Abstract
This study examines a new formulation of non-equilibrium thermodynamics, which gives a conditional derivation of the ‘maximum entropy production’ (MEP) principle for flow and/or chemical reaction systems at steady state. The analysis uses a dimensionless potential function ϕst for non-equilibrium systems, analogous to the free energy concept of equilibrium thermodynamics. Spontaneous reductions in ϕst arise from increases in the ‘flux entropy’ of the system—a measure of the variability of the fluxes—or in the local entropy production; conditionally, depending on the behaviour of the flux entropy, the formulation reduces to the MEP principle. The inferred steady state is also shown to exhibit high variability in its instantaneous fluxes and rates, consistent with the observed behaviour of turbulent fluid flow, heat convection and biological systems; one consequence is the coexistence of energy producers and consumers in ecological systems. The different paths for attaining steady state are also classified.
Keywords: maximum entropy, maximum entropy production, thermodynamics, steady-state, irreversible, biological system
1. Introduction
Since the seminal book ‘What is Life’ by Schrödinger (1944), scientists have pondered the existence of life and its compatibility with the second law of thermodynamics. Ridiculing the popular notion that the primary purpose of biological metabolism is to extract matter and energy from the environment, he moves to the crux of the issue (p. 71):
… a living organism continually increases its entropy … and thus tends to approach the dangerous state of maximum entropy, which is death. It can only keep aloof from it, i.e. alive, by continually drawing from its environment negative entropy … What an organism feeds upon is negative entropy.
(The argument is qualified in a footnote, to refer to free energy instead of negative entropy.) The topic was taken up in more detail by Prigogine (1967, 1980), who described living organisms—along with heat-transporting convection cells, turbulent fluid flow vortices and oscillatory chemical reactions—as dissipative structures, which continually dissipate heat and thus generate and export entropy to the environment. However, Prigogine's main quantitative result, his minimum entropy production (MinEP) principle—valid in the linear or Onsager (1931a,b) transport regime—seems diametrically opposed to life (Martyushev et al. 2007), as was recognized by Prigogine (1980, p. 88) himself. Bacteria in a microcosm, organisms in an ecosystem or humans on a planet do not try to minimize their entropy production, but instead grow, reproduce and consume all available resources as rapidly as possible. More recently, other thermodynamics-inspired perspectives on biological systems have been advanced, including the use of biological measures of entropy and information (e.g. Ayres 1994); the non-mathematical gradient theory of Schneider & Sagan (2005); and exergy-based treatments of ecological systems and processes (e.g. Jørgensen 2006).
Over the past 30 years, a new principle has been proposed, the maximum entropy production (MEP) principle, which states that a flow system subject to various flows or gradients will tend towards a steady-state position of maximum thermodynamic entropy production, σ̇ (Ozawa et al. 2003; Kleidon & Lorenz 2005; Martyushev & Seleznev 2006; Bruers 2007). The MEP principle has been successfully applied—in a heuristic sense—to the prediction of steady states of a wide range of systems, including the Earth's climate system (e.g. Paltridge 1975, 1978; Kleidon 2004; Kleidon & Lorenz 2005); thermal (Bénard) convection (Ozawa et al. 2001); mantle convection (Vanyo & Paltridge 1981; Lorenz 2001); electrical currents (Županović et al. 2004; Botrić et al. 2005; Christen 2006; Bruers et al. 2007a); plasmas (Christen 2007a; Yoshida & Mahajan 2008); crystalline solids (Martyushev & Axelrod 2003; Christen 2007b); ecological systems (Meysman & Bruers 2007) and biochemical processes (Juretić & Županović 2003; Dewar et al. 2006). The MEP principle therefore offers a new approach for the analysis of biological systems at the cellular, organism, ecosystem and biosphere levels. Most importantly, it is a quantitative principle, based on precisely defined, rigorous thermodynamic concepts; it does not rest upon vague, non-mathematical notions such as ‘order’, ‘disorder’, ‘randomness’ or ‘complexity’ often seen in discussions of biological systems.
Several theoretical justifications of the MEP principle have been advanced, including approaches based on path or transition probabilities (Dewar 2003, 2005; Attard 2006a,b) and two more generalistic arguments (Županović et al. 2006; Martyushev 2007). Recently, a rather different derivation was presented to directly determine the steady state of a flow system, based on an entropy defined on the set of local instantaneous fluxes and reaction rates through or within each infinitesimal element; this reduces to a local form of the MEP principle in some circumstances (Niven 2009b). The analysis invokes a generalized potential function (negative Massieu function) obtained from Jaynes' maximum entropy method, somewhat analogous to the free energy concept used in equilibrium thermodynamics, which attains a minimum at steady state. The aim of this study is to explore the implications of this derivation in somewhat simpler terms than in Niven (2009b), using terminology adapted from chemical and statistical thermodynamics. In particular, the nature of the inferred steady state of a flow system, and the various means by which it can be attained, are examined in detail. The analysis has important implications for the modelling of flow systems, including the Earth's climatic-biosphere system and all biological systems.
2. The generalized free energy concept
(a). Jaynes' maximum entropy principle
The maximum entropy (MaxEnt) principle of Jaynes (1957, 1963, 2003, see also Tribus 1961a,b; Kapur & Kesevan 1992) provides a powerful technique with which to infer the most probable position of a probabilistic system. Consider a system composed of N distinguishable entities allocated to s equiprobable, distinguishable categories (a multinomial system: Niven 2005, 2006, 2007, 2009a; Niven & Grendar 2009). In the asymptotic limit N → ∞, the most probable position can be obtained by maximizing the relative entropy function (the negative of the Kullback & Leibler (1951) function, D):
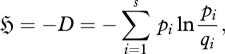 |
2.1 |
where pi is the probability of an entity in the ith category and qi is the source or ‘prior’ probability of category i. For equiprobable categories, this reduces to the Shannon (1948) entropy function:
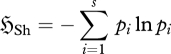 |
2.2 |
plus a constant. Equation (2.1) (or equation (2.2)) is maximized subject to the natural (normalization) and any moment constraints on the system:
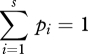 |
2.3 |
and
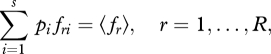 |
2.4 |
where fri is the value of the ith category of property fr and 〈fr〉 is the expectation (average) of fri. This yields the most probable (stationary) distribution of the system:
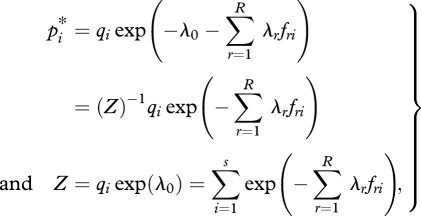 |
2.5 |
and the maximum entropy position (Jaynes 1957, 1963, 2003):
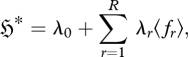 |
2.6 |
where λr is the rth Lagrangian multiplier, λ0 is the ‘Massieu function’ (Massieu 1869), Z is the partition function and an asterisk denotes the stationary position. It is emphasized that the above derivation is generic, and applies to any probabilistic system of multinomial form; it need not refer to a thermodynamic system.
(b). Generalized heat, work and potential function
We now consider any conserved quantity fr, for which we adopt the definition:
| 2.7 |
where the path differentials 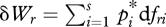 and
and 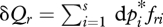 can be termed the ‘generalized work’ and ‘generalized heat’ associated respectively with a change in 〈fr〉. It can be shown (Jaynes 1957, 1963, 2003) that
can be termed the ‘generalized work’ and ‘generalized heat’ associated respectively with a change in 〈fr〉. It can be shown (Jaynes 1957, 1963, 2003) that
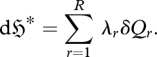 |
2.8 |
This is a ‘generalized Clausius equality’ (cf. Clausius 1865), applicable to all multinomial systems. Substituting equation (2.8) into the differential of equation (2.6) and rearranging gives:
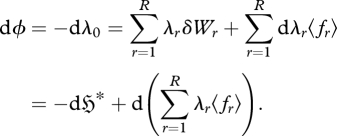 |
2.9 |
We therefore obtain a potential function ϕ (negative Massieu function) which captures all possible changes in the system, due to changes in the entropy 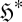 or in the ‘constraint set’
or in the ‘constraint set’ 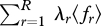 . If the multipliers {λr} are constant, dϕ reduces to the multiplier-weighted total generalized work on the system,
. If the multipliers {λr} are constant, dϕ reduces to the multiplier-weighted total generalized work on the system, 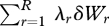 . We therefore see that ϕ is a dimensionless, weighted, extended version of the free energy function, applicable to any probabilistic system of multinomial form (Jaynes 1957, 1963, 2003; Tribus 1961a,b).
. We therefore see that ϕ is a dimensionless, weighted, extended version of the free energy function, applicable to any probabilistic system of multinomial form (Jaynes 1957, 1963, 2003; Tribus 1961a,b).
How should we interpret equation (2.9)? Consider some form of ‘open system’, consisting of a defined region or collection of discrete entities in contact with some surroundings (or the rest of the universe). The internal structure of the system may be described by some probability function pi, giving rise to some relative entropy function 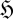 for the system (not necessarily the thermodynamic entropy S, but any entropy). From a purely probabilistic formulation of the second law (Niven 2009a,b):
for the system (not necessarily the thermodynamic entropy S, but any entropy). From a purely probabilistic formulation of the second law (Niven 2009a,b):
The entropy of the universe,
univ, however defined, can only increase,
it is evident that any spontaneous event must be driven by an increase of the entropy of the system 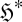 and/or an increase in entropy produced and exported by the system to its surroundings,
and/or an increase in entropy produced and exported by the system to its surroundings, 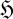 prod. Quantitatively, this can be written as1:
prod. Quantitatively, this can be written as1:
| 2.10 |
However, the only means by which a system can produce and export entropy—thereby increasing 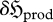 —is by a reduction in the magnitude of one or more constraints (or multipliers) which govern the system, {〈fr〉} (or {λr}). For this to produce a change in a quantity in (dimensionless) entropy units, we therefore establish that
—is by a reduction in the magnitude of one or more constraints (or multipliers) which govern the system, {〈fr〉} (or {λr}). For this to produce a change in a quantity in (dimensionless) entropy units, we therefore establish that 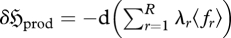 . Comparing equations (2.9) and (2.10), we thus see that dϕ expresses, in a negative sense, the change in entropy of the universe. This can be written as
. Comparing equations (2.9) and (2.10), we thus see that dϕ expresses, in a negative sense, the change in entropy of the universe. This can be written as
| 2.11 |
Equations (2.9) and (2.11) thus provide a mathematical formulation of a generalized second law (with sign reversed), expressing the interplay between changes in the entropy—however defined—of a system, and changes in entropy produced and exported by a system to its surroundings. This is again consistent with the interpretation of ϕ as a dimensionless, weighted, extended version of the free energy concept (Jaynes 1957, 1963, 2003; Tribus 1961a,b).
3. Applications
(a). Equilibrium systems example
The above discussion is best illustrated by an example from thermodynamics. Whilst a broader free energy concept is considered in Niven (2009b), most readers will be more familiar with the Gibbs (1875–1878) free energy function for systems of constant composition:
| 3.1 |
where S* is the maximum thermodynamic entropy, U is internal energy, V is volume, P is pressure, T is absolute temperature and H is the enthalpy. Equation (3.1) can be derived by applying Jaynes' method to an equilibrium thermodynamic system subject to the constraints U and V, wherein pi is the joint probability that a molecule will occupy a specified energy level and volume element. Equation (2.9) then gives the potential function (Jaynes 1957, 1963, 2003; Tribus 1961a,b):
| 3.2 |
Recognizing 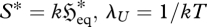 and λV = P/kT, where k is Boltzmann's constant, gives
and λV = P/kT, where k is Boltzmann's constant, gives
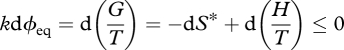 |
3.3 |
equivalent to equation (3.1). This form reveals the true meaning of the Gibbs free energy concept: it expresses—in a negative sense—the interplay between the change in entropy within the system dS*, and the change in entropy exported by the system, −d(H/T), due to transfers of heat (Planck 1922, 1932; Fermi 1956; Strong & Halliwell 1970; Craig 1988). From the (classical) second law, their sum must be positive, and so a system will spontaneously approach a position of minimum G/T (for constant T, it will approach minimum G). From equation (2.11), we can rewrite equation (3.3) as (Niven 2009b):
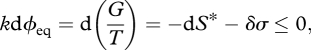 |
3.4 |
where 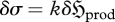 is the increment of thermodynamic entropy produced and exported by the system to its surroundings.
is the increment of thermodynamic entropy produced and exported by the system to its surroundings.
(b). Flow system example
Now consider a second example, of an infinitesimal fluid element in a control volume of a flowing fluid, subject to local mean values of the heat flux jQ, diffusive mass fluxes jc of each chemical species c, stress tensor τ and chemical reaction rates 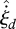 of each reaction d, plus the natural constraint (2.3). This model encompasses all fluid flow, heat flow, biological and ecological systems. Such a system can be analysed by Jaynes' method using the local flux relative entropy (Niven 2009b):
of each reaction d, plus the natural constraint (2.3). This model encompasses all fluid flow, heat flow, biological and ecological systems. Such a system can be analysed by Jaynes' method using the local flux relative entropy (Niven 2009b):
| 3.5 |
where πI is the joint probability that the fluid element experiences a set of instantaneous local fluxes of heat, species c, momentum and rates of chemical reactions d, and γI is the joint prior probability. For this system, it can be shown that equation (2.9) gives the increment in the local potential function (Niven 2009b):
| 3.6 |
where 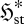 is the local flux entropy at steady state, θ and
is the local flux entropy at steady state, θ and 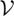 are characteristic time and volume scales of the system, and
are characteristic time and volume scales of the system, and 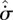 is the local thermodynamic entropy production of the element per unit volume:
is the local thermodynamic entropy production of the element per unit volume:
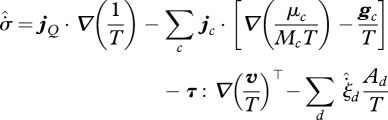 |
3.7 |
in which μc, Mc and gc are the molar chemical potential, molar mass and specific body force on species c, v is the mass-average fluid velocity and Ad is the molar chemical affinity of reaction d, with Ad < 0 indicating spontaneous forwards reaction. Equation (3.7) can be summarized in the form:
| 3.8 |
where jX is the local mean flux or mean reaction rate of quantity or species X, and FX is the corresponding local ‘thermodynamic force’ (gradient or affinity term). Not coincidentally, equation (3.6) has the same form as equation (2.11), expressing (with sign reversed) the sum of changes in the flux entropy within the element plus its export out of the system. A flow element will therefore try to approach a steady-state position of minimum ϕst, for the same reason that an equilibrium thermodynamic system tries to approach an equilibrium position of minimum ϕeq (minimum G/T).
Comparing equations (3.3) and (3.6), we see that the entropy production 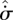 within a fluid element of a steady-state system plays a similar role (with change of sign and units) to the enthalpy function H in equilibrium systems. This is an important insight, which has perhaps been hindered by the lack of popular understanding of the free energy concept (Strong & Halliwell 1970; Craig 1988). The common feature is that H and
within a fluid element of a steady-state system plays a similar role (with change of sign and units) to the enthalpy function H in equilibrium systems. This is an important insight, which has perhaps been hindered by the lack of popular understanding of the free energy concept (Strong & Halliwell 1970; Craig 1988). The common feature is that H and 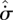 both serve as (modified) measures of the export of entropy—however defined—by a system to its surroundings. Many previous authors have erred in considering
both serve as (modified) measures of the export of entropy—however defined—by a system to its surroundings. Many previous authors have erred in considering 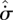 to be the non-equilibrium analogue of S*; while this may seem reasonable at first glance, it is not correct.
to be the non-equilibrium analogue of S*; while this may seem reasonable at first glance, it is not correct.
4. Implications
(a). Meaning of the flux entropy
To understand equation (3.6), it is necessary to appreciate the meaning of the flux entropy 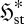 . To do this, we need to consider the mathematical properties of the relative entropy (2.1) and Shannon entropy (2.2) (e.g. Kapur & Kesavan 1992). In essence,
. To do this, we need to consider the mathematical properties of the relative entropy (2.1) and Shannon entropy (2.2) (e.g. Kapur & Kesavan 1992). In essence, 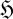 indicates the spread of the distribution pi amongst its categories i; the thermodynamic entropy S* therefore reflects the spread of the equilibrium distribution pi* over energy levels and volume elements, with low S* indicating a narrow distribution and high S* a broad one. In the same way,
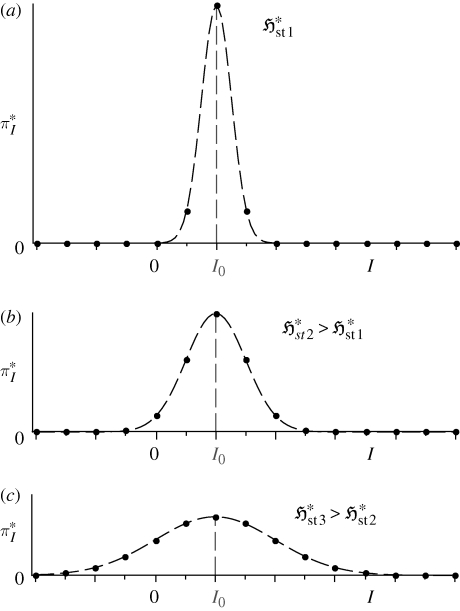
indicates the spread of the distribution pi amongst its categories i; the thermodynamic entropy S* therefore reflects the spread of the equilibrium distribution pi* over energy levels and volume elements, with low S* indicating a narrow distribution and high S* a broad one. In the same way, 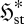 reflects the spread of the steady-state probability π*I over the set of instantaneous local fluxes and reaction rates. This is illustrated by the schematic plots in figure 1a–c, for a univariate parameter I = I (e.g. a single flux or reaction rate of quantity X). All three plots have the same mean flux or rate jX—represented schematically by a fixed ‘mean category’ I0—but the variance, and therefore the flux entropy, increases from (a) to (c).
reflects the spread of the steady-state probability π*I over the set of instantaneous local fluxes and reaction rates. This is illustrated by the schematic plots in figure 1a–c, for a univariate parameter I = I (e.g. a single flux or reaction rate of quantity X). All three plots have the same mean flux or rate jX—represented schematically by a fixed ‘mean category’ I0—but the variance, and therefore the flux entropy, increases from (a) to (c).
Figure 1.
Effect of increasing 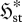 on a univariate steady-state distribution
on a univariate steady-state distribution 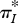 (schematic only).
(schematic only).
The plots reveal an additional, extraordinary feature of flow systems. In equilibrium systems, the categories (e.g. energy levels) are generally taken to start from a ‘zero’ or reference level, for which the value of the index is unimportant. In contrast, flow systems have no such minimum, since we must allow for positive and negative flux or rate levels I = 0, ±1, ±2, … . In consequence, as 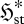 increases, the system is more likely to access its states of reverse flow or reverse chemical reaction I < 0, even if the mean value jX is high (figure 1b,c). In other words, a high flux entropy is associated with greater variability in the fluxes and rates, which therefore implies oscillatory or chaotic processes. We immediately see the connection between steady states of high
increases, the system is more likely to access its states of reverse flow or reverse chemical reaction I < 0, even if the mean value jX is high (figure 1b,c). In other words, a high flux entropy is associated with greater variability in the fluxes and rates, which therefore implies oscillatory or chaotic processes. We immediately see the connection between steady states of high 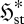 and the defining features of many ‘far from equilibrium’ systems, such as fluid turbulence, heat-induced convection cells, nonlinear diffusion phenomena and oscillatory chemical reactions (Prigogine 1967, 1980); indeed, the latter are prevalent in biochemical processes such as nutrient degradation processes (Meysman & Bruers 2007) and the photosynthesis cycle (Juretić & Županović 2003; Dewar et al. 2006).
and the defining features of many ‘far from equilibrium’ systems, such as fluid turbulence, heat-induced convection cells, nonlinear diffusion phenomena and oscillatory chemical reactions (Prigogine 1967, 1980); indeed, the latter are prevalent in biochemical processes such as nutrient degradation processes (Meysman & Bruers 2007) and the photosynthesis cycle (Juretić & Županović 2003; Dewar et al. 2006).
The importance of the above analysis can be illustrated by its application to the species population structure within an ecosystem. Consider a small element of a (rudimentary) ecosystem of s species, identified only by their energy usage, such that each organism of species i has the energy consumption εi, and the overall system has mean energy consumption 〈E〉. This model dramatically simplifies the MaxEnt ecosystem model given by Dewar & Porté (2008). To infer the steady-state species population distribution 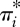 , we maximize the relative entropy
, we maximize the relative entropy 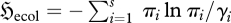 (3.5), subject to known prior probabilities γi and constraints
(3.5), subject to known prior probabilities γi and constraints 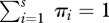 and
and 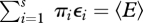 , giving:
, giving:
| 4.1 |
and
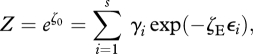 |
4.2 |
where ζ0 and ζE are, respectively, the Lagrangian multipliers for the two constraints, and Z is the partition function. The analysis (3.6)–(3.8) then follows from equation (4.2), with the energetic multiplier identified as ζE∝ FE = ∇T−1. Although equation (4.2) has the appearance of a Boltzmann distribution akin to that of chemical thermodynamics, in an ecosystem the ‘energy levels’ i are actually ‘energy consumption levels’, which can be positive or negative, corresponding, respectively, to net energy consumers (i > 0) and net energy producers (i < 0). At a high ecological flux entropy 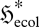 , the ‘most probable’ ecosystem will therefore be forced to contain both energy producers and consumers, rather than just energy consumers. Similarly, in turbulent fluid flow, some energetic structures will be net energy consumers (dissipating energy as heat), while others will be net energy producers (transferring energy from its incoming source to the consumers). We therefore recover—at least in a qualitative sense—the essence of the trophic structure (food chain or food web) of ecological systems and the energy cascade of turbulent flow systems.
, the ‘most probable’ ecosystem will therefore be forced to contain both energy producers and consumers, rather than just energy consumers. Similarly, in turbulent fluid flow, some energetic structures will be net energy consumers (dissipating energy as heat), while others will be net energy producers (transferring energy from its incoming source to the consumers). We therefore recover—at least in a qualitative sense—the essence of the trophic structure (food chain or food web) of ecological systems and the energy cascade of turbulent flow systems.
(b). Classification of spontaneous processes
We can now return to the equilibrium (3.3) and steady-state (3.6) potential functions. For simplicity, we first confine the discussion to processes with monotonic changes in the entropy S* and entropy produced σ = −H/T. We see that in equilibrium systems, the path towards equilibrium dϕeq ≤ 0 will depend on the relative changes in S* and σ, leading to three possible scenarios for a spontaneous process, as listed in table 1. In Case E1, the process is driven by changes in both entropy terms, while in Cases E2 and E3, a reduction in one entropy is ‘paid for’ by a greater and opposite gain in the other. In all cases, since the entropy changes are monotonic, the equilibrium position (minimum ϕeq = minimum G/T) must coincide with extrema (a minimum or maximum) in both S* and σ, as set out in the table.
Table 1.
List of possible spontaneous processes in equilibrium and steady-state systems, for monotonically varying parameters (terminology from ancient Greek: exo-, external; endo-, internal; pan-, everywhere; tropos, transformation (Clausius 1865); -genic, generating or producing).
| case | conditions | entropic driving force | label | extrema at stationary position |
||
|---|---|---|---|---|---|---|
| equilibrium systems (kdϕeq = d(G/T) ≤ 0) | ||||||
| E1 | dS* ≥ 0, | δσ ≥ 0 | universal | panentropic | max S*, | max σ (= min H/T) |
| E2 | dS* ≥ |δσ| ≥ 0, | δσ ≤ 0 | internal dominant | endoentropic | max S*, | min σ (= max H/T) |
| E3 | dS* ≤ 0, | δσ ≥ |dS*| ≥ 0 | external dominant | exoentropic | min S*, | max σ (= min H/T) |
steady-state systems (Kdϕst≤ 0 with K = k/θ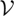 ) ) | ||||||
| S1 |
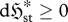 , , |
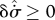 |
universal | panentropogenic | max 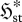 , , |
max 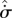
|
| S2 |
 , , |
 |
internal dominant | endoentropogenic | max 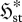 , , |
min 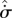
|
| S3 |
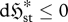 , , |
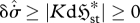 |
external dominant | exoentropogenic | min 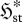 , , |
max 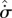
|
Similarly, from equation (3.6), in a flow system subject to monotonic changes in 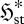 and
and 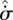 , each increment towards steady state
, each increment towards steady state 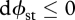 could be achieved by one of the three cases listed in table 1. The corresponding extrema at steady state are also listed. As shown, Cases S1 and S3 are consistent with a position of MEP. Case S2, on the other hand, involves convergence towards a position of MinEP. The three cases therefore encompass the two major (seemingly contradictory) principles of non-equilibrium thermodynamics (Prigogine 1967, 1980; Martyushev et al. 2007).
could be achieved by one of the three cases listed in table 1. The corresponding extrema at steady state are also listed. As shown, Cases S1 and S3 are consistent with a position of MEP. Case S2, on the other hand, involves convergence towards a position of MinEP. The three cases therefore encompass the two major (seemingly contradictory) principles of non-equilibrium thermodynamics (Prigogine 1967, 1980; Martyushev et al. 2007).
We further note that if passage to equilibrium or steady state is not monotonic, many more scenarios are possible. In thermodynamics, this is handled by considering only the net change in Gibbs free energy ΔG = −TΔS* + ΔH at constant T and P. In light of equation (3.3), this is more appropriately written as:
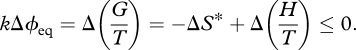 |
4.3 |
This rests on the fact that G, S*, U, V and H are state functions, so we can disregard the path taken by the system. Although such systems could follow any of the paths E1–E3 in table 1 during different stages of the process—or even temporarily deviate from d(G/T) ≤ 0 to overcome an activation energy barrier—they must approach a position of minimum G/T, leading to a net change Δ(G/T) ≤ 0. The system can still be said to follow one of Cases E1–E3, but now only in a net sense (using Δ's rather than d's). In Case E3, for example, we can still speak of the system tending towards a position of minimum S* and maximum σ (= minimum H/T), provided this is understood to refer to their net changes rather than the path taken by the system.
In a similar vein, if an unsteady flow system is not restricted to purely monotonic changes, it must still approach a steady-state position of minimum 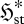 , and thus undergo the net change
, and thus undergo the net change 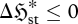 . Presuming that
. Presuming that 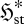 and
and 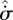 can be considered as state functions, the system can still be identified as following—now in a net sense—one of the three Cases S1–S3 in table 1.
can be considered as state functions, the system can still be identified as following—now in a net sense—one of the three Cases S1–S3 in table 1.
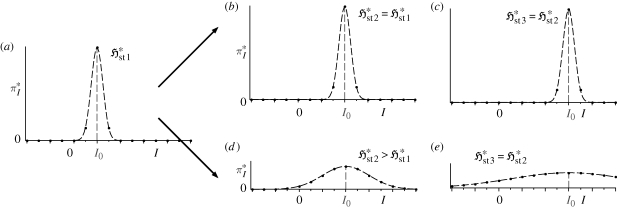
Can we infer anything more about flow systems? Indeed, we can. Consider a flow element which experiences a gradual increase in the local thermodynamic force FX conjugate to the mean local flux or rate jX. Such an element may undergo two types of changes: (i) an increase in jX without any corresponding increase (or even a decrease) in 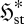 , illustrated in figure 2a–c; and (ii) increases in both jX and
, illustrated in figure 2a–c; and (ii) increases in both jX and 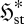 , illustrated in figure 2a,d,e. From equation (3.7), both scenarios involve identical increases in the entropy production,
, illustrated in figure 2a,d,e. From equation (3.7), both scenarios involve identical increases in the entropy production, 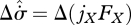 . Which is more likely? From our knowledge of flow systems, the first scenario seems less credible, since it requires the fluxes to remain within a narrow range of instantaneous values at all times, even though the driving force has increased. A decrease in
. Which is more likely? From our knowledge of flow systems, the first scenario seems less credible, since it requires the fluxes to remain within a narrow range of instantaneous values at all times, even though the driving force has increased. A decrease in 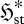 seems even more unlikely. The second scenario permits greater variability (fluctuations) of the fluxes, consistent with the formation of a nonlinear mechanism to enable greater transport or production of X. A similar argument applies if the flux, rather than the gradient, is the control variable. Although this is not a proof, it does lend support to the argument (Niven 2009b) that fluid elements tend to undergo concurrent increases in
seems even more unlikely. The second scenario permits greater variability (fluctuations) of the fluxes, consistent with the formation of a nonlinear mechanism to enable greater transport or production of X. A similar argument applies if the flux, rather than the gradient, is the control variable. Although this is not a proof, it does lend support to the argument (Niven 2009b) that fluid elements tend to undergo concurrent increases in 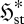 and
and 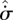 , and thus converge to steady state by a (net) panentropogenic process. In such cases, the steady-state position can be determined by the (net) MEP principle, without concern over contrary effects due to decreases in
, and thus converge to steady state by a (net) panentropogenic process. In such cases, the steady-state position can be determined by the (net) MEP principle, without concern over contrary effects due to decreases in 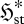 .
.
Figure 2.
Possible responses of a flow system to an increasing force FX or mean flux jX (increasing I0): (a,b,c) constant 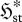 or (a,d,e) increasing
or (a,d,e) increasing 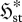 .
.
5. The MEP ‘heuristic’
We now turn to a discussion of current practice in the application of the MEP principle to flow or chemical reaction systems, including biological systems. From the pioneering works of Paltridge (1975, 1978) and three decades of further experience (e.g. Ozawa et al. 2001, 2003; Juretić & Županović 2003; Kleidon 2004; Kleidon & Lorenz 2005; Dewar et al. 2006; Martyushev & Seleznev 2006; Bruers 2007; Meysman & Bruers 2007), this has evolved into a set of practices which can be termed the ‘MEP heuristic’:
— Divide the control volume into very large subdomains (or even consider the entire domain).
— Set up the set of mass, chemical species, energy, momentum and/or charge balance equations for the system, based on the bulk flow rates between subdomains, using linear (Onsager-like) transport equations with adjustable, whole-subdomain transport coefficient(s), and chemical reaction rate equations with adjustable first-order rate constant(s).
— Calculate the thermodynamic entropy production of the system, as a function of the adjustable parameter(s).
— The inferred steady state of the system is given by the position of MEP with respect to the adjustable parameter(s).
How does this heuristic work? In effect, it selects the highest allowable entropy production consistent with the set of allowable bulk net fluxes JX,Γ and bulk thermodynamic forces FX,Γ in and between subdomains Γ of the system:
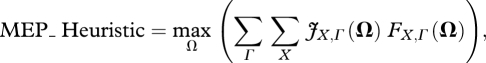 |
5.1 |
where the bulk fluxes and/or forces are functions of the set of subdomain-wide adjustable parameters Ω = {Ω}. It must be recognized, however, that the adjustable parameters are secondary variables, which do not represent fundamental physical processes. The true maximum must therefore be given by a ‘system maximum entropy production’ (SMEP) principle:
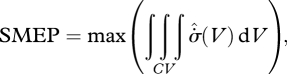 |
5.2 |
where the maximum is taken with respect to the instantaneous fluxes jXI, conditioned by the constraints on the system, and the integral is calculated over the control volume. The MEP heuristic therefore makes the assumption that equations (5.1) and (5.2) are equivalent, which is correct if and only if there exists a set of local physical mechanisms by which the maximum in equation (5.1) can be physically realized. Using the terminology of MEP practitioners, the MEP heuristic equation (5.1) must be considered to apply only to ‘many-degree-of-freedom’ systems (Ozawa et al. 2001, 2003; Juretić & Županović 2003; Kleidon 2004; Kleidon & Lorenz 2005; Dewar et al. 2006; Martyushev & Seleznev 2006; Bruers 2007; Meysman & Bruers 2007).
In contrast, the analysis herein (§§2–4) and in Niven (2009b) gives the optimization principle:
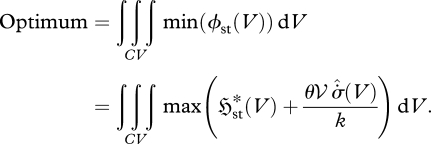 |
5.3 |
If the parameters 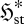 and
and 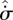 are positively correlated—as argued in §4—then equation (5.3) becomes functionally equivalent to a ‘local maximum entropy production’ (LMEP) principle, which gives for the overall system:
are positively correlated—as argued in §4—then equation (5.3) becomes functionally equivalent to a ‘local maximum entropy production’ (LMEP) principle, which gives for the overall system:
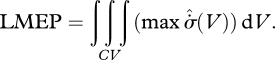 |
5.4 |
This is a much stronger condition than equation (5.2). By considerations of integral calculus (Zwillinger 2003), the two bounds are related by
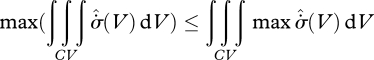 |
5.5 |
since the left-hand side could possess regions of 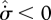 , compensated by other regions of greater
, compensated by other regions of greater 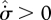 . This, however, runs against an argument used by Prigogine (1967, 1980): how can a system possibly ‘know’ that it can consume entropy in some regions, which will be compensated by greater entropy production in others? Indeed, we could construct a smaller control volume containing only the entropy-consuming elements, which would continuously violate the second law of thermodynamics. It is for this sound reason that the MEP principle must be a local principle, applicable at all volume scales. With the restriction
. This, however, runs against an argument used by Prigogine (1967, 1980): how can a system possibly ‘know’ that it can consume entropy in some regions, which will be compensated by greater entropy production in others? Indeed, we could construct a smaller control volume containing only the entropy-consuming elements, which would continuously violate the second law of thermodynamics. It is for this sound reason that the MEP principle must be a local principle, applicable at all volume scales. With the restriction 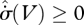 , we see that the two maxima in equation (5.5) coincide, and so the MEP heuristic (with its assumption of many degrees of freedom) becomes equivalent to the local formulation.
, we see that the two maxima in equation (5.5) coincide, and so the MEP heuristic (with its assumption of many degrees of freedom) becomes equivalent to the local formulation.
6. Conclusions
This study examines the meaning and implications of a new formulation of non-equilibrium thermodynamics applicable to flow and/or chemical reaction systems at steady state (Niven 2009b). This provides a very different, conditional derivation of the MEP principle, based on minimization of a dimensionless, local, free-energy-like potential function ϕst. The analysis encompasses all biological and ecological systems. Firstly, the basis of the derivation and the meaning of ϕst are examined. The flux entropy 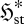 used in the analysis is then shown to represent the ‘spread’ of the distribution of instantaneous fluxes and/or reaction rates through or within the element. Since a flow system can access states of reverse flow or reaction, a high flux entropy is consistent with higher variability and thus with chaotic or oscillatory processes. In this respect, the term ‘steady state’ is therefore something of a misnomer, since it refers only to the constancy of the mean bulk flows and not their temporal and spatial variability. One consequence, examined through a specific example, is the coexistence of energy producers and consumers in ecological systems.
used in the analysis is then shown to represent the ‘spread’ of the distribution of instantaneous fluxes and/or reaction rates through or within the element. Since a flow system can access states of reverse flow or reaction, a high flux entropy is consistent with higher variability and thus with chaotic or oscillatory processes. In this respect, the term ‘steady state’ is therefore something of a misnomer, since it refers only to the constancy of the mean bulk flows and not their temporal and spatial variability. One consequence, examined through a specific example, is the coexistence of energy producers and consumers in ecological systems.
The effects of reinforcement or competition between changes in flux entropy 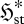 and entropy production
and entropy production 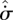 are then examined and classified. It is argued that in many systems, these two parameters should increase concurrently, enabling the steady-state position to be determined by the MEP principle. The ‘MEP heuristic’ used by MEP practitioners is then shown to be consistent with the present local formulation, with the additional assumption that the system has sufficient dynamic degrees of freedom that the MEP state can be physically realized.
are then examined and classified. It is argued that in many systems, these two parameters should increase concurrently, enabling the steady-state position to be determined by the MEP principle. The ‘MEP heuristic’ used by MEP practitioners is then shown to be consistent with the present local formulation, with the additional assumption that the system has sufficient dynamic degrees of freedom that the MEP state can be physically realized.
Acknowledgements
The author thanks the participants of the MEP workshops hosted by the Max-Planck-Institut für Biogeochemie, Jena, Germany, in 2007 and 2008, for valuable discussions; The University of New South Wales and the above Institute for financial support; and the European Commission for financial support as a Marie Curie Incoming International Fellow (2007–2008) under Framework Programme 6.
Endnote
One contribution of 17 to a Theme Issue ‘Maximum entropy production in ecological and environmental systems: applications and implications’.
Technically, the variation in 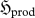 is written with a δ, since it is a ‘non-property’ of the system; however, for a reproducible phenomenon, it will be expressible in terms of other state functions of the system.
is written with a δ, since it is a ‘non-property’ of the system; however, for a reproducible phenomenon, it will be expressible in terms of other state functions of the system.
References
- Attard P.2006aStatistical mechanical theory for steady state systems. VI. Variational principles. J. Chem. Phys. 125, 214 502 (doi:10.1063/1.2400859) [DOI] [PubMed] [Google Scholar]
- Attard P.2006bTheory for non-equilibrium statistical mechanics. Phys. Chem. Chem. Phys. 8, 3585–3611 (doi:10.1039/b604284h) [DOI] [PubMed] [Google Scholar]
- Ayres R. U.1994Information, entropy and progress. Woodbury, NY: AIP [Google Scholar]
- Bénard H.1901Les tourbillons cellulaires dans une nappe liquide transportant de la chaleur par convection en régime permanent. Ann. Chim. Phys. 23, 62–144 [Google Scholar]
- Botrić S., Županović P., Juretić D.2005Is the stationary current distribution in a linear planar electric network determined by the principle of maximum entropy production? Croatica Chemica Acta 78, 181–184 [Google Scholar]
- Bruers S.2007Classification and discussion of macroscopic entropy production principles. (http://arxiv.org/cond-mat/0604482v3) [Google Scholar]
- Bruers S., Maes C., Netočný K.2007aOn the validity of entropy production principles for linear electrical circuits. J. Stat. Phys. 129, 725–740 (doi:10.1007/s10955-007-9412-z) [Google Scholar]
- Christen T.2006Application of the maximum entropy production principle to electrical systems. J. Phys. D: Appl. Phys. 39, 4497–4503 (doi:10.1088/0022-3727/39/20/030) [Google Scholar]
- Christen T.2007aA maximum entropy production model for Teflon ablation by arc radiation. J. Phys. D: Appl. Phys. 40, 5719–5722 (doi:10.1088/0022-3727/40/18/031) [Google Scholar]
- Christen T.2007bModelling diffusion in nonuniform solids using entropy production rate. J. Phys. D: Appl. Phys. 40, 5723–5726 (doi:10.1088/0022-3727/40/18/032) [Google Scholar]
- Clausius R.1865Über verschiedene für die Anwendung bequeme Formen der Hauptgleichungen der mechanischen WŠrmetheorie. Poggendorfs Annalen 125, 335–400 [Google Scholar]
- Craig N. C.1988Entropy analysis of four familiar processes. J. Chem. Ed. 65, 760–764 (doi:10.1021/ed065p760) [Google Scholar]
- Dewar R. C.2003Information theory explanation of the fluctuation theorem, maximum entropy production and self-organized criticality in non-equilibrium stationary states. J. Phys. A 36, 631–641 (doi:10.1088/0305-4470/36/3/303) [Google Scholar]
- Dewar R. C.2005Maximum entropy production and the fluctuation theorem, J. Phys. A 38, L371–L381 (doi:10.1088/0305-4470/38/21/L01) [Google Scholar]
- Dewar R. C., Porté A.2008Statistical mechanics unifies different ecological patterns. J. Theor. Biol. 251, 389–403 (doi:10.1016/j.jtbi.2007.12.007) [DOI] [PubMed] [Google Scholar]
- Dewar R. C., Juretić D., Županović P.2006The functional design of the rotary enzyme ATP synthase is consistent with maximum entropy production. Chem. Phys. Lett 430, 177–182 (doi:10.1016/j.cplett.2006.08.095) [Google Scholar]
- Edelstein-Keshet E.1988Mathematical models in biology Philadelphia, PA: SIAM [Google Scholar]
- Fermi E.1956Thermodynamics Mineola, NY: Dover [Google Scholar]
- Gibbs J. W.1875–1878On the equilibrium of heterogeneous substances. Trans. Connecticut Acad. 3, 108–248; 343–524 [Google Scholar]
- Jaynes E. T.1957Information theory and statistical mechanics. Phys. Rev. 106, 620–630 (doi:10.1103/PhysRev.106.620) [Google Scholar]
- Jaynes E. T.1963Information theory and statistical mechanics. In Brandeis University Summer Institute, Lectures in Theoretical Physics, vol. 3, Statistical Physics (ed Ford K. W.), pp. 181–218 Menlo Park, CA: Benjamin-Cummings [Google Scholar]
- Jaynes E. T.2003Probability theory: the logic of science (ed. Bretthorst G. L.). Cambridge, UK: Cambridge University Press [Google Scholar]
- Jørgensen S. E.2006Eco-exergy as sustainability Southampton, UK: WIT Press [Google Scholar]
- Juretić D., Županović P.2003Photosynthetic models with maximum entropy production in irreversible charge transfer steps. Comput. Biol. Chem. 27, 541–553 (doi:10.1016/j.compbiolchem.2003.09.001) [DOI] [PubMed] [Google Scholar]
- Kapur J. N., Kesevan H. K.1992Entropy optimization principles with applications Boston, MA: Academic Press [Google Scholar]
- Kleidon A.2004Beyond Gaia: thermodynamics of life and Earth system functioning. Clim. Change 66, 271–319 (doi:10.1023/B:CLIM.0000044616.34867.ec) [Google Scholar]
- Kleidon A., Lorenz R. D. (eds) 2005Non-equilibrium thermodynamics and the production of entropy: life, earth and beyond Heidelberg, Germany: Springer [Google Scholar]
- Kullback S., Leibler R. A.1951On information and sufficiency. Ann. Math. Stat. 22, 79–86 (doi:10.1214/aoms/1177729694) [Google Scholar]
- Lorenz R. D.2001Of course Ganymede and Callisto have oceans: application of a principle of maximum entropy production to icy satellite convection. Proc. Lunar Planet Sci. Conf. vol. 32, abstract no.1160. [Google Scholar]
- Martyushev L. M. Do nonequilibrium processes have features in common? 2007 (http://arxiv.org/abs/0709.0152v1. ) [Google Scholar]
- Martyushev L. M., Axelrod E. G.2003From dendrites and S-shaped growth curves to the maximum entropy production principle. J. Exp. Theor. Phys. Lett. 78, 476–479 (doi:10.1134/1.1637697) [Google Scholar]
- Martyushev L. M., Seleznev V. D.2006Maximum entropy production principle in physics, chemistry and biology. Phys. Rep. 426, 1–45 (doi:10.1016/j.physrep.2005.12.001) [Google Scholar]
- Martyushev L. M., Nazarova A. S., Seleznev V. D.2007On the problem of the minimum entropy production in the nonequilibrium stationary state. J. Phys. A: Math. Theor. 40, 371–380 (doi:10.1088/1751-8113/40/3/002) [Google Scholar]
- Massieu M.1869Thermodynamique—Sur les fonctions caractéristiques des divers fluides. Comptes Rendus 69, 858–862; 1057–1061 [Google Scholar]
- Meysman F. J. R., Bruers S.2007A thermodynamic perspective on food webs: quantifying entropy production within detrital-based ecosystems. J. Theor. Biol. 249, 124–139 [DOI] [PubMed] [Google Scholar]
- Moran M. J., Shapiro H. N.2006Fundamentals of engineering thermodynamics, 5th edn New York, NY: Wiley [Google Scholar]
- Niven R. K.2005Exact Maxwell–Boltzmann, Bose–Einstein and Fermi–Dirac statistics. Phys. Lett. A 342, 286–293 (doi:10.1016/j.physleta.2005.05.063) [Google Scholar]
- Niven R. K.2006Cost of s-fold decisions in exact Maxwell–Boltzmann, Bose–Einstein and Fermi–Dirac statistics. Physica A 365, 142–149 (doi:10.1016/j.physa.2006.01.021) [Google Scholar]
- Niven R. K.2007Origins of the combinatorial basis of entropy. In MaxEnt07, AIP Conf. Proc. vol. 954 (eds Knuth K. H., Caticha A., Center J. L., Giffon A., Rodriguez C. C.), pp. 133–142 Melville, NY: Amercian Institute of Physics [Google Scholar]
- Niven R. K.2009aCombinatorial entropies and statistics. Eur. Phys. J. B 70, 49–63 (doi:10.1140/epjb/e2009-00168-5) [Google Scholar]
- Niven R. K.2009bSteady state of a dissipative flow-controlled system and the maximum entropy production principle. Phys. Rev. E 80, 021113 (doi:10.1103/PhysRevE.80.021113) [DOI] [PubMed] [Google Scholar]
- Niven R. K., Grendar M.2009Generalized classical, quantum and intermediate statistics and the Polya urn model. Phys. Lett. A 373, 621–626 [Google Scholar]
- Onsager L.1931aReciprocal relations in irreversible processes I. Phys. Rev. 37, 405–426 (doi:10.1103/PhysRev.37.405) [Google Scholar]
- Onsager L.1931bReciprocal relations in irreversible processes II. Phys. Rev. 38, 2265–2279 (doi:10.1103/PhysRev.38.2265) [Google Scholar]
- Ozawa H., Shikokawa S., Sakuma H.2001Thermodynamics of fluid turbulence: a unified approach to the maximum transport properties. Phys. Rev. E 64, 026 303 (doi:10.1103/PhysRevE.64.026303) [DOI] [PubMed] [Google Scholar]
- Ozawa H., Ohmura A., Lorenz R. D., Pujol T.2003The second law of thermodynamics and the global climate system: a review of the maximum entropy production principle, Rev. Geophys. 41, 1018 (doi:10.1029/2002RG000113) [Google Scholar]
- Paltridge G. W.1975Global dynamics and climate—a system of minimum entropy exchange. Q. J. R. Meteorol. Soc. 101, 475–484 (doi:10.1002/qj.49710142906) [Google Scholar]
- Paltridge G. W.1978The steady-state format of global climate. Q. J. R. Meteorol. Soc. 104, 927–945 (doi:10.1002/qj.49710444206) [Google Scholar]
- Planck M.1922Treatise on thermodynamics (Engl. transl.), 3rd edn Mineola, NY: Dover Publications [Google Scholar]
- Planck M.1932Introduction to theoretical physics, Vol. V: theory of heat (Engl. transl Brose H. L.). New York, NY: Macmillan [Google Scholar]
- Prigogine I.1967Introduction to thermodynamics of irreversible processes, 3rd edn New York, NY: Interscience [Google Scholar]
- Prigogine I.1980From being to becoming: time and complexity in the physical sciences San Francisco, CA: W.H. Freeman [Google Scholar]
- Schneider E. D., Sagan D.2005Into the cool: energy flow, thermodynamics and life Chicago, IL: University of Chicago Press [Google Scholar]
- Schrödinger E.1944What is life? Cambridge, UK: Cambridge University Press; (see chapter 6 and following Note) [Google Scholar]
- Shannon C. E.1948A mathematical theory of communication. Bell Sys. Tech. J 27, 379–423; 623–659 [Google Scholar]
- Strong L. E., Halliwell H. F.1970An alternative to free energy for undergraduate instruction. J. Chem. Ed. 47, 347–352 (doi:10.1021/ed047p347) [Google Scholar]
- Tribus M.1961aInformation theory as the basis for thermostatics and thermodynamics. J. Appl. Mech. Trans. ASME 28, 1–8 [Google Scholar]
- Tribus M.1961bThermostatics and thermodynamics Princeton, NJ: D. Van Nostrand [Google Scholar]
- Vanyo J. P., Paltridge G. W.1981A model for energy dissipation at the mantle-core boundary. Geophys. J. R. Astron. Soc 66, 677–690 [Google Scholar]
- Yoshida Z., Mahajan S. M.2008Maximum entropy production in self-organized plasma boundary layer: a thermodynamic discussion about turbulent heat transport. Phys. Plasmas 15, 032 307 (doi:10.1063/1.2890189) [Google Scholar]
- Županović P., Juretić D., Botrić S.2004Kirchhoffs loop law and the maximum entropy production principle. Phys. Rev. E 70, 056 108 (doi:10.1103/PhysRevE.70.056108) [DOI] [PubMed] [Google Scholar]
- Županović P., Botrić S., Juretić D.2006Relaxation processes, MaxEnt formalism and Einstein's formula for the probability of fluctuations. Croatica Chemica Acta 79, 335–338 [Google Scholar]
- Zwillinger D.2003CRC Standard mathematical tables and formulae Boca Raton, FL: CRC Press/Chapman & Hall [Google Scholar]