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387, 589–592 (doi:10.1038/42451) [Google Scholar]
- Sætre G.-P., Král M., Bures S.1997bDifferential species recognition abilities of males and females in a flycatcher hybrid zone. J. Avian Biol. 28, 259–263 (doi:10.2307/3676978) [Google Scholar]
- Sætre G.-P., Posty E., Král M.1999Can environmental fluctuations prevent competitive exclusion in sympatric flycatchers? Proc. R. Soc. Lond. B 266, 1247–1251 (doi:10.1098/rspb.1999.0770) [Google Scholar]
- Sætre G.-P., Borge T., Lindell J., Moum T., Primmer C. R., Sheldom B. C., Haavie J., Johnson A., Ellegren H.2001Speciation, introgressive hybridization and nonlinear rate of molecular evolution on flycatchers. Mol. Ecol. 10, 737–749 (doi:10.1046/j.1365-294x.2001.01208.x) [DOI] [PubMed] [Google Scholar]
- Sætre G.-P., Borge T., Lindroos K., Haavie J., Sheldon B. C., Primmer C., Syvänen C.2003Sex chromosome evolution and speciation in Ficedula flycatchers. Proc. R. Soc. Lond. B 270, 53–59 (doi:10.1098/rspb.2002.2204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D.2000The ecology of adaptive radiation. New York, NY: Oxford University Press [Google Scholar]
- Schluter D., Grant P. R.1984Ecological correlates of morphological evolution in a Darwin's finch Geospiza difficilis. Evolution 38, 856–869 (doi:10.2307/2408396) [DOI] [PubMed] [Google Scholar]
- Seehausen O., Takimoto G., Roy D., Jokela J.2008Speciation reversal and biodiversity dynamics with hybridization in changing environments. Mol. Ecol. 17, 30–44 (doi:10.1111/j.1365-294X.2007.03529.x) [DOI] [PubMed] [Google Scholar]
- Servedio M. R., Noor M. A. F.2003The role of reinforcement in speciation: theory and data meet. Annu. Rev. Ecol. Syst. 34, 339–364 (doi:10.1146/annurev.ecolsys.34.011802.132412) [Google Scholar]
- Servedio M. R., Sæther S. A., Sætre P.2009Reinforcement and learning. Evol. Ecol. 23, 109–123 (doi:10.1007/s10682-007-9188-2) [Google Scholar]
- Sheldon B. C., Ellegren H.1996Offspring sex and paternity in the collared flycatcher. Proc. R. Soc. Lond. B 263, 1017–1021 (doi:10.1098/rspb.1996.0150) [Google Scholar]
- Sheldon B. C., Ellegren H.1998Sexual selection resulting from extra-pair paternity in collared flycatchers. Anim. Behav. 57, 285–298 (doi:10.1006/anbe.1998.0968) [DOI] [PubMed] [Google Scholar]
- Sheldon B. C., Ellegren H.1999Paternal effort related to experimentally manipulated paternity of male collared flycatchers. Proc. R. Soc. Lond. B 265, 1737–1742 (doi:10.1098/rspb.1998.0496) [Google Scholar]
- Slagsvold T., Sætre G. P.1991Evolution of plumage colour in male pied flycatchers (Ficedula hypoleuca)—evidence for female mimicry. Evolution 45, 910–917 (doi:10.2307/2409698) [DOI] [PubMed] [Google Scholar]
- Svedin N., Wiley C., Veen T., Gustafsson L., Qvarnström A.2008Natural and sexual selection against hybrid flycatchers. Proc. R. Soc. B 275, 735–744 (doi:10.1098/rspb.2007.0967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedin N., Edström B., Wiley C., Qvarnström A.Submitted Relative frequency of two hybridizing Ficedula flycatchers predicts their song similarity. [Google Scholar]
- Svensson L.1992Identification guide to European passerines, 4th edn Stockholm, Sweden: Märstatryck [Google Scholar]
- Svensson E., Sheldon B. C.1998The social context of life history evolution. Oikos 83, 466–477 (doi:10.2307/3546674) [Google Scholar]
- Teeter K. C., Payseur B. A., Harris L. W., Bakewell M. A., ÓBrien J. E., Sans-Fuentes M. A., Nachman M. W., Tucker P. K.2008Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Res. 18, 67–76 (doi:10.1101/gr.6757907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. K., Sage R. D., Warner J., Wilson A. C., Eicher E. M.1992Abrupt cline for sex chromosomes in a hybrid zone between two species of mice. Evolution 46, 1146–1163 (doi:10.2307/2409762) [DOI] [PubMed] [Google Scholar]
- Vallin N., Feng J., Thörngren H., Qvarnström A.Submitted Life history divergence and relative fitness of nestling Ficedula hybrids across environmental conditions. [Google Scholar]
- Veen T., Borge T., Griffith S. C., Sætre G.-P., Bures S., Gustafsson L., Sheldon B. C.2001Hybridization and adaptive mate choice in flycatchers. Nature 411, 45–50 (doi:10.1038/35075000) [DOI] [PubMed] [Google Scholar]
- Veen T., Svedin N., Forsman J., Hjernquist M., Qvarnström A., Hjernquist T., Träff J., Klaasen M.2007Does migration of hybrids contribute to post-zygotic isolation in flycatchers? Proc. R. Soc. B 274, 707–712 (doi:10.1098/rspb.2006.0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen T., Sheldon B. C., Klaassen M., Weissing F. J., Qvarnström A., Sætre P.In press Temporal differences in food abundance promotes coexistence between two congeneric passerines. Oecologia [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Wheat C. W., Fescemyer H. W., Frilander M. J., Crawford D. L., Hanski I., Marden J. H.2008Rapid transcriptome characterization for nonmodel organism using 454 pyrosequencing. Mol. Ecol. 17, 1636–1647 (doi:10.1111/j.1365-294X.2008.03666.x) [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J.1983Sexual selection, social competition, and speciation. Quart. Rev. Biol. 58, 155–183 (doi:10.1086/413215) [Google Scholar]
- Wiley C., Bengtson J. M., Svedin N., Qvarnström A.2005Hybridization cost of delayed maturation of secondary sexual traits in the collared flycatcher. Evolution 59, 2311–2316 [PubMed] [Google Scholar]
- Wiley C., Fogelberg N., Sæther S. A., Veen T., Svedin N., Vogel Kehlenbeck J., Qvarsntröm A.2007Direct benefits and costs for hybridizing Ficedula flycatchers. J. Evol. Biol. 20, 854–864 (doi:10.1111/j.1420-9101.2007.01316.x) [DOI] [PubMed] [Google Scholar]
- Wiley C., Qvarnström A., Gustafsson L.2009aEffects of hybridization on the immunity of collared (Ficedula albicollis) and pied (F. hypoleuca) flycatchers, and their infection by haemosporidians. J. Avian Biol. 40, 352–357 (doi:10.1111/j.1600-048X.2009.04741.x) [Google Scholar]
- Wiley C., Qvarnström A., Andersson G., Borge T., Sætre P.2009bPostzygotic isolation over multiple generations of hybrid descendents in a natural hybrid zone: how well do single-generation estimates reflect reproductive isolation? Evolution 63, 1731–1739 (doi:10.1111/j.1558-5646.2009.00674.x) [DOI] [PubMed] [Google Scholar]