Abstract
The high incidence of low sperm counts in young (European) men and evidence for declining sperm counts in recent decades mean that the environmental/lifestyle impact on spermatogenesis is an important health issue. This review assesses potential causes involving adverse effects on testis development in perinatal life (primarily effects on Sertoli cell number), which are probably irreversible, or effects on the process of spermatogenesis in adulthood, which are probably mainly reversible. Several lifestyle-related (obesity, smoking) and environmental (exposure to traffic exhaust fumes, dioxins, combustion products) factors appear to negatively affect both the perinatal and adult testes, emphasizing the importance of environmental/lifestyle impacts throughout the life course. Apart from this, public concern about adverse effects of environmental chemicals (ECs) (pesticides, food additives, persistent pollutants such as DDT, polychlorinated biphenyls) on spermatogenesis in adult men are, in general, not supported by the available data for humans. Where adverse effects of ECs have been shown, they are usually in an occupational setting rather than applying to the general population. In contrast, a modern Western lifestyle (sedentary work/lifestyle, obesity) is potentially damaging to sperm production. Spermatogenesis in normal men is poorly organized and inefficient so that men are poorly placed to cope with environmental/lifestyle insults.
Keywords: Sertoli cells, spermatogenesis, obesity, smoking, scrotal heating, environmental chemicals
1. Introduction and background
In comparison to most animals, human fertility is remarkably low. The prevalence of couple infertility is extremely high and in many countries affects one in seven couples, with the most commonly identified cause being ‘male factor’. Indeed, in a series of prospective and well-standardized studies undertaken across Europe over the past 10 years, it has been found that the prevalence of an abnormally low sperm count (less than 20 million sperm ml−1; the cut-off for normal based on WHO standards) in young men (18–25 years of age) is as high as 15–20% (Jørgensen et al. 2006; Andersson et al. 2008). Another remarkable feature about semen quality in normal young men is that only a low percentage of the sperm that are actually produced can be classified as normal (5–15% depending on how strict the criteria of normality used are; WHO 1999), which is remarkably lower than in domestic (bull, ram) or laboratory (rat, mouse) animals in which greater than 90 per cent of sperm can usually be classified as normal. This suggests there are fundamental differences between spermatogenesis in the human and other species that result in production of lower quality sperm overall. This may make spermatogenesis in humans inherently more vulnerable to disruption by outside factors, as there is little room for manoeuvre in terms of maintaining the production of adequate numbers of normal sperm, and thus fertility. Such concerns are reinforced by the evidence that sperm counts in humans may have declined substantially over the past 50 years or so, although this remains controversial (Swan et al. 2000). There is also good evidence for considerable geographical variation in sperm counts, which could indicate variation in environmental exposures and/or in genetic/ethnic influences (reviewed in Sharpe 2009).
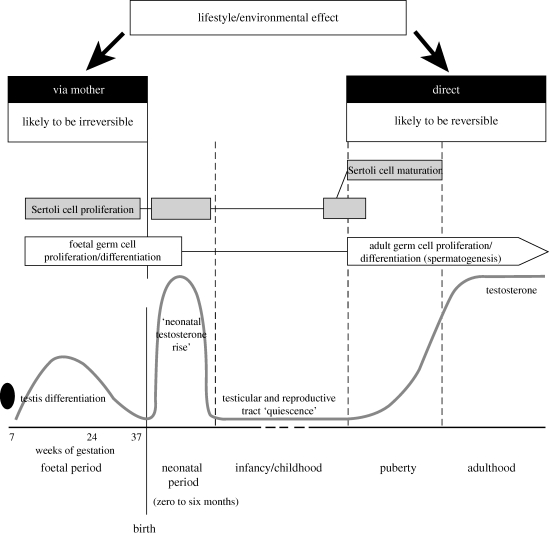
The process of spermatogenesis is not initiated until puberty and is then maintained throughout the rest of life in normal men. It is, therefore, only during this period that the spermatogenic process itself is directly vulnerable to adverse effects resulting from lifestyle of the man and/or his exposure to toxic agents from the general environment or as a result of his occupation. However, the foundations for spermatogenesis are laid during foetal development, and it is recognized increasingly that disturbance of events at this time may have subsequent impact on the scale or the quality of spermatogenesis in adulthood. Therefore, in this chapter, the impact of environmental and lifestyle factors on spermatogenesis will consider also the effects that may occur in foetal life and how this might then impact on spermatogenesis in adulthood (figure 1).
Figure 1.
Known times at which environmental/lifestyle factors can negatively impact on spermatogenesis and sperm counts in human males in relation to the major relevant events in testis development and function. Details are provided in the text.
2. Foetal determinants of spermatogenesis in adulthood
The testis differentiates from a sexually indifferent genital ridge, and it is this differentiation process that sets up the structure and cellular organization of the testis. The first cells to differentiate, and to set the wider process of testis differentiation underway, are the Sertoli cells, and it is thought that it is via signals from these cells that much of the subsequent organization within the testis is directed and coordinated, although the mechanisms involved are poorly understood (Brennan & Capel 2004). A critical event is the formation of seminiferous cords, the precursors of the seminiferous tubules (Combes et al. 2009). The first step in this process involves the Sertoli cells surrounding the germ cells that have migrated into the gonad, and the process is completed by the attraction of peritubular myoid cells which encircle the Sertoli–germ cell complex to form a seminiferous cord (Brennan & Capel 2004; Combes et al. 2009). These cords anchor themselves at both ends in the rete testis and subsequently elongate enormously as the Sertoli cells and germ cells proliferate. At the same time, foetal Leydig cells differentiate in the interstitial spaces between the seminiferous cords and begin to produce testosterone, which initiates masculinization of the foetus (Sharpe 2006); this will not be discussed further here.
(a). Sertoli cell proliferation and final number
There are important effects of the testosterone produced by the Leydig cells within the developing testis itself, notably to stimulate the proliferation of Sertoli cells. Thus, knock-out of the androgen receptor (AR) results in approximately 50 per cent reduction in Sertoli cell numbers at birth, and further experimental studies in rats have since confirmed the importance of foetal testosterone for determining Sertoli cell number (Johnston et al. 2004; Scott et al. 2007, 2008). It had not previously been suspected that testosterone might have this effect because ARs are not expressed in foetal Sertoli cells in any species studied (Sharpe 2005). Testosterone effects on the Sertoli cells are probably mediated via the adjacent peritubular myoid cells that express ARs strongly during foetal life (Scott et al. 2007); however, direct evidence for this and the signalling mechanisms involved remain unknown.
The importance of Sertoli cell proliferation in foetal life is that the number of sperm produced in adulthood is critically determined by the number of Sertoli cells within the testis, as each Sertoli cell can support only a finite number of germ cells through development into sperm (Orth et al. 1988; Sharpe et al. 2003); the number of germ cells supported varies between species (Sharpe 1994). Sertoli cells proliferate in foetal life, in the immediate postnatal/neonatal period and just prior to puberty (figure 1) (Sharpe et al. 2003), although the relative importance of proliferation during these three periods may not be equal and the factors that regulate Sertoli cell proliferation may also vary; testosterone appears to be most important in the foetal and early neonatal period, whereas follicle-stimulating hormone (FSH) is probably more important subsequent to this and especially during puberty (Sharpe et al. 2003). It is possible that lifestyle/environmental factors acting during any of these three periods could interfere with Sertoli cell proliferation and thus delimit the final Sertoli cell number. This would reduce the maximum number of sperm that could then be produced, and in humans in whom there is little, if any, sperm storage, this would directly impact on sperm counts (Sharpe et al. 2003). It is established that the marked variation in sperm counts found between individual normal men in adulthood is likely to be determined by extraordinarily high variation in the number of Sertoli cells (Sharpe et al. 2003), but otherwise details for humans are lacking. However, as detailed below, maternal lifestyle factors in pregnancy can have quite substantial effects on sperm counts in sons in adulthood and the most logical mechanism via which this could occur is via reducing Sertoli cell number (figure 1).
(b). Perinatal germ cell development and relationship to spermatogenesis
Sperm cannot be made in adulthood unless there is a sufficient pool of normal germ cells in the form of spermatogonia and spermatogonial stem cells. These have to differentiate from foetal germ cells. Therefore, as with the Sertoli cells, it is important that germ cells proliferate and differentiate normally during foetal life and in the postnatal period to ensure that adequate numbers are in place to facilitate normal spermatogenesis in adulthood (figure 1). Germ cell differentiation in perinatal life and the regulatory processes involved are poorly understood (Orth et al. 2000; Culty 2009). Based on rodent studies, there are three important events (Orth et al. 2000; Culty 2009). First, the foetal germ cells have to lose their pluripotency, typified by expression of a number of protein markers shared with embryonic stem cells (e.g. OCT4). Failure to execute this differentiation step results in persistence of pluripotency characteristics, which is dangerous as such cells can potentially differentiate into various tissues. Such a failure is thought to be the underlying mechanism via which carcinoma-in situ (CIS) cells are formed in the human, and it is from these CIS cells that testicular germ cell cancer (TGCC) subsequently develops in young adulthood (Rajpert-De Meyts 2006). Second, the germ cells enter a period of quiescence, i.e. cease proliferation; this is well established in rodents, but a period of quiescence has not yet been defined in the marmoset and humans (Mitchell et al. 2008). Third, the differentiated germ cells must migrate to the basal lamina and position themselves underneath the Sertoli cells so as to provide the spermatogonial stock from which spermatogenesis will be delivered thereafter. In rodents, this migration takes place at around birth, and initially involves the Kit ligand-ckit system (Orth et al. 2000; Culty 2009), but it is uncertain when migration occurs in the human and in other primates as the arrangement of germ cells at birth and in the neonatal period is quite haphazard and the majority are not located at the basal lamina (Mitchell et al. 2008). It is possible that this migration may not occur until near puberty. Failure to execute the first and last of these three steps would be prejudicial to future spermatogenesis, but it is uncertain whether the period of quiescence is also essential.
(c). Testicular germ cell cancer and testicular dysgenesis syndrome
The incidence of TGCC has increased progressively in many Western nations over the past 60 years, and among Caucasians is the commonest cancer of young men (Bray et al. 2006). This increase is too rapid to be explained by genetic factors and is therefore presumed to have environmental/lifestyle causes. This is reinforced by studies of migrants from a country with a high incidence of TGCC (e.g. Denmark) to a country with a lower incidence (e.g. Finland, Sweden; Hemminki & Chen 2006). Risk of TGCC in migrant males is comparable to their country of origin, but incidence of risk in children born in the country to which their parents have emigrated is the same as in that country. The causes of the increase in TGCC and the between-country differences are unexplained, but differential exposure to environmental chemicals (ECs) has been suggested as one possibility for which there is limited evidence (Sharpe 2009). Other disorders of male reproductive development, such as cryptorchidism and hypospadias, are associated with increased risk of TGCC, and individuals with low sperm counts/infertility in adulthood are also at increased risk of TGCC (Skakkebaek et al. 2001; Sharpe 2009). Such observations have given rise to the testicular dysgenesis syndrome (TDS) hypothesis which proposes that each of these disorders may have a common foetal origin triggered by maldevelopment of the testis, leading to malfunction of the somatic cells and thence to increased risk of the TDS disorders (Skakkebaek et al. 2001). However, only a proportion of cases of low sperm counts, cryptorchidism and hypospadias will originate because of TDS.
(d). Maternal lifestyle/environmental effects on son's sperm counts/Sertoli cell number
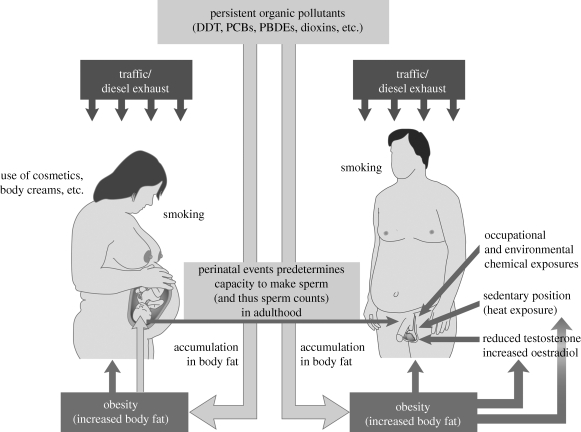
Impaired testosterone production or action is thought to be a likely factor in TDS, and this might alter Sertoli cell proliferation as outlined above and thus lower sperm counts in adulthood (Skakkebaek et al. 2001; Sharpe 2009). The mechanisms that interlink TDS disorders and the exogenous factors that can impinge on and disrupt these remain largely unknown. However, the increase in incidence of TGCC during a period when there have been dramatic changes in Western lifestyle, diet, exercise, etc. suggests that one or more of these changes could be aetiologically involved (figure 2). For example, the incidence of female obesity has increased considerably, and one preliminary study has suggested that high maternal BMI negatively affects semen quality in resulting sons when they grow to adulthood, with indirect evidence pointing towards reduced Sertoli cell number (Ramlau-Hansen et al. 2007a). Over the same time period, exposure to a wide range of ECs has increased, several of which are clearly documented to have intrinsic endocrine-disrupting activity, in particular anti-androgenic activity. Studies in laboratory animals have shown that such chemicals on their own or in mixtures can disrupt masculinization, lending credence to the possibility that they might exert similar effects in humans (Sharpe 2009). Supporting evidence for this is beginning to emerge, but is by no means definitive, and the evidence linking such exposures in perinatal life to low sperm counts in adulthood is, with one notable exception (see below), non-existent. Interactions between lifestyle/diet and chemical exposures also need to be considered (figure 2). For example, many of the persistent organochlorine compounds, including various pesticides and polychlorinated biphenyls (PCBs), are lipophilic and accumulate in fat. In theory, obese women will have accumulated more of such compounds, which are then mobilized in pregnancy/lactation and delivered to the baby/infant (Chevrier et al. 2000), although other studies suggest that (older) age is a far more important determinant of the body burden of organochlorine contaminants (Hue et al. 2007), consistent with the progressive decline in environmental levels of such compounds (and thus of human exposure) in recent decades.
Figure 2.
Diagrammatic illustration of the main environmental/lifestyle factors established to negatively impact on spermatogenesis and sperm counts in human males, whether via the mother during foetal development of the testis or directly on the testis during adulthood. Note that several factors have similarly negative effects at both life stages. Details are provided in the text.
Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) is a highly toxic by-product of combustion processes such as incineration, and certain other polychlorinated compounds (certain PCBs, dibenzofurans) also have dioxin-like activity. Within the body, dioxins interact with the aryl hydrocarbon (Ah) receptor, and a number of other environmental compounds also interact with this receptor, such as polycyclic aromatic hydrocarbons (PAHs) that are constituents of exhaust fumes, smoke and cooking processes. Human exposure during pregnancy to dioxin as a result of the Seveso accident in 1976 resulted in lower sperm counts in ‘exposed’ (foetal) males in adulthood whereas those who were exposed to dioxin as adult men showed no effect (Mocarelli et al. 2008). In laboratory animal studies, similar effects of dioxin exposure in foetal life on adult sperm counts have been found (Gray et al. 1995). Similarly, foetal exposure of laboratory animals to diesel exhaust results in reduced sperm production in adulthood (Takeda et al. 2004), an effect partly explained by reduced Sertoli cell numbers (Watanabe 2005), and which is thought to be mediated by activation of the Ah receptor (Izawa et al. 2007). Evidence from cell transfection experiments suggests that activation of the Ah receptor can antagonize AR-mediated action (Kizu et al. 2003; Barnes-Ellerbe et al. 2004), providing a potential pathway for dioxin-induced reduction in Sertoli cell number. Furthermore, exposure of pregnant women to diesel/car exhaust fumes could have affected Sertoli cell number and lowered sperm counts in resulting male offspring in adulthood via Ah receptor-mediated actions (figure 2). If so, this effect will have been higher (in mothers) in the past in Western countries in which atmospheric pollution has improved considerably in recent decades, but may have worsened in some developing countries. There is no direct evidence to support this possibility, but there is evidence that smoking by mothers during pregnancy can dramatically lower their sons’ sperm counts in adulthood.
In several large studies, substantial reductions in sperm counts (approx. 40% in three studies) were found in men whose mothers had smoked heavily in pregnancy (Storgaard et al. 2003; Jensen et al. 2004a,b, 2005; Ramlau-Hansen et al. 2007b). As most of these studies did not find significant effects on the quality of sperm produced, the tentative interpretation was that this reflected a reduction in Sertoli cell number. Whatever the explanation, the initial trigger will probably have involved interaction of PAHs (or other constitutents) in the cigarette smoke with the Ah receptor, although other mechanisms are possible. A similar pathway might explain the results of one study in the USA that showed that high consumption of beef by mothers during pregnancy was associated with a significant reduction in sperm counts in their sons in adulthood (Swan et al. 2007). However, this could also be due to saturated fats in meats or to other compounds, such as exposure of the foetus to anabolic steroids present in the carcasses of the beef as these were used widely to enhance growth in cattle in the USA in the relevant period; at the time, diethylstilboestrol was a widely used growth promoter.
Other than the above, no other studies have identified a specific lifestyle or environmental exposure during pregnancy with an effect on sperm counts in the human male offspring in adulthood, although several lines of investigation show associations between maternal exposure to a range of persistent ECs (e.g. PCBs, polybrominated compounds) or compounds (e.g. certain phthalates) present in cosmetics/toiletries/medications during pregnancy and increased risk of TDS disorders other than low sperm counts (figure 2) (reviewed in Sharpe 2009). However, there is little consistency between studies in associations between exposure to a specific chemical or chemical class and risk of TDS disorders. Therefore, if there are such effects on the developing foetal testis, they are likely to occur as the result of exposure to a mixture of ECs. Emerging data for mixtures studies in laboratory animals show that these have pronounced adverse effects on male reproductive development (Howdeshell et al. 2008; Rider et al. 2008), although sperm counts have not yet been studied in this context.
One interesting finding to have emerged from studies involving exposure of foetal rats to anti-androgenic chemicals during specific foetal time windows is that exposure during what is termed the ‘masculinization programming window’ is associated in adulthood with reduced testicular size and presumably therefore reduced sperm production, although the basis for this relationship has yet to be established as it does not appear to involve a straightforward relationship with Sertoli cell number (Scott et al. 2008). Nevertheless, this finding could indicate that any EC that affects testosterone production by the foetal testis during this time period may have later consequences in terms of reduced sperm production/reduced sperm counts. This sort of mechanism may underlie the effects described above for dioxin/maternal smoking, as there is indirect evidence that dioxin exposure at least may result in reduced androgen action during the masculinization programming window (Ohsako et al. 2002) based on measurement of anogenital distance (Welsh et al. 2008).
3. Lifestyle effects on spermatogenesis in adulthood
Numerous studies in laboratory animals have investigated whether exposure in adulthood to a wide range of chemical compounds can impair spermatogenesis. Many have used high dose levels that probably have no counterpart in terms of human environmental exposure, and their relevance to effects on spermatogenesis in human males is therefore difficult to evaluate. This literature is too vast and diverse to be considered here, so the focus is on the evidence for environmental/lifestyle effects on spermatogenesis, sperm counts or fertility in human males, with reference to the experimental animal studies where appropriate to provide better understanding for the basis for the human effects. In taking this approach, it is recognized that demonstration of cause and effect in human studies is inordinately more difficult than in laboratory animal studies because of the greater ease of intervention and measurement in the latter, but also because laboratory animals are very much less variable in their phenotype and spermatogenesis profile in comparison to the huge variation seen among normal adult human males (WHO 1999; Sharpe 2000). Additionally, human males are exposed in reality to a complex mixture of ECs that may cause additive or interactive effects on spermatogenesis, and this may confound interpretation of studies in which exposure to only one chemical is evaluated (Sharpe 2009). Even when considering exposure to one EC, men will be variably exposed and will have variation in sperm counts between them to begin with, in contrast to the situation in experimental animals where they are exposed to a constant dose under test circumstances and there is little variation between animals in spermatogenesis. Therefore, it is not a comparison of ‘like with like’ and account has to be taken of the very different situation in which human studies are undertaken in comparison to laboratory animal studies; accordingly, a different level of proof is probably required to establish cause and effect. Furthermore, there are important differences between species in the set-up and organization of spermatogenesis that may affect vulnerability of the testis to disruption by exogenous factors (Sharpe 1994).
(a). Species differences in organization and efficiency of spermatogenesis
Spermatogenesis in the adult human male is differently organized from that in laboratory animals (table 1). The most notable difference is that in laboratory animals, and in most domestic animals and most non-human primates, spermatogenesis is a highly organized process in which synchronized development of germ cells occurs in an apparently highly organized and efficient manner (Sharpe 1994). Thus, in each seminferous tubule cross section, the collection of germ cells that are present in association with the Sertoli cells is homogeneous and is commonly referred to as radial organization of the stages of the spermatogenic cycle (Sharpe 1994). In contrast, in the human, organization of the spermatogenic stages appears haphazard, such that within any seminiferous tubule cross section, there may be two to five stages of the spermatogenic cycle present as ‘islands’ next to each other. This ‘asynchronous organization’ is similar to what is observed in late foetal and early postnatal life in humans when there is asynchronous proliferation and differentiation of foetal germ cells (Mitchell et al. 2008), whereas in laboratory animals, these events are highly synchronous (Orth et al. 2000; Mitchell et al. 2008; Culty 2009). Whether there is a relationship between the ‘asynchrony’ of germ cell development/organization in foetal life and that in adulthood is unknown.
Table 1.
Comparison of the phases, pace and other features of testis development and spermatogenesis in rodents and humans relevant to sperm count/quality in adulthood and which may affect predisposition to lifestyle/environmental effects and limit usefulness of rodents as a model for humans.
| parameter | rodents | humans |
|---|---|---|
| Sertoli cell proliferation | foetal, neonatal/prepubertal; maturation at puberty | foetal, neonatal, prepubertal; maturation at puberty |
| perinatal germ cell proliferation and differentiation | synchronous proliferation and differentiation (rapid changes) | asynchronous proliferation and differentiation (spread over foetal and early postnatal life) |
| neonatal/infant period of hypothalamic-pituitary-testis hormone activity | lasts approximately 6 h | lasts four to five months |
| childhood testis ‘quiescence’ | none | lasts 11–14 years |
| puberty | reactivation of hypothalamic-pituitary-testis hormone activity | reactivation of hypothalamic-pituitary-testis hormone activity |
| spermatogenesis | synchronous (‘organized’); highly efficient | asynchronous (‘disorganized’); inefficient |
| sperm count/sperm storage | sperm stored in epididymis, so high sperm count can be maintained during repeated ejaculation | minimal/no sperm storage, so ejaculatory frequency is an important determinant of sperm count |
Asynchronous organization of spermatogenesis has been described also in chimpanzees (Smithwick & Young 1996) and marmosets (Millar et al. 2000), but only the latter have been investigated in detail. In the marmoset, as in the human (Sharpe 1994), the efficiency of spermatogenesis is poor when compared with animals with a radial/synchronized organization of spermatogenic stages (Sharpe et al. 2000); the term ‘efficiency’ is used here to describe the number of germ cells that each Sertoli cell supports during their development into sperm. The asynchronous nature of spermatogenic organization is a potential explanation for the poor semen quality in humans (Sharpe 1994). The basis for this is not entirely clear, but as it is the Sertoli cells that create the environment in which the germ cells can develop normally through spermatogenesis, the heterogeneity of the environment within a seminiferous tubule containing mixed seminiferous tubule stages may not be as conducive to efficient germ cell development within individual stages as is the case in laboratory animals when all Sertoli cells are doing the same thing.
It is unclear whether the species difference in organization of spermatogenesis renders the human more susceptible to perturbation of spermatogenesis by exogenous factors, but common sense dictates that inefficient spermatogenesis has less room in which to compensate for any such effects when compared with highly efficient spermatogenesis. An additional factor is that humans, in contrast to rodents and most domestic species, do not store sperm to any extent (table 1), with the result that ejaculatory frequency can have a profound effect on sperm count in the ejaculate (Irvine 1998). If sperm production is reduced by any environmental exposures in humans, it may be that the consequences in terms of fertility potential are exacerbated by the lack of sperm storage. If this is the case, then, dose for dose, ECs or lifestyle could have a proportionately greater effect in humans than in laboratory animals, and this may need to be factored into risk assessment.
There are other important differences between humans and laboratory animals in terms of the phases and duration of testis development (table 1). Two features in humans that particularly differ from laboratory animals are the occurrence of an extended postnatal period of hypothalamic-pituitary-testicular hormone activation during which testosterone levels can rise to within the low adult range, while in laboratory animals any such activation lasts for a matter of hours at around the time of birth (Mann & Fraser 1996; Grumbach 2005). After this period, in the human, there is an extended phase of childhood testicular quiescence during which relatively little happens in terms of testis development, but also about which little is known. There is no corresponding period in laboratory animals, but there is in non-human primates, including in the marmoset (Kelnar et al. 2002). To what extent these differences may affect the development of spermatogenesis or its susceptibility to perturbation by exogenous factors is unknown.
(b). Scrotal heating and sedentary position
Testicular descent into the scrotum normally occurs by birth in boys and failure of testicular descent, especially when this extends into puberty and adulthood, results in absence of spermatogenesis. The testes descend into the scrotum in order that their temperature can be kept 3–4°C below core body temperature, as maintenance at normal body temperature is incompatible with spermatogenesis (Mieusset & Bujan 1995b; Setchell 1998). It is probably also important that the testes are descended into the bottom of the scrotum rather than being placed at the top where their proximity to the body surface is likely to impair cooling of the testis. This is mentioned because it is reckoned that failure of the testes to descend into the bottom of the scrotum should probably be classified as a form of cryptorchidism (Boisen et al. 2004). As well as testis position, the two other key elements in ensuring cooling of the testis are the presence of a vascular-rich corrugated scrotal surface via which heat loss can occur and the presence of an arterio-venous plexus (the pampiniform plexus) in the spermatic cord and which functions as a heat exchanger to cool incoming blood to the testis by heat exchange with the cooler venous blood that is exiting the testis (Maddocks et al. 1993; Piner et al. 2002). Normal functioning of this plexus is important for maintaining testicular coolness, and it is potentially susceptible to disruption by chemicals or by vascular-active drugs (Piner et al. 2002) or by disorders such as varicocele in which the veins in the plexus are varicosed (Turner 2001). However, even if the pampiniform plexus is functioning normally, it cannot cool the incoming arterial blood to the testis unless the blood leaving the testis is already itself cool, and this requires heat loss via the scrotal surface and its transmission to the underlying testes. Therefore, anything that impedes scrotal heat loss will affect testicular temperature and in turn any elevation of testicular temperature will have a harmful effect on spermatogenesis. In general, the more prolonged is the elevation in testicular temperature, then the greater will be the detrimental effect on spermatogenesis (Mieusset & Bujan 1995b; Setchell 1998).
The most obvious things that can affect scrotal heat loss are a febrile illness such as influenza, exposure to an exogenous heat source, such as occupationally (bakers, welders, foundry workers) or via taking a hot bath (Mieusset & Bujan 1995b; Thonneau et al. 1998). Based on experimental studies in laboratory animals, a 30 min soak in a moderately hot bath (40–42°C) impairs spermatogenesis (Setchell 1998) and, more importantly, it can induce germ cell apoptosis, DNA damage to the sperm and impair embryo development and fertility when ‘affected’ males are mated with normal females (Paul et al. 2008a,b). Follow-up studies have provided insight into the mechanisms involved (Paul et al. 2009). These have shown that heat exposure causes hypoxia and oxidative stress responses in the germ cells, manifest as increased expression of hypoxia inducible factor 1α, haem oxygenase 1, glutathione peroxidase 1 and glutathione-S-transferase-α, which push the germ cells towards apoptosis (Paul et al. 2009). Perhaps of more concern is if mild oxidative DNA damage is induced such that the germ cells continue their development into sperm, as this is associated with increased time for such sperm to initiate a pregnancy in humans (Loft et al. 2003). The adverse effects of scrotal heating on spermatogenesis and fertility are equally evident in non-human primates (Lue et al. 2002). Exposure to heat in other situations, such as in a hot shower, would have minimal effect as the scrotum is still able to thermo-regulate (it is not immersed in water) and a similar situation applies to saunas, although spending a long time in very hot saunas is detrimental.
Arguably of more concern are lifestyle and occupational factors that cause men to spend a long time in a sedentary position, something that has become common for many men working in Western countries today (figure 2). When seated, air does not circulate so easily around the scrotum and therefore there is less-efficient cooling, an effect likely to be exacerbated if wearing tight underpants or trousers. In studies of men in whom scrotal temperature was measured continuously in relation to position and activity, scrotal temperature increased progressively with duration of sedentation, and this was associated with lower sperm counts (Hjollund et al. 2000, 2002a,b). Studies in lorry and taxi drivers, who spend a long time seated, have also produced evidence for detrimental effects on semen quality (Figa-Talamanca et al. 1996; Bujan et al. 2000). However, overall, the relationship between time spent seated and poor semen quality is not suggestive of a major impact on fertility (Hjollund et al. 2000, 2002b; Stoy et al. 2004). Other studies have investigated the impact of wearing tight versus loose underwear and reached similar conclusions (Mieusset & Bujan 1995b). The most recent scenario investigated has been the impact on scrotal temperature of using a laptop computer (Sheynkin et al. 2005). It is perhaps more likely that scrotal heating may combine with or exacerbate adverse effects of other environmental/lifestyle factors and that only then will there be a significant impact on fertility (Lue et al. 2000).
Scrotal heating has been investigated as a potential contraceptive method in men and shown to be effective (Mieusset & Bujan 1995a). However, other studies that have tried to link more modest elevations in scrotal temperature (such as those associated with sedentary position) to infertility have not shown major or consistent associations, as outlined above. Nevertheless, it is common sense that any factor that impedes normal cooling of the scrotum/testes can only have an adverse effect on spermatogenesis, and it is therefore prudent to advise all men who are attempting to father a pregnancy, especially if they are known to have low sperm counts or low sperm motility, to take steps to minimize scrotal heating by any of the pathways mentioned above—where this has been done in a controlled way, the results have been positive (Jung et al. 2001). Such small lifestyle changes can only have a beneficial effect on spermatogenesis.
(c). Obesity
An important lifestyle-dependent factor that adversely affects spermatogenesis is obesity (figure 2). As 10–30% of adult men in Western countries are now obese, it is likely that this will have an increasing impact on male fertility (Hammoud et al. 2006, 2008; Nielsen et al. 2007). Several studies have shown up to a threefold higher incidence of obesity in infertile men than in those with normal semen quality (Magnusdottir et al. 2005; Hammoud et al. 2008); a BMI of more than 25 is associated with an average 25 per cent reduction in sperm count and sperm motility (Jensen et al. 2004a,b; Kort et al. 2006). A variety of explanations have been put forward to explain this association. The strongest evidence is that the alterations in sperm production are secondary to altered hormone changes. Obesity in men is associated with reduced blood testosterone levels, this reduction being proportional to the degree of obesity (e.g. Tchernof et al. 1995; Gould et al. 2007; Nielsen et al. 2007). In addition, there may be an increase in circulating oestradiol levels, leading to an altered testosterone : oestradiol ratio (Hammoud et al. 2006, 2008). As such patients often show reduced blood levels of LH (and FSH), when an increase might be expected in the face of reduced testosterone levels, one interpretation is that there is decreased intratesticular testosterone levels and thus reduced androgen drive to spermatogenesis. The best evidence supporting this interpretation is that suppression of oestradiol levels in obese men using aromatase inhibitors normalizes the testosterone : oestradiol ratio and improves semen quality (Raman & Schlegel 2002), and there are similar results for oligozoospermic dogs (Kawakami et al. 2004). However, there may also be intratesticular effects that are unrelated to altered gonadotrophin levels because the reduction in inhibin B levels in obese men is disproportionately larger than the change in FSH levels, suggesting there may be direct effects of the increased obesity on Sertoli function (Jensen et al. 2004a,b; Winters et al. 2006; Hammoud et al. 2008). Alternatively, it could indicate reduced Sertoli cell number in obese (young) men (Winters et al. 2006). The latter is a far more serious possibility as reduced Sertoli cell number would permanently lower sperm counts as discussed earlier; it is unclear how, or when, obesity would lead to a reduction in Sertoli cell number.
Another explanation for reduced spermatogenesis in obese men could be deposition of fat around the scrotal blood vessels, leading to impaired blood cooling and elevated testicular temperature (Shafik & Olfat 1981); the more sedentary life of obese men would probably exacerbate any temperature increase. Another potential argument to explain reduced sperm counts in obese men is that they accumulate increased amounts of toxicants in their adipose tissue because many of the persistent ECs are lipophilic (Pelletier et al. 2002; Hammoud et al. 2008), although present evidence does not support this idea (Magnusdottir et al. 2005). Nevertheless, considering the high prevalence of obesity among young men today and the equally high prevalence of low sperm counts, it is possible that the obesity epidemic may be having an impact on spermatogenesis among young men, and it may also render such individuals more susceptible to damaging effects by other lifestyle or environmental exposures. For certain, obesity looks set to play an important role in determining the hormonal and fertility profile of Western men over the coming decades (Jensen et al. 2004a,b; Nielsen et al. 2007).
(d). Smoking, alcohol and drugs
Of the Western lifestyle factors commonly suspected to have adverse effects on health, smoking and alcohol consumption usually come top of the list. There is little evidence that either of these has a major impact on spermatogenesis, although meta-analysis supports the view that smoking has a small negative impact (figure 2) (Vine et al. 1994; Vine 1996). Physiologically, the testis is considered to be poised on the brink of hypoxia, in part because of its high metabolic requirements owing to spermatogenesis but also because of its vascular supply in which approximately 50 per cent of incoming arterial blood is siphoned off via arterio-venous anastomoses in the spermatic cord (Maddocks et al. 1993; Piner et al. 2002). Therefore, factors that compromise delivery of oxygen to the testis would be suspected to have a detrimental effect. The negative impact of smoking on sperm counts in men is therefore consistent with this thinking. However, in comparison with the fairly dramatic decrease (up to 40%) in sperm counts that is induced in sons by maternal smoking in pregnancy, the reduction (10–17%) in sperm counts in adult men who smoke heavily (Ramlau-Hansen et al. 2007c) is modest, and in many individual studies no significant effects were found (Vine 1996; Martini et al. 2004). Although interference with oxygen supply is an obvious mechanism via which smoking could reduce spermatogenesis, other mechanisms may also operate, including exposure to cadmium (see below) and activation of the Ah receptor, as is thought to occur with maternal smoking in pregnancy (see above).
Most studies that included alcohol as a point of investigation have failed to show a significant impact on sperm counts, at least among those with moderate alcohol consumption (e.g. Marinelli et al. 2004; Martini et al. 2004). In contrast, in chronic alcoholics, there is good evidence for impairment of spermatogenesis and reductions in sperm counts and testosterone levels (Villalta et al. 1997; Muthusami & Chinnaswamy 2005). The mechanisms for these effects have not been studied in detail.
There are surprisingly few studies of the effects of recreational, sports (anabolic steroids) and therapeutic drugs on spermatogenesis in men. Animal studies have demonstrated adverse effects of cannabinoids, such as marijuana, on testicular steroidogenesis (Brown & Dobs 2002), sperm maturation and motility (Ricci et al. 2007) and in some studies on sperm production (Abel 1981; Patra & Wadsworth 1991). These effects work via endogenous cannabinoid-type receptors (CB1, CB2; Brown & Dobs 2002; Ricci et al. 2007) that are expressed also in humans (Brown & Dobs 2002), including on sperm (Rossato et al. 2005). However, although there are bits of evidence pointing to adverse effects of cannabis use on testosterone levels (Diamond et al. 1986; Brown & Dobs 2002) and on sperm motility, overall there is no convincing evidence for major effects of cannabis use on spermatogenesis in humans (Brown & Dobs 2002). Similarly, chronic cocaine use may be associated with low sperm counts (Bracken et al. 1990), but detailed evidence for effects and mechanisms is lacking.
Administration of androgenic steroids to men results in reduced spermatogenesis because it causes suppression of LH secretion from the pituitary gland and a resulting suppression of intratesticular testosterone levels; this has been widely evaluated as an approach to male contraception (Anderson & Baird 2002). It is therefore not surprising that use of anabolic steroids by athletes, weightlifters and bodybuilders can have similar adverse effects (Knuth et al. 1989; Karila et al. 2004); as is the case with male contraception, cessation of anabolic steroid use results in recovery of spermatogenesis (Knuth et al. 1989).
Prescription drugs of numerous types are used widely by normal males, and although effects of such compounds on spermatogenesis cannot really be classified as lifestyle/environmental, if they adversely impact on spermatogenesis such effects may be suspected as having an environmental cause, especially if the drug is taken chronically. An example is sulfasalazine, which has been widely used for the chronic treatment of irritable bowel disorders and which can induce infertility in men (O'Morian et al. 1984; Feagins & Kane 2009) and in rats (O'Morian et al. 1984) via effects probably late in spermatogenesis (O'Morian et al. 1984). Similarly, some chemotherapeutic agents (anti-mitotics such as cyclophosphamide) used for treatment of cancers or of some kidney diseases have well-documented adverse effects on spermatogenesis and/or fertility (Buchanan & Davis 1984; Nudell et al. 2002). Of more concern from an environmental perspective is for (largely unsuspected) effects of commonly prescribed drugs that are not necessarily considered as anti-spermatogenic. This is highlighted by a recent study of 165 infertile men (selected from a group of 1768 infertile men) who were taking medications of various sorts at the time of investigation and in whom no hormonal, historical or other cause for the infertility could be found (Hayashi et al. 2008). The most common medications being taken by these men were H1 receptor antagonists (for allergy relief, such as hayfever), anti-epileptics and antibiotics. When half of the 165 patients switched or ceased their treatment, there was a 93 per cent improvement in semen quality and an 85 per cent increase in conception rate, compared with values of 12 and 10 per cent, respectively, in the control group (men who continued on treatment). Other studies indicate reduced fertility in epileptic men and one explanation is that the drugs used for treatment (carbamazepine, oxcarbazepine, valproate) are associated with adverse effects on sperm number, morphology or motility (Isojarvi et al. 2004). For valproate at least, there is supporting data in rats showing adverse effects on spermatogenesis (Nishimura et al. 2000), although such effects are only obvious with supra-therapeutic dose levels, while at therapeutic dose levels only effects on reproductive hormone levels (reduced oestradiol and LH) are found (Sveberg Roste et al. 2002). Some drugs (e.g. the H2-receptor antagonist, cimetidine) that affect spermatogenesis (Van Thiel et al. 1979) do so by altering androgen action, which has led to the development of substitute compounds that lack this side effect.
4. Effects of environmental chemicals on spermatogenesis in adulthood
There are three sorts of exposures to ECs: those that occur occupationally, those that occur in the general or home environment (e.g. pollutants) and those that occur because of our lifestyle choices (e.g. use of skin creams, deodorants, etc.). There is a widespread belief that human exposure to ECs via one or more of these routes can impair spermatogenesis in adult men and lead to reduced sperm counts. This belief has probably been triggered by the coincidence of concerns about ‘falling sperm counts’ in men (Swan et al. 2000) with concerns about the high prevalence of ECs in the modern environment (Sharpe 2009). However, evidence to support this belief is remarkably thin on the ground.
(a). Occupational exposures
There are one or two well-documented examples of occupationally induced infertility resulting from EC exposure, the best known being that of dibromochloropropane (DBCP), a nematocide used on crops such as bananas and pineapple. Exposure of men to DBCP during manufacture or application caused severe impairment of spermatogenesis and resulting infertility in a high proportion of highly exposed men (Whorton et al. 1977), and recovery after stopping exposure did not always occur (Whorton & Foliart 1988). A less dramatic example is occupational exposure to glycol ethers such as ethylene glycol monomethyl ether, which are highly volatile compounds used as solvents in many different processes. Several studies in infertility clinics have identified occupational exposure to glycol ethers as a potential cause (e.g. Multigner et al. 2007; Cherry et al. 2008). Numerous studies in laboratory animals (e.g. rats, rabbits) have demonstrated similar adverse effects of glycol ethers on spermatogenesis (e.g. Berndtson & Foote 1997; Watanabe et al. 2000). As a result, changes in the types of glycol ethers being used have occurred, and recent evidence suggests that the substitute compounds may be without effect on spermatogenesis and/or fertility in men (Watanabe et al. 2000).
In light of experience with DBCP (above), numerous studies have addressed whether occupational exposure to pesticides in general (e.g. in crop sprayers, greenhouse workers) can affect spermatogenesis or fertility in men; in most such studies, individuals will have been exposed to multiple rather than single pesticides, making interpretation less straightforward. Although several studies have shown evidence for detrimental effects (Rupa et al. 1991; Abell et al. 2000; Ayotte et al. 2001), most studies, including several large prospective studies, found no evidence for any major impact in Western countries (Larsen et al. 1999; Thonneau et al. 1999; Bonde & Storgaard 2002).
Other occupational exposures that have been shown in epidemiological studies to negatively affect spermatogenesis and/or sperm counts in men include inorganic lead and other heavy metals (cadmium, mercury), metal welding fumes and carbon disulphide (Tas et al. 1996; Figa-Talamanca et al. 2001; Bonde & Storgaard 2002). Of these, exposure to heavy metals is perhaps the most interesting for two reasons. First, recent studies have shown significantly higher levels of cadmium in blood and seminal plasma in infertile than in fertile men or men from the normal population (Benoff et al. 2009); in infertile men, there was a negative correlation between cadmium levels and sperm concentration and motility. Such an association can also be recapitulated experimentally in adult rats (Benoff et al. 2008). Second, wider exposure of the general population to lead has occurred via paints and gasoline, prior to its removal from these products, and exposure continues via consumption of certain fish (e.g. tuna) in which these metals may concentrate (Figa-Talamanca et al. 2001). For example, exposure to vehicle exhaust in tollgate workers has been associated with reduced sperm counts and quality, these changes correlating with higher blood levels of lead and methaemoglobin (De Rosa et al. 2003). Exposure to hexanedione (an ingredient of gasoline) may also occur via this route or in other occupational settings, and this has been shown in laboratory animal studies to disrupt Sertoli–germ cell junctions and/or adhesion and impair spermatogenesis (Boekelheide et al. 2003). Similarly, for cadmium at least, recent studies in rats have shown that at relatively low doses (though still above environmental levels), it can specifically disrupt inter-Sertoli cell tight junctions and thus lead to disruption of spermatogenesis (Siu et al. 2009). Whether other heavy metals can target the same cellular processes in the testis as cadmium remains unknown.
(b). Environmental exposure to pollutants
Exposure to ozone as the result of general atmospheric pollution has been negatively associated with sperm counts in a large group of sperm donors (Sokol et al. 2006), and such effects might reinforce effects resulting from traffic pollution mentioned above. Exactly how such effects occur is unclear, as ozone does not appear to impair oxygen transport by erythrocytes in the same way as carbon monoxide.
There is a widespread belief that environmental pesticide exposures via fruit and vegetables or the general environment can adversely affect spermatogenesis in men at large. However, this seems largely untenable, based on the studies of men occupationally exposed to such agents (above), as it is reasonable to assume that the general population will be less highly exposed than these workers. An exception to this logic may be exposure to organochlorine pesticides (e.g. DDT) that have been banned in Western countries, as there will no longer be occupational exposure to such compounds, whereas there is exposure of the general population because of their persistence in the environment and food chain. However, although some studies point towards effects of DDT exposure on semen parameters, these are generally from developing countries in which DDT is still used, and the effects reported are generally small (Dalvie et al. 2004; Aneck-Hahn et al. 2007). In contrast, studies in North America (Hauser et al. 2003) and in Europe, including in the highly exposed Inuit population (Bonefeld-Jorgensen et al. 2006; Elzanaty et al. 2006; Toft et al. 2006, 2007; Krüger et al. 2007), have shown no evidence for major effects on semen parameters or on fertility. This conclusion applies also to other persistent environmental contaminants such as PCBs, although in some studies there are trends towards reduced sperm quality with high PCB exposure (Rignell-Hydbom et al. 2004; Hauser 2006) or after exposure to perfluorinated chemicals (Joensen et al. 2009). The overall conclusion based on these and other data is that although such persistent pollutants may have some minor effects on spermatogenesis (Bonde et al. 2008), they have no impact on fertility (Giwercman et al. 2007; Bonde et al. 2008).
In contrast to the foregoing conclusions, one study of US men (Swan et al. 2003) reported a highly significant association between urinary metabolite levels of three currently used pesticides (alachlor, atrazine, diazinon) and occurrence of low sperm counts in male partners of pregnant women. Further studies of these compounds are warranted, but it is evident that any effects on spermatogenesis in these men were not sufficient to affect fertility. Interestingly, exposure to non-persistent, pyrethroid insecticides has also been associated with reproductive hormone changes in adult men (Meeker et al. 2009) as well as with reductions in sperm concentration, motility and DNA integrity (Meeker et al. 2008), and the use of these pesticides is likely to increase owing to restrictions on the use of certain other pesticides.
(c). Environmental chemical exposure resulting from lifestyle choices
Although men are assumed to be considerably less exposed to chemicals via cosmetics and/or body creams than are women, there are trends for more substantial use of such products among young men in Western countries, and use of suncreams and basic toiletries will be more generally widespread. Such products contain numerous chemicals, at least two of which (various types of parabens or phthalates) have been implicated in potentially causing adverse effects on testosterone and/or sperm production in animal studies (e.g. David et al. 2000; Ishihara et al. 2000; Oishi 2001, 2002), although for phthalates such effects have only been found after exposure to extremely high levels (500–1500 mg kg−1 d−1). There are no data relating parabens exposure to sperm counts in men, but recent in-depth safety assessments (based on animal studies) concluded that effects are unlikely (Golden et al. 2005; CIR 2008).
Three studies have assessed whether phthalate exposure is associated with semen quality in men from infertile couples and reported significant or near-significant associations between urinary levels of monobutyl phthalate and low sperm counts and/or motility and/or velocity (Duty et al. 2003, 2004; Hauser 2006); similar trends were found for certain other phthalate metabolites (monobenzyl phthalate) in some of the studies. However, a similar study of young Swedish military conscripts from the normal population (fertility status unknown) found no association between urinary phthalate metabolite levels and semen parameters (Jönsson et al. 2005). Overall, the present view is that there is no firm evidence that exposure of adult men to common ECs, whether persistent or not, has any major impact on their fertility or semen quality (Hauser 2006), although further studies of perfluorinated chemicals (alachlor, atrazine and diazinon) are warranted.
5. Concluding remarks
The high prevalence of low sperm counts in young men across Europe today is a cause for concern, especially when considered together with the trend for ever-later age for first pregnancy in the female partner (and thus reduced female fertility). The evidence for increasing incidence of other male reproductive disorders reinforces this concern. Abnormally low sperm counts mean that sperm production is subnormal, a change that can derive either from an adverse impact of factors in adulthood or as the result of a developmental problem. These two possibilities are fundamentally different as the former carries the possibility of being resolved/recovered from (by identification and removal of the ‘insult’), whereas the developmental problem is most likely to be irrecoverable in an affected individual.
As this review has shown, evidence for widespread or major effects of individual lifestyle or environmental factors on spermatogenesis in adulthood is largely lacking, whereas there is growing evidence that prenatal exposures of males (reflective of maternal lifestyle and/or exposures) can have major impact on capacity to produce sperm in adulthood, although the overall importance of such effects is difficult to gauge because of the inherent difficulties in accurately relating events that are separated by two or more decades. However, it seems intuitively likely that the major changes to our lifestyles, diets and activity levels over recent decades will have impacted negatively on spermatogenesis in adulthood, as all available evidence points towards negative effects of sedentary lifestyles and obesity on testis function (testosterone levels and sperm production); potential effects of traffic/atmospheric pollutants can only exacerbate such effects. Realistically, the likelihood is that small effects of several different factors may combine together to induce more substantial negative effects on spermatogenesis, although this is difficult and expensive to prove or test. Whatever the reality of effects in adulthood, any negative effects prenatally on sperm-producing capacity in adulthood can only exacerbate such ‘adult’ effects, especially when it is recognized that several of the same factors that impact negatively on spermatogenesis in adulthood also impact negatively (via the mother) in foetal life (figure 2).
Despite the practical difficulties in identifying what, when and how, environmental and/or lifestyle factors can impact negatively on testis development and function, the high current incidence of low sperm counts in young men and its major implications for fertility and population renewal in the West provide the strongest possible incentive to strengthen research in this area. Identification and removal and/or moderation of such effects can only have positive effects on spermatogenesis, without the need for lengthy development and testing of any new therapeutic drugs.
Acknowledgements
The author's work was supported in part by the UK Medical Research Council (WBS U.1276.00.003.00003.01) and by the European Union (DEER; FP7-ENV-2007-1-212844).
Footnotes
One contribution of 17 to a Theme Issue ‘The biology and regulation of spermatogenesis’.
References
- Abel E. L.1981Marihuana and sex: a critical survey. Drug Alcohol Depend. 8, 1–22 (doi:10.1016/0376-8716(81)90082-X) [DOI] [PubMed] [Google Scholar]
- Abell A., Ernst E., Bonde J. P.2000Semen quality and sexual hormones in greenhouse workers. Scand. J. Work Environ. Health 26, 492–500 [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Baird D. T.2002Male contraception. Endocr. Rev. 23, 735–762 (doi:10.1210/er.2002-0002) [DOI] [PubMed] [Google Scholar]
- Andersson A. M., Jørgensen N., Main K. M., Toppari J., Rajpert-De Meyts E., Leffers H., Juul A., Jensen T. K., Skakkebaek N. E.2008Adverse trends in male reproductive health: we may have reached a crucial ‘tipping point’. Int. J. Androl. 31, 74–80 (doi:10.1111/j.1365-2605.2007.00853.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneck-Hahn N. H., Schulenburg G. W., Bornman M. S., Farias P., de Jager C.2007Impaired semen quality associated with environmental DDT exposure in young men living in a malaria area in the Limpopo Province, South Africa. J. Androl. 28, 423–434 (doi:10.2164/jandrol.106.001701) [DOI] [PubMed] [Google Scholar]
- Ayotte P., Giroux S., Dewailly E., Hernandez Avila M., Farias P., Danis R., Villanueva Diaz C.2001DDT spraying for malaria control and reproductive function in Mexican men. Epidemiology 12, 366–367 (doi:10.1097/00001648-200105000-00022) [DOI] [PubMed] [Google Scholar]
- Barnes-Ellerbe S., Knudsen K. E., Puga A.20042,3,7,8-tetrachlorodibenzo-p-dioxin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol. Pharmacol. 66, 502–511 (doi:10.1124/mol.104.000356) [DOI] [PubMed] [Google Scholar]
- Benoff S., Auborn K., Marmar J. L., Hurley I. R.2008Link between low-dose environmentally relevant cadmium exposures and asthenozoospermia in a rat model. Fertil. Steril. 89(Suppl. 2), e73–e79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Hauser R., Marmar J. L., Hurley I. R., Napolitano B., Centola G. M.2009Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, articial insemination donors, and unselected volunteers). Mol. Med. 15, 248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndtson W. E., Foote R. H.1997Disruption of spermatogenesis in rabbits consuming ethylene glycol monomethyl ether. Reprod. Toxicol. 11, 29–36 (doi:10.1016/S0890-6238(96)00194-3) [DOI] [PubMed] [Google Scholar]
- Boekelheide K., et al. 20032,5-Hexanedione-induced testicular injury. Ann. Rev. Pharmacol. Toxicol. 43, 125–147 (doi:10.1146/annurev.pharmtox.43.100901.135930) [DOI] [PubMed] [Google Scholar]
- Boisen K. A., et al. 2004Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet 363, 1264–1269 (doi:10.1016/S0140-6736(04)15998-9) [DOI] [PubMed] [Google Scholar]
- Bonde J. P., Storgaard L.2002How work-place conditions, environmental toxicants and lifestyle affect male reproductive function. Int. J Androl. 25, 262–268 (doi:10.1046/j.1365-2605.2002.00373.x) [DOI] [PubMed] [Google Scholar]
- Bonde J. P., et al. 2008Fertility and markers of male reproductive function in Inuit and European populations spanning large contrasts in blood levels of persistent organochlorines. Environ. Health Perspect. 116, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen E. C., et al. 2006Xenoestrogenic activity in blood of European and Inuit populations. Environ. Health 5, 12 (doi:10.1186/1476-069X-5-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken M. B., Eskenazi B., Sachse K., McSharry J. E., Hellenbrand K., Leo-Summers L.1990Association of cocaine use with sperm concentration, motility and morphology. Fertil. Steril. 53, 315–322 [DOI] [PubMed] [Google Scholar]
- Bray F., Richiardi L., Ekbom A., Pukkala E., Cuninkova M., Møller H.2006Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int. J. Cancer 118, 3099–3111 (doi:10.1002/ijc.21747) [DOI] [PubMed] [Google Scholar]
- Brennan J., Capel B.2004One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat. Rev. Genet. 7, 509–521 [DOI] [PubMed] [Google Scholar]
- Brown T. T., Dobs A. S.2002Endocrine effects of marijuana. J. Clin. Pharmacol. 42(Suppl. 11), 90S–96S [DOI] [PubMed] [Google Scholar]
- Buchanan J. F., Davis L. J.1984Drug-induced infertility. Drug Intell. Clin. Pharm. 18, 122–132 [DOI] [PubMed] [Google Scholar]
- Bujan L., Daudin M., Charlet J. P., Thonneau P., Mieusset R.2000Increase in scrotal temperature in car drivers. Hum. Reprod. 15, 1355–1357 [DOI] [PubMed] [Google Scholar]
- Cherry N., Moore H., McNamee R., Pacey A., Burgess G., Clyma J. A., Dippnall M., Baillie H., Povey A.2008Occupation and male infertility: glycol ethers and other exposures. Occup. Environ. Med. 65, 708–714 [DOI] [PubMed] [Google Scholar]
- Chevrier J., Dewaillyt E., Ayotte P., Mauriege P., Despres J. P., Tremblay A.2000Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int. J. Obes. Relat. Metab. Disord. 24, 1272–1278 (doi:10.1038/sj.ijo.0801380) [DOI] [PubMed] [Google Scholar]
- CIR 2008Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben and benzylparaben as used in cosmetic products. Int. J. Toxicol. 27(Suppl. 4), 1–82 [DOI] [PubMed] [Google Scholar]
- Combes A. N., Wilhelm D., Davidson T., Dejana E., Harley V., Sinclair A., Koopman P.2009Endothelial cell migration directs testis cord formation. Dev. Biol. 326, 112–120 (doi:10.1016/j.ydbio.2008.10.040) [DOI] [PubMed] [Google Scholar]
- Culty M.2009Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res. (Part C) 87, 1–26 (doi:10.1002/bdrc.20142) [DOI] [PubMed] [Google Scholar]
- Dalvie M. A., et al. 2004The long-term effects of DDT exposure on semen, fertility and sexual function of malaria vector-control workers in Limpopo Province, South Africa. Environ. Res. 96, 1–8 (doi:10.1016/j.envres.2003.09.002) [DOI] [PubMed] [Google Scholar]
- David R. M., Moore M. R., Finney D. C., Guest D.2000Chronic toxicity of di(2-ethylhexyl)phthalate in mice. Toxicol. Sci. 58, 377–385 (doi:10.1093/toxsci/58.2.377) [DOI] [PubMed] [Google Scholar]
- De Rosa M., et al. 2003Traffic pollutants affect fertility in men. Hum. Reprod. 18, 1055–1061 [DOI] [PubMed] [Google Scholar]
- Diamond F., Jr, Ringenberg L., MacDonald D., Barnes J., Hu C. S., Duckett G., Sweetland M., Root A.1986Effects of drug and alcohol abuse upon pituitary-testicular function in adolescent males. J. Adolesc. Health Care 7, 28–33 [DOI] [PubMed] [Google Scholar]
- Duty S. M., Silva M. J., Barr D. B., Brock J. W., Ryan L., Chen Z., Herrick R. F., Christiani D. C., Hauser R.2003Phthalate exposure and human semen parameters. Epidemiology 14, 269–277 [PubMed] [Google Scholar]
- Duty S. M., Calafat A. M., Silva M. J., Brock J. W., Ryan L., Chen Z., Overstreet J., Hauser R.2004The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J. Androl. 25, 293–302 [DOI] [PubMed] [Google Scholar]
- Elzanaty S., et al. 2006Association between exposure to persistent organohalogen pollutants and epididymal and accessory sex gland function: multicentre study in Inuit and European populations. Reprod. Toxicol. 22, 765–773 (doi:10.1016/j.reprotox.2006.07.005) [DOI] [PubMed] [Google Scholar]
- Feagins L. A., Kane S. V.2009Sexual and reproductive issues for men with inflammatory bowel disease. Am. J. Gastroenterol. 104, 768–773 (doi:10.1038/ajg.2008.90) [DOI] [PubMed] [Google Scholar]
- Figa-Talamanca I., et al. 1996Effects of prolonged autovehicle driving on male reproductive function: a study among taxi drivers. Am. J. Ind. Med. 30, 750–758 (doi:10.1002/(SICI)1097-0274(199612)30:6<750::AID-AJIM12>3.0.CO;2-1) [DOI] [PubMed] [Google Scholar]
- Figa-Talamanca I., Traina M. E., Urbani E.2001Occupational exposures to metals, solvents and pesticides: recent evidence on male reproductive effects and biological markers. Occup. Med. 51, 174–188 (doi:10.1093/occmed/51.3.174) [DOI] [PubMed] [Google Scholar]
- Giwercman A., Rylander L., Lundberg Giwercman Y. L.2007Influence of endocrine disruptors on human male fertility. Reprod. Biomed. Online 15, 633–642 [DOI] [PubMed] [Google Scholar]
- Golden R., Gandy J., Vollmer G.2005A review of the endocrine activity of parabens and implications for potential risk to human health. Crit. Rev. Toxicol. 35, 435–458 (doi:10.1080/10408440490920104) [DOI] [PubMed] [Google Scholar]
- Gould D. C., Kirby R. S., Amoroso P.2007Hypoandrogen-metabolic syndrome: a potentially common and underdiagnosed condition in men. Int. J. Clin. Pract. 61, 341–344 (doi:10.1111/j.1742-1241.2006.01239.x) [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Kelce W. R., Monosson E., Ostby J. S., Birnbaum L. S.1995Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol. Appl. Pharmacol. 131, 108–118 [DOI] [PubMed] [Google Scholar]
- Grumbach M.2005A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J. Clin. Endocrinol. Metab. 90, 3122–3127 [DOI] [PubMed] [Google Scholar]
- Hammoud A. O., Gibson M., Petersen C. M., Hamilton B. D., Carrell D. T.2006Obesity and male reproductive potential. J. Androl. 27, 619–626 (doi:10.2164/jandrol.106.000125) [DOI] [PubMed] [Google Scholar]
- Hammoud A. O., Gibson M., Petersen C. M., Meikle A. W., Carrell D. T.2008Impact of male obesity on infertility: a critical review. Fertil. Steril. 90, 897–904 (doi:10.1016/j.fertnstert.2008.08.026) [DOI] [PubMed] [Google Scholar]
- Hauser R.2006The environment and male fertility: recent research on emerging chemicals and semen quality. Semin. Reprod. Med. 24, 156–167 (doi:10.1055/s-2006-944422) [DOI] [PubMed] [Google Scholar]
- Hauser R., Chen Z., Potheir L., Ryan L., Altshul L.2003The relationship between human semen parameters and environmental exposure to polychlorinated biphenyls and p,p′-DDE. Environ. Health Perspect. 111, 1505–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Miyata A., Yamada T.2008The impact of commonly prescribed drugs on male fertility. Hum. Fertil. 11, 191–196 (doi:10.1080/14647270701739566) [DOI] [PubMed] [Google Scholar]
- Hemminki K., Chen B.2006Familial risks in testicular cancer as aetiological clues. Int. J. Androl. 29, 205–210 (doi:10.1111/j.1365-2605.2005.00599.x) [DOI] [PubMed] [Google Scholar]
- Hjollund N. H., Bonde J. P., Jensen T. K., Olsen J.2000Diurnal scrotal skin temperature and semen quality. The Danish first pregnancy planner study team. Int. J. Androl. 23, 309–318 (doi:10.1046/j.1365-2605.2000.00245.x) [DOI] [PubMed] [Google Scholar]
- Hjollund N. H., Storgaard L., Ernst E., Bonde J. P., Olsen J.2002aThe relations between daily activities and scrotal temperature. Reprod. Toxicol. 16, 209–214 (doi:10.1016/S0890-6238(02)00026-6) [DOI] [PubMed] [Google Scholar]
- Hjollund N. H., Storgaard L., Ernst E., Bonde J. P., Olsen J.2002bImpact of diurnal scrotal temperature on semen quality. Reprod. Toxicol. 16, 215–221 (doi:10.1016/S0890-6238(02)00025-4) [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Wilson V. S., Furr J., Lambright C. R., Rider C. V., Blystone C. R., Hotchkiss A. K., Gray L. E., Jr2008A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague–Dawley rat in a cumulative, dose-additive manner. Toxicol. Sci. 105, 153–165 (doi:10.1093/toxsci/kfn077) [DOI] [PubMed] [Google Scholar]
- Hue O., Marcotte J., Berrigan F., Simoneau M., Dore J., Marceau P., Marceau S., Tremblay A., Teasdale N.2007Plasma concentration of organochlorine compounds is associated with age and not obesity. Chemosphere 67, 1463–1467 (doi:10.1016/j.chemosphere.2006.10.033) [DOI] [PubMed] [Google Scholar]
- Irvine D. S.1998Epidemiology and aetiology of male infertility. Hum. Reprod. 13, 33–44 [DOI] [PubMed] [Google Scholar]
- Ishihara M., Itoh M., Miyamoto K., Suna S., Takeuchi Y., Takenata I., Jitsunari F.2000Spermatogenic disturbance induced by di(2-ethylhexyl) phthalate is significantly prevented by treatment with antioxidant vitamins in the rat. Int. J. Androl. 23, 85–94 (doi:10.1046/j.1365-2605.2000.00212.x) [DOI] [PubMed] [Google Scholar]
- Isojarvi J. I., Lofgren E., Juntunen K. S., Pakarinen A. J., Paivansato M., Rautakorpi I., Tuomivaara L.2004Effect of epilepsy and antiepileptic drugs on male reproductive health. Neurology 62, 247–253 [DOI] [PubMed] [Google Scholar]
- Izawa H., Kohara M., Watanbe G., Taya K., Sagai M.2007Effects of diesel exhaust particles on the male reproductive system in strains of mice with different aryl hydrocarbon receptor responsiveness. J. Reprod. Dev. 53, 1191–1197 (doi:10.1262/jrd.19114) [DOI] [PubMed] [Google Scholar]
- Jensen T. K., Andersson A.-M., Jørgensen N., Andersen A.-G., Carlsen E., Petersen J. H., Skakkebaek N. E.2004aBody mass index in relation to semen quality and reproductive hormones among 1558 Danish men. Fertil. Steril. 82, 863–870 (doi:10.1016/j.fertnstert.2004.03.056) [DOI] [PubMed] [Google Scholar]
- Jensen T. K., et al. 2004bAssociation of in utero exposure smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1770 young men from the general population in five European countries. Am. J. Epidemiol. 159, 49–58 (doi:10.1093/aje/kwh002) [DOI] [PubMed] [Google Scholar]
- Jensen M. S., Mabeck L. M., Toft G., Thulstrup A. M., Bonde J. P.2005Lower sperm counts following prenatal tobacco exposure. Hum. Reprod. 20, 2559–2566 (doi:10.1093/humrep/dei110) [DOI] [PubMed] [Google Scholar]
- Joensen U. N., Bossi R., Leffers H. J., Jensen A. A., Skakkebaek N. E., Jogensen N.2009Do perfluoroalkyl compounds impair human semen quality? Environ. Health Perspect. 117, 923–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H., Baker P. J., Abel M., Charlton H. M., Jackson G., Fleming L., Kumar T. R., O'Shaughnessy P. J.2004Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 145, 318–329 (doi:10.1210/en.2003-1055) [DOI] [PubMed] [Google Scholar]
- Jönsson B. A., Richthoff J., Rylander L., Giwercman A., Hagmar L.2005Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology 16, 487–493 [DOI] [PubMed] [Google Scholar]
- Jørgensen N., Asklund C., Carlsen E., Skakkebaek N. E.2006Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int. J. Androl. 29, 54–61 (doi:10.1111/j.1365-2605.2005.00635.x) [DOI] [PubMed] [Google Scholar]
- Jung A., Eberl M., Schill W. B.2001Improvement of semen quality by nocturnal scrotal cooling and moderate behavioural change to reduce genital heat stress in men with oligoasthenoteratozoospermia. Reproduction 121, 595–603 (doi:10.1530/rep.0.1210595) [DOI] [PubMed] [Google Scholar]
- Karila T., Hovatta O., Seppala T.2004Concomitant abuse of anabolic androgenic steroids and human chorionic gonadotrophin impairs spermatogenesis in power athletes. Int. J. Sports Med. 25, 257–263 [DOI] [PubMed] [Google Scholar]
- Kawakami E., Hirano T., Hori T., Tsutsui T.2004Improvement in spermatogenic function after subcutaneous implantation of a capsule containing an aromatase inhibitor in four oligozoospermic dogs and one azoospermic dog with high plasma estradiol-17 beta concentrations. Theriogenology 62, 165–178 (doi:10.1016/j.theriogenology.2003.09.021) [DOI] [PubMed] [Google Scholar]
- Kelnar C. J., McKinnell C., Walker M., Morris K. D., Wallace W. H., Saunders P. T., Fraser H. M., Sharpe R. M.2002Testicular changes during infantile ‘quiescence’ in the marmoset and their gonadotrophin dependence: a model for investigating susceptibility of the prepubertal human testis to cancer therapy? Hum. Reprod. 17, 1367–1378 [DOI] [PubMed] [Google Scholar]
- Kizu R., Okamura K., Toriba A., Kakishima H., Mizokami A., Burnstein K. L., Hayakawa K.2003A role for aryl hydrocarbon receptor in the antiandrogenic effects of polycyclic aromatic hydrocarbons in LNCaP human prostate carcinoma cells. Arch. Toxicol. 77, 335–343 [DOI] [PubMed] [Google Scholar]
- Knuth U. A., Maniera H., Nieschlag E.1989Anabolic steroids and semen parameters in bodybuilders. Fertil. Steril. 52, 1041–1047 [DOI] [PubMed] [Google Scholar]
- Kort H. I., Massey J. B., Elsner C. W., Mitchell-Leef D., Shapiro D. B., Witt M. A., Roudebush W. E.2006Impact of body mass index values on sperm quality and quantity. J. Androl. 27, 450–452 (doi:10.2164/jandrol.05124) [DOI] [PubMed] [Google Scholar]
- Krüger T., et al. 2007Xenoandrogenic activity in serum differs across Europe and Inuit populations. Environ. Health Perspect. 115, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. B., Spano M., Giwercman A., Bonde J. P.1999Semen quality and sex hormones among organic and traditional Danish farmers. ASCLEPIOS Study Group. Occup. Environ. Med. 56, 139–144 (doi:10.1136/oem.56.2.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft S., et al. 2003Oxidative DNA damage in human sperm influences time to pregnancy. Hum. Reprod. 18, 1265–1272 (doi:10.1093/humrep/deg202) [DOI] [PubMed] [Google Scholar]
- Lue Y. H., Hikim A. P., Wang C., Im A., Leung A., Swerdloff R. S.2000Testicular heat exposure enhances the suppression of spermatogenesis by testosterone in rats: the ‘two-hit’ approach to male contraceptive development. Endocrinology 141, 1414–1424 (doi:10.1210/en.141.4.1414) [DOI] [PubMed] [Google Scholar]
- Lue Y. H., Lasley B. L., Laughlin L. S., Swerdloff R. S., Hikim A. P., Leung A., Overstreet J. W., Wang C.2002Mild testicular hyperthermia induces profound transitional spermatogenic suppression through increased germ cell apoptosis in adult cynomolgus monkeys (Macaca fascicularis). J. Androl. 23, 799–805 [PubMed] [Google Scholar]
- Maddocks S., Hargreave T. B., Reddie K., Fraser H. M., Kerr J. B., Sharpe R. M.1993Intratesticular hormone levels and the route of secretion of hormones from the testis of the rat, guinea pig, monkey and human. Int. J. Androl. 16, 272–278 (doi:10.1111/j.1365-2605.1993.tb01191.x) [DOI] [PubMed] [Google Scholar]
- Magnusdottir E. V., Thorsteinsson T., Thorsteindottir S., Heimisdottir M., Olafsdottir K.2005Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Hum. Reprod. 20, 208–215 (doi:10.1093/humrep/deh569) [DOI] [PubMed] [Google Scholar]
- Mann D. R., Fraser H. M.1996The neonatal period: a critical interval in male primate development. J. Endocrinol. 149, 191–197 [DOI] [PubMed] [Google Scholar]
- Marinelli D., Gaspari L., Pedotti P., Taioli E.2004Mini-review of studies on the effect of smoking and drinking habits on semen parameters. Int. J. Hyg. Environ. Health 207, 185–192 (doi:10.1078/1438-4639-00283) [DOI] [PubMed] [Google Scholar]
- Martini A. C., Molina R. I., Estofan D., Senestrari D., Fiol de Cuneo M., Ruiz R. D.2004Effects of alcohol and cigarette consumption on human seminal quality. Fertil. Steril. 82, 374–377 (doi:10.1016/j.fertnstert.2004.03.022) [DOI] [PubMed] [Google Scholar]
- Meeker J. D., Barr D. B., Hauser R.2008Human semen quality and sperm DNA damage in relation to urinary metabolites of pyrethroid insecticides. Hum. Reprod. 23, 1932–1940 (doi:10.1093/humrep/den242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Barr D. B., Hauser R.2009Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod. Toxicol. 27, 155–160 (doi:10.1016/j.reprotox.2008.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieusset R., Bujan L.1995aThe potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Int. J. Androl. 17, 186–191 (doi:10.1111/j.1365-2605.1994.tb01241.x) [DOI] [PubMed] [Google Scholar]
- Mieusset R., Bujan L.1995bTesticular heating and its possible contributions to male infertility: a review. Int. J. Androl. 18, 169–184 (doi:10.1111/j.1365-2605.1995.tb00408.x) [DOI] [PubMed] [Google Scholar]
- Millar M. R., Sharpe R. M., Weinbauer G. F., Fraser H. M., Saunders P. T. K.2000Marmoset spermatogenesis: organizational similarities to the human. Int. J. Androl. 23, 266–277 (doi:10.1046/j.1365-2605.2000.00236.x) [DOI] [PubMed] [Google Scholar]
- Mitchell R., et al. 2008Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum. Reprod. 23, 2755–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P., et al. 2008Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ. Health Perspect. 116, 70–77 (doi:10.1289/ehp.10399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multigner L., Ben Brik E., Arnaud I., Haguenoer J. M., Jouannet P., Auger J., Eustache F.2007Glycol ethers and semen quality: a cross-sectional study among male workers in the Paris municipality. Occup. Environ. Med. 64, 467–473 (doi:10.1136/oem.2005.023952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusami K. R., Chinnaswamy P.2005Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil. Steril. 84, 919–924 (doi:10.1016/j.fertnstert.2005.04.025) [DOI] [PubMed] [Google Scholar]
- Nielsen T. L., Hagen C., Wraae K., Brixen K., Petersen P. H., Haug E., Larsen R., Andersen M.2007Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J. Clin. Endocrinol. Metab. 92, 2696–2705 [DOI] [PubMed] [Google Scholar]
- Nishimura T., Sakai M., Yonezawa H.2000Effects of valproic acid on fertility and reproductive organs in male rats. J. Toxicol. Sci. 25, 85–93 [DOI] [PubMed] [Google Scholar]
- Nudell D. M., Monoski M. M., Lipshultz L. I.2002Common medications and drugs: how they affect male fertility. Urol. Clin. North Am. 29, 965–973 (doi:10.1016/S0094-0143(02)00079-4) [DOI] [PubMed] [Google Scholar]
- Ohsako S., Miyabara Y., Nishimura N., Sakaue M., Ishimura R., Kakeyama M., Izumi H., Yonemoto J., Tohyama C.2002Developmental stage-specific effects of perinatal 2,3,7,8-tetrachlorodebenzo-p-dioxin exposure on reproductive organs of male rat offspring. Toxicol. Sci. 66, 283–292 (doi:10.1093/toxsci/66.2.283) [DOI] [PubMed] [Google Scholar]
- Oishi S.2001Effect of butyl paraben on the male reproductive system in rats. Toxicol. Ind. Health 17, 31–39 [DOI] [PubMed] [Google Scholar]
- Oishi S.2002Effect of propyl paraben on the male reproductive system. Food Chem. Toxicol. 40, 1807–1813 [DOI] [PubMed] [Google Scholar]
- O'Morian C., Smethurst P., Dore C. J., Levi A. J.1984Reversible male infertility due to sulphasalazine: studies in man and rat. Gut 25, 1078–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth J. M., Gunsalus G. L., Lamperti A. A.1988Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 122, 787–794 (doi:10.1210/endo-122-3-787) [DOI] [PubMed] [Google Scholar]
- Orth J. M., Jester W. F., Li L. H., Laslett A. L.2000Gonocyte-Sertoli cell interactions during development of the neonatal rodent testis. Curr. Top Dev. Biol. 50, 103–124 (doi:10.1016/S0070-2153(00)50006-4) [DOI] [PubMed] [Google Scholar]
- Patra P. B., Wadsworth R. M.1991Quantitative evaluation of spermatogenesis in mice following chronic exposure to cannabinoids. Andrologia 23, 151–156 [DOI] [PubMed] [Google Scholar]
- Paul C., Murray A. A., Spears N., Saunders P. T. K.2008aDo heat stress and deficits in DNA repair pathways have a negative impact on male fertility? Mol. Hum. Reprod. 14, 1–8 (doi:10.1093/molehr/gam089) [DOI] [PubMed] [Google Scholar]
- Paul C., Melton D. W., Saunders P. T. K.2008bA single, mild transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 136, 73–84 (doi:10.1530/REP-08-0036) [DOI] [PubMed] [Google Scholar]
- Paul C., Teng S., Saunders P. T. K.2009A single, mild transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol. Reprod. 80, 913–919 (doi:10.1095/biolreprod.108.071779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier C., Despres J. P., Tremblay A.2002Plasma organochlorine concentrations in endurance athletes and obese individuals. Med. Sci. Sports Exerc. 34, 1971–1975 [DOI] [PubMed] [Google Scholar]
- Piner J., Sutherland M., Millar M., Turner K. J., Newall D., Sharpe R. M.2002Changes in vascular dynamics of the adult rat testis leading to transient accumulation of seminiferous tubule fluid after administration of a novel 5-hydroxytryptamine (5-HT) agonist. Reprod. Toxicol. 16, 141–150 (doi:10.1016/S0890-6238(02)00008-4) [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E.2006Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum. Reprod. Update 12, 303–323 [DOI] [PubMed] [Google Scholar]
- Raman J. D., Schlegel P. N.2002Aromatase inhibitors for male infertility. J. Urol. 167, 624–629 (doi:10.1016/S0022-5347(01)69099-2) [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen C. H., Nohr E. A., Bonde J. P., Storgaard L., Olsen J.2007aIs maternal obesity related to semen quality in the male offspring? A pilot study. Hum. Reprod. 22, 2758–2762 (doi:10.1093/humrep/dem219) [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen C. H., Thulstrup A. M., Storgaard L., Toft G., Olsen J., Bonde J. P.2007bIs prenatal exposure to tobacco smoking a cause of poor semen quality? Am. J. Epidemiol. 165, 1372–1379 (doi:10.1093/aje/kwm032) [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen C. H., Thulstrup A. M., Aggerholm A. S., Jensen M. S., Toft G., Bonde J. P.2007cIs smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum. Reprod. 22, 188–196 (doi:10.1093/humrep/del364) [DOI] [PubMed] [Google Scholar]
- Ricci G., Cacciola G., Altucci L., Meccariello R., Pierantoni R., Fasano S., Cobellis G.2007Endocannabinoid control of sperm motility: the role of the epididymis. Gen. Comp. Endocrinol. 153, 320–322 (doi:10.1016/j.ygcen.2007.02.003) [DOI] [PubMed] [Google Scholar]
- Rider C. V., Furr J., Wilson V. S., Gray L. E., Jr2008A mixture of seven antiandrogens induces reproductive malformations in rats. Int. J. Androl. 31, 249–262 (doi:10.1111/j.1365-2605.2007.00859.x) [DOI] [PubMed] [Google Scholar]
- Rignell-Hydbom A., Rylander L., Giwercman A., Jönsson B. A., Nilsson-Ehle P., Hagmar L.2004Exposure to CB-153 and p,p′DDE and male reproductive function. Hum. Reprod. 19, 2066–2075 (doi:10.1093/humrep/deh362) [DOI] [PubMed] [Google Scholar]
- Rossato M., Ion Popa F., Ferigo M., Clari G., Foresta C.2005Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction and mitochondrial function. J. Clin. Endocrinol. Metab. 90, 984–991 (doi:10.1210/jc.2004-1287) [DOI] [PubMed] [Google Scholar]
- Rupa D. S., Reddy P. P., Reddi O. S.1991Reproductive performance in population exposed to pesticides in cotton fields in India. Environ. Res. 55, 123–128 (doi:10.1016/S0013-9351(05)80168-9) [DOI] [PubMed] [Google Scholar]
- Scott H. M., Hutchison G. R., Mahood I. K., Hallmark N., Welsh M., De Gendt K., Verhoeven G., O'Shaughnessy P. J., Sharpe R. M.2007Role of androgens in fetal testis development and dysgenesis. Endocrinology 148, 2027–2036 (doi:10.1210/en.2006-1622) [DOI] [PubMed] [Google Scholar]
- Scott H. M., Hutchison G. R., Jobling M. S., McKinnell C., Drake A. J., Sharpe R. M.2008Relationship between androgen action in the ‘male programming window’, fetal Sertoli cell number and adult testis size in the rat. Endocrinology 149, 5280–5287 (doi:10.1210/en.2008-0413) [DOI] [PubMed] [Google Scholar]
- Setchell B. P.1998Heat and the testis. J. Reprod. Fertil. 114, 179–184 [DOI] [PubMed] [Google Scholar]
- Shafik A., Olfat S.1981Scrotal lipomatosis. Br. J. Urol. 53, 50–54 [DOI] [PubMed] [Google Scholar]
- Sharpe R. M.1994Regulation of spermatogenesis. In The physiology of reproduction (eds Knobil E., Neill J. D.), pp. 1363–1434 New York, NY: Raven Press [Google Scholar]
- Sharpe R. M.2000Lifestyle and environmental contribution to male infertility. Br. Med. Bull. 56, 630–642 (doi:10.1258/0007142001903436) [DOI] [PubMed] [Google Scholar]
- Sharpe R. M.2005Sertoli cell endocrinology and signal transduction: androgen regulation. In Sertoli cell biology (eds Skinner M. K., Griswold M. D.), pp. 199–216 London, UK: Elsevier/Academic Press [Google Scholar]
- Sharpe R. M.2006Pathways of endocrine disruption during male sexual differentiation and masculinisation. Best Pract. Res. Clin. Endocrinol. Metab. 20, 91–110 (doi:10.1016/j.beem.2005.09.005) [DOI] [PubMed] [Google Scholar]
- Sharpe R. M.2009Male reproductive health disorders and the potential role of exposure to environmental chemicals. Report for ChemTrust UK. http://www.chemtrust.org.uk/Press_and_Media.php
- Sharpe R. M., Walker M., Millar M. R., Morris K., McKinnell C., Saunders P. T., Fraser H. M.2000Effect of neonatal GnRH antagonist administration on Sertoli cell number and testicular development in the marmoset: comparison with the rat. Biol. Reprod. 62, 1685–1693 [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., McKinnell C., Kivlin C., Fisher J. S.2003Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125, 769–784 (doi:10.1530/rep.0.1250769) [DOI] [PubMed] [Google Scholar]
- Sheynkin Y., Jung M., Yoo P., Schulsinger D., Komaroff E.2005Increase in scrotal temperature in laptop computer users. Hum. Reprod. 20, 452–455 (doi:10.1093/humrep/deh616) [DOI] [PubMed] [Google Scholar]
- Siu E. R., Mruk D. D., Porto C. S., Cheng C. Y.2009Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 238, 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek N. E., Rajpert-De Meyts E., Main K. M.2001Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 [DOI] [PubMed] [Google Scholar]
- Smithwick E. B., Young L. G.1996Germ cell maturation and cellular associations in the seminiferous epithelial cycle of the chimpanzee. Tissue Cell 28, 137–148 (doi:10.1016/S0040-8166(96)80002-4) [DOI] [PubMed] [Google Scholar]
- Sokol R. Z., Kraft P., Fowler I. M., Mamet R., Kim E., Berhane K. T.2006Exposure to environmental ozone affects semen quality. Environ. Health Perspect. 114, 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storgaard L., Bonde J. P., Ernst E., Spanô M., Andersen C. Y., Frydenberg M., Olsen J.2003Does smoking during pregnancy affect sons' sperm counts? Epidemiology 14, 278–286 (doi:10.1097/00001648-200305000-00006) [PubMed] [Google Scholar]
- Stoy J., Hjollund N. H., Mortensen J. T., Burr H., Bonde J. P.2004Semen quality and sedentary work position. Int. J. Androl. 27, 5–11 (doi:10.1046/j.0105-6263.2003.00428.x) [DOI] [PubMed] [Google Scholar]
- Sveberg Roste L., Tauboli E., Isojarvi J. I., Pakarinen A. J., Huhtaniemi I. P., Knip M., Gjerstad L.2002Effects of chronic valproate treatment on reproductive endocrine hormones in female and male Wistar rats. Reprod. Toxicol. 16, 767–773 [DOI] [PubMed] [Google Scholar]
- Swan S. H., Elkin E. P., Fenster L.2000The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ. Health Perspect. 108, 961–966 (doi:10.2307/3435055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H., Kruse R. L., Liu F., Barr D. B., Drobnis E. Z., Redmon J. B., Wang C., Brazil C., Overstreet J. W. & the Study for Future Families Research Group. 2003Semen quality in relation to biomarkers of pesticide exposure. Environ. Health Perspect. 111, 1478–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H., Liu F., Overstreet J. W., Brazil C., Skakkebaek N. E.2007Semen quality of fertile US males in relation to their mothers' beef consumption during pregnancy. Hum. Reprod. 22, 1497–1502 (doi:10.1093/humrep/dem068) [DOI] [PubMed] [Google Scholar]
- Takeda K., Tsukue N., Yoshida S.2004Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ. Sci. 11, 33–45 [PubMed] [Google Scholar]
- Tas S., Lauwerys R., Lison D.1996Occupational hazards for the male reproductive system. Crit. Rev. Toxicol. 26, 261–307 (doi:10.3109/10408449609012525) [DOI] [PubMed] [Google Scholar]
- Tchernof A., Despres J. P., Belanger A., Dupont A., Prud'homme D., Moorjani S., Lupien P. J., Labrie F.1995Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism 44, 513–519 (doi:10.1016/0026-0495(95)90060-8) [DOI] [PubMed] [Google Scholar]
- Thonneau P., Bujan L., Multigner L., Mieussset R.1998Occupational heat exposure and male infertility: a review. Hum. Reprod. 13, 2122–2125 (doi:10.1093/humrep/13.8.2122) [DOI] [PubMed] [Google Scholar]
- Thonneau P., et al. 1999Effects of pesticide exposure on time to pregnancy: results of a multicenter study in France and Denmark. ASCLEPIOS study group. Am. J. Epidemiol. 150, 157–163 [DOI] [PubMed] [Google Scholar]
- Toft G., et al. 2006Semen quality and exposure to persistent organochlorine pollutants. Epidemiology 17, 450–458 (doi:10.1097/01.ede.0000221769.41028.d2) [DOI] [PubMed] [Google Scholar]
- Toft G., et al. 2007Semen quality in relation to xenohormone and dioxin-like semen activity among the Inuits and three European populations. Environ. Health Perspect. 115, 15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. T.2001The study of varicocele through the use of animal models. Hum. Reprod. Update 7, 78–84 (doi:10.1093/humupd/7.1.78) [DOI] [PubMed] [Google Scholar]
- Van Thiel D. H., Gavaler J. S., Smith W. I., Paul G.1979Hypothalamic-pituitary-gonadal dysfunction in men using cimetidine. N. Engl. J. Med. 300, 1012–1015 [DOI] [PubMed] [Google Scholar]
- Villalta J., Ballesca J. L., Nicolas J. M., Martinez de Osaba M. J., Antunez E., Pimentel C.1997Testicular function in asymptomatic chronic alcoholics: relation to ethanol intake. Alcohol Clin. Exp. Res. 21, 128–133 [PubMed] [Google Scholar]
- Vine M. F.1996Smoking and male reproduction: a review. Int. J. Androl. 19, 323–337 (doi:10.1111/j.1365-2605.1996.tb00523.x) [DOI] [PubMed] [Google Scholar]
- Vine M. F., Margolin B. H., Morrison H. I., Hulka B. S.1994Cigarette smoking and sperm density: a meta-analysis. Fertil. Steril. 61, 35–43 [PubMed] [Google Scholar]
- Watanabe N.2005Decreased number of sperms and Sertoli cells in mature rats exposed to diesel exhaust as fetuses. Toxciol. Lett. 15, 51–58 [DOI] [PubMed] [Google Scholar]
- Watanabe A., Nakano Y., Endo T., Sato N., Kai K., Shiraiwa K.2000Collaborative work to evaluate toxicity on male reproductive organs by repeated dose studies in rats 27. Repeated toxicity study on ethylene glycol monomethyl ether for 2 and 4 weeks to detect effects on male reproductive organs in rats. J. Toxicol. Sci. 25, 259–266 [DOI] [PubMed] [Google Scholar]
- Welsh M., Saunders P. T. K., Fisken M., Scott H. M., Hutchison G. R., Smith L. B., Sharpe R. M.2008Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Invest. 118, 1479–1490 (doi:10.1172/JCI34241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organisation) 1999Laboratory manual for the examination of human semen and sperm-cervical mucus interaction, 4th edn.Cambridge, UK: Cambridge University Press [Google Scholar]
- Whorton M. D., Foliart D.1988DBCP: eleven years later. Reprod. Toxicol. 2, 155–161 (doi:10.1016/0890-6238(88)90016-0) [DOI] [PubMed] [Google Scholar]
- Whorton M. D., Krauss R. M., Marshall S., Milby T. H.1977Infertility in male pesticide workers. Lancet 2, 1259–1261 [DOI] [PubMed] [Google Scholar]
- Winters S. J., Wang C., Abdelrahaman E., Hadeed V., Dyky M. A., Brufsky A.2006Inhibin-B levels in healthy young adult men and prepubertal boys: is obesity the cause for the comtemporary decline in sperm count because of fewer Sertoli cell? J. Androl. 27, 560–564 (doi:10.2164/jandrol.05193) [DOI] [PubMed] [Google Scholar]