Abstract
Spermatogenesis is a process that involves an array of cellular and biochemical events, collectively culminating in the formation of haploid spermatids from diploid precursor cells known as spermatogonia. As germ cells differentiate from spermatogonia into elongated spermatids, they also progressively migrate across the entire length of the seminiferous epithelium until they reach the luminal edge in anticipation of spermiation at late stage VIII of spermatogenesis. At the same time, these germ cells must maintain stable attachment with Sertoli cells via testis-unique intermediate filament- (i.e. desmosome-like junctions) and actin- (i.e. ectoplasmic specializations, ESs) based cell junctions to prevent sloughing of immature germ cells from the seminiferous epithelium, which may result in infertility. In essence, both desmosome-like junctions and basal ESs are known to coexist between Sertoli cells at the level of the blood–testis barrier where they cofunction with the well-studied tight junction in maintaining the immunological barrier. However, the type of anchoring device that is present between Sertoli and germ cells depends on the developmental stage of the germ cell, i.e. desmosome-like junctions are present between Sertoli and germ cells up to, but not including, step 8 spermatids after which this junction type is replaced by the apical ES. While little is known about the biology of the desmosome-like junction in the testis, we have a relatively good understanding of the molecular architecture and the regulation of the ES. Here, we discuss recent findings relating to these two junction types in the testis, highlighting prospective areas that should be investigated in future studies.
Keywords: testis, Sertoli cell, germ cell, cell junctions
1. Introduction
Spermatogenesis is a highly regulated and complex process that initiates shortly after birth under the regulation of follicle-stimulating hormone, luteinizing hormone, testosterone and oestradiol 17β and continues until old age in males (de Kretser & Kerr 1988; Kerr et al. 2006). It involves four key cellular events, namely (i) spermatogoniogenesis (a continuous process that involves division of type A spermatogonia, which maintains a pool of stem cells, and the production of type B spermatogonia whose fate is to develop into spermatozoa), (ii) spermatocyte differentiation, (iii) spermiogenesis (a process in which spermatids undergo morphogenesis to become mature and motile spermatozoa) and (iv) spermiation (the release of elongated spermatids or spermatozoa, the end-product of spermatogenesis) into the lumen of the seminiferous epithelium (Holstein et al. 2003). Besides developing germ cells, the seminiferous epithelium is also composed of a somatic constituent: the Sertoli cell, a ‘nurse-like’ cell known to provide nutritional and structural support to developing germ cells (Bardin et al. 1988; Griswold & McLean 2006). As germ cells differentiate and travel progressively towards the tubule lumen throughout spermatogenesis, Sertoli and germ cells form dynamic associations within the seminiferous epithelium defined as stages of the seminiferous epithelium, and 14 distinct stages (denoted by roman numerals) have been described in the rat (Leblond & Clermont 1952; Clermont 1972). Spermiogenesis, on the other hand, involves different morphological changes (i.e. development of polarity, condensation of chromosomes and formation of the acrosome, tail and residual body) within spermatids, and this process is divided into 19 steps in the rat. These transformations, in turn, are accompanied by distinct changes in spermatid position within the seminiferous epithelium (Leblond & Clermont 1952).
Throughout spermatogenesis, developing germ cells remain in close contact with Sertoli cells, which is essential for their development. Crucial to these cell–cell interactions in the seminiferous epithelium is the production of several molecules such as hormones, growth factors, proteases, protease inhibitors and components of the extracellular matrix (ECM) by both Sertoli and germ cells, and there are several reports to support the direct role of both cell types in the maintenance of spermatogenesis (Cheng & Mruk 2002; Mruk & Cheng 2004). Additionally, Sertoli cells are known to contribute to the formation of the blood–testis barrier (BTB), an impermeable barrier that divides the seminiferous epithelium into basal and adluminal compartments, thereby sequestering post-meiotic germ cell development from the systemic circulation (Dym & Fawcett 1970; Mruk & Cheng 2004; Hedger & Hales 2006) (figure 1). This is important because spermatozoa and their cell-surface antigens appear long after ‘self’ tolerance is established, and a compromise in BTB function would result in the host producing antibodies against its own sperm. Ultrastructurally, the BTB is composed of coexisting tight junctions (TJs), ectoplasmic specializations (ESs), desmosome-like junctions and gap junctions, and these junctions are known to function collectively in the maintenance of BTB integrity, which is essential for spermatogenesis and fertility. Throughout spermatogenesis, these junctions undergo remodelling to facilitate the transit of preleptotene spermatocytes across the BTB starting at late stage VIII of the seminiferous epithelial cycle. At the same time, Sertoli–germ cell junctions, namely desmosome-like junctions and ESs pachytene spermatocytes, are also restructured to promote the progressive migration of developing germ cells towards the tubule lumen (figure 1). In this review, we discuss briefly recent findings, as well as challenges and prospects for future studies, relating to Sertoli–germ cell desmosome-like junctions and ESs.
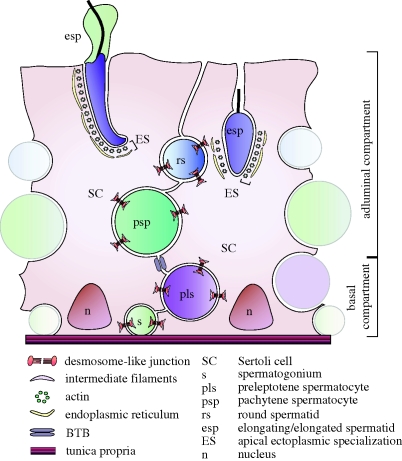
Figure 1.
Different cell–cell adhesion junctions in the adult rodent testis. This schematic drawing illustrates anchoring junctions found between Sertoli and germ cells. A cross section of the seminiferous epithelium, showing two Sertoli cells sitting atop the tunica propria with germ cells at different stages of development (i.e. spermatogonia, preleptotene spermatocytes, round and elongating spermatids). The BTB physically divides the seminiferous epithelium into basal and adluminal compartments, and it forms the immunological barrier. Desmosome-like junctions are present between Sertoli cells and all germ cells up to, but not including, step 8 spermatids (see also figure 2), whereas the apical ES is found between Sertoli cells and germ cells at step 8 of development and beyond (see also figure 3).
2. Sertoli–germ cell anchoring junctions
As briefly discussed above, two types of testis-unique anchoring junctions are present in the seminiferous epithelium: desmosome-like junctions and ESs. Desmosome-like junctions (defined as such in the testis only because they lacked characteristics of true desmosomes found in other organs such as the heart and skin; Holthofer et al. 2007) are found between Sertoli cells at the BTB, as well as between Sertoli and all germ cells up to, but not including, step 8 spermatids. On the other hand, the basal ES is found between Sertoli cells coexisting with desmosome-like junctions at the BTB, whereas the apical ES is found between Sertoli cells and all elongating/elongated spermatids (Russell 1993) (figure 1). In this context, several important observations should be emphasized. First, the Sertoli–germ cell desmosome-like junction and the apical ES do not coexist in the seminiferous epithelium. This is in contrast to all other cell types where desmosomes cofunction and coexist with adherens junctions (AJs) to mediate adhesion. Second, it is not completely known at this point why the desmosome-like junction would need to be replaced by the apical ES, except that the latter junction type facilitates spermatid orientation, and its presence within the seminiferous epithelium correlates precisely with spermatid elongation and the acquisition of polarity. Finally, both junction types are known to mediate stable adhesion throughout spermatogenesis. For instance, perfusion of testes with a hypertonic fixative solution did not affect the integrity of desmosome-like junctions between Sertoli and germ cells, but adjacent regions of cell contact were clearly damaged (Russell 1977). Adhesion mediated by the ES was shown to be equally robust. When the force required to detach different types of germ cells (i.e. spermatocytes, pre-step 8 and step 8 spermatids) from Sertoli cells in vitro was measured with a micropipette pressure transducing system, step 8 spermatids were shown to exhibit the strongest adhesive force (Wolski et al. 2005), suggesting that Sertoli–germ cell adhesion is strengthened as germ cells approach the tubule lumen in anticipation of spermiation at late stage VIII. In the remainder of this section on Sertoli–germ cell anchoring junctions, we discuss the desmosome-like junction and the ES in greater detail, highlighting important insights from in vitro and in vivo model systems that have helped to expand our knowledge of their biology and regulation in the testis.
(a). Desmosomes/desmosome-like junctions
Desmosomes are cell junctions mediating stable and robust adhesion between epithelial cells via the intermediate filament cytoskeleton. They are prominent in organs subjected to mechanical stress (i.e. heart and skin), but they are also found elsewhere (i.e. liver, kidney and testis) (Holthofer et al. 2007). Ultrastructurally, desmosomes appear to be composed of two components: (i) an extracellular space filled with electron-dense material (i.e. the desmoglea) with distinct midline and (ii) two dense cytoplasmic plaques (i.e. inner and outer dense plaques), and desmosomes lacking these components are assumed to be simple, immature and not hyper-adhesive (see discussion below) (Garrod et al. 2005; Holthofer et al. 2007; Scothern & Garrod 2008). The molecular backbone of desmosomes is composed of integral membrane proteins of the cadherin family, namely desmogleins (Dsg) and desmocollins (Dsc), which in contrast to classic cadherins (e.g. E- or N-cadherin) interact both homo- and heterotypically to mediate cell adhesion (Delva et al. 2009). Desmosomal cadherins are then linked to the intermediate filament network via armadillo (i.e. plakoglobin and plakophilins) and plakin (i.e. desmoplakins) family proteins to form a multi-protein complex (Holthofer et al. 2007) (figure 2). Desmosome assembly in confluent cell cultures in vitro has been shown to require Ca++, but as desmosomes mature, they become Ca++-independent and resistant to disruption even by chelation of Ca++ ions (i.e. hyper-adhesive), in turn facilitating strong and stress-resistant adhesion. Recent evidence indicates that desmosomes also function outside of cell adhesion as important hubs to organize and regulate signalling events relating to cell proliferation, differentiation, migration and morphogenesis (Garrod & Chidgey 2008).
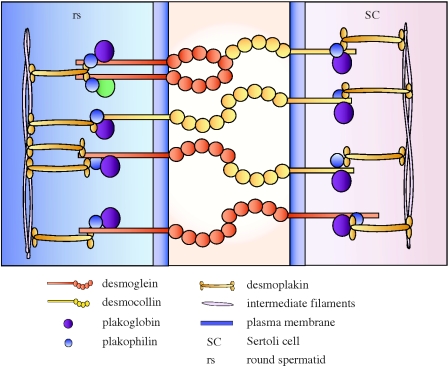
Figure 2.
Desmosome-like junction in the testis. This schematic drawing is based on a recently completed study from our laboratory (Lie et al. in press), and it generalizes the different types of proteins found at the Sertoli–germ cell desmosome-like junction. In this study, desmoglein, desmocollin, plakoglobin, plakophilin, desmoplakin and vimentin were all shown to be expressed by the testis. Additional studies are now underway to better understand the regulation of desmosome-like junctions.
Recent studies have shown protein phosphorylation to play a critical role in the regulation of desmosome function, and some interesting results have emerged in recent years (Yin & Green 2004). In most cases, but not all, phosphorylation of cell junction-associated proteins, namely those of the TJ and AJ, is followed by a compromise in junction function (Gonzalez-Mariscal et al. 2008; Mruk et al. 2008; Nelson 2008). For instance, Tyr phosphorylation of Dsg-2 and plakoglobin in response to epidermal growth factor (EGF) treatment resulted in weakened adhesion in keratinocytes and A431 cells (epithelial cell carcinoma) (Gaudry et al. 2001; Yin et al. 2005). In line with these findings, blocking EGF receptor (EGFR) function in SCC68 cells (oral squamous cell carcinoma) by using either an EGFR tyrosine kinase inhibitor or blocking antibody recruited Dsg-2 and Dsc-2 to cell–cell contact sites, thereby enhancing adhesion (Lorch et al. 2004). Desmosome-mediated cell adhesion was also strengthened following treatment of epithelial cells with sodium pervanadate, a Tyr phosphatase inhibitor, thereby inducing hyper-adhesion (Garrod et al. 2008). Moreover, protein kinase C (PKC), a family of Ser/Thr kinases, has been implicated in desmosome function (Wallis et al. 2000). Specifically, phosphorylation of desmosome plaque proteins by PKCα was demonstrated to switch desmosomes from a state of Ca++-independence (i.e. hyper-adhesion) to one of Ca++-dependence (Wallis et al. 2000). Interestingly, PKCα localized by confocal microscopy to the plasma membrane in Madin–Darby canine kidney (MDCK) cells and keratinocytes with Ca++-dependent desmosomes (Garrod & Kimura 2008), and knockdown of PKCα by RNA interference (RNAi) increased the number of cells with hyper-adhesive desmosomes (Wallis et al. 2000). Likewise, in plakophilin-2-deficient cells, PKCα failed to associate with desmoplakin, thereby affecting the delivery of desmoplakin to nascent desmosomes (Bass-Zubek et al. 2008). These data clearly reveal protein kinases such as PKCα to be critical for desmosome regulation.
Although the presence of desmosome-like structures in the testis has been known for over three decades (Russell 1977), our understanding of their biology and regulation is still surprisingly poor. A striking feature of desmosome-like junctions in the testis is that they lack a clearly defined dense midline, which is characteristic of conventional desmosomes, suggesting that these structures are Ca++-dependent and not likely to mediate robust adhesion. However, this observation is seemingly in disagreement with the in vivo study discussed previously, which showed that desmosome-like junctions were unaffected following use of a hypertonic buffer for perfusion (Russell 1977; Russell & Peterson 1985). Additional biochemical and cellular studies would be needed to address this disparity in morphological observations. Based on a recently completed study from this laboratory, we know that several desmosomal genes are expressed by the testis and that functional desmosome-like junctions are assembled between Sertoli cells in vitro (Lie et al. in press). If desmosome-like junctions in the testis are indeed Ca++-dependent and not hyper-adhesive, a logical next step would be to investigate their function in vitro in the presence of selective PKC, as well as other kinase inhibitors. Moreover, desmosome-like junctions in the testis have been shown to share ultrastructural features of gap junctions (Russell 1993). This is underscored by the recent finding that plakophilin-2 (a cytoplasmic protein of the armadillo family that links desmosomal cadherins to intermediate filaments) structurally associates with connexin 43 (a transmembrane protein of the gap junction that is widely expressed), and that connexin 43, in turn, interacts with constituent proteins of the BTB (i.e. N-cadherin and ZO-1) and plays a role in the maintenance of BTB integrity (Li et al. 2009). In support of these results, there is at least one report that describes coimmunoprecipitation and colocalization of connexins with TJ proteins (i.e. occludin, claudin and ZO-1) in endothelial cells (Nagasawa et al. 2006). Interestingly, inhibition of gap junction function by 18β-glycyrrhetinic acid or oleamide adversely affected barrier function as demonstrated by the measurement of transepithelial electrical resistance across the cell epithelium (Nagasawa et al. 2006). In light of these engaging results, it is important that future studies investigate signalling events that mediate crosstalk between different junction types in the testis.
(b). Ectoplasmic specializations
(i). Cadherin–catenin multi-protein complex
The cadherin–catenin multi-protein complex is the best studied actin-based adhesion unit in epithelial and endothelial cells (table 1); hence, it is discussed first in this section, which focuses on ES dynamics. Although very well known for its role in cell adhesion, the cadherin–catenin protein complex also functions in other cellular processes, i.e. in the regulation of the cytoskeleton and cell polarity, control of cell division and tumour suppression. Cadherins are a large family of Ca++-dependent transmembrane proteins that can be further categorized into five distinct subfamilies: (i) type I and type II, (ii) desmosomal, (iii) atypical (note: atypical cadherins are GPI-anchored and do not link to the cytoskeleton), (iv) protocadherins and (v) cadherin-like. Catenins (i.e. α-, β- and γ-catenins), on the other hand, are cytoplasmic proteins that bind to the C-terminus of type I/II and desmosomal cadherins, thereby regulating cadherin-mediated adhesion via the actin and intermediate filament (as is the case for desmosomes/desmosome-like junctions) cytoskeletons (figures 2 and 3). In this section, we aim our discussion on type I cadherins because they have been studied most extensively in the testis.
Table 1.
Different AJ, focal contact and TJ proteins/multi-protein complexes present at the apical ES. This table summarizes different proteins/multi-protein complexes, as well as their interacting partners, present at the apical ES. Each protein/multi-protein complex listed here is classified as an AJ, focal contact or TJ protein, illustrating that the apical ES is a hybrid-like junction composed of diverse proteins. For additional background information, readers are asked to refer to the following review articles: Cheng & Mruk (2002), Mruk & Cheng (2004) and Yan et al. (2007). aPKC, atypical protein kinase C; CAR, coxsackievirus and adenovirus receptor; Src, protein tyrosine kinase of the transforming gene of Rous sarcoma virus; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; ILK, integrin-linked kinase; IQGAP1, IQ motif containing GTPase-activating protein; JAM-C, junctional adhesion molecule-C; LIMK1, lin-11 isl-1 mec3 kinase 1; MMP-2, matrix metalloprotease-2; MT1-MMP, membrane-type 1-MMP; MTMR2, myotubularin-related protein 2; NOS, nitric oxide synthase; p130Cas, p130 Crk-associated protein; PAK, p21-activated kinase; Par3, partitioning defective 3; Par6, partitioning defective 6; PATJ, Pals1-associated tight junction protein; p-FAK, phosphorylated FAK; PI3K, phosphatidylinositol 3-kinase; PKB, protein kinase B; PKG, protein kinase G; PRKG, cGMP-dependent protein kinase; p-Src, phosphorylated Src; PTEN, phosphatase and tensin homologue deleted on chromosome 10; RA175, IGSF4A/RA175/TSLC1/SynCAM/SgIGSF/Necl2; ROCK1, Rho-associated protein kinase 1; sGC, soluble guanylate cyclase; TIMP-2, tissue inhibitor of metalloproteases-2 and WASP, Wiskott–Aldrich syndrome protein.
| protein/multi-protein complex | interacting proteins | reference(s) |
|---|---|---|
| AJ | ||
| N-cadherin–β-catenin | PKG, α-catenin, β-catenin, NOS, c-Src, Cdc42, IQGAP1, dynamin-2, sGC, p-p120ctn, Fer kinase, zyxin, axin, WASP, β1-integrin, α4-integrin, cortactin, MTMR2, p-Src, p-ERK, Rab 8 | Wine & Chapin (1999), Chapin et al. (2001), Johnson & Boekelheide (2002), Chen et al. (2003), Lau & Mruk (2003), Lee et al. (2004, 2005), Lui et al. (2005), Zhang et al. (2005), Lie et al. (2006) and Sarkar et al. (2006) |
| nectin–afadin | ponsin, αT-catenin, sGC | Ozaki-Kuroda et al. (2002), Sarkar et al. (2006) and Goossens et al. (2007) |
| focal contact | ||
| α6β1-integrin–laminin α3β3γ3 | ILK, c-Src, p-Src, FAK, p-FAK, PI3K, PKB, PAK, ERK, vinculin, paxillin, cofilin, gelsolin, PTEN, p130Cas, Rho B, ROCK1, LIMK1, MMP-2, MT1-MMP, TIMP-2, N-cadherin | Palombi et al. (1992), Salanova et al. (1995), Chapin et al. (2001), Mulholland et al. (2001), Lui et al. (2003), Siu et al. (2003, 2005), Siu & Cheng (2004), Beardsley et al. (2006) and Yan & Cheng (2006) |
| tight junction (TJ) | ||
| JAM-C | JAM-B, Par3, Pals1, Par6, Cdc42, PKCλ, PATJ, RA175 (associated via Par3) | Gliki et al. (2004), Fujita et al. (2007) and Wong et al. (2008) |
| CAR | c-Src, vinculin, α-catenin, β-catenin | Wang et al. (2007) |
| aPKC–Par3/Par6 and Crb–Pals1–PATJ | JAM-C, Src, RA175 | Gliki et al. (2004), Fujita et al. (2007) and Wong et al. (2008) |
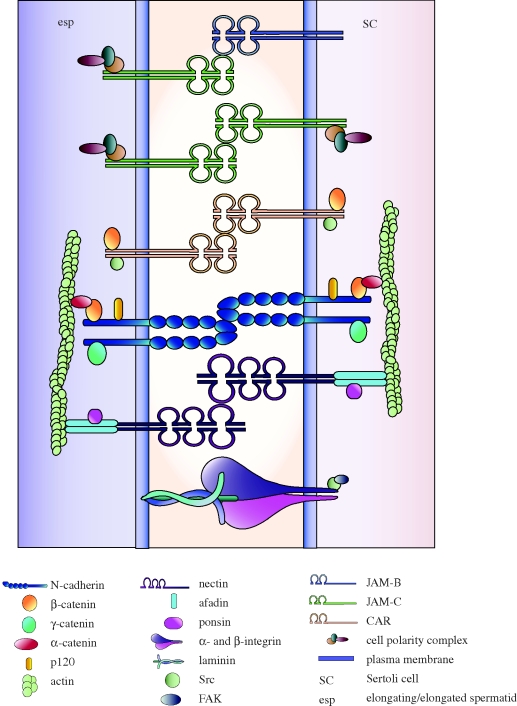
Figure 3.
Apical ES. This illustration depicts several proteins that have been described to exist at the apical ES, namely the cadherin–catenin, nectin–afadin, α6β1 integrin–laminin α3β3γ3 and JAM–Par/CAR multi-protein complexes (see also table 1). The apical ES is unique with hybrid-like characteristics because it is composed of several proteins that are generally found in focal contacts and TJs. For simplicity, only proteins that were discussed in the text are included in this figure.
A number of different cadherins have been identified in the testis, and although this was an arguable topic in the past, it is now well established that N-cadherin is present at the apical ES (Wine & Chapin 1999; Johnson & Boekelheide 2002; Lee et al. 2003, 2004) (table 1, figure 3). As in other epithelial cells that use conventional AJs for adhesion, the ES is regulated by various mechanisms and constantly remodelled. For instance, both N- and E-cadherin are known to be expressed by Sertoli and germ cells (Lee et al. 2003), and in vitro and in vivo studies have demonstrated their steady-state levels to be regulated by cytokines and testosterone (Lee et al. 2003; Yan et al. 2008a). Generally speaking, the turnover of AJs, including the ES, is achieved in part by the endocytosis of cadherin, followed by its recycling back to the cell surface (Yan et al. 2008a; Delva & Kowalczyk 2009). Indeed, a recent study demonstrated an increase in the kinetics of N-cadherin (as well as in two TJ proteins: occludin and JAM-A) internalization via a clathrin-dependent pathway when Sertoli cells were cultured in the presence of transforming growth factor-β2 (Yan et al. 2008b). The addition of testosterone was also shown to enhance the rate of N-cadherin endocytosis, as well as to promote its recycling back to the Sertoli cell surface. These findings seemingly illustrate that movement of preleptotene spermatocytes across the BTB is facilitated by cytokine-mediated internalization of ES and TJ proteins that resulted in the transient disassembly of the BTB, whereas testosterone promoted its assembly after spermatocytes crossed the barrier and entered into the adluminal compartment of the seminiferous epithelium. Interestingly, the Cdc42-atypical protein kinase C (aPKC)–Par6 multi-protein complex known to function in cell polarity has been shown to regulate AJ dynamics in epithelial cells in Drosophila by controlling E-cadherin endocytosis via actin regulatory proteins (i.e. WASp, Arp2/3 and dynamin) (Georgiou et al. 2008; Leibfried et al. 2008). In essence, loss of Cdc42, aPKC or Par6 was shown to result in AJ disruption, and Cdc42 in particular was thought to function with the Par complex to slow down the entry of proteins into the endocytic pathway (Harris & Tepass 2008) (see also table 1). This finding is supported to some extent by another study which demonstrated that homophilic interaction of E-cadherin molecules activates Rho GTPases, which in turn mediates cadherin–actin association, and this can protect E-cadherin from endocytosis (Izumi et al. 2004). A next step would be to investigate how polarity proteins present at the BTB take part in TGF-β3- and testosterone-mediated endocytosis of N-cadherin, occludin and JAM-A in light of published results that Par6 immunoreactivity was weakest at the BTB at stage VIII when preleptotene spermatocytes cross the barrier (Wong et al. 2008). Whether a mechanism similar to the one described in Drosophila is at work in the seminiferous epithelium remains to be examined in future studies.
(ii). Nectin–afadin multi-protein complex
Nectins (nectins 1–4) and nectin-like molecules (Necls, Necls1–5) comprise a small family of Ca++-independent, immunoglobulin (Ig)-like molecules known to have roles in cell adhesion, proliferation, differentiation, cell survival, migration and cell polarity (Takai & Nakanishi 2003; Takai et al. 2003, 2008c; Irie et al. 2004). The major difference between nectins and Necls lies in their ability to bind afadin, an F-actin binding protein (Takai et al. 2008b); nectins bind afadin, but Necls do not. The assembly of nectin-based adhesive structures is a complex, multi-step process. Nectins are known to first form homo-cis dimers, followed by the assembly of homo- or hetero-trans dimers, and the existence of nectin 1–nectin 3, nectin 1–nectin 4 and nectin 2–nectin 3 hetero-trans dimers has been reported (Takai et al. 2008a,b). These trans-interactions then activate other proteins, namely Cdc42, Rac1 and Rap1 GTPases via Src (Fukuhara et al. 2004; Fukuyama et al. 2005; Kawakatsu et al. 2005), followed by the phosphorylation of FGD1-related Cdc42-guanine nucleotide exchange factor and Vav2, guanine nucleotide exchange factors specific for Cdc42 and Rac1, respectively (Fukuhara et al. 2004; Kawakatsu et al. 2005). This is followed by the recruitment of cadherins, which also promote the assembly of the AJ (Ogita & Takai 2008).
As mentioned above, nectins and Necls are not simply cell adhesion molecules. They also play an important role in cell movement (Takai et al. 2003, 2008b). Studies have shown that nectins 1 and 3 (Sakamoto et al. 2006) and Necl-5 (Ikeda et al. 2004; Kakunaga et al. 2004) associate with integrin αvβ3, and that integrin αvβ3 activation was essential for nectin-mediated AJ assembly (Sakamoto et al. 2006). However, logical reasoning would suggest integrin activation to support cell junction disassembly and cell migration—and not cell adhesion. This implies that nectin-bound integrin αvβ3 would have to be inactivated or downregulated to mediate cell adhesion. While this mechanism is unique in its ability to regulate cell adhesion, it remains to be known whether nectins bind to additional integrin family members.
All four members of the nectin family are present in the testis, but only two of them—nectin 2 and nectin 3—are found at the apical ES, with nectin 2 and nectin 3 residing on the Sertoli cell and spermatid cell surface, respectively (Ozaki-Kuroda et al. 2002) (table 1, figure 3). Studies have shown that heterotypic interactions between these two nectins are essential for apical ES function, as well as for the proper development and positioning of elongated spermatids within the seminiferous epithelium (Ozaki-Kuroda et al. 2002; Toyama et al. 2008). Nectin 2 and nectin 3 knockout mice displayed abnormalities in actin distribution and defects in spermatid morphology, including irregularities in nuclear shape and mislocalization of mitochondria (Bouchard et al. 2000; Ozaki-Kuroda et al. 2002; Inagaki et al. 2006). These malformations resulted in male sterility (Ozaki-Kuroda et al. 2002), illustrating the importance of nectin-mediated Sertoli cell–spermatid adhesion for spermatogenesis and fertility. It would be interesting to know whether nectin 2 and/or nectin 3 interacts with the integrin–laminin multi-protein complex (see discussion below) at the apical ES to regulate adhesion (figure 3).
(iii). Integrin–laminin multi-protein complex
Generally speaking, integrins are well-studied proteins of the focal contact (a type of actin-based cell junction that connects a cell to the ECM) and hemidesmosome (a type of intermediate filament-based cell junction that connects a cell to the ECM) (Margadant et al. 2008; Geiger et al. 2009), whereas laminins are constituents of the basement membrane (Miner & Yurchenco 2004; Miner 2008). Both protein families are known to have an important role in cell adhesion, but also in cell migration and invasion during metastasis (Barczyk et al. 2009; Durbeej 2009; Huveneers & Danen 2009; Moser et al. 2009), and at least eight integrins have been reported to interact with laminins to produce ‘inside-out’ and ‘outside-in’ signals at the level of the cell–ECM (Huveneers & Danen 2009; Moser et al. 2009). For example, interaction of α3β1 integrin with laminin α3β2γ2 is known to regulate Src via focal adhesion kinase (FAK) (see also table 1), in turn promoting Rac1 activation and keratinocyte movement (Choma et al. 2007). Another integrin (i.e. α6β4 integrin) was also reported to coimmunoprecipitate with Rac1 from extracts of keratinocytes (Sehgal et al. 2006), illustrating the critical role of integrin in cell migration.
In the testis, the focal contact is absent from the Sertoli cell–ECM interface, and the only adhesive structure mounting Sertoli cells to the tunica propria is the hemidesmosome (Wrobel et al. 1979). While β1 integrin staining was recently observed at the hemidesmosome (Yan et al. 2008a), the α6β1 integrin–laminin α3β3γ3 multi-protein complex did not localize to this junction type in the testis. Instead, it was shown to localize at the opposite end of the seminiferous epithelium (i.e. at the apical ES), with α6β1 integrin and laminin α3β3γ3 being expressed by Sertoli cells and elongated spermatids, respectively (Siu & Cheng 2004; Yan & Cheng 2006) (table 1, figure 3). Interestingly, laminin β3 domain I and laminin γ3 domain IV recombinant protein fragments were recently shown to reduce the level of occludin at the Sertoli cell barrier and β1 integrin at the hemidesmosome, probably revealing precise coordination of junction restructuring during spermiation and transit of preleptotene spermatocytes across the BTB at stage VIII of the seminiferous epithelial cycle via signalling that also involved the hemidesmosome (Yan et al. 2008a). These findings are in line with at least another report demonstrating that cleavage of laminin α3β3γ2 by matrix metalloprotease 2 (MMP-2) or membrane type 1 metalloprotease (MT1-MMP) produces a γ2 fragment of 80 kDa that can facilitate migration of human breast epithelial cells (Giannelli et al. 1997; Koshikawa et al. 2000). In a separate study, however, the reverse was reported: proteolytic cleavage of the α3 chain of laminin α3β3γ2 by plasmin was shown to support hemidesmosome assembly, resulting in decreased cell motility (Goldfinger et al. 1998). Taken collectively, these findings reveal that laminin fragments elicit a broad repertoire of biological effects to regulate different aspects of cell function.
On a final note, a recent study has reported basolateral colocalization of Par1 with laminin in MDCK cells (Masuda-Hirata et al. 2009). Par1 was also reported to regulate the localization of dystroglycan, a laminin receptor essential for basement membrane formation. Interestingly, knockdown of Par1 by RNAi upregulated the secretion of β1 and γ1 chains (Masuda-Hirata et al. 2009), illustrating that polarity proteins take active part in ECM organization, which is required for epithelial cell polarity. Overexpression of a dominant-negative Rac1 mutant also resulted in the accumulation of extracellular laminin (O'Brien et al. 2002; Yu et al. 2005). The connection between key polarity determinants and ECM proteins at the apical ES in the testis is not yet known but well worth investigating because elongated spermatids express both Par3 and Par6, and laminin (Yan & Cheng 2006; Fujita et al. 2007; Wong et al. 2008) (table 1, figure 3). The role of the α6β1 integrin–laminin α3β3γ3 multi-protein complex in coordinating cell movement (i.e. spermiation) and germ cell polarity should also be addressed.
(iv). JAM–Par/CAR multi-protein complex
Although TJs are restricted to the BTB in the seminiferous epithelium, TJ proteins have been shown to localize at the apical ES, and these include junctional adhesion molecule (JAM), coxsackievirus and adenovirus receptor (CAR) and Par proteins (table 1, figure 3). It is also worthy to note that these proteins are expressed by elongating/elongated spermatids, even though the ES, as well as its unique ultrastructural characteristics (e.g. hexagonally arranged actin filament bundles and cisternae of endoplasmic reticulum), has never been described to exist within germ cells. JAMs (i.e. JAM-A, -B, -C, -D and JAML) are single-span transmembrane proteins of the Ig superfamily known to mediate homo- and heterophilic interactions with diverse transmembrane ligands, including CAR and integrin (Bazzoni et al. 2000; Ostermann et al. 2002; Bazzoni 2003; Zen et al. 2005; Mirza et al. 2006; Luissint et al. 2008). In addition to facilitating cell adhesion, JAMs are also understood to function in the migration of cells across the endothelium (Martin-Padura et al. 1998; Chavakis et al. 2004; Bradfield et al. 2007; Weber et al. 2007). The expression of all three JAMs has been reported in the testis: both JAM-A and -B are present in Sertoli cells at the BTB, and in round and elongated spermatids (Gliki et al. 2004; Shao et al. 2008), whereas JAM-C is only found at the apical ES where it probably functions in Sertoli cell–spermatid adhesion and germ cell positioning and polarization (Gliki et al. 2004). JAM-A is also present in spermatozoa and essential for sperm motility, as supported by a study using JAM-A−/− mice (Shao et al. 2008). Interestingly, Zen and colleagues reported JAM-C to be a component of the desmosome (i.e. JAM-C colocalized precisely with desmoplakin, a cytoplasmic protein of the desmosome), but not the TJ, in intestinal epithelial cells, as well as to participate in the transepithelial migration of leucocytes (Zen et al. 2004). This led the authors to speculate that desmosomes, similar to TJs, may also need to be opened transiently to allow leucocytes to cross the epithelium. This is apparently in line with a recently completed study by our laboratory which demonstrated that knockdown of Dsg-2 and Dsc-2 by RNAi in Sertoli cells in vitro downregulated the steady-state level of JAM-A, as well as ZO-1 (Lie et al. in press). These findings were corroborated by a functional in vitro experiment that showed a partial compromise in barrier function when TER was quantified across the epithelium of Dsg-2 and Dsc-2 silenced-Sertoli cells, revealing that desmosomes contribute to barrier function possibly by opening up TJs during the passage of preleptotene spermatocytes. JAMs are also known to interact with the Par–aPKC–Cdc42 multi-protein complex, which plays an important role in TJ dynamics (Assemat et al. 2008) (see also table 1), illustrating extensive but yet-to-be understood crosstalk among different junction types.
CAR, on the other hand, was initially characterized as a cell-surface protein required for the entry of coxsackie B and adenoviruses into cells (Coyne & Bergelson 2005). Subsequently, CAR was reported to be a component of the TJ complex and a regulator of TJ assembly when it was shown to colocalize with occludin and to coimmunoprecipitate ZO-1 (Cohen et al. 2001; Coyne et al. 2004; Excoffon et al. 2004; Mirza et al. 2005; Raschperger et al. 2006). CAR has also been assigned a role in cell adhesion (Cohen et al. 2001; Philipson & Pettersson 2004). In human airway epithelia, CAR staining overlapped with that of β-catenin, and immunoprecipitation revealed a direct interaction between these two proteins (Walters et al. 2002). Similar results were also reported in the testis, and CAR was shown to be expressed by both Sertoli and germ cells (Wang et al. 2007) (table 1, figure 3). Furthermore, homophilic interactions are known to underlie CAR function, but as mentioned above, there are at least two reports in the literature illustrating a heterophilic interaction with JAML (Zen et al. 2005; Luissint et al. 2008). Generally speaking, loss of CAR expression results in weakened cell adhesion, thereby promoting cell migration (Okegawa et al. 2001; Bruning & Runnebaum 2004; Matsumoto et al. 2005). The role of CAR in the testis is being actively investigated in light of its possible role in the movement of preleptotene spermatocytes across the BTB. However, it will be several years before reproductive biologists have a clearer understanding of the biochemical and molecular events that underlie germ cell movement in the seminiferous epithelium of the testis.
3. Future perspectives
Although the biology of the desmosome-like junction and ES is not yet clearly understood, the number of proteins present at these two structures has increased systematically. Recently, carcino-embryogenic antigen-related cell adhesion molecule (CEACAM6) was described as a novel component of the ES in the rat testis (Kurio et al. 2008), and it is likely that other important cell adhesion proteins will be identified in the years to come. However, the challenges that remain are those that address desmosome-like junction and ES regulation in the testis. For instance, are desmosomal cadherins endocytosed during germ cell migration which involves extensive restructuring of Sertoli–germ cell junctions? What is the identity of the signal that initiates desmosome-like junction disassembly and dissolution in late step 7 spermatids, followed by apical ES assembly in step 8 spermatids? Is the signal a polarity-related protein kinase? Finally, is there any crosstalk between cadherin–catenin, nectin–afadin, integrin–laminin and JAM–Par/CAR multi-protein complexes at the apical ES, and how do they contribute to one of the most important events taking place in the seminiferous epithelium: spermiation at late stage VIII of the seminiferous epithelial cycle? With recent technological advancements in cell and molecular biology such as RNAi and fluorescence recovery after photobleaching, we expect the next few decades to be an exciting time in the field as reproductive biologists find answers to some of the most important questions in testis biology.
Acknowledgements
Research supported by NICHD, NIH (R03HD061401 to D.D.M.; R01HD056034 and U54HD029990, Project 5 to C.Y.C.) and by the Foundation for Polish Science (an Academic Grant 2008 from the Mistrz Programme to B.B.).
Footnotes
One contribution of 17 to a Theme Issue ‘The biology and regulation of spermatogenesis’.
References
- Assemat E., Bazellieres E., Pallesi-Pocachard E., Le Bivic A., Massey-Harroche D.2008Polarity complex proteins. Biochim. Biophys. Acta 1778, 614–630 [DOI] [PubMed] [Google Scholar]
- Barczyk M., Carracedo S., Gullberg D.2009Integrins. Cell Tissue Res. 339, 269–280 (doi:10.1007/s00441-009-0834-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin C. W., Cheng C. Y., Musto N. A., Gunsalus G. L.1988The Sertoli cell. In The physiology of reproduction (eds Knobil E., Neill J. D., Ewing L. L., Greenwald G. S., Markert C. L., Pfaff D. W.), pp. 933–974 New York, NY: Raven Press [Google Scholar]
- Bass-Zubek A. E., Hobbs R. P., Amargo E. V., Garcia N. J., Hsieh S. N., Chen X., Wahl J. K., III, Denning M. F., Green K. J.2008Plakophilin 2: a critical scaffold for PKCα that regulates intercellular junction assembly. J. Cell Biol. 181, 605–613 (doi:10.1083/jcb.200712133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G.2003The JAM family of junctional adhesion molecules. Curr. Opin. Cell Biol. 15, 525–530 (doi:10.1016/S0955-0674(03)00104-2) [DOI] [PubMed] [Google Scholar]
- Bazzoni G., Martinez-Estrada O. M., Mueller F., Nelboeck P., Schmid G., Bartfai T., Dejana E., Brockhaus M.2000Homophilic interaction of junctional adhesion molecule. J. Biol. Chem. 275, 30 970–30 976 (doi:10.1074/jbc.M003946200) [DOI] [PubMed] [Google Scholar]
- Beardsley A., Robertson D. M., O'Donnell L.2006A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J. Endocrinol. 190, 759–770 (doi:10.1677/joe.1.06867) [DOI] [PubMed] [Google Scholar]
- Bouchard M. J., Dong Y., McDermott B. M., Jr, Lam D. H., Brown K. R., Shelanski M., Bellve A. R., Racaniello V. R.2000Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell–cell adherens junctions. Mol. Cell. Biol. 20, 2865–2873 (doi:10.1128/MCB.20.8.2865-2873.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield P. F., et al. 2007JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood 110, 2545–2555 (doi:10.1182/blood-2007-03-078733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning A., Runnebaum I. B.2004The coxsackie adenovirus receptor inhibits cancer cell migration. Exp. Cell Res. 298, 624–631 (doi:10.1016/j.yexcr.2004.05.001) [DOI] [PubMed] [Google Scholar]
- Chapin R. E., Wine R. N., Harris M. W., Borchers C. H., Haseman J. K.2001Structure and control of a cell–cell adhesion complex associated with spermiation in rat seminiferous epithelium. J. Androl. 22, 1030–1052 [DOI] [PubMed] [Google Scholar]
- Chavakis T., Keiper T., Matz-Westphal R., Hersemeyer K., Sachs U. J., Nawroth P. P., Preissner K. T., Santoso S.2004The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J. Biol. Chem. 279, 55 602–55 608 (doi:10.1074/jbc.M404676200) [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Lee N. P., Mruk D. D., Lee W. M., Cheng C. Y.2003Fer kinase/FerT and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol. Reprod. 69, 656–672 (doi:10.1095/biolreprod.103.016881) [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Mruk D. D.2002Cell junction dynamics in the testis: Sertoli–germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874 [DOI] [PubMed] [Google Scholar]
- Choma D. P., Milano V., Pumiglia K. M., DiPersio C. M.2007Integrin α3β1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J. Invest. Dermatol. 127, 31–40 (doi:10.1038/sj.jid.5700505) [DOI] [PubMed] [Google Scholar]
- Clermont Y.1972Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 52, 198–236 [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Shieh J. T., Pickles R. J., Okegawa T., Hsieh J. T., Bergelson J. M.2001The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl Acad. Sci. USA 98, 15 191–15 196 (doi:10.1073/pnas.261452898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne C. B., Bergelson J. M.2005CAR: a virus receptor within the tight junction. Adv. Drug Deliv. Rev. 57, 869–882 (doi:10.1016/j.addr.2005.01.007) [DOI] [PubMed] [Google Scholar]
- Coyne C. B., Voelker T., Pichla S. L., Bergelson J. M.2004The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J. Biol. Chem. 279, 48 079–48 084 (doi:10.1074/jbc.M409061200) [DOI] [PubMed] [Google Scholar]
- de Kretser D. M., Kerr J. B.1988The cytology of the testis. In The physiology of reproduction (eds Knobil E., Neill J. D., Ewing L. L., Greenwald G. S., Markert C. L., Pfaff D. W.), pp. 837–932 New York, NY: Raven Press [Google Scholar]
- Delva E., Kowalczyk A. P.2009Regulation of cadherin trafficking. Traffic 10, 259–267 (doi:10.1111/j.1600-0854.2008.00862.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva E., Tucker D. K., Kowalczyk A. P.2009The desmosome. Cold Spring Harb. Perspect. Biol. 1, a002543 (doi:10.1101/cshperspect.a002543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M.2009Laminins. Cell Tissue Res. 339, 259–268 (doi:10.1007/s00441-009-0838-2) [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W.1970The blood–testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol. Reprod. 3, 308–326 [DOI] [PubMed] [Google Scholar]
- Excoffon K. J., Hruska-Hageman A., Klotz M., Traver G. L., Zabner J.2004A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J. Cell Sci. 117, 4401–4409 (doi:10.1242/jcs.01300) [DOI] [PubMed] [Google Scholar]
- Fujita E., Tanabe Y., Hirose T., Aurrand-Lions M., Kasahara T., Imhof B. A., Ohno S., Momoi T.2007Loss of partitioning-defective-3/isotype-specific interacting protein (Par-3/ASIP) in the elongating spermatid of RA175 (IGSF4A/SynCAM)-deficient mice. Am. J. Pathol. 171, 1800–1810 (doi:10.2353/ajpath.2007.070261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara T., et al. 2004Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. J. Cell Biol. 166, 393–405 (doi:10.1083/jcb.200401093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama T., et al. 2005Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J. Biol. Chem. 280, 815–825 [DOI] [PubMed] [Google Scholar]
- Garrod D., Chidgey M.2008Desmosome structure, composition and function. Biochim. Biophys. Acta 1778, 572–587 [DOI] [PubMed] [Google Scholar]
- Garrod D., Kimura T. E.2008Hyper-adhesion: a new concept in cell–cell adhesion. Biochem. Soc. Trans. 36, 195–201 (doi:10.1042/BST0360195) [DOI] [PubMed] [Google Scholar]
- Garrod D. R., Berika M. Y., Bardsley W. F., Holmes D., Tabernero L.2005Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. J. Cell Sci. 118, 5743–5754 (doi:10.1242/jcs.02700) [DOI] [PubMed] [Google Scholar]
- Garrod D. R., Fisher C., Smith A., Nie Z.2008Pervanadate stabilizes desmosomes. Cell Adh. Migr. 2, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry C. A., Palka H. L., Dusek R. L., Huen A. C., Khandekar M. J., Hudson L. G., Green K. J.2001Tyrosine-phosphorylated plakoglobin is associated with desmogleins but not desmoplakin after epidermal growth factor receptor activation. J. Biol. Chem. 276, 24 871–24 880 (doi:10.1074/jbc.M102731200) [DOI] [PubMed] [Google Scholar]
- Geiger B., Spatz J. P., Bershadsky A. D.2009Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol 10, 21–33 (doi:10.1038/nrm2593) [DOI] [PubMed] [Google Scholar]
- Georgiou M., Marinari E., Burden J., Baum B.2008Cdc42, Par6 and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr. Biol. 18, 1631–1638 (doi:10.1016/j.cub.2008.09.029) [DOI] [PubMed] [Google Scholar]
- Giannelli G., Falk-Marzillier J., Schiraldi O., Stetler-Stevenson W. G., Quaranta V.1997Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277, 225–228 (doi:10.1126/science.277.5323.225) [DOI] [PubMed] [Google Scholar]
- Gliki G., Ebnet K., Aurrand-Lions M., Imhof B. A., Adams R. H.2004Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature 431, 320–324 (doi:10.1038/nature02877) [DOI] [PubMed] [Google Scholar]
- Goldfinger L. E., Stack M. S., Jones J. C. R.1998Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J. Cell Biol. 141, 255–265 (doi:10.1083/jcb.141.1.255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Tapia R., Chamorro D.2008Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 1778, 729–756 [DOI] [PubMed] [Google Scholar]
- Goossens S., Janssens B., Vanpoucke G., De Rycke R., van Hengel J., van Roy F.2007Truncated isoform of mouse αT-catenin is testis-restricted in expression and function. FASEB J. 21, 647–655 (doi:10.1096/fj.06-6066com) [DOI] [PubMed] [Google Scholar]
- Griswold M. D., McLean D. J.2006The Sertoli cell. In Knobil and Neill's physiology of reproduction (ed. Neill J. D.), pp. 949–975 New York, NY: Elsevier [Google Scholar]
- Harris K. P., Tepass U.2008Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 183, 1129–1143 (doi:10.1083/jcb.200807020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger M. P., Hales D. B.2006Immunophysiology of the male reproductive tract. In Knobil and Neill's physiology of reproduction (ed. Neill J. D.), pp. 1195–1286 New York, NY: Elsevier [Google Scholar]
- Holstein A. F., Schulze W., Davidoff M.2003Understanding spermatogenesis is a prerequisite for treatment. Reprod. Biol. Endocrinol. 1, 107 (doi:10.1186/1477-7827-1-107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthofer B., Windoffer R., Troyanovsky S., Leube R. E.2007Structure and function of desmosomes. Int. Rev. Cytol. 264, 65–163 (doi:10.1016/S0074-7696(07)64003-0) [DOI] [PubMed] [Google Scholar]
- Huveneers S., Danen E. H. J.2009Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 122, 1059–1069 (doi:10.1242/jcs.039446) [DOI] [PubMed] [Google Scholar]
- Ikeda W., Kakunaga S., Takekuni K., Shingai T., Satoh K., Morimoto K., Takeuchi M., Imai T., Takai Y.2004Nectin-like molecule-5/Tage4 enhances cell migration in an integrin-dependent, nectin-3-independent manner. J. Biol. Chem. 279, 18 015–18 025 (doi:10.1074/jbc.M312969200) [DOI] [PubMed] [Google Scholar]
- Inagaki M., Irie K., Ishizaki H., Tanaka-Okamoto M., Miyoshi J., Takai Y.2006Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells 11, 1125–1132 (doi:10.1111/j.1365-2443.2006.01006.x) [DOI] [PubMed] [Google Scholar]
- Irie K., Shimizu K., Sakisaka T., Ikeda W., Takai Y.2004Roles and modes of action of nectins in cell–cell adhesion. Semin. Cell Dev. Biol. 15, 643–656 [DOI] [PubMed] [Google Scholar]
- Izumi G., Sakisaka T., Baba T., Tanaka S., Morimoto K., Takai Y.2004Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J. Cell Biol. 166, 237–248 (doi:10.1083/jcb.200401078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Boekelheide K.2002Dynamic testicular adhesion junctions are immunologically unique. II. Localization of classic cadherins in rat testis. Biol. Reprod. 66, 992–1000 (doi:10.1095/biolreprod66.4.992) [DOI] [PubMed] [Google Scholar]
- Kakunaga S., Ikeda W., Shingai T., Fujito T., Yamada A., Minami Y., Imai T., Takai Y.2004Enhancement of serum- and platelet-derived growth factor-induced cell proliferation by Necl-5/Tage4/poliovirus receptor/CD155 through the Ras-Raf-MEK-ERK signaling. J. Biol. Chem. 279, 36 419–36 425 (doi:10.1074/jbc.M406340200) [DOI] [PubMed] [Google Scholar]
- Kawakatsu T., Ogita H., Fukuhara T., Fukuyama T., Minami Y., Shimizu K., Takai Y.2005Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J. Biol. Chem. 280, 4940–4947 (doi:10.1074/jbc.M408710200) [DOI] [PubMed] [Google Scholar]
- Kerr J. B., Loveland K. L., O'Bryan M. K., de Kretser D. M.2006Cytology of the testis and intrinsic control mechanisms. In Knobil and Neill's physiology of reproduction (ed. Neill J. D.), pp. 827–947 New York, NY: Elsevier [Google Scholar]
- Koshikawa N., Giannelli G., Cirulli V., Miyazaki K., Quaranta V.2000Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin 5. J. Cell Biol. 148, 615–624 (doi:10.1083/jcb.148.3.615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurio H., Murayama E., Kaneko T., Shibata Y., Inai T., Iida H.2008Intron retention generates a novel isoform of CEACAM6 that may act as an adhesion molecule in the ectoplasmic specialization structures between spermatids and Sertoli cells in rat testis. Biol. Reprod. 79, 1062–1073 (doi:10.1095/biolreprod.108.069872) [DOI] [PubMed] [Google Scholar]
- Lau A. S. N., Mruk D. D.2003Rab8B GTPase and junction dynamics in the testis. Endocrinology 144, 1549–1563 (doi:10.1210/en.2002-220893) [DOI] [PubMed] [Google Scholar]
- Leblond C. P., Clermont Y.1952Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. NY Acad. Sci. 55, 548–573 (doi:10.1111/j.1749-6632.1952.tb26576.x) [DOI] [PubMed] [Google Scholar]
- Lee N. P., Mruk D. D., Lee W. M., Cheng C. Y.2003Is the cadherin/catenin complex a functional unit of cell–cell-actin-based adherens junctions (AJ) in the rat testis? Biol. Reprod. 68, 489–508 (doi:10.1095/biolreprod.102.005793) [DOI] [PubMed] [Google Scholar]
- Lee N. P., Mruk D. D., Conway A. M., Cheng C. Y.2004Zyxin, axin, and Wiskott–Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J. Androl. 25, 200–215 [DOI] [PubMed] [Google Scholar]
- Lee N. P., Mruk D. D., Wong C. H., Cheng C. Y.2005Regulation of Sertoli–germ cell adherens junction dynamics in the testis via the nitric oxide synthase (NOS)/cGMP/protein kinase G (PRKG)/β-catenin (CATNB) signaling pathway: an in vitro and in vivo study. Biol. Reprod. 73, 458–471 (doi:10.1095/biolreprod.105.040766) [DOI] [PubMed] [Google Scholar]
- Leibfried A., Fricke R., Morgan M. J., Bogdan S., Bellaiche Y.2008Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr. Biol. 18, 1639–1648 (doi:10.1016/j.cub.2008.09.063) [DOI] [PubMed] [Google Scholar]
- Li M. W., Mruk D. D., Lee W. M., Cheng C. Y.2009Connexin 43 and plakophilin-2 as a protein complex that regulates blood–testis barrier dynamics. Proc. Natl Acad. Sci. USA 106, 10 213–10 218 (doi:10.1073/pnas.0901700106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie P. P., Xia W., Wang C. Q., Mruk D. D., Yan H. H., Wong C. H., Lee W. M., Cheng C. Y.2006Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood–testis barrier in adult rat testes. J. Endocrinol. 191, 571–586 (doi:10.1677/joe.1.06996) [DOI] [PubMed] [Google Scholar]
- Lie P. P. Y., Cheng C. Y., Mruk D. D.In press.Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes regulates blood–testis barrier dynamics. Int. J. Biochem. Cell. Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch J. H., Klessner J., Park J. K., Getsios S., Wu Y. L., Stack M. S., Green K. J.2004Epidermal growth factor receptor inhibition promotes desmosome assembly and strengthens intercellular adhesion in squamous cell carcinoma cells. J. Biol. Chem. 279, 37 191–37 200 (doi:10.1074/jbc.M405123200) [DOI] [PubMed] [Google Scholar]
- Lui W. Y., Lee W. M., Cheng C. Y.2003Sertoli–germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol. Reprod. 68, 2189–2206 (doi:10.1095/biolreprod.102.011379) [DOI] [PubMed] [Google Scholar]
- Lui W. Y., Mruk D. D., Cheng C. Y.2005Interactions among IQGAP1, Cdc42, and the cadherin/catenin protein complex regulate Sertoli–germ cell adherens junction dynamics in the testis. J. Cell. Physiol. 202, 49–66 (doi:10.1002/jcp.20098) [DOI] [PubMed] [Google Scholar]
- Luissint A. C., Lutz P. G., Calderwood D. A., Couraud P. O., Bourdoulous S.2008JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by α4β1 integrin activation. J. Cell Biol. 183, 1159–1173 (doi:10.1083/jcb.200805061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C., Frijns E., Wilhelmsen K., Sonnenberg A.2008Regulation of hemidesmosome disassembly by growth factor receptors. Curr. Opin. Cell Biol 20, 1–8 [DOI] [PubMed] [Google Scholar]
- Martin-Padura I., et al. 1998Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142, 117–127 (doi:10.1083/jcb.142.1.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Hirata M., Suzuki A., Amano Y., Yamashita K., Ide M., Yamanaka T., Sakai M., Imamura M., Ohno S.2009Intracellular polarity protein PAR-1 regulates extracellular laminin assembly by regulating the dystroglycan complex. Genes Cells 14, 835–850 (doi:10.1111/j.1365-2443.2009.01315.x) [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Shariat S. F., Ayala G. E., Rauen K. A., Lerner S. P.2005Loss of coxsackie and adenovirus receptor expression is associated with features of aggressive bladder cancer. Urology 66, 441–446 (doi:10.1016/j.urology.2005.02.033) [DOI] [PubMed] [Google Scholar]
- Miner J. H.2008Laminins and their roles in mammals. Microsc. Res. Tech. 71, 349–356 (doi:10.1002/jemt.20563) [DOI] [PubMed] [Google Scholar]
- Miner J. H., Yurchenco P. D.2004Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 20, 255–284 (doi:10.1146/annurev.cellbio.20.010403.094555) [DOI] [PubMed] [Google Scholar]
- Mirza M., Raschperger E., Philipson L., Pettersson R. F., Sollerbrant K.2005The cell surface protein coxsackie- and adenovirus receptor (CAR) directly associates with the ligand-of-Numb Protein-X2 (LNX2). Exp. Cell Res. 309, 110–120 (doi:10.1016/j.yexcr.2005.05.023) [DOI] [PubMed] [Google Scholar]
- Mirza M., Hreinsson J., Strand M. L., Hovatta O., Soder O., Philipson L., Pettersson R. F., Sollerbrant K.2006Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp. Cell Res. 312, 817–830 (doi:10.1016/j.yexcr.2005.11.030) [DOI] [PubMed] [Google Scholar]
- Moser M., Legate K. R., Zent R., Fassler R.2009The tail of integrins, talins, and kindlins. Science 324, 895–899 (doi:10.1126/science.1163865) [DOI] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y.2004Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 (doi:10.1210/er.2003-0022) [DOI] [PubMed] [Google Scholar]
- Mruk D. D., Silvestrini B., Cheng C. Y.2008Anchoring junctions as drug targets: role in contraceptive development. Pharmacol. Rev. 60, 146–180 (doi:10.1124/pr.107.07105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland D. J., Dedhar S., Vogl A. W.2001Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol. Reprod. 64, 396–407 (doi:10.1095/biolreprod64.1.396) [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Chiba H., Fujita H., Kojima T., Saito T., Endo T., Sawada N.2006Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol. 208, 123–132 (doi:10.1002/jcp.20647) [DOI] [PubMed] [Google Scholar]
- Nelson W. J.2008Regulation of cell–cell adhesion by the cadherin–catenin complex. Biochem. Soc. Trans. 36, 149–155 (doi:10.1042/BST0360149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L. E., Zegers M. M., Mostov K. E.2002Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 3, 531–537 (doi:10.1038/nrm859) [DOI] [PubMed] [Google Scholar]
- Ogita H., Takai Y.2008Cross-talk among integrin, cadherin, and growth factor receptor: roles of nectin and nectin like molecule. Int. Rev. Cytol. 265, 1–54 (doi:10.1016/S0074-7696(07)65001-3) [DOI] [PubMed] [Google Scholar]
- Okegawa T., Li Y., Pong R. C., Bergelson J. M., Zhou J., Hsieh J. T.2001The dual impact of coxsackie and adenovirus receptor expression on human prostate cancer gene therapy. Cancer Res. 60, 5031–5036 [PubMed] [Google Scholar]
- Ostermann G., Weber K. S., Zernecke A., Schroder A., Weber C.2002JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 3, 151–158 (doi:10.1038/ni755) [DOI] [PubMed] [Google Scholar]
- Ozaki-Kuroda K., et al. 2002Nectin couples cell–cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr. Biol. 12, 1145–1150 (doi:10.1016/S0960-9822(02)00922-3) [DOI] [PubMed] [Google Scholar]
- Palombi F., Salanova M., Tarone G., Farini D., Stefanini M.1992Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol. Reprod. 47, 1173–1182 (doi:10.1095/biolreprod47.6.1173) [DOI] [PubMed] [Google Scholar]
- Philipson L., Pettersson R. F.2004The coxsackie–adenovirus receptor—a new receptor in the immunoglobulin family involved in cell adhesion. Curr. Top. Microbiol. Immunol. 273, 87–111 [DOI] [PubMed] [Google Scholar]
- Raschperger E., Thyberg J., Pettersson S., Philipson L., Fuxe J., Pettersson R. F.2006The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp. Cell Res. 312, 1566–1580 (doi:10.1016/j.yexcr.2006.01.025) [DOI] [PubMed] [Google Scholar]
- Russell L. D.1977Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am. J. Anat. 148, 301–312 (doi:10.1002/aja.1001480302) [DOI] [PubMed] [Google Scholar]
- Russell L. D.1993Morphological and functional evidence for Sertoli–germ cell relationships. In The Sertoli cell (eds Russell L. D., Griswold M. D.), pp. 365–390 Clearwater, FL: Cache River Press [Google Scholar]
- Russell L. D., Peterson R. N.1985Sertoli cell junctions: morphological and functional correlates. Int. J. Cytol. 94, 177–211 (doi:10.1016/S0074-7696(08)60397-6) [DOI] [PubMed] [Google Scholar]
- Sakamoto Y., Ogita H., Hirota T., Kawakatsu T., Fukuyama T., Yasumi M., Kanzaki N., Ozaki M., Takai Y.2006Interaction of integrin avβ3 with nectin. Implication in cross-talk between cell–matrix and cell–cell junctions. J. Biol. Chem. 281, 19 631–19 644 (doi:10.1074/jbc.M600301200) [DOI] [PubMed] [Google Scholar]
- Salanova M., Stefanini M., De Curtis I., Palombi F.1995Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol. Reprod. 52, 79–87 (doi:10.1095/biolreprod52.1.79) [DOI] [PubMed] [Google Scholar]
- Sarkar O., Xia W., Mruk D. D.2006Adjudin-mediated junction restructuring in the seminiferous epithelium leads to displacement of soluble guanylate cyclase from adherens junctions. J. Cell. Physiol. 208, 175–187 (doi:10.1002/jcp.20651) [DOI] [PubMed] [Google Scholar]
- Scothern A., Garrod D.2008Visualization of desmosomes in the electron microscope. Methods Cell Biol. 88, 347–366 (doi:10.1016/S0091-679X(08)00418-4) [DOI] [PubMed] [Google Scholar]
- Sehgal B. U., DeBiase P. J., Matzno S., Chew T. L., Claiborne J. N., Hopkinson S. B., Russell A., Marinkovich M. P., Jones J. C. R.2006Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J. Biol. Chem. 281, 35 487–35 498 (doi:10.1074/jbc.M606317200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M., Ghosh A., Cooke V. G., Naik U. P., Martin-DeLeon P. A.2008JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Dev. Biol. 313, 246–255 (doi:10.1016/j.ydbio.2007.10.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu M. K., Cheng C. Y.2004Interactions of proteases, protease inhibitors, and the β1 integrin/laminin protein complex in the regulation of ectoplasmic specialization dynamics in the testis. Biol. Reprod. 70, 945–964 (doi:10.1095/biolreprod.103.023606) [DOI] [PubMed] [Google Scholar]
- Siu M. K., Mruk D. D., Lee W. M., Cheng C. Y.2003Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex-associated proteins. Endocrinology 144, 2141–2163 (doi:10.1210/en.2002-221035) [DOI] [PubMed] [Google Scholar]
- Siu M. K., Wong C. H., Lee W. M., Cheng C. Y.2005Sertoli–germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J. Biol. Chem. 280, 25 029–25 047 (doi:10.1074/jbc.M501049200) [DOI] [PubMed] [Google Scholar]
- Takai Y., Nakanishi H.2003Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116, 17–27 (doi:10.1242/jcs.00167) [DOI] [PubMed] [Google Scholar]
- Takai Y., Irie K., Shimizu K., Sakisaka T., Ikeda W.2003Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 94, 655–667 (doi:10.1111/j.1349-7006.2003.tb01499.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Ikeda W., Ogita H., Rikitake Y.2008aThe immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol 24, 309–342 (doi:10.1146/annurev.cellbio.24.110707.175339) [DOI] [PubMed] [Google Scholar]
- Takai Y., Miyoshi J., Ikeda W., Ogita H.2008bNectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 9, 603–615 (doi:10.1038/nrm2457) [DOI] [PubMed] [Google Scholar]
- Takai Y., Ikeda W., Ogita H., Rikitake Y.2008cThe immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 24, 309–342 (doi:10.1146/annurev.cellbio.24.110707.175339) [DOI] [PubMed] [Google Scholar]
- Toyama Y., Suzuki-Toyota F., Maekawa M., Ito C., Toshimori K.2008Disruption of ectoplasmic specializations between Sertoli cells and maturing spermatids by anti-nectin and anti-nectin-3 antibodies. Asian J. Androl. 10, 577–584 (doi:10.1111/j.1745-7262.2008.00357.x) [DOI] [PubMed] [Google Scholar]
- Wallis S., Lloyd S., Wise I., Ireland G., Fleming T. P., Garrod D.2000The α isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol. Biol. Cell 11, 1077–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. W., Freimuth P., Moninger T. O., Ganske I., Zabner J., Welsh M. J.2002Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110, 789–799 (doi:10.1016/S0092-8674(02)00912-1) [DOI] [PubMed] [Google Scholar]
- Wang C. Q. F., Mruk D. D., Lee W. M., Cheng C. Y.2007Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli–Sertoli and Sertoli–germ cell interface. Exp. Cell Res. 313, 1373–1392 (doi:10.1016/j.yexcr.2007.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C., Fraemohs L., Dejana E.2007The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. Immunol. 7, 467–477 (doi:10.1038/nri2096) [DOI] [PubMed] [Google Scholar]
- Wine R. N., Chapin R. E.1999Adhesion and signaling proteins spatiotemporally associated with spermiation in the rat. J. Androl. 20, 198–213 [PubMed] [Google Scholar]
- Wolski K. M., Perrault C., Tran-Son-Tay R., Cameron D. F.2005Strength measurement of the Sertoli–spermatid junctional complex. J. Androl. 26, 354–359 (doi:10.2164/jandrol.04142) [DOI] [PubMed] [Google Scholar]
- Wong E. W., Mruk D. D., Lee W. M., Cheng C. Y.2008Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood–testis barrier restructuring during spermatogenesis. Proc. Natl Acad. Sci. USA 105, 9657–9662 (doi:10.1073/pnas.0801527105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel K. H., Mademann R., Sinowatz F.1979The lamina propria of the bovine seminiferous tubule. Cell Tissue Res. 202, 357–377 [DOI] [PubMed] [Google Scholar]
- Yan H. H., Cheng C. Y.2006Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J. Biol. Chem. 281, 17 286–17 303 (doi:10.1074/jbc.M513218200) [DOI] [PubMed] [Google Scholar]
- Yan H. H., Mruk D. D., Lee W. M., Cheng C. Y.2007Ectoplasmic specialization: a friend or foe of spermatogenesis? Bioessays 29, 36–48 (doi:10.1002/bies.20513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H., Mruk D. D., Wong E. W., Lee W. M., Cheng C. Y.2008aAn autocrine axis in the testis that coordinates spermiation and blood–testis barrier restructuring during spermatogenesis. Proc. Natl Acad. Sci. USA 105, 8950–8955 (doi:10.1073/pnas.0711264105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H., Mruk D. D., Lee W. M., Cheng C. Y.2008bBlood–testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 22, 1945–1959 (doi:10.1096/fj.06-070342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T., Green K. J.2004Regulation of desmosome assembly and adhesion. Semin. Cell Dev. Biol. 15, 665–677 [DOI] [PubMed] [Google Scholar]
- Yin T., Getsios S., Caldelari R., Godsel L. M., Kowalczyk A. P., Muller E. J., Green K. J.2005Mechanisms of plakoglobin-dependent adhesion: desmosome-specific functions in assembly and regulation by epidermal growth factor receptor. J. Biol. Chem. 280, 40 355–40 363 (doi:10.1074/jbc.M506692200) [DOI] [PubMed] [Google Scholar]
- Yu W., Datta A., Leroy P., O'Brien L. E., Mak G., Jou T. S., Matlin K. S., Mostov K. E., Zegers M. M.2005β1-Integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell 16, 433–445 (doi:10.1091/mbc.E04-05-0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K., Babbin B. A., Liu Y., Whelan J. B., Nusrat A., Parkos C. A.2004JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol. Biol. Cell 15, 3926–3937 (doi:10.1091/mbc.E04-04-0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K., Liu Y., McCall I. C., Wu T., Lee W. M., Babbin B. A., Nusrat A., Parkos C. A.2005Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Mol. Biol. Cell 16, 2694–2703 (doi:10.1091/mbc.E05-01-0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wong C. H., Xia W., Mruk D. D., Lee N. P., Lee W. M., Cheng C. Y.2005Regulation of Sertoli–germ cell adherens junction dynamics via changes in protein–protein interactions of the N-cadherin–β-catenin protein complex which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology 146, 1268–1284 (doi:10.1210/en.2004-1194) [DOI] [PubMed] [Google Scholar]