Abstract
Plant ecologists have proposed a variety of optimization theories to explain the adaptive behaviour and evolution of plants from the perspective of natural selection (‘survival of the fittest’). Optimization theories identify some objective function—such as shoot or canopy photosynthesis, or growth rate—which is maximized with respect to one or more plant functional traits. However, the link between these objective functions and individual plant fitness is seldom quantified and there remains some uncertainty about the most appropriate choice of objective function to use. Here, plants are viewed from an alternative thermodynamic perspective, as members of a wider class of non-equilibrium systems for which maximum entropy production (MEP) has been proposed as a common theoretical principle. I show how MEP unifies different plant optimization theories that have been proposed previously on the basis of ad hoc measures of individual fitness—the different objective functions of these theories emerge as examples of entropy production on different spatio-temporal scales. The proposed statistical explanation of MEP, that states of MEP are by far the most probable ones, suggests a new and extended paradigm for biological evolution—‘survival of the likeliest’—which applies from biomacromolecules to ecosystems, not just to individuals.
Keywords: entropy production, natural selection, optimization, plants
1. Introduction
Just as large-scale, parameter-intensive global circulation models currently dominate modelling of climate dynamics, modelling of plant and terrestrial ecosystem dynamics is currently dominated by complex, numerical simulation models that attempt to represent explicitly the many physical, successional and biogeochemical processes governing plant and ecosystem function. To some extent, this approach reflects the demand from the global change research community for land-surface models that operate over a wide range of vegetation types, environments and time scales.
In this ‘bottom-up’ approach, plausible assumptions are introduced about each process for each plant type, requiring typically hundreds of parameters to be specified, few of which are identifiable from available data (e.g. Wang et al. 2001). These processes are then coupled together in various ways, leading to a wide range of model structures. Crucially, when it comes to modelling the adaptive responses of plants to global change—e.g. the responses of stomatal conductance, plant nitrogen content, leaf biomass and leaf–root growth allocation to changes in CO2, nitrogen and water availability—complex models generally offer no explanation of those responses; they are usually represented empirically, if at all. Consequently, uncertainties in model parameter values, differences among model structures and the empirical treatment or omission of key adaptive plant processes have led to a great divergence in the predicted responses of complex vegetation models to elevated [CO2] (e.g. Cramer et al. 2001), nitrogen (N) enrichment (e.g. Levy et al. 2004) and combined changes in [CO2], precipitation and temperature (e.g. Luo et al. 2008).
Theories of optimal plant function offer an alternative ‘top-down’ approach to modelling in plant ecology (e.g. Givnish 1986; Kull 2002; Mäkelä et al. 2002; Dewar et al. 2009; Schymanski et al. 2009). Optimization models identify an apparent goal or objective function F that is maximized with respect to one or more plant functional traits f. The maximization of F is usually subjected to one or more physiological or environmental constraints C. The advantage of this approach is that it avoids the need for an explicit sub-model for f with its attendant parameters; instead, f is simply determined by the optimality condition that F is stationary with respect to variations in f permitted by the constraints C.
Functional traits to which the optimization approach has been applied include stomatal conductance (e.g. Cowan & Farquhar 1977), leaf and canopy N content (e.g. Dewar 1996; Haxeltine & Prentice 1996), shoot/root biomass ratio (e.g. Reynolds & Thornley 1982), N allocation within canopies (e.g. Field 1983), allocation between height and diameter growth in trees (Mäkelä & Sievänen 1992) and leaf-area index (e.g. McMurtrie 1985; Franklin & Ågren 2002). Unlike complex vegetation models, optimization models explain—not only qualitatively but also quantitatively—many of the plant trait responses to changes in CO2, N and water supply observed in multiple-resource manipulation experiments and other empirical studies as consequences of the maximization of various objective functions (e.g. Dewar et al. 2009). While this is encouraging, optimization models have yet to be adopted as mainstream modelling tools in global change research.
One reason for this might be because we do not yet have an unambiguous answer to the key question: What do plants maximize? Often the objective function F is proposed as some proxy for individual fitness—such as shoot or canopy photosynthesis, net primary productivity or net growth rate (e.g. Dewar 1996; Dewar et al. 1998; Ackerly 1999; Hikosaka 2003; Anten 2005; Franklin 2007; Mäkelä et al. 2008; McMurtrie et al. 2008; Dewar et al. 2009)—although the link with individual fitness is seldom quantified and usually only a verbal justification of F is offered. In particular, if natural selection operates uniquely at the level of individuals, the use of canopy-scale objective functions (e.g. canopy photosynthesis) may be called into question. As a result, there remains some uncertainty about the most appropriate choice of objective function to use.
Recently, however, an alternative thermodynamic perspective on biological adaptation and evolution has emerged (Dewar 2004; Whitfield 2005, 2007; Martyushev & Seleznev 2006), which identifies a fundamental objective function based on entropy concepts. Within this perspective, living systems are viewed as examples of a wider class of non-equilibrium structures—including non-living systems such as growing crystals and weather cyclones—that import energy in one form and export it in a higher entropy form. The hypothesis of maximum entropy production (MEP) conjectures that these systems self-organize (adapt, evolve) under given constraints so as to maximize this rate of entropic export.
The theoretical basis of MEP remains a subject of open debate (e.g. Dewar 2004, 2009; Martyushev & Seleznev 2006; Bruers 2007; Grinstein & Linsker 2007; Niven 2009). One proposition is that MEP has a statistical explanation (Dewar 2003, 2005, 2009): when a system is forced out of thermodynamic equilibrium, the non-equilibrium state of MEP is selected simply because it is by far the most probable one—that is, the MEP state can be realized microscopically in an overwhelmingly greater number of ways than any other non-equilibrium state. In this sense, MEP is a statistical principle, rather than a physical principle open to experimental falsification (Dewar 2009).
MEP has previously been applied to biological systems, for example, the evolution of biomacromolecules (e.g. Dewar et al. 2006) and food web functioning in detrital-based ecosystems (Meysman & Bruers 2007). In this paper, I apply MEP to plant function. The aim is to demonstrate how MEP unifies and extends various plant optimization theories that have been proposed previously on the basis of ad hoc natural selection arguments (‘survival of the fittest’). Specifically, using simple models of the carbon balance of plants and ecosystems, I show that the different objective functions of these theories emerge as examples of entropy production on different spatio-temporal scales. MEP thus predicts optimal plant behaviour that is reasonable from the perspective of natural selection, but, in addition, offers a novel statistical reinterpretation of that behaviour—‘survival of the likeliest’ (Whitfield 2007)—which extends beyond individual plants to vegetation canopies and whole ecosystems.
The paper is organized as follows. Section 2 outlines the basic formalism for calculating the rate of chemical entropy production in plants and ecosystems (σchem), involving a simplifying approximation that the system is in a quasi-steady state. In §3, MEP is applied successively to three systems that can be considered to be in a quasi-steady state on different time scales. In each case, the expression for σchem is related to the objective functions of previously proposed plant optimization theories, and a brief illustration is given of how MEP can lead to realistic optimal plant function. Section 4 presents some conclusions.
2. Chemical entropy production by plants and ecosystems
The instantaneous rate of chemical entropy production (σchem, J K−1 s−1) of a system (e.g. a plant or ecosystem) within a prescribed volume V is given by (e.g. Dewar 2003)
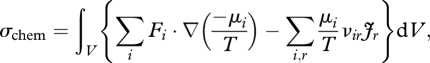 |
2.1 |
where Fi (a vector) is the molar flux density (mol m−2 s−1) of chemical species i, μi (J mol−1) is the chemical potential of species i, T (K) is the temperature, νir is the stoichiometric coefficient of species i in reaction r (positive for products and negative for reactants) and Jr (mol m−3 s−1) is the rate of reaction r per unit volume. In general, Fi, μi, T and Jr are functions of space and time. The first term in curly brackets is the local rate of entropy production due to mass flow across chemical gradients (i.e. gradients in −μi/T); the second term is the local rate of entropy production due to chemical reactions.
The local mass balance of chemical species i is described by the continuity equation
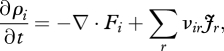 |
2.2 |
where ρi (mol m−3) is the molar density. When the system is in a steady state (∂ρi/∂t = 0), equation (2.2) gives 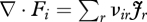 and equation (2.1) then simplifies to
and equation (2.1) then simplifies to
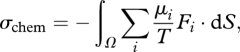 |
2.3 |
where Ω is the system boundary, and dS is the local surface element (a vector of magnitude |dS| pointing in the direction outwardly normal to the surface). Equation (2.3) only involves contributions from species i whose mass flux across the boundary (Fi) is non-zero, and σchem may be interpreted as the rate of entropy export by those boundary fluxes.
In §3, I focus on the chemical entropy export from plants and ecosystems associated with three carbon species—photosynthates (sugars), carbon dioxide (CO2) and structural biomass (proteins, cellulose, etc.). I ignore other potential contributions to σchem in plants (e.g. due to radiative exchange, plant water transport, evaporation of liquid water, etc.). I consider the maximization of σchem on different time scales. At each time scale, I identify a system of ‘fast’ carbon pools that can be considered to be in an approximate steady state on that time scale, i.e. the net carbon exchange between the ‘fast’ system and its environment is approximately zero. This approximation is useful because then σchem can be approximated by equation (2.3) in which Ω is the boundary of the ‘fast’ system. I will assume for simplicity that T is a constant in equation (2.3) (isothermal boundary conditions).
3. Maximum chemical entropy production at different scales
(a). MEP applied to plant photosynthates and CO2
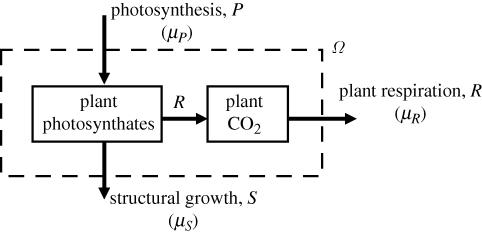
Figure 1 schematically depicts the carbon balance of plant photosynthates (CH2O) and CO2. This system may be considered to be in an approximate steady state on a time scale of the order of 1 year (i.e. approximately zero net annual accumulation of CH2O and CO2).
Figure 1.
Carbon balance of plant photosynthates (CH2O) and CO2. The dashed box indicates the system boundary Ω. The internal distributions of CH2O and CO2 within Ω are not represented. Plant structural biomass (protein, cellulose, etc.) lies outside Ω. The direction of the arrows indicates the sense in which fluxes are taken to be positive: P, photosynthesis; R, respiration for plant maintenance and growth; S, conversion of photosynthate carbon to new plant structure. Values of chemical potentials refer to the boundary Ω: μP, chemical potential of source photosynthate; μS, chemical potential of photosynthate at the sites of growth (sinks); μR, chemical potential of respired CO2. In the steady state, R = P − S.
The input flux is identified with the end products of photosynthesis (P). Some of the photosynthate is converted to CO2 during plant respiration (R) and exported to the environment; the remainder is incorporated into various carbon products (proteins, cellulose, etc.) during structural growth (S). For each carbon flux across the system boundary, the chemical potential of the associated carbon species is indicated: μP, chemical potential of source photosynthate; μS, chemical potential of photosynthate at the sites of growth (sinks); μR, chemical potential of respired CO2.
Applying equation (2.3) (noting the minus sign) to the system in figure 1 (noting the flux sign convention) gives
| 3.1 |
Substituting the steady-state flux relation R = P − S into equation (3.1) then gives
| 3.2 |
If one assumes that photosynthate is an ideal solute, then μP = μref + RGT ln(ρP/ρref) and μS = μref + RGT ln(ρS/ρref), where RG is the universal gas constant, ρP and ρS are the source and sink photosynthate concentrations, and μref and ρref are the chemical potential and concentration of photosynthate in some reference state. Similarly, for CO2 one may assume μR = μR,ref + RGT ln(ρR/ρR,ref). The chemical potentials μP, μS and μR will vary in time to some degree due to variations in ρP, ρS and ρR (which depend on the fluxes P, S and R). As a first approximation, I will ignore these variations and treat μP, μS and μR as fixed parameters. Then, from equation (3.2) and recalling that T is also assumed to be constant, we have
| 3.3 |
where
| 3.4 |
is a constant. According to the Münch hypothesis of phloem transport (e.g. Christy & Ferrier 1973), the internal movement of photosynthates between sources and sinks—represented, respectively, by the upper and lower system boundaries in figure 1—occurs from high to low concentrations (i.e. ρP ≥ ρS), implying μP ≥ μS. Also, we have μS ≥ μR since respiration involves the dissipation of high-quality substrates (CH2O) to low-quality products (CO2). Therefore, μP ≥ μS ≥ μR and so, from equation (3.4), 0 ≤ λC ≤ 1. Note that the second law of thermodynamics (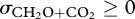 ) is satisfied so long as R ≥ 0 (since R = P − S ≥ 0 and λC ≤ 1 imply P ≥ S ≥ λCS).
) is satisfied so long as R ≥ 0 (since R = P − S ≥ 0 and λC ≤ 1 imply P ≥ S ≥ λCS).
When λC = 0, equation (3.3) implies that MEP is equivalent to maximizing P, which is a realistic goal from the perspective of natural selection; however, this lower limit is physiologically and thermodynamically unrealistic (μS = μR). The upper limit λC = 1 (i.e. μP = μS) is possibly a reasonable approximation for small plants (small internal gradients in CH2O concentration). In this case, MEP is equivalent to maximizing plant respiration, R = P − S. At first sight, maximizing R might seem a less obvious goal for plant survival than maximizing P because R is often viewed negatively (especially by modellers) as a carbon ‘cost’ for plant growth (since S = P − R). This view neglects the fact that R drives all the metabolic processes that are crucial to plant function and survival (including growth, S) so that maximizing R is reasonable on fitness grounds. Moreover, in the steady state, maximizing R (= P − S) cannot be sustained without also maximizing P, because photosynthesis provides the substrate for respiration. Consideration of the hypothetical limiting cases λC = 0 and λC = 1 therefore suggests that, across the entire range 0 ≤ λC ≤ 1, maximization of σCH2O + CO2 (equation (3.3)) is reasonable from the perspective of natural selection.
To explore this suggestion in more detail, I now consider the biological implications of maximizing σCH2O+CO2 ∝ P − λCS for the more realistic intermediate values 0 < λC < 1. In general, P is a saturating function of plant light absorption (I) and plant nitrogen content (N) (e.g. Dewar 1996), while S is more nearly proportional to N (e.g. Ågren & Franklin 2003). It follows that there is an optimal value of N which maximizes P − λCS; as N increases, the ‘benefit’ of increased P is eventually offset by the ‘cost’ of increased λCS. It should be remembered, however, that the benefit and cost here are being interpreted in terms of chemical entropy production (=entropy export) rather than carbon gain.
As a simple example, let us assume the rectangular hyperbolic relationship P = hαIkN/(αI + kN) (Dewar 1996) and the linear relationship S = gN (I, plant light absorption; N, plant nitrogen content; h, daylength; α, quantum yield; k, carboxylation coefficient; g, nitrogen growth efficiency). Maximization of P − λCS then predicts that the optimal plant nitrogen content is
| 3.5 |
where 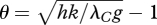 . The optimal rate of structural growth is then predicted to be directly proportional to plant light interception, SMEP = ɛSI, where ɛS = αgθ/k can be interpreted as the plant growth ‘light-use efficiency’. A linear relationship between plant growth rate and light absorption has been observed empirically over a wide range of different plant types (e.g. Dewar 1996 and references therein). The above equations imply that a similar result also applies to photosynthesis itself: PMEP = ɛPI, where ɛP = αhθ/(1 + θ) is the photosynthetic light-use efficiency—I will use this result in §3b.
. The optimal rate of structural growth is then predicted to be directly proportional to plant light interception, SMEP = ɛSI, where ɛS = αgθ/k can be interpreted as the plant growth ‘light-use efficiency’. A linear relationship between plant growth rate and light absorption has been observed empirically over a wide range of different plant types (e.g. Dewar 1996 and references therein). The above equations imply that a similar result also applies to photosynthesis itself: PMEP = ɛPI, where ɛP = αhθ/(1 + θ) is the photosynthetic light-use efficiency—I will use this result in §3b.
The nitrogen-based trade-off here between P and S is mathematically equivalent to the nitrogen-based trade-off between P and maintenance respiration proposed previously under the assumption that plants maximize their net primary productivity (Dewar 1996; Haxeltine & Prentice 1996). Only a verbal justification for that assumption was given—it seems reasonable for plant survival. Here, this trade-off is given a novel thermodynamic interpretation—it is the result of MEP applied to plant photosynthates and CO2.
(b). MEP applied to whole plants
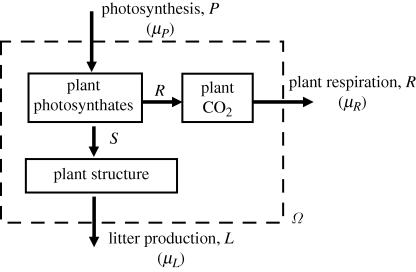
Figure 2 depicts the carbon balance of whole plants. Here, the system boundary (Ω) in figure 1 has been extended to include plant structure. This extended system may be considered to be in an approximate steady state on a time scale of 1–10 years (i.e. of the order of the lifetime of plant structural biomass, which depends on plant type). Structural growth S is now an internal flux; litter production (L) takes the place of S as an external flux, and the associated chemical potential is μL.
Figure 2.
Whole-plant carbon balance. Notation as in figure 1, except that the system boundary (Ω) has been extended to include plant structure (proteins, cellulose, etc.). L, plant litter production; μL, chemical potential of plant litter. In the steady state, R = P − L.
Analogous to equation (3.1), the plant entropy export rate is given by
| 3.6 |
Substituting the steady-state flux relation R = P − L into equation (3.6) then gives a result analogous to equation (3.3)
| 3.7 |
where
| 3.8 |
Here, again, I have ignored variations in the chemical potentials μP, μL and μR, and the temperature T. As before, I assume that μP ≥ μR (CH2O → CO2 representing dissipation of chemical free energy), and also μL ≥ μP (plant structure being more reduced than sugars), so that λP ≥ 1. Then, the second law of thermodynamics (σplant ≥ 0) is satisfied so long as R ≥ (λP − 1)L (since R = P − L ≥ (λP − 1)L implies P ≥ λPL); this condition reflects the fact that structural growth is an active process (i.e. μL ≥ μP) that is driven by the free energy generated by respiration (in the form of non-equilibrium ATP/ADP and NADPH/NADP ratios).
The chemical potentials μP and μR may be modelled as before in terms of the respective concentrations of CH2O and CO2 on the system boundary Ω. The chemical potential of litter (μL) is well defined theoretically as a function of its chemical composition by μL = ∑kνkμk (νk and μk being, respectively, the fraction and chemical potential of component k). However, the practical determination of μL remains challenging due to the compositional complexity of biomass (e.g. Meysman & Bruers 2007 and references therein); for the purposes of this study, I simply assume that μL is a given constant.
I now consider a simple example of the application of MEP to equation (3.7), which leads to the prediction of an optimal leaf biomass Bl. We have already seen from the maximization of σCH2O+CO2 on shorter time scales (§3a) that plant photosynthesis at the optimal nitrogen content is given by P = ɛPI, where ɛP is the photosynthetic light-use efficiency and I is plant light absorption. In general, I is a saturating function of Bl due to the effect of leaf mutual shading; a simple model of this is given by the Beer–Lambert law I = Iin(1 − e−ksBl), where Iin is the incident radiation at the top of the canopy, s is the leaf area per unit leaf biomass (inversely related to leaf thickness) and k (a function of leaf orientation and clumping) describes the exponential extinction of light within the canopy. In contrast, plant litter production L is more appropriately modelled as a linear function of each biomass compartment, L = ∑j mjBj, where mj is the specific mortality rate of biomass compartment j.
With reference to equation (3.7), as Bl increases (all other Bj being held fixed), the entropic ‘benefit’ of increased P (via increased light absorption I) is eventually offset by the entropic ‘cost’ of increased λPL. The optimal leaf biomass that maximizes σplant is easily calculated as
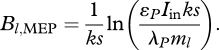 |
3.9 |
The optimal rate of photosynthesis is then found to be PMEP = ɛPIin − λPml/ks.
The interpretation of observed leaf biomass in terms of an optimal trade-off between canopy photosynthesis and leaf litter production has been proposed previously (e.g. McMurtrie 1985 and references therein). The objective function given by equation (3.7) is also mathematically similar to that adopted by Franklin (2007). In either case, only a verbal justification for the choice of objective function was given. Here, the use of equation (3.7) as an objective function for plant optimization models is given a new thermodynamic interpretation—it is the result of MEP applied at the whole-plant scale.
(c). MEP applied to ecosystems
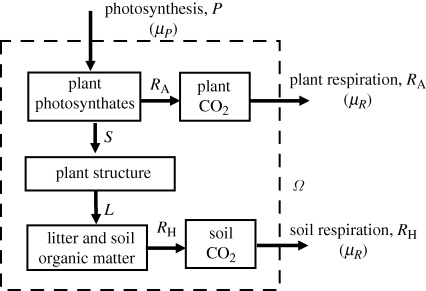
Figure 3 depicts the carbon balance of an ecosystem. At this scale, the system boundary now includes litter and soil organic carbon. This system may be considered to be in an approximate steady state on a time scale of the order of 10–100 years (i.e. the residence time of carbon in litter and soil organic matter).
Figure 3.
Ecosystem carbon balance. Notation as in figure 2, except that the system boundary (Ω) has been extended to include litter and soil organic matter. RA, autotrophic (plant) respiration; RH, heterotrophic (soil) respiration. In the steady state, RA + RH = P.
The ecosystem entropy export rate is (notation as in figure 3)
| 3.10 |
Substituting the steady-state flux relation RA + RH = P into equation (3.10) then yields (cf. equations (3.3) and (3.7))
| 3.11 |
where again I have assumed that μP, μR and T are fixed. At the ecosystem scale, therefore, the steady-state chemical entropy export is associated with the overall dissipative reaction that converts photosynthates (CH2O) to CO2 (μP ≥ μR), the second law (σecosystem ≥ 0) is automatically satisfied since P ≥ 0 and MEP is equivalent to maximizing canopy photosynthesis. Maximization of canopy photosynthesis has been proposed previously as a plant optimization goal (e.g. Field 1983; Anten 2005; McMurtrie et al. 2008). MEP provides a thermodynamic justification for maximizing P—it is the result of MEP applied at the ecosystem scale.
The application of optimization theories is traditionally confined to the plant or canopy scales, reflecting the popular view that natural selection acts uniquely at the level of individual organisms. But as the analysis here suggests, MEP provides an alternative thermodynamic interpretation of optimization theories that can be extended beyond individual plants to whole ecosystems.
As a simple example of applying MEP at this scale, recall the result PMEP = ɛPIin − λPml/ks obtained previously by maximizing whole-plant entropy export (§3b). The maximization of P may thus be accomplished in part by maximizing ɛPIin. Kleidon (2004) has demonstrated that a maximum in ɛPIin exists with respect to variations in stomatal conductance, when large-scale vegetation–atmosphere feedbacks are taken into account. Specifically, increasing stomatal conductance leads, on the one hand, to increased plant CO2 uptake (hence increased ɛP) and, on the other hand, to increased transpiration (hence increased cloud cover and reduced Iin at the land surface). Optimization of stomatal conductance was found to predict realistic vegetation–climate states (Kleidon 2004).
We may envisage MEP (maximum PMEP = ɛPIin − λPml/ks) also operating through minimization of the term λPml/ks, involving co-adaptation of leaf lifespan (affecting ml), leaf orientation (affecting k) and leaf thickness (affecting s). Observed correlations between leaf traits (e.g. Reich et al. 1992; Wright et al. 2004) offer a fertile testing ground for MEP and other candidate optimization theories of plant function (e.g. McMurtrie & Dewar submitted). Finally, extending MEP to ecosystems also raises the possibility of predicting optimal soil characteristics (e.g. soil depth, moisture content and nutrient cycling), since the long-term maximization of photosynthesis may involve trade-offs that depend on plant–soil feedbacks.
4. Conclusions
Within well-defined approximations (fixed chemical potentials and fixed temperature), MEP is closely related to various plant optimization theories that have been proposed previously on the basis of ad hoc measures of individual fitness. Like traditional plant optimization theories, MEP can predict optimal plant behaviour that is reasonable from the perspective of natural selection. The different objective functions of these theories emerge as examples of entropy production on different spatio-temporal scales. Moreover, as a system-level thermodynamic principle, MEP extends the traditional optimization approach beyond individual plants to vegetation canopies and whole ecosystems. This suggests that MEP offers a unifying optimization principle for plant and ecosystem function, and that entropy production might be considered as a general objective function for biological systems (e.g. Dewar et al. 2006; Meysman & Bruers 2007).
Further work is needed to determine whether MEP improves on previous optimization theories in predicting observed plant function. To this end, the simple analysis presented here for illustrative purposes might be developed further to include variable chemical potentials (e.g. Meysman & Bruers 2007). An outstanding practical issue here is how to estimate the chemical potential of plant litter (μL) required when applying MEP at the whole-plant scale (figure 2). Also, the simple applications of MEP presented in §3 do not incorporate resource supply constraints such as nitrogen and water availability. Some recent plant optimization models incorporating resource supply constraints are reviewed in Dewar et al. (2009), see also McMurtrie & Dewar (submitted). These constraints might also be introduced into the MEP framework presented here.
Conceptually, MEP offers a radically new perspective on the adaptive behaviour and evolution of plants. The proposed statistical explanation of MEP—a subject of open debate (Dewar 2003, 2005, 2009; Martyushev & Seleznev 2006; Bruers 2007; Grinstein & Linsker 2007; Niven 2009)—is that the MEP state is selected by nature because it can be realized microscopically in an overwhelmingly greater number of ways than any other non-equilibrium state. MEP therefore suggests a new and extended paradigm for biological evolution—survival of the likeliest (Whitfield 2007)—which applies at scales ranging from biomacromolecules to ecosystems (e.g. Dewar et al. 2006; Dewar & Porté 2008), not just to individual organisms, and which encompasses both living and non-living structures.
Acknowledgements
I thank Axel Kleidon and co-organizers of the 2007, 2008 and 2009 MEP workshops held at the Max-Planck-Institute for Biogeochemistry, Jena, for their kind hospitality and financial assistance, and the participants for the many stimulating discussions that contributed to the development of the ideas presented here. I thank Yadvinder Mahli and two anonymous reviewers for helpful comments on the manuscript.
Footnotes
One contribution of 17 to a Theme Issue ‘Maximum entropy production in ecological and environmental systems: applications and implications’.
References
- Ackerly D. D.1999Self-shading, carbon gain and leaf dynamics: a test of alternative optimality models. Oecologia 119, 300–310 (doi:10.1007/s004420050790) [DOI] [PubMed] [Google Scholar]
- Ågren G. I., Franklin O.2003Root : shoot ratios, optimization and nitrogen productivity. Ann. Bot. 92, 795–800 (doi:10.1093/aob/mcg203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten N. P. R.2005Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Ann. Bot. 95, 495–506 (doi:10.1093/aob/mci048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruers S.2007A discussion on maximum entropy production and information theory. J. Phys. A 40, 7441–7450 (doi:10.1088/1751-8113/40/27/003) [Google Scholar]
- Christy A. L., Ferrier J. M.1973A mathematical treatment of Munch's pressure-flow hypothesis of phloem translocation. Plant Physiol. 52, 531–538 (doi:10.1104/pp.52.6.531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan I. R., Farquhar G. D.1977Stomatal function in relation to leaf metabolism and environment. Soc. Exp. Biol. Symp. 31, 471–505 [PubMed] [Google Scholar]
- Cramer W., et al. 2001Global responses of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Global Change Biol. 7, 357–373 (doi:10.1046/j.1365-2486.2001.00383.x) [Google Scholar]
- Dewar R. C.1996The correlation between plant growth and intercepted radiation: an interpretation in terms of optimal plant nitrogen content. Ann. Bot. 78, 125–136 (doi:10.1006/anbo.1996.0104) [Google Scholar]
- Dewar R. C.2003Information theory explanation of the fluctuation theorem, maximum entropy production and self-organized criticality in non-equilibrium stationary states. J. Phys. A 36, 631–641 (doi:10.1088/0305-4470/36/3/303) [Google Scholar]
- Dewar R. C.2004Maximum entropy production and non-equilibrium statistical mechanics. In Non-equilibrium thermodynamics and entropy production: life, earth and beyond (eds Kleidon A., Lorenz R.), pp. 41–55 Heidelberg, Germany: Springer Publishers [Google Scholar]
- Dewar R. C.2005Maximum entropy production and the fluctuation theorem. J. Phys. A 38, L371–L381 (doi:10.1088/0305-4470/38/21/L01) [Google Scholar]
- Dewar R. C.2009Maximum entropy production as an algorithm that translates physical assumptions into macroscopic predictions: don't shoot the messenger. Entropy (Special Issue. What is maximum entropy production and how should we apply it?)11, 931–944 (doi:10.3390/e11040931) [Google Scholar]
- Dewar R. C., Porté A.2008Statistical mechanics unifies different ecological patterns. J. Theor. Biol. 251, 389–403 (doi:10.1016/j.jtbi.2007.12.007) [DOI] [PubMed] [Google Scholar]
- Dewar R. C., Medlyn B. E., McMurtrie R. E.1998A mechanistic analysis of light and carbon use efficiencies. Plant Cell Environ. 21, 573–588 (doi:10.1046/j.1365-3040.1998.00311.x) [Google Scholar]
- Dewar R. C., Juretić D., Županović P.2006The functional design of the rotary enzyme ATP synthase is consistent with maximum entropy production. Chem. Phys. Lett. 430, 177–182 (doi:10.1016/j.cplett.2006.08.095) [Google Scholar]
- Dewar R. C., Franklin O., Mäkelä A., McMurtrie R. E., Valentine H. T.2009Optimal function explains forest responses to global change. BioScience 59, 127–139 (doi:10.1525/bio.2009.59.2.6) [Google Scholar]
- Field C. B.1983Allocating leaf nitrogen for the maximization of carbon gain—leaf age as a control on the allocation program. Oecologia 56, 341–347 (doi:10.1007/BF00379710) [DOI] [PubMed] [Google Scholar]
- Franklin O.2007Optimal nitrogen allocation controls tree responses to elevated CO2. New Phytol. 174, 811–822 (doi:10.1111/j.1469-8137.2007.02063.x) [DOI] [PubMed] [Google Scholar]
- Franklin O., Ågren G. I.2002Leaf senescence and resorption as mechanisms of maximising photosynthetic production during canopy development at N limitation. Funct. Ecol. 16, 727–733 (doi:10.1046/j.1365-2435.2002.00674.x) [Google Scholar]
- Givnish T. J. (ed.) 1986On the economy of plant form and function. Cambridge, UK: Cambridge University Press [Google Scholar]
- Grinstein G., Linsker R.2007Comments on a derivation and an application of the ‘maximum entropy production’ principle. J. Phys. A 40, 9717–9720 (doi:10.1088/1751-8113/40/31/N01) [Google Scholar]
- Haxeltine A., Prentice I. C.1996A general model for the light use efficiency of primary production. Funct. Ecol. 10, 551–561 (doi:10.2307/2390165) [Google Scholar]
- Hikosaka K.2003Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann. Bot. 95, 521–533 (doi:10.1093/aob/mci050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleidon A.2004Optimized stomatal conductance of vegetated land surfaces and its effect on simulated productivity and climate. Geophys. Res. Lett. 31, L21203 (doi:10.1029/2004GL020769) [Google Scholar]
- Kull O.2002Acclimation of photosynthesis in canopies: models and limitations. Oecologia 133, 267–279 (doi:10.1007/s00442-002-1042-1) [DOI] [PubMed] [Google Scholar]
- Levy P. E., Wendler R., Van Oijen M., Cannell M. G. R., Millard P.2004The effects of nitrogen enrichment on the carbon sink in coniferous forests: uncertainty and sensitivity analyses of three ecosystem models. Wat. Air Soil Poll.: Focus 4, 67–74 (doi:10.1007/s11267-004-3015-3) [Google Scholar]
- Luo Y., et al. 2008Modeled interactive effects of precipitation, temperature, and [CO2] on ecosystem carbon and water dynamics in different climatic zones. Global Change Biol. 14, 1986–1999 (doi:10.1111/j.1365-2486.2008.01629.x) [Google Scholar]
- Mäkelä A., Sievänen R.1992Height growth strategies in open-grown trees. J. Theor. Biol. 159, 443–467 (doi:10.1016/S0022-5193(05)80690-3) [Google Scholar]
- Mäkelä A., Givnish T. J., Berninger F., Buckley T. N., Farquhar G. D., Hari P.2002Challenges and opportunities of the optimality approach in plant ecology. Silv. Fenn. 36, 605–614 [Google Scholar]
- Mäkelä A., Valentine H., Helmisaari S.2008Optimal co-allocation of carbon and nitrogen in a closed forest stand at steady state. New Phytol. 180, 114–123 (doi:10.1111/j.1469-8137.2008.02558.x) [DOI] [PubMed] [Google Scholar]
- Martyushev L. M., Seleznev V. D.2006Maximum entropy production principle in physics, chemistry and biology. Phys. Rep. 426, 1–45 (doi:10.1016/j.physrep.2005.12.001) [Google Scholar]
- McMurtrie R. E.1985Forest productivity in relation to carbon partitioning and nutrient cycling: a mathematical model. In Attributes of trees as crop plants (eds Cannell M. G. R., Jackson J. E.), pp. 194–207 Huntingdon, UK: Institute of Terrestrial Ecology [Google Scholar]
- McMurtrie R. E., Dewar R. C.Submitted Leaf trait variations explained by the hypothesis that plants maximise their canopy carbon export over the lifespan of leaves. Proc. Natl Acad. Sci. USA [DOI] [PubMed] [Google Scholar]
- McMurtrie R. E., Norby R. J., Medlyn B. E., Dewar R. C., Pepper D. A., Reich P. B., Barton C. V. M.2008Why is plant-growth response to elevated CO2 amplified when water is limiting, but reduced when nitrogen is limiting? A growth optimisation hypothesis. Funct. Biol. 35, 521–534 (doi:10.1071/FP08128) [DOI] [PubMed] [Google Scholar]
- Meysman F. J. R., Bruers S.2007A thermodynamic perspective on food webs: quantifying entropy production within detrital-based ecosystems. J. Theor. Biol. 249, 124–139 (doi:10.1016/j.jtbi.2007.07.015) [DOI] [PubMed] [Google Scholar]
- Niven R. K.2009Steady state of a dissipative flow-controlled system and the maximum entropy production principle. Phys. Rev. E 80, 021 113 (doi:10.1103/PhysRevE.80.021113) [DOI] [PubMed] [Google Scholar]
- Reich P. B., Walters M. B., Ellsworth D. S.1992Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 62, 365–392 (doi:10.2307/2937116) [Google Scholar]
- Reynolds J. F., Thornley J. H. M.1982A shoot : root partitioning model. Ann. Bot. 49, 585–597 [Google Scholar]
- Schymanski S. J., Sivapalan M., Roderick M. L., Hutley L. B., Beringer J.2009An optimality-based model of the dynamic feedbacks between natural vegetation and the water balance. Wat. Resour. Res. 45, W01412 (doi:10.1029/2008WR006841) [Google Scholar]
- Wang Y.-P., Leuning R., Cleugh H. A., Coppin P. A.2001Parameter estimation in surface exchange models using nonlinear inversion: how many parameters can we estimate and which measurements are most useful? Global Change Biol. 7, 495–510 (doi:10.1046/j.1365-2486.2001.00434.x) [Google Scholar]
- Whitfield J.2005Order out of chaos. Nature 436, 905–907 (doi:10.1038/436905a) [DOI] [PubMed] [Google Scholar]
- Whitfield J.2007Survival of the likeliest? PLoS (Biol) 5, e142 (doi:10.1371/journal.pbio.0050142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I. J., et al. 2004The worldwide leaf economics spectrum. Nature 428, 821–827 (doi:10.1038/nature02403) [DOI] [PubMed] [Google Scholar]