Abstract
Key components of the cell cycle machinery are the regulatory subunits, the cyclins, and their catalytic partners the cyclin-dependent kinases. Regulating the cell cycle in the male germ line cells represents unique challenges for this machinery given the constant renewal of gametes throughout the reproductive lifespan and the induction of the unique process of meiosis, a highly specialized kind of cell division. With challenges come opportunities to the critical eye, recognizing that understanding these specialized modes of regulation will provide considerable insight into both normal differentiation as well as disease conditions, including infertility and oncogenesis.
Keywords: cell cycle regulation, mitosis, meiosis, germ cell differentiation, cyclin-dependent kinases
1. The basics of cell cycle machinery in mitotic cells
A basic requirement for cellular proliferation is that the cell replicates its DNA and then segregates the resulting products in an orderly, cyclic manner. This progression through the cell cycle is controlled in part by the sequential activity of the basic components of the cell cycle machinery, the cyclins and the cyclin-dependent serine–threonine kinases, collectively called the cyclin-dependent kinases (Cdks). The cyclins are regulatory subunits that bind, activate and provide substrate specificity for their catalytic partner Cdks (for reviews, see Murray 2004; Sherr & Roberts 2004; Bloom & Cross 2007). The activity of cyclin–Cdk complexes is tightly regulated by a complex network of proteins that function as activators and inhibitors of their kinase activity as well as influencing their transcription, sub-cellular localization and degradation.
Several classes of cyclins have been described in mammalian cells, designated A to I, and also T. Progression through the mitotic cell cycle is governed by the sequential activation and function of these different cyclins, in partnership with Cdks. During the mitotic cell cycle, cyclins from the D-type family regulate progression of cells through the first gap phase, G1 (Sherr & Roberts 1999). D-type cyclins bind and activate Cdk4 and Cdk6 which phosphorylate and functionally inactivate the retinoblastoma protein and related family members. Cyclin E family members are expressed during late G1 and during S-phase progression. E-type cyclins associate with and activate mainly Cdk2, but they can also associate with Cdk1 and Cdk3. Following E-type cyclin activation during S-phase, the A-type cyclin, cyclin A2, becomes functional. Entry into M-phase progression is driven primarily by the B-type cyclins, which associate with and activate Cdk1; however, cyclin A2 also functions prior to cyclin B/Cdk1 activation.
While best known for their association with, and regulation of, Cdks, it is increasingly recognized that several of the cyclins also have functions that are kinase-independent. For example, cyclin D1 has been reported to have a Cdk-independent role as a co-activator of tissue-specific transcription factors (e.g. Zwijsen et al. 1997). Studies using mutational analysis have shown that this regulation, specifically the transactivation of p300, PPARγ (Fu et al. 2005) and DMP1 (Inoue & Sherr 1998), is independent of the residues required for binding Cdk. Further, recent studies on the function of the E-type cyclins also implicate kinase-independent functions (Geng et al. 2007). Specifically, cyclin E1 that is incapable of activating its Cdk2 partner is nonetheless able to support the cell's entry to S-phase, although at a reduced rate. Also, the expression of kinase-deficient cyclin E mutant in cyclin E-null cells was able to rescue MCM loading, to levels similar to those in wild-type cyclin E cells.
2. Basics of the meiotic cell cycle: another layer of complexity
Meiosis is restricted to germ line cells and has features of cell division that simply do not exist in somatic cells. One striking unique feature, for example, is that there are two metaphase segregation events that occur without an intervening round of DNA synthesis. Another aspect of meiosis that distinguishes it from mitosis is the behaviour of sister chromatids during the first meiotic division. That is, in mitosis, the sister chromatids separate in the single phase of chromosome segregation, while in meiosis, sister chromatids do not separate from one another until the second round of segregation—the first stage involves segregation of homologous chromosomes. In addition, it is critical that meiosis-unique processes, including the synapsis or pairing of homologous chromosome, recombination and formation of chiasmata, be strictly coordinated with cell cycle progression, as it would be catastrophic for the gamete cell to attempt to move into the cell cycle without these other processes having been completed.
Higher organisms are characterized by having sexually dimorphic gametes. While male and female germ cells have stages of cell cycle regulation in common, including a mitotic proliferative stage, entry into meiosis, completion of a reductive division and entry into a quiescent state prior to fertilization, the timing of these events and the stage of development at which they occur differ in the two sexes (reviewed in Wolgemuth et al. 1995, 2002; Handel & Eppig 1998). In the mouse model, the germ line is specified early in embryonic development, probably as early as embryonic day 6.0–6.5. The progenitor germ cells migrate from the proximal epiblast to the gonadal ridge, at which point sexual determination based upon genotype occurs. The germ cells then follow either a male pathway, in which the cells enter into a mitotic arrest, or alternatively, a female pathway, in which they enter into pre-meiotic DNA synthesis and meiotic prophase. Thus, germ cells of both sexes undergo mitotic divisions in the embryonic gonad, but the female germ cells enter meiosis during foetal development, whereas this is a postnatal event in the male.
Once the male germ cell has entered meiosis, the process continues without interruption until the haploid sperm is produced. In contrast, the oocyte is arrested in the diplotene stage of meiotic prophase I, where it can remain for months or years depending on the species. Following a growth period, which begins at puberty, the oocyte resumes meiosis, but arrests a second time, at metaphase II. Fertilization then triggers the completion of meiosis and extrusion of the second polar body. Given these striking differences in the sequence of mitotic and meiotic events, it is almost given that the genetic programme underlying this regulation will be distinct between the male and female and will be reflected in a sexual dimorphism in the genes involved in regulating these processes (Handel 1998; Wolgemuth et al. 2002).
Mouse spermatogenesis has been a particularly powerful in vivo model system to pursue the regulation of mitosis and meiosis in higher eukaryotes, specifically in mammals, for several reasons. First, the biology of spermatogenesis in the rodents is particularly amenable to precise histological analysis. The adult mouse testis contains germ cells in various stages of differentiation, including self-renewing stem cells, mitotically dividing spermatogonia, meiotically dividing spermatocytes as well as spermatids, which are genetically haploid and undergo the remarkable morphological changes and chromatin remodelling that characterize spermiogenesis. Second, the progression of differentiation of these events occurs in a strict temporally and spatially regulated manner, such that classical cellular associations within this cycle of the seminiferous epithelium have been defined. Comparison of changes in such cellular associations upon loss of expression and function of specific genes can provide insight into the stages of differentiation that are affected. Third, the mouse model can be relatively easily manipulated genetically by several experimental strategies. There are also naturally existing mutations in mice that have provided considerable insight into the control of germ cell differentiation, although they have not been particularly useful for understanding the control of cell cycle in the germ line. For example, mutations in the W alleles encoding the c-kit tyrosine kinase receptor result in aberrant germ cell differentiation in both males and females and can result, for example, in complete loss of the germ cell lineage and, of course, sterility (e.g. Rossi et al. 2000, 2003). A series of studies using both a naturally occurring mutation in parallel with targeted mutagenesis approaches revealed the critical role of the gene encoding the transcription factor Plzf in the maintenance of the spermatogonial stem cell population (Plzf knockout (Costoya et al. 2004) while concomitantly identifying the spontaneous mutation luxoid (Buaas et al. 2004) as in fact being the Plzf locus).
Conventional gene targeting of both germ-cell-specific genes and genes more widely expressed has been a powerful tool to identify the roles that specific genes play in these processes but has only revealed the ‘tip of the iceberg’ regarding the complexity of the genetic control of these processes (reviewed in Venables & Cooke 2000; Matzuk & Lamb 2002; de Rooij & de Boer 2003). A compilation of the phenotypes observed from conventional knockouts of the major cyclins (which are the focus of this review) and several of their partner Cdks is found in table 1.
Table 1.
Effects of the absence of specific cyclins and CDKs on male fertility. n.d., not determined.
| cyclin/CDK knockout | expression in WT testis | effect on male fertility | arrest point if infertile | reference |
|---|---|---|---|---|
| cyclin A1 | late pachytene to diplotene | infertile | diplotene | Liu et al. (1998a,b) |
| cyclin A2 | spermatogonia to preleptotene | embryonic lethal | Sweeney et al. (1996), Ravnik & Wolgemuth (1999), Murphy et al. (1997) | |
| cyclin B1 | spermatocytes and post-meiotic spermatids | embryonic lethal | Chapman & Wolgemuth (1992), Brandeis et al. (1998) | |
| cyclin B2 | spermatocytes and round spermatids | fertile | Chapman & Wolgemuth (1992), Brandeis et al. (1998) | |
| cyclin B3 | leptotene and zygotene | misexpression leads to infertility | Nguyen et al. (2002), Refik-Rogers et al. (2006) | |
| cyclin D1 | spermatogonia | fertile | Sicinski et al. (1995), Beumer et al. (2000) | |
| cyclin D2 | spermatogonia, pachytene, diplotene, round spermatids to stage V | hypoplastic testis, remain fertile | Sicinski et al. (1996), Beumer et al. (2000) | |
| cyclin D3 | spermatogonia, pachytene, diplotene, round spermatids, elongating spermatids | fertile | Sicinska et al. (2003), Beumer et al. (2000), Zhang et al. (1999) | |
| cyclin E1 | n.d. | fertile | Geng et al. (2003) | |
| cyclin E2 | n.d. | reduced fertility | no consistent arrest point | Geng et al. (2003) |
| Cdk1 | differentiating cells | embryonic lethal | Santamaria et al. (2007), Ravnik & Wolgemuth (1999) | |
| Cdk2 | differentiating cells | infertile | mid-pachytene | Ortega et al. (2003), Berthet et al. (2003), Ravnik & Wolgemuth (1999) |
| Cdk4 | n.d. | low sperm count, 80% infertile | no consistent arrest point | Rane et al. (1999) |
| Cdk6 | n.d. | fertile male | Malumbres et al. (2004) |
In the paragraphs below, we will summarize our current understanding of the expression and function of the A-, B-, D- and E-type cyclins in the male mammalian germ line. It will be obvious that our understanding of the expression and function of the A-type cyclins is more advanced than for the three other families, which should not be interpreted as reflecting their relative importance but rather our limited knowledge.
3. A-type cyclins
There are two distinct A-type cyclins in the mouse (and human) genome, cyclin A1 and cyclin A2 (designated genetically as Ccna1/CCNA1 and Ccna2/CCNA2, respectively). These two A-type cyclins exhibit strikingly different patterns of expression that have been documented in both mice and humans: cyclin A2 is ubiquitously expressed in mitotically dividing cells, whereas expression of cyclin A1 is highly restricted, being most abundant in the testis (Ravnik & Wolgemuth 1996; Sweeney et al. 1996; Yang et al. 1997). Mouse cyclin A2 has also been shown to be expressed in a broad variety of tissues in the adult mouse and during embryogenesis (Ravnik & Wolgemuth 1996; Sweeney et al. 1996). The originally identified A-type cyclin, human cyclin A2, is ubiquitously expressed in vitro in tissue culture cells and is upregulated in many cancers (Pines & Hunter 1990; Wang et al. 1990).
(a). A-type cyclins in the mammalian germ line: distinct and sexually dimorphic patterns of expression
In the mouse testis, cyclin A1 expression has been detected at both the mRNA and protein levels specifically in pachytene and diplotene spermatocytes in stages IX to XII tubules (Sweeney et al. 1996; Liu et al. 1998a). That the mRNA and proteins are detected in close temporal proximity suggests that regulation of cyclin A1 expression is primarily at the level of transcription and that there is little if any regulation at the level of translation. On the other hand, cyclin A2 is expressed in spermatogonia and pre-leptotene spermatocytes and its expression is specifically downregulated early in meiotic prophase, well before cyclin A1 is expressed (Sweeney et al. 1996; Ravnik & Wolgemuth 1999).
It is interesting to contrast the expression of the two A-type cyclins between the testis and ovary: in the mouse ovary, cyclin A1 is completely repressed and cyclin A2 is expressed in both oogonia and oocytes in a developmentally regulated manner (Persson et al. 2005). The overall levels of expression of cyclin A2 protein decrease from embryonic oocytes to oocytes in postnatal and adult ovaries. Also, cyclin A2 protein expression is nuclear from embryonic days 13.5 to 15.5 and then changes to predominantly cytoplasmic from embryonic day 16.5 to postnatal and to adult ovaries. There are also high expression levels of cyclin A2, Cdk1 and Cdk2 in granulosa cells. Although there had been conflicting reports of cyclin A1 expression in mouse oocytes (Sweeney et al. 1996), there was subsequent agreement from gene targeting studies that loss of cyclin A1 function has no effect on oogenesis (Liu et al. 1998a; van der Meer et al. 2004). This question was resolved in a subsequent study that used the cyclin A1-deficient mouse model to unequivocally demonstrate that cyclin A1 is not expressed to any significant levels in oocytes (Persson et al. 2005). It is therefore likely that the two A-type cyclins play unique functions in cell cycle progression in the male and female germ lines.
(b). Regulation of expression of the A-type cyclins in the germ line
Given the distinct patterns of expression of the A-type cyclins that differ between the two genes and which exhibit such striking differences between the two sexes, there must be specific regulatory elements unique to each A-type cyclin that are critical for their distinct regulation of expression, and further, there may be important post-transcriptional regulation that is critical as well. There were several studies that have begun to identify critical transcriptional regulatory elements of the human CCNA1 gene. In one such study, transient transfection of CV-1 cells with a c-Myb expression vector and CCNA1 reporter constructs suggested that Myb was able to induce CCNA1 expression and that sequences in a 335 bp fragment upstream of the promoter, which contains several c-Myb binding sites, may be involved in this expression (Muller et al. 1999).
However, the most definitive studies are those using a transgenic mouse model and examining the expression of reporter constructs in vivo (Lele & Wolgemuth 2004). Transgenic mice carrying constructs consisting of varying lengths of the mouse cyclin A1 regulatory region fused with the reporter gene lacZ were generated. Analysis of tissue-specific and testicular cell-type-specific transgene expression indicated that sequences within approximately 1.3 kb of the Ccna1 putative transcriptional start site were sufficient to direct transgene expression uniquely to late spermatocytes, while maintaining repression in other tissues. However, sequences located between approximately 4.8 and 1.3 kb of the putative transcriptional start site were apparently required to transcribe the reporter at levels needed for consistent X-gal staining. Comparison of the mouse, rat and human proximal promoters revealed regions of high sequence conservation and consensus sequences both for known transcription factors, some of which are co-expressed with Ccna1, such as A-myb and Hsf2, and for elements that control expression of genes in somatic cell cycles, such as CDE, CHR and CCAAT elements. Thus, the promoter region within 1.3 kb upstream of the putative Ccna1 transcriptional start can direct expression of lacZ to spermatocytes, while sequences located further upstream may enhance expression.
Although our understanding of the regulation of Ccna1 is limited, even less is known about the transcriptional regulation of Ccna2 in either the male or female germ line. There have been several studies, however, examining its regulation in mitotic cell division, mostly in cultured cells (Blanchard 2000). Cyclin A2 expression is repressed in quiescent (G0) cells and early in G1 and is then rapidly induced as cells approach S-phase. In vivo foot printing analysis revealed the presence of several putative regulatory regions within a relatively short region (approx. 70 bp) upstream of the initiation site. Two of these elements, designated CRE and CAAT boxes, are bound constitutively throughout the cell cycle by transcription factors of the CREB/ATF and NF-Y families, respectively. The other two sites, known as CCRE/CDE and CHR elements, have been suggested to be negative regulatory elements in the cyclin A2 promoter, and in the promoters of several other cell cycle-related genes, including Cdc25C, Cdk1 and members of the Myb family (Lucibello et al. 1995; Huet et al. 1996). Transcriptional regulatory factors such as E2F, Rb, CDF-1 and perhaps the SWI/SNF chromatin remodelling complex have all been implicated in the function of these elements (Schulze et al. 1995; Liu et al. 1998b; Philips et al. 1999; Coisy et al. 2004), but they remain poorly characterized. It is of interest that the CDE/CHR elements are also found in the promoter proximal region of both the human and mouse cyclin A1 gene (Lele & Wolgemuth 2004).
As mentioned above, there does not appear to be significant regulation of either Ccna1 or Ccna2 at the level of post-transcription in the male germ line. However, this may be different for Ccna2 in the female germ line. While clearly transcriptional regulation is critical, as evidenced from the complete repression of Ccna1 expression in oogonia and oocytes, regulation at the level of translation and perhaps sub-cellular distribution may be involved for Ccna2 in the female germ line. That is, during embryonic days 13.5–15.5, the localization of cyclin A2 protein was predominantly nuclear (Persson et al. 2005). At embryonic day 16.5, the localization of cyclin A2 in about 50 per cent of the oocytes began to shift to the cytoplasm. By embryonic day 18.5 only a few clusters of oocytes exhibited nuclear localization of cyclin A2 and the vast majority of expression was in the cytoplasm.
Another interesting example of post-translational regulation of an A-type cyclin has been recently discussed in the context of the Drosophila model system (Vardy et al. 2009). Although there is only a single A-type cyclin in this organism, its modes of regulation of expression may be relevant to both A-type cyclin's regulation in mammalian germ cells. In Drosophila oogenesis, the expression and levels of cyclin A protein must be tightly regulated for proper oocyte differentiation, including the mitotic divisions of the oogonia that give rise to the oocyte and its sister nurse cells, and the induction of meiosis (Lilly et al. 2000). Repression of cyclin A translation has been shown to be important early in prophase I in order to maintain the oocyte in meiosis, a repression that is mediated by a combination of deadenylation of the mRNA and function of the inhibitor protein Bruno (Morris et al. 2005; Sugimura & Lilly 2006). As meiosis resumed during oocyte maturation, cyclin A protein was concomitantly detected with the polyadenylation of its message. There are also multiple regulatory phosphorylations of the cyclin A protein during the progression of meiosis I, which may in fact involve autophosphoryation and be involved in regulating its stability or rate of degradation (Vardy et al. 2009).
(c). Targeted mutagenesis of the A-type cyclins in the mouse model
It has been known for over 10 years that mice homozygous null for the Ccna2 gene die in utero, with lethality occurring around the peri-implantation stage (Murphy et al. 1997); however, very little is known about the physiological basis of the lethality and even less about the molecular mechanisms involved. These observations demonstrated the essential nature of cyclin A2 function in mammalian development but yield little insight into the cellular and molecular basis of its function and only complicated efforts for understanding its putative function in adult lineages.
In contrast, homozygous null Ccna1 mice grow and develop normally with the exception of male-specific sterility in the adult animals (Liu et al. 1998a; van der Meer et al. 2004). Examination of the testes of the mutant mice revealed that they exhibited normal meiotic progression until mid-diplotene, with normal formation and resolution of chiasmata (Liu et al. 1998a; Nickerson et al. 2007). However, in late diplotene, rather than undergoing diakinesis and proceeding to a metaphase I arrangement, spermatocytes in the cyclin A1-deficient mice arrested and, as assessed by TUNEL staining, underwent apoptosis (Liu et al. 1998a; Salazar et al. 2005). Interestingly, this arrest and induction of apoptosis occurred later than the meiotic arrest observed in mice deficient in the putative cyclin A1 kinase partner, Cdk2 (Berthet et al. 2003; Ortega et al. 2003).
(d). Possible targets and downstream events of cyclin A1 function in male germ cells
As is the goal of genetic approaches involving loss of function mutations, several features of the phenotype that results from the loss of cyclin A1 function may indeed provide insight into some of the cellular processes and components involved. Changes in timing and progression of various nuclear events that accompany the cell cycle arrest and apoptosis in cyclin A1-deficient spermatocytes have been analysed by Nickerson et al. (2007). For example, it has been reported that the Ser139-phosphorylated form of H2AX, or γH2AX, localizes in a characteristic staining pattern at each stage of meiotic prophase in spermatocytes, in particular, in the XY body at the pachytene stage (Mahadevaiah et al. 2001). In cyclin A1-deficient spermatocytes, the localization of γH2AX was indistinguishable from that of the control spermatocytes (Nickerson et al. 2007). However, at the point of meiotic arrest in diplotene, γH2AX foci were observed first at the centromere and subsequently along the length of the chromosomes. The appearance of γH2AX foci was concurrent with an aggregation of centromeric heterochromatin, which led to the speculation that it may be involved with signalling the induction of apoptosis (Nickerson et al. 2007).
The pronounced phosphorylation of histone H3 at serine 10 that has been reported in the late diplotene stage of meiosis (Handel et al. 1999) was noticeably reduced in heterozygous Ccna1± spermatocytes and undetectable in spermatocytes totally lacking cyclin A1 function (Nickerson et al. 2007). Concomitantly, cyclin A1-deficient spermatocytes show reduced staining of aurora B kinase at the pericentromeric heterochromatin. While immunoblot analysis of whole testicular lysates did not indicate a significant difference in levels of aurora B protein between control and A1-deficient testicular lysates, the amount of aurora B protein associated with meiotic chromosomes was clearly different. Interestingly, histone H3 serine 10 is a known target of phosphorylation by the aurora B component of the passenger protein complex (Chen et al. 2003) and the point of meiotic arrest in cyclin A1 mice also overlaps with the assembly of the passenger protein complex.
Finally, it is also of interest that Cdk2, a major kinase partner for both A- and E-type cyclins, has been shown to be essential for meiosis in both the male and female germ lines (Ortega et al. 2003). Like cyclin A1-deficient spermatocytes, spermatocytes lacking Cdk2 also arrest in meiotic prophase; however, the arrest was observed in mid-pachytene spermatocytes and was accompanied by thin threads of SCP3 staining, perhaps indicating aberrant pairing (Ortega et al. 2003). In contrast, obvious differences have not been detected in the appearance of the meiotic chromosomes, notably the appearance of synaptonemal complexes (Liu et al. 1998a) or in staining of SCP3 (Nickerson et al. 2007) in spermatocytes lacking cyclin A1. Further, when cyclin A1-deficicient pachytene spermatocytes were driven into a meiotic configuration by treatment with the phosphatase inhibitor okadaic acid, metaphase I preparations from mutant and normal spermatocytes appeared similar, with no obvious defects in chiasmata (Liu et al. 2000).
It has been suggested that a possible reason underlying the two distinct phenotypes may involve differences in the temporal expression and nuclear distribution of cyclin A1 versus Cdk2. Cdk2 protein is localized in the centromeric region, at telomeres, and at foci along chromosomes during pachytene to diplotene (Ashley et al. 2001) and is not altered in cyclin A1-deficient mice (Nickerson et al. 2007). However, cyclin A1 did not completely co-localize with its putative Cdk2 partner at the centromeres (Nickerson et al. 2007). Further, although cyclin A1 is capable of activating Cdk2 (Liu et al. 2000), it cannot represent the regulatory subunit for Cdk2 at these early stages of meiotic prophase as it is not expressed until later in meiotic prophase (Sweeney et al. 1996). Other Cdk family members have also been studied by targeted mutagenesis, including Cdk1 (Santamaria et al. 2007), Cdk4 (Rane et al. 1999) and Cdk6 (Malumbres et al. 2004; table 1). Not unexpectedly, Cdk1-deficient mice are embryonic lethal while both Cdk4- and Cdk6-deficient mice are viable. There is a quite apparent fertility defect seen in Cdk4-deficient mice, with the females being completely sterile and the males exhibiting an age-dependent infertility. Although the exact causes of the fertility defects remain to be elucidated, it is more likely to be involved with their concomitant diabetic conditions than to primary functions for Cdk4 in the germ cells (Mettus & Rane 2003).
4. E-type cyclins
There are also two members of the E-type cyclin family in mammals, cyclin E1 and cyclin E2. Targeted mutagenesis of each of the E-type cyclins revealed an unanticipated function for one of these genes, Ccne2, in male fertility (Geng et al. 2003; Parisi et al. 2003). Surprisingly, reduced fertility in the cyclin E2-deficient male mice was the only obvious phenotype in the single knock-out models of either cyclin E1 or E2. The cyclin E2-deficient males exhibited reduced fertility, with approximately 50 per cent of the males being sterile, and there was an accompanying reduction (approximately fourfold) in their sperm counts. Although only very preliminary histological analysis was presented in the initial publications, it was clear that there was a reduced cellularity within the tubules and giant cells were observed in the lumens (Geng et al. 2003). We have recently undertaken more detailed analysis and observed that spermatogenesis is not arrested at a unique stage, as it is in cyclin A1-deficient testes, and further, that there is a gradation in the level of disruption of spermatogenesis and the loss of cells among the testes of the infertile group (S. S. W. Chung, S. S. Roberts, Y. Geng, P. Sincinski and D. J. Wolgemuth, unpublished data). In the most severely disrupted tubules, later stage spermatocytes and subsequent stages of spermatids were missing. In the less severely affected tubules, more advanced stages, including fully elongated spermatids, could be found.
Mice lacking both cyclin E genes have also been generated and shown to be embryonic lethal, dying during mid-gestation (Geng et al. 2003). This lethality could be partially rescued (to birth) by complementation with wild-type tetraploid blastocysts. Mouse embryonic fibroblasts from the doubly cyclin E1- and E2-deficient embryos proliferated normally under conditions of continuous cell cycling, but were unable to re-enter the cell cycle from quiescence. At the molecular level, cyclin E was shown to be loaded into DNA pre-replication complexes, interestingly, in a CDK-independent manner (Geng et al. 2007).
5. B-type cyclins
There are also two B-type cyclins, which have been well characterized in terms of their mitotic functions, and there may be as many as nine B1-related sequences in the mouse genome (Hanley-Hyde et al. 1992; Lock et al. 1992). Targeted mutagenesis of Ccnb1 showed that it is an essential gene, as cyclin B1-deficient mice are embryonic lethal (Brandeis et al. 1998). In contrast, Ccnb2−/− mice are viable and fertile (Brandeis et al. 1998). Although there was an indication that the litter size of homozygous Ccnb2−/− matings are slightly smaller, the reason for this observation has not been investigated further; thus its biological significance, if any, is unclear.
Studies of the B-type cyclins in reproduction had demonstrated distinct, developmentally regulated patterns of expression of the mouse Ccnb1 and Ccnb2 in both the male and female germ line (Chapman & Wolgemuth 1992, 1993). In the adult testis, Ccnb2 was present at highest levels in the meiotically dividing spermatocytes, and while Ccnb1 transcripts are also found in spermatocytes, they are most abundant in post-meiotic round spermatids. Whether this indicates a non-cell cycle progression-related function for cyclin B1 remains to be determined. Lower levels of Ccnb2 mRNAs were also detected in the early round spermatids. Curiously, neither Ccnb1 nor Ccnb2 transcripts were detected in the mitotically dividing spermatogonia. While this could be explained by the sensitivity of the assay, it could also mean that another cyclin could be responsible for activating Cdk1 in these cells.
Although not well studied, a third mouse B-type cyclin, cyclin B3, has also been identified (Nguyen et al. 2002). No endogenous cyclin B3 could be detected in a variety of mammalian cell lines that were examined; however, Ccnb3 mRNA and protein were quite readily detected in leptotene and zygotene spermatocytes and in clusters of oocytes in embryonic ovaries. Studies in which cyclin B3 was ectopically expressed in cultured cells revealed that the protein is predominantly nuclear and that it is degraded upon anaphase entry, following cyclin B1 degradation. Interestingly, it was also shown to be a poor activator of Cdk2 kinase, at least in this cultured cell model (Nguyen et al. 2002). In Drosophila, cyclin B3 is expressed in both mitotic and meiotic cells (Jacobs et al. 1998) and was shown to be essential for fertility, but only in the female. There have not been any reports of the consequences of loss of cyclin B3 function in the mouse model; however, in transgenic mice that express cyclin B3 throughout meiosis, there are severe defects in spermatogenesis and reduced sperm production (Refik-Rogers et al. 2006). It thus appears that cyclin B3 is present in a relatively narrow window of meiotic prophase in which the cells are in fact not progressing in the cell cycle but are rather undergoing the business of recombination, and that it is turned over in order to resume the cell cycle in preparation for the first meiotic division. If cyclin B3–Cdk complexes are also poorly functional in vivo, one might speculate that its function is to interact with the non-cycling Cdks that are present, essentially preventing them from forming active complexes that would drive division.
6. D-type cyclins
The D-type cyclins, D1, D2 and D3, play crucial roles for cells to be able to enter into G1/S of the mitotic cell cycle (Sherr 1994; Kozar et al. 2004). Although best known (and studied) for their role in mitosis, they have been implicated in diverse cellular events, including differentiation (Kato et al. 1993; Sicinski et al. 1995; Bartkova et al. 1998), apoptosis (Freeman et al. 1994) and as mentioned above, in non-cell cycle-related functions in activating transcription factors (Zwijsen et al. 1998). Each of the D-type cyclins has been characterized for their expression patterns in the adult mouse testis (figure 1) and each has been knocked out in mouse models (Sicinski et al. 1995, 1996; Sicinska et al. 2003; Kozar et al. 2004; table 1). Not surprisingly, all three D-type cyclins are expressed in proliferating spermatogonia. However, the expression of cyclins D2 and D3 was also detected in spermatocytes, low-level expression in round spermatids (Ravnik et al. 1995; Zhang et al. 1999; Beumer et al. 2000) and quite clear expression of cyclin D3 in elongating spermatids (Zhang et al. 1999). There is also expression of the D cyclins in the somatic testicular cells, with cyclin D3 appearing to be the preferred cyclin D in Sertoli cells (Zhang et al. 1999) and D1 and D3 being more abundant in Leydig cells (Beumer et al. 2000). Interestingly, cyclin D3 expression persists even after the Sertoli cells are no longer dividing (as in the adult testis) and it is readily detected in the slowly dividing Leydig cells (Beumer et al. 2000).
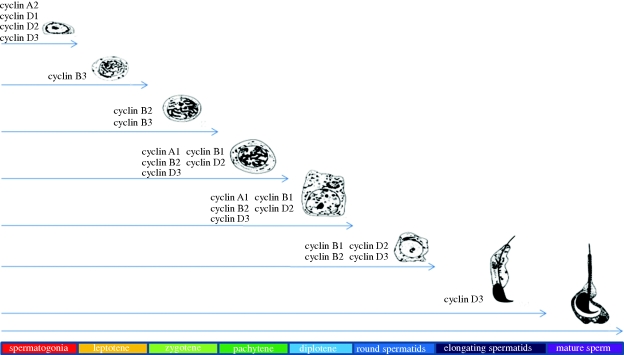
Figure 1.
Cartoon summarizing the meiotic stages of spermatogenesis in which cyclin expression has been documented. The arrows represent cellular progression through spermatogenesis. Cyclins listed are expressed as protein in that specific stage. The coloured bars at the bottom denote the cell type shown directly above.
As noted above, each of the D-type cyclins has been studied by targeted mutagenesis and, as in other tissues in which their function would have been predicted to be very important, their individual loss of function had little effect on testis development and spermatogenesis in general (Sherr & Roberts 2004; table 1). Whether there is functional redundancy among the D-type cyclins in testicular cells that express all three cyclin Ds, such as spermatogonia, or whether they are simply not critical in the male germ line remains to be determined. There was mention that although cyclin D2-deficient males are fertile, their testes are hypoplastic (Sicinski et al. 1996). Loss of function of all three D-type cyclins resulted in embryonic lethality, although much later in development than for loss of function of cyclin B1 and cyclin A2 (Kozar et al. 2004).
7. Cyclins in oncogenesis and infertility
It is evident that the cyclins play a critical role in regulation and control of spermatogenesis. Therefore, deregulation of the cell cycle that causes testicular cancer might be expected to involve some of the same cyclins dictating spermatogenic control. Cyclins A1, A2 and D2 have been shown to have a role in various types of germ cell tumours (GCTs). We have shown that cyclin A2 protein levels are elevated in the majority of GCTs and expression levels were strongly correlated with the severity of the tumour (Liao et al. 2004). In contrast, cyclin A1 was virtually undetectable in carcinoma in situ, and seminomas, but aberrantly expressed in all non-seminomatous GCTs. Histone kinase activities of both cyclin A1/Cdks and cyclin A2/Cdks were found to be elevated in the tumours. One of the D-type cyclins, cyclin D2, has also been implicated in testicular cancer. The role of cyclin D2 in testicular cancer has focused on mutations in chromosome 12 and the effects on the G1/S-phase transition. In GCT cell lines, the levels of expression of cyclin D2 were inversely correlated with the extent of differentiation properties characteristic of the cell line (Houldsworth et al. 1997). In human germ cells cyclin D2 protein levels were not detectable; however, cyclin D2 was expressed in abnormal germ cells of all of the human GCTs examined. In another study examining the role of cyclin D2 in GCTs, there was increased expression of cyclin D2 that interacts with p27, consistent with its known ability to sequester and block the cyclin E inhibitory function of p27 (Kukoski et al. 2003). In mice lacking inhibin alpha, cyclin D2 apparently promoted gonadal growth and tumour development, and male mice died as early as 12 weeks of age (Burns et al. 2003). Interestingly, male mice that are doubly deficient for both inhibin alpha and cyclin D2 were markedly less susceptible to tumour development, with a 50 per cent survival at 40–41 weeks of age and almost one-third of the mice were still alive at 1 year of age.
Given the clear essential role for cyclin A1 in the male germ line, and the likely important function for several of the others, including cyclins E2, B1 and A2, it is of interest to ask whether mutations in these genes might be implicated in human infertility. This is particularly true for cyclin A1, since male sterility is the only phenotype observed; that is, men harbouring mutations in the human CCNA1 gene would be predicted to be otherwise healthy with infertility as the only phenotype. As the only phenotype observed in Cdk2-deficient mice was male (and female) sterility, human CDK2 might also be a candidate gene for screening in cases of unexplained infertility in healthy men. A similar phenotype might be predicted for defects in other genes uniquely involved in meiosis. Indeed, the examination of such candidate genes in unexplained cases of human infertility has been the subject of some interest (Mandon-Pepin et al. 2002) and warrants further attention.
8. Summary
Understanding the genetic programme controlling the mitotic and meiotic divisions of the germ line presents a unique opportunity for providing insight into cell cycle control in vivo, during development and differentiation. Elucidating the key control points and proteins involved in their regulation may also enhance our understanding of the etiology of human infertility and, ultimately, provide new directions for contraception.
Acknowledgements
This work was supported in part by a grant from the NIH to D.J.W., HD034915.
Footnotes
One contribution of 17 to a Theme Issue ‘The biology and regulation of spermatogenesis’.
References
- Ashley T., Walpita D., De Rooij D. G.2001Localization of two mammalian cyclin dependent kinases during mammalian meiosis. J. Cell Sci. 114, 685–693 [DOI] [PubMed] [Google Scholar]
- Bartkova J., Lukas J., Strauss M., Bartek J.1998Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 17, 1027–1037 (doi:10.1038/sj.onc.1202016) [DOI] [PubMed] [Google Scholar]
- Berthet C., Aleem E., Coppola V., Tessarollo L., Kaldis P.2003Cdk2 knockout mice are viable. Curr. Biol. 13, 1775–1785 (doi:10.1016/j.cub.2003.09.024) [DOI] [PubMed] [Google Scholar]
- Beumer T. L., Roepers-Gajadien H. L., Gademan I. S., Kal H. B., De Rooij D. G.2000Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol. Reprod. 63, 1893–1898 (doi:10.1095/biolreprod63.6.1893) [DOI] [PubMed] [Google Scholar]
- Blanchard J.2000Cyclin A2 transcriptional regulation: modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem. Pharmacol. 60, 1179–1184 (doi:10.1016/S0006-2952(00)00384-1) [DOI] [PubMed] [Google Scholar]
- Bloom J., Cross F. R.2007Multiple levels of cyclin specificity in cell-cycle control. Nat. Rev. Mol. Cell Biol. 8, 149–160 (doi:10.1038/nrm2105) [DOI] [PubMed] [Google Scholar]
- Brandeis M., Rosewell I., Carrington M., Crompton T., Jacobs M. A., Kirk J., Gannon J., Hunt T.1998Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc. Natl Acad. Sci. USA 95, 4344–4349 (doi:10.1073/pnas.95.8.4344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas F. W., Kirsh A. L., Sharma M., Mclean D. J., Morris J. L., Griswold M. D., De Rooij D. G., Braun R. E.2004Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 36, 647–652 (doi:10.1038/ng1366) [DOI] [PubMed] [Google Scholar]
- Burns K. H., Agno J. E., Sicinski P., Matzuk M. M.2003Cyclin D2 and p27 are tissue-specific regulators of tumorigenesis in inhibin alpha knockout mice. Mol. Endocrinol. 17, 2053–2069 (doi:10.1210/me.2003-0038) [DOI] [PubMed] [Google Scholar]
- Chapman D. L., Wolgemuth D. J.1992Identification of a mouse B-type cyclin which exhibits developmentally regulated expression in the germ line. Mol. Reprod. Dev. 33, 259–269 (doi:10.1002/mrd.1080330305) [DOI] [PubMed] [Google Scholar]
- Chapman D. L., Wolgemuth D. J.1993Isolation of the murine cyclin B2 cDNA and characterization of the lineage and temporal specificity of expression of the B1 and B2 cyclins during oogenesis, spermatogenesis and early embryogenesis. Development 118, 229–240 [DOI] [PubMed] [Google Scholar]
- Chen J., et al. 2003Survivin enhances Aurora-B kinase activity and localizes Aurora-B in human cells. J. Biol. Chem. 278, 486–490 (doi:10.1074/jbc.M211119200) [DOI] [PubMed] [Google Scholar]
- Coisy M., Roure V., Ribot M., Philips A., Muchardt C., Blanchard J. M., Dantonel J. C.2004Cyclin A repression in quiescent cells is associated with chromatin remodeling of its promoter and requires Brahma/SNF2alpha. Mol. Cell 15, 43–56 (doi:10.1016/j.molcel.2004.06.022) [DOI] [PubMed] [Google Scholar]
- Costoya J. A., Hobbs R. M., Barna M., Cattoretti G., Manova K., Sukhwani M., Orwig K. E., Wolgemuth D. J., Pandolfi P. P.2004Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653–659 Epub 2004 May 23 (doi:10.1038/ng1367) [DOI] [PubMed] [Google Scholar]
- de Rooij D. G., de Boer P.2003Specific arrests of spermatogenesis in genetically modified and mutant mice. Cytogenet. Genome Res. 103, 267–276 (doi:10.1159/000076812) [DOI] [PubMed] [Google Scholar]
- Freeman R. S., Estus S., Johnson E. M., Jr1994Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron 12, 343–355 (doi:10.1016/0896-6273(94)90276-3) [DOI] [PubMed] [Google Scholar]
- Fu M., et al. 2005Cyclin D1 represses p300 transactivation through a cyclin-dependent kinase-independent mechanism. J. Biol. Chem. 280, 29 728–29 742 (doi:10.1074/jbc.M503188200) [DOI] [PubMed] [Google Scholar]
- Geng Y., et al. 2003Cyclin E ablation in the mouse. Cell 114, 431–443 (doi:10.1016/S0092-8674(03)00645-7) [DOI] [PubMed] [Google Scholar]
- Geng Y., et al. 2007Kinase-independent function of cyclin E. Mol. Cell 25, 127–139 (doi:10.1016/j.molcel.2006.11.029) [DOI] [PubMed] [Google Scholar]
- Handel M. A.1998Monitoring meiosis in gametogenesis. Theriogenology 49, 423–430 (doi:10.1016/S0093-691X(97)00414-7) [DOI] [PubMed] [Google Scholar]
- Handel M. A., Eppig J. J.1998Sexual dimorphism in the regulation of mammalian meiosis. Curr. Top. Dev. Biol. 37, 333–358 (doi:10.1016/S0070-2153(08)60179-9) [DOI] [PubMed] [Google Scholar]
- Handel M. A., Cobb J., Eaker S.1999What are the spermatocyte's requirements for successful meiotic division? J. Exp. Zool. 285, 243–250 (doi:10.1002/(SICI)1097-010X(19991015)285:3<243::AID-JEZ7>3.0.CO;2-#) [PubMed] [Google Scholar]
- Hanley-Hyde J., Mushinski J. F., Sadofsky M., Huppi K., Krall M., Kozak C. A., Mock B.1992Expression of murine cyclin B1 mRNAs and genetic mapping of related genomic sequences. Genomics 13, 1018–1030 (doi:10.1016/0888-7543(92)90015-K) [DOI] [PubMed] [Google Scholar]
- Houldsworth J., Reuter V., Bosl G. J., Chaganti R. S.1997Aberrant expression of cyclin D2 is an early event in human male germ cell tumorigenesis. Cell Growth Differ. 8, 293–299 [PubMed] [Google Scholar]
- Huet X., Rech J., Plet A., Vie A., Blanchard J. M.1996Cyclin A expression is under negative transcriptional control during the cell cycle. Mol. Cell Biol. 16, 3789–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Sherr C. J.1998Gene expression and cell cycle arrest mediated by transcription factor DMP1 is antagonized by D-type cyclins through a cyclin-dependent-kinase-independent mechanism. Mol. Cell. Biol. 18, 1590–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. W., Knoblich J. A., Lehner C. F.1998Drosophila cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 12, 3741–3751 (doi:10.1101/gad.12.23.3741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Matsushime H., Hiebert S. W., Ewen M. E., Sherr C. J.1993Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7, 331–342 (doi:10.1101/gad.7.3.331) [DOI] [PubMed] [Google Scholar]
- Kozar K., et al. 2004Mouse development and cell proliferation in the absence of D-cyclins. Cell 118, 477–491 (doi:10.1016/j.cell.2004.07.025) [DOI] [PubMed] [Google Scholar]
- Kukoski R., Blonigen B., Macri E., Renshaw A. A., Hoffman M., Loda M., Datta M. W.2003p27 and cyclin E/D2 associations in testicular germ cell tumors: implications for tumorigenesis. Appl. Immunohistochem. Mol. Morphol. 11, 138–143 [DOI] [PubMed] [Google Scholar]
- Lele K. M., Wolgemuth D. J.2004Distinct regions of the mouse cyclin A1 gene, Ccna1, confer male germ-cell specific expression and enhancer function. Biol. Reprod. 71, 1340–1347 (doi:10.1095/biolreprod.104.030387) [DOI] [PubMed] [Google Scholar]
- Liao C., Li S. Q., Wang X., Muhlrad S., Bjartell A., Wolgemuth D. J.2004Elevated levels and distinct patterns of expression of A-type cyclins and their associated cyclin-dependent kinases in male germ cell tumors. Int. J. Cancer 108, 654–664 (doi:10.1002/ijc.11573) [DOI] [PubMed] [Google Scholar]
- Lilly M. A., De Cuevas M., Spradling A. C.2000Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev. Biol. 218, 53–63 (doi:10.1006/dbio.1999.9570) [DOI] [PubMed] [Google Scholar]
- Liu D., Matzuk M. M., Sung K. W., Guo Q., Wang P., Wolgemuth D. J.1998aCyclin A1 is required for meiosis in the male mouse. Nat. Genet. 20, 377–380 (doi:10.1038/3855) [DOI] [PubMed] [Google Scholar]
- Liu N., Lucibello F. C., Engeland K., Muller R.1998bA new model of cell cycle-regulated transcription: repression of the cyclin A promoter by CDF-1 and anti-repression by E2F. Oncogene 16, 2957–2963 (doi:10.1038/sj.onc.1201838) [DOI] [PubMed] [Google Scholar]
- Liu D., Liao C., Wolgemuth D. J.2000A role for cyclin A1 in the activation of MPF and G2-M transition during meiosis of male germ cells in mice. Dev. Biol. 224, 388–400 (doi:10.1006/dbio.2000.9776) [DOI] [PubMed] [Google Scholar]
- Lock L. F., Pines J., Hunter T., Gilbert D. J., Gopalan G., Jenkins N. A., Copeland N. G., Donovan P. J.1992A single cyclin A gene and multiple cyclin B1-related sequences are dispersed in the mouse genome. Genomics 13, 415–424 (doi:10.1016/0888-7543(92)90262-Q) [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Truss M., Zwicker J., Ehlert F., Beato M., Muller R.1995Periodic cdc25C transcription is mediated by a novel cell cycle-regulated repressor element (CDE). EMBO J 14, 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah S. K., et al. 2001Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 27, 271–276 (doi:10.1038/85830) [DOI] [PubMed] [Google Scholar]
- Malumbres M., Sotillo R., Santamaria D., Galan J., Cerezo A., Ortega S., Dubus P., Barbacid M.2004Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118, 493–504 (doi:10.1016/j.cell.2004.08.002) [DOI] [PubMed] [Google Scholar]
- Mandon-Pepin B., Derbois C., Matsuda F., Cotinot C., Wolgemuth D. J., Smith K., Mcelreavey K., Nicolas A., Fellous M.2002Human infertility: meiotic genes as potential candidates. Gynecol. Obstet. Fertil. 30, 817–821 (doi:10.1016/S1297-9589(02)00444-7) [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Lamb D. J.2002Genetic dissection of mammalian fertility pathways. Nat. Cell Biol. 4(Suppl.), s41–s49 (doi:10.1038/ncb-nm-fertilityS41) [DOI] [PubMed] [Google Scholar]
- Mettus R. V., Rane S. G.2003Characterization of the abnormal pancreatic development, reduced growth and infertility in Cdk4 mutant mice. Oncogene 22, 8413–8421 (doi:10.1038/sj.onc.1206888) [DOI] [PubMed] [Google Scholar]
- Morris J. Z., Hong A., Lilly M. A., Lehmann R.2005Twin, a CCR4 homolog, regulates cyclin poly(A) tail length to permit Drosophila oogenesis. Development 132, 1165–1174 (doi:10.1242/dev.01672) [DOI] [PubMed] [Google Scholar]
- Muller C., Yang R., Idos G., Tidow N., Diederichs S., Koch O. M., Verbeek W., Bender T. P., Koeffler H. P.1999c-myb transactivates the human cyclin A1 promoter and induces cyclin A1 gene expression. Blood 94, 4255–4262 [PubMed] [Google Scholar]
- Murphy M., Stinnakre M. G., Senamaud-Beaufort C., Winston N. J., Sweeney C., Kubelka M., Carrington M., Brechot C., Sobczak-Thepot J.1997Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nature Genetics 15, 83–86 [Erratum in Nat. Genet. 1999 23, 481.] (doi:10.1038/ng0197-83) [DOI] [PubMed] [Google Scholar]
- Murray A. W.2004Recycling the cell cycle: cyclins revisited. Cell 116, 221–234 (doi:10.1016/S0092-8674(03)01080-8) [DOI] [PubMed] [Google Scholar]
- Nguyen T. B., et al. 2002Characterization and expression of mammalian cyclin b3, a prepachytene meiotic cyclin. J. Biol. Chem. 277, 41960–41969 (doi:10.1074/jbc.M203951200) [DOI] [PubMed] [Google Scholar]
- Nickerson H. D., Joshi A., Wolgemuth D. J.2007Cyclin A1-deficient mice lack histone H3 serine 10 phosphorylation and exhibit altered aurora B dynamics in late prophase of male meiosis. Dev. Biol. 306, 725–735 (doi:10.1016/j.ydbio.2007.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega S., Prieto I., Odajima J., Martin A., Dubus P., Sotillo R., Barbero J. L., Malumbres M., Barbacid M.2003Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 35, 25–31 (doi:10.1038/ng1232) [DOI] [PubMed] [Google Scholar]
- Parisi T., Beck A. R., Rougier N., Mcneil T., Lucian L., Werb Z., Amati B.2003Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J 22, 4794–4803 (doi:10.1093/emboj/cdg482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J., Zhang Q., Wang X. Y., Ravnik S. E., Muhlrad S., Wolgemuth D. J.2005Distinct roles for the mammalian A-type cyclins during oogenesis. Reproduction 130, 411–422 [DOI] [PubMed] [Google Scholar]
- Philips A., Huet X., Plet A., Rech J., Vie A., Blanchard J. M.1999Anchorage-dependent expression of cyclin A in primary cells requires a negative DNA regulatory element and a functional Rb. Oncogene 18, 1819–1825 (doi:10.1038/sj.onc.1202530) [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T.1990Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature 346, 760–763 (doi:10.1038/346760a0) [DOI] [PubMed] [Google Scholar]
- Rane S. G., Dubus P., Mettus R. V., Galbreath E. J., Boden G., Reddy E. P., Barbacid M.1999Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat. Genet. 22, 44–52 (doi:10.1038/8751) [DOI] [PubMed] [Google Scholar]
- Ravnik S. E., Wolgemuth D. J.1996The developmentally restricted pattern of expression in the male germ line of a murine cyclin A, cyclin A2, suggests roles in both mitotic and meiotic cell cycles. Dev. Biol. 173, 69–78 (doi:10.1006/dbio.1996.0007) [DOI] [PubMed] [Google Scholar]
- Ravnik S. E., Wolgemuth D. J.1999Regulation of meiosis during mammalian spermatogenesis: the A-type cyclins and their associated cyclin-dependent kinases are differentially expressed in the germ-cell lineage. Dev. Biol. 207, 408–418 (doi:10.1006/dbio.1998.9156) [DOI] [PubMed] [Google Scholar]
- Ravnik S. E., Rhee K., Wolgemuth D. J.1995Distinct patterns of expression of the D-type cyclins during testicular development in the mouse. Dev. Genet. 16, 171–178 (doi:10.1002/dvg.1020160209) [DOI] [PubMed] [Google Scholar]
- Refik-Rogers J., Manova K., Koff A.2006Misexpression of cyclin B3 leads to aberrant spermatogenesis. Cell Cycle 5, 1966–1973 [DOI] [PubMed] [Google Scholar]
- Rossi P., Sette C., Dolci S., Geremia R.2000Role of c-kit in mammalian spermatogenesis. J. Endocrinol. Invest. 23, 609–615 [DOI] [PubMed] [Google Scholar]
- Rossi P., Dolci S., Sette C., Geremia R.2003Molecular mechanisms utilized by alternative c-kit gene products in the control of spermatogonial proliferation and sperm-mediated egg activation. Andrologia 35, 71–78 (doi:10.1046/j.1439-0272.2003.00539.x) [DOI] [PubMed] [Google Scholar]
- Salazar G., Joshi A., Liu D., Wei H., Persson J. L., Wolgemuth D. J.2005Induction of apoptosis involving multiple pathways is a primary response to cyclin A1-deficiency in male meiosis. Dev. Dyn. 234, 114–123 (doi:10.1002/dvdy.20533) [DOI] [PubMed] [Google Scholar]
- Santamaria D., et al. 2007Cdk1 is sufficient to drive the mammalian cell cycle. Nature 448, 811–815 (doi:10.1038/nature06046) [DOI] [PubMed] [Google Scholar]
- Schulze A., Zerfass K., Spitkovsky D., Middendorp S., Berges J., Helin K., Jansen-Durr P., Henglein B.1995Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc. Natl Acad. Sci. USA 92, 11 264–11 268 (doi:10.1073/pnas.92.24.11264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J.1994G1 phase progression: cycling on cue (see comments). Cell 79, 551–555 (doi:10.1016/0092-8674(94)90540-1) [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M.1999CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (doi:10.1101/gad.13.12.1501) [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M.2004Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18, 2699–2711 (doi:10.1101/gad.1256504) [DOI] [PubMed] [Google Scholar]
- Sicinska E., et al. 2003Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4, 451–461 (doi:10.1016/S1535-6108(03)00301-5) [DOI] [PubMed] [Google Scholar]
- Sicinski P., et al. 1995Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82, 621–630 (doi:10.1016/0092-8674(95)90034-9) [DOI] [PubMed] [Google Scholar]
- Sicinski P., et al. 1996Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384, 470–474 (doi:10.1038/384470a0) [DOI] [PubMed] [Google Scholar]
- Sugimura I., Lilly M. A.2006Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev. Cell 10, 127–135 (doi:10.1016/j.devcel.2005.10.018) [DOI] [PubMed] [Google Scholar]
- Sweeney C., Murphy M., Kubelka M., Ravnik S. E., Hawkins C. F., Wolgemuth D. J., Carrington M.1996A distinct cyclin A is expressed in germ cells in the mouse. Development 122, 53–64 [DOI] [PubMed] [Google Scholar]
- van der Meer T., Chan W. Y., Palazon L. S., Nieduszynski C., Murphy M., Sobczak-Thepot J., Carrington M., Colledge W. H.2004Cyclin A1 protein shows haplo-insufficiency for normal fertility in male mice. Reproduction 127, 503–511 (doi:10.1530/rep.1.00131) [DOI] [PubMed] [Google Scholar]
- Vardy L., Pesin J. A., Orr-Weaver T. L.2009Regulation of Cyclin A protein in meiosis and early embryogenesis. Proc. Natl Acad. Sci. USA 106, 1838–1843 (doi:10.1073/pnas.0813237106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables J. P., Cooke H. J.2000Lessons from knockout and transgenic mice for infertility in men. J. Endocrinol. Invest. 23, 584–591 [DOI] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Brechot C.1990Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature 343, 555–557 (doi:10.1038/343555a0) [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Rhee K., Wu S., Ravnik S. E.1995Genetic control of mitosis, meiosis and cellular differentiation during mammalian spermatogenesis. Reprod. Fertil. Dev. 7, 669–683 (doi:10.1071/RD9950669) [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Laurion E., Lele K. M.2002Regulation of the mitotic and meiotic cell cycles in the male germ line. Recent Prog. Horm. Res. 57, 75–101 (doi:10.1210/rp.57.1.75) [DOI] [PubMed] [Google Scholar]
- Yang R., Morosetti R., Koeffler H. P.1997Characterization of a second human cyclin A that is highly expressed in testis and in several leukemic cell lines. Cancer Res. 57, 913–920 [PubMed] [Google Scholar]
- Zhang Q., Wang X., Wolgemuth D. J.1999Developmentally regulated expression of cyclin D3 and its potential in vivo interacting proteins during murine gametogenesis. Endocrinology 140, 2790–2800 (doi:10.1210/en.140.6.2790) [DOI] [PubMed] [Google Scholar]
- Zwijsen R. M., Wientjens E., Klompmaker R., van der Sman J., Bernards R., Michalides R. J.1997CDK-independent activation of estrogen receptor by cyclin D1. Cell 88, 405–415 (doi:10.1016/S0092-8674(00)81879-6) [DOI] [PubMed] [Google Scholar]
- Zwijsen R. M., Buckle R. S., Hijmans E. M., Loomans C. J., Bernards R.1998Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 12, 3488–3498 (doi:10.1101/gad.12.22.3488) [DOI] [PMC free article] [PubMed] [Google Scholar]