Abstract
Different cellular events occur during spermatogenesis, and these include (i) mitosis for self-renewal of spermatogonia, (ii) differentiation of type A spermatogonia into type B and commitment of type B spermatogonia to develop into preleptotene primary spermatocytes, (iii) transit of preleptotene/leptotene spermatocytes across the blood–testis barrier in coordination with germ cell cycle progression and meiosis, (iv) spermiogenesis and spermiation. These events also associate with extensive changes in cell shape and size, and germ cell movement. The cytoskeleton, which comprises actin, microtubules and intermediate filaments, is believed to function in these cellular events. However, few studies have been conducted by investigators in the past decades to unfold the role of the cytoskeleton during spermatogenesis. This review summarizes recent advances in the field relating to cytoskeletal dynamics in the testis, and highlights areas of research that require additional emphasis so that new approaches for male contraception, as well as therapeutic approaches to alleviate environmental toxicant-induced reproductive dysfunction in men, can possibly be developed.
Keywords: testis, spermatogenesis, actin, blood–testis barrier, ectoplasmic specialization
1. Introduction
In the adult mammalian testis, Sertoli cells are the somatic epithelial cells that nurse and structurally support developing germ cells in the seminiferous epithelium which eventually develop into spermatozoa by the process known as spermatogenesis (Bardin et al. 1988). While spermatogenesis is composed of different cellular events, including mitosis, meiosis and spermiogenesis, an integrated aspect of spermatogenesis is the dynamic restructuring of cell junctions at the Sertoli–Sertoli and Sertoli–germ cell interface. For instance, Sertoli cell junctions at the blood–testis barrier (BTB) must restructure at stage VIII of the seminiferous epithelial cycle to allow the transit and entry of preleptotene spermatocytes into the adluminal compartment, which is a prerequisite for meiosis that takes place at stage XIV of the epithelial cycle and post-meiotic development known as spermiogenesis. Thus, extensive restructuring is envisioned to occur at the cell–cell interface throughout spermatogenesis. Since cytoskeletal components are known to serve as the foundation for different protein complexes within cell junctions, the underlying cytoskeleton is of paramount importance for the integrity of cell junctions, as well as for their dynamic restructuring.
The cytoskeleton in eukaryotic cells comprises actin, microtubule (MT) and intermediate filament (IF) networks. Cytoskeletal proteins are known to have numerous roles, such as determination of cell shape, cell motility, maintenance of cell junctions and intracellular trafficking, all of which help to maintain normal epithelial function and morphology. Since the role of IFs in spermatogenesis has not been studied extensively in the testis and their function is less understood when compared with actin and MTs, the latter two cytoskeletal components will be the primary focus of this review. We first provide an overview of the organization and regulation of actin and MTs in Sertoli cells (see figure 1), followed by a discussion on the crosstalk between these two networks during spermatogenesis. Finally, we discuss the use of small molecules to study cytoskeletal dynamics during spermatogenesis, as well as their possible use for male contraception.
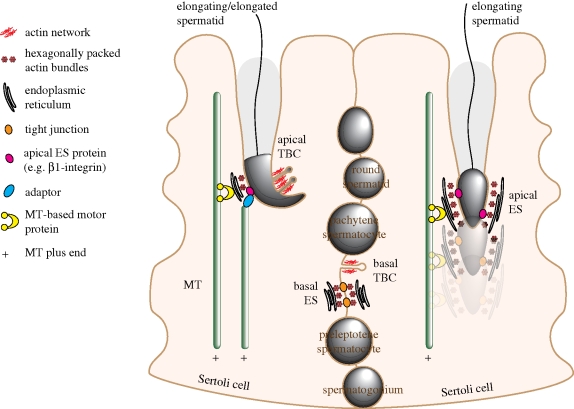
Figure 1.
A schematic drawing to illustrate the organization of F-actin and microtubules (MTs) in Sertoli cells in the seminiferous epithelium and their possible crosstalk during spermatogenesis. F-actin is abundantly found at sites of cell–cell contact, including the basal ES and TBC at the BTB formed between Sertoli cells, and the apical ES and TBC at the Sertoli cell–spermatid interface. Actin filaments found at the ES and the TBC are arranged in a different manner, being packed in hexagonal arrays in the ES, but exist as a branched network at the TBC. MTs run vertically in parallel to the longitudinal axis of Sertoli cells with their minus ends pointing apically. The translocation of the apical ES in both directions in the seminiferous epithelium is proposed to be mediated by MT-based motors using MTs as tracks. Based on studies in other epithelial cells, MT minus ends may interact with junction protein complexes at the apical ES.
2. Actin dynamics in the seminiferous epithelium
(a). Actin organization in Sertoli cells
Filamentous actin (F-actin) is a polymer that possesses intrinsic polarity, and it is assembled by globular actin subunits (G-actin) with the fast-growing end known as the barbed end, and the slow-growing end defined as the pointed end (Pellegrin & Mellor 2007). Eukaryotic cells are equipped with an array of actin-binding proteins to produce rapid changes in actin cytoskeletal dynamics, such as polymerization, depolymerization, bundling, severing, capping and branching (see table 1). In polarized epithelial cells, actin filaments form a prominent contractile actomyosin ring which encircles each cell in the epithelium, and this ring associates with the cadherin-based junction complex, together forming a mature zonula adherens/adhesion belt (Mege et al. 2006; Cavey et al. 2008). Interestingly, the seminiferous epithelium in adult mammalian testes lacks classic zonula adherens. However, F-actin can be found at the BTB, where different types of Sertoli cell junctions coexist with one another (Vogl et al. 2008). As integral components of the BTB, the basal ectoplasmic specialization (ES) and the basal tubulobulbar complex (TBC) are specialized actin-rich structures unique to the testis, but they exhibit drastically different F-actin organization. Figure 2 illustrates actin filaments visualized in primary Sertoli cells isolated from rat testes and cultured in vitro. Apart from the BTB, F-actin is also found to be abundant at two other sites of Sertoli–germ cell adhesion, namely the apical ES and the apical TBC. These structures are complementary to the basal ES and the basal TBC, respectively, but are only found within Sertoli cells at the Sertoli cell–elongating/elongated spermatid interface. Although the functional significance of the ES and TBC is not fully understood, these structures are thought to contribute as least in part to cell adhesion.
Table 1.
Different proteins that affect actin dynamics in the testis. Abbreviations: BTB, blood–testis barrier; ES, ectoplasmic specialization; GC, germ cell; SC, Sertoli cell; n.d., not determined; TBC, tubulobulbar complex.
| function of protein/family | cellular expression | localization | possible function in testis | references | |
|---|---|---|---|---|---|
| actin-binding proteins | |||||
| espin | actin bundling | n.d. | apical ESa, BTB | formation of actin bundles at ES | Bartles et al. (1996) |
| fimbrin | actin bundling | SC | apical ESa | formation of actin bundles at ES | Grove & Vogl (1989) |
| α-actinin | actin bundling | n.d. | BTB | formation of actin bundles at ES | Yazama et al. (1991) |
| vinculin | membrane anchor; shows actin cross-linking activity | SC | apical ESa, BTB | formation of actin bundles at ES | Grove & Vogl (1989) |
| myosin-VIIa | actin based motor protein | SC | apical ES | scaffold for adhesion proteins | Hasson et al. (1997) |
| Eps8 | actin capping; regulation of bundling and Rac GTPase | SC > GC | apical ES, BTB, apical TBC | formation of actin bundles and network | Lie et al. (2009) |
| cofilin (non-muscle) | actin depolymerization | SC | apical TBCa | increase of actin turnover at TBC | Guttman et al. (2004b) |
| cortactin | Arp2/3 complex activation | n.d. | apical TBCa | formation of actin network at TBC | Young et al. (2009) |
| mDia1/2 | actin nucleation | SC and GC | cytoplasmic/nuclear | n.d. | Mironova & Millette (2008) |
| testis fascin | actin bundling | spermatid (elongating) | spermatid head | GC morphogenesis | Tubb et al. (2002) |
| profilin III | actin monomer binding | spermatid | nuclear | GC morphogenesis | Hara et al. (2008) |
| profilin IV | actin monomer binding | spermatid | perinuclear | GC morphogenesis | Obermann et al. (2004) |
| WASP family and associated proteins | |||||
| N-WASP | Arp2/3 complex activation | n.d. | apical TBCa | formation of actin network at TBC | Young et al. (2009) |
| WAVE1 | Arp2/3 complex activation | spermatocyte/spermatid | Golgi/mitochondrial sheath | GC morphogenesis/ sperm function | Rawe et al. (2004) |
| WAVE3 | Arp2/3 complex activation | n.d. | n.d. | n.d. | Sossey-Alaoui et al. (2002) |
| WISH | N-WASP and Arp2/3 complex activation | n.d. | n.d. | n.d. | Fukuoka et al. (2001) |
| small GTPases and kinases | |||||
| RhoB | actin reorganization | SC > GC | apical ES, BTB | regulation of Sertoli–germ cell adhesion | Lui et al. (2003b) |
| Rac1 | actin reorganization | n.d. | apical TBC, BTB | n.d. | Chapin et al. (2001) |
| Cdc42 | actin reorganization | n.d. | basal, peri-round spermatid | n.d. | Chapin et al. (2001) |
| TESK2 | cofilin inactivation | SC | basal | n.d. | Toshima et al. (2001) |
| LIMK2 | cofilin inactivation | spermatocyte/ spermatid | nuclear | GC development | Takahashi et al. (2002) |
| actin-related proteins | |||||
| Arp3 | actin nucleation and branching | n.d. | apical TBCa | formation of actin network at TBC | Young et al. (2009) |
| ArpM1 | n.d. | spermatid | nuclear | GC morphogenesis | Hara et al. (2008) |
| T-actin 1/2 | n.d. | spermatid | spermatid head and tail | GC morphogenesis/ sperm function | Tanaka et al. (2003) |
| ArpT1/2 | n.d. | spermatozooa | sperm head calyx | GC morphogenesis/ sperm function | Heid et al. (2002) |
aLocalization at ES or TBC structures has been directly demonstrated by electron microscopy or fluorescence microscopy following specialized tissue processing.
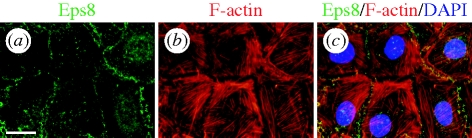
Figure 2.
Co-localization of Eps8 and F-actin in Sertoli cells cultured in vitro. Sertoli cells isolated from 20-day-old rat testes were cultured on Matrigel-coated glass coverslips for 4 days at 0.04 × 106 cells cm−2. Eps8 (green) and F-actin (red) were visualized with an anti-Eps8 antibody and rhodamine conjugated phalloidin, respectively. Sertoli cell nuclei were visualized by DAPI (blue). Eps8 is concentrated at the cell–cell interface, co-localizing with the tips of cortical F-actin. Orderly bundles of actin filaments are also found inside the Sertoli cell. Scale bar, (a) 20 µM, which applies to (b) and (c).
(i). Ectoplasmic specialization
The ES is a tripartite structure recognizable under the electron microscope as a layer of actin bundles packed in parallel hexagonal arrays and sandwiched in between cisternae of endoplasmic reticulum and the plasma membrane of Sertoli cells (Vogl et al. 2000). With recent technological advances which have helped to better understand the molecular composition of the ES, this structure is regarded as an atypical, testis-specific adherens junction (AJ) because its function is constituted by nectin-, cadherin-, and integrin-based protein complexes (Mruk & Cheng 2004a; Yan et al. 2006). The basal ES is also an integrated component of the BTB, and it co-exists with tight- and gap-junctions, and desmosomes which collectively maintain the immunological barrier in the testis. Having the same ultrastructural features as the basal ES, the apical ES, however, can only be visible by electron microscopy within the Sertoli cell at the site of elongating/elongated spermatid attachment to Sertoli cells, even though studies have demonstrated that elongating/elongated spermatids express apical ES proteins similar to the ones expressed by Sertoli cells (Vogl et al. 2000; Mruk & Cheng 2004b). Similar to other polarized epithelia, when the seminiferous epithelium is viewed as a continuous sheet of Sertoli cells, actin bundles at the basal ES encircle the borders of Sertoli cells forming a honeycomb pattern (Vogl & Soucy 1985). However, in contrast to classic AJs, ES-associated actin bundles are believed to be non-contractile, which is in line with the unipolar arrangement of hexagonally packed actin filaments characteristic to the ES (Vogl et al. 2000). This lack of contractility was illustrated by the failure to produce contractions in actin at the ES (Vogl & Soucy 1985). Interestingly, an actin-based motor protein myosin VIIa has been found to localize to actin bundles at the apical ES by electron microscopy, and it was also detected at the BTB by immunofluorescence microscopy (Velichkova et al. 2002). Studies in other epithelia suggest the participation of myosin VIIa during AJ formation. For instance, in MDCK cells, myosin VIIa is recruited to the cadherin-based protein complex together with vezatin, where it is proposed to generate a tensile force between the plasma membrane and the actin cytoskeleton to strengthen cell–cell adhesion (Kussel-Andermann et al. 2000). The significance of myosin VIIa at the ES is yet to be investigated.
(ii). Tubulobulbar complex
TBCs are found at the BTB, termed basal TBC, and at the ventral (or concave) side of the heads of elongated spermatids (steps 18–19) attached to the Sertoli cell at sites formerly occupied by the apical ES, termed apical TBC. Ultrastructurally, apical TBCs are tubular extensions derived from the plasma membrane of spermatid heads that protrude into the corresponding Sertoli cell plasma membrane as invaginations, and are visible under electron and confocal microscopy. The tubular portion ends with a bulbar end which has a bristle-coated pit. Unlike the orderly array of tightly packed actin bundles found at the ES, actin filaments at the TBC exist as highly branched networks surrounding the tubular portion, but not the bulbar end (Russell 1979; Vogl et al. 2008). Since apical TBCs are only found at sites previously occupied by apical ES, it has been hypothesized that TBCs function to internalize components from disassembled apical ES junctions in preparation for the release of spermatids during spermiation (Guttman et al. 2004a). For example, dynamin III, which is implicated in the fission of endocytic vesicles, localized to the apical TBC (Vaid et al. 2007a). The timing of apical TBC formation, its localization, and its ultrastructural features also suggest that the apical TBC is a transitional ultrastructure of the apical ES which must be disassembled and is lost from the seminiferous epithelium before spermiation. Recent reports have demonstrated an efficient mechanism of BTB protein internalization by clathrin-mediated endocytosis, which may facilitate the transit of primary spermatocytes across the BTB at stage VIII of the epithelial cycle (Yan et al. 2008; Xia et al. 2009). Likewise, the TBC-associated double membraneous invagination is reminiscent of a ‘giant’ endocytic vesicle. As such, the apical TBC is probably composed of residual apical ES structures that were engulfed by Sertoli cells via protein endocytosis, so that structural proteins (e.g. laminins, integrins, cadherins, catenins, nectins, afadins) can be ‘transcytased’ and ‘recycled’ for the assembly of ‘new’ apical ES at the Sertoli cell–elongating spermatid interface at spermiogenesis in the seminiferous epithelium. This possibly provides an efficient system to facilitate spermatogenesis without involving unnecessary de novo synthesis of proteins and/or organelles. Indeed recent studies have suggested that apical TBC serves as a platform for extensive protein endocytosis with the abundant presence of clathrin at the apical TBC (Young et al. 2009), which is a crucial endocytic vesicle constituent protein. Nonetheless, the precise function, molecular composition and mechanism(s) by which the apical ES transforms into the TBC require further investigation. For instance, it is unclear at present whether the TBC is constituted by adhesion protein complexes derived from the ES, such as nectin- and integrin-based protein complexes and their peripheral adaptors/protein kinases (note: these are the two major constituent protein complexes at the apical ES since the appearance of cadherin-based complexes at the apical ES is stage-specific, being highest at stage VI–VII, and cadherin was found at apical TBC) or some additional specific proteins found at the site. It is noted that a protein complex containing α6β1- integrin and focal adhesion kinase (FAK)-Tyr397, an integrated component of the apical ES, persisted at the Sertoli cell–spermatid interface long after apical ES disassembly until the time of spermatid disengagement (Beardsley et al. 2006). Although the staining pattern of this particular protein complex at the dorsal side of spermatid heads does not suggest its localization to the TBC, some other ES-associated proteins retained after apical ES disassembly (e.g. cadherins) may be used to constitute the apical TBC. While several hypotheses have been postulated regarding the function of the apical TBC, very few functional studies have been conducted on basal TBCs. Thus, this is an important area of research to be pursued in future studies.
(b). Actin regulation in Sertoli cells
Sertoli cells are equipped with different types of actin regulating proteins at the F-actin-rich ES and TBC, which are found at the Sertoli cell–spermatid interface and the BTB (see table 1). Currently, the functional significance of most actin regulatory proteins in the seminiferous epithelium has not been directly demonstrated. However, generally speaking, the local organization of F-actin is largely consistent with the reported functions of actin regulators identified at a particular site. For instance, several actin bundling proteins are found at the ES, whose actin filaments are arranged into parallel bundles, whereas actin branching proteins are associated with the highly branched actin network at the TBC. Because of the relative ease of isolation and identification of the apical ES and the apical TBC, more effort has been placed on studying these structures at the Sertoli–germ cell interface, and less is known about their basal counterparts at the BTB.
(i). Ectoplasmic specialization formation and maintenance
For the formation and maintenance of characteristic actin bundles at the ES, several actin bundling/crosslinking proteins have been identified at this site. These include espin (Chen et al. 1999), fimbrin (Grove & Vogl 1989), vinculin (Grove & Vogl 1989) and α-actinin (Yazama et al. 1991). Espin, the founding member of the espin family of bundling proteins consisting of four isoforms to date, was first identified in the submembraneous plaque of the apical ES and also at the BTB (Bartles et al. 1996; Sekerkova et al. 2006b). Espin displays high binding affinity for F-actin with actin bundling activity that is insensitive to Ca2+ in vitro, and also with actin elongating activity (Sekerkova et al. 2006a). The timing of espin accumulation at the Sertoli cell–spermatid interface coincides with the appearance of actin bundles at the apical ES at the onset of spermatid elongation which occurs at step 8 of spermiogenesis (Chen et al. 1999). The role of espin during BTB establishment in rat pups has also been illustrated by its delayed appearance at the BTB site following adjudin treatment, a drug that perturbs the apical ES in adult rat testes, and this observation correlated with the delayed assembly of a functional BTB (Kopera et al. 2009). However, the intrinsic activity of espin and other actin bundlers/crosslinkers at the ES has not been investigated. Thus, it is unclear how they contribute individually, or how they work together to regulate actin dynamics in Sertoli cells. In other systems, the coexistence of two or more non-redundant actin bundling proteins has been demonstrated (Revenu et al. 2004). For instance, in Drosophila bristles, the bundling protein forked is required for the initial phase of actin bundle aggregation, while fascin is needed for a higher order crosslinking to assemble compact and rigid bundles (Tilney et al. 1998). Moreover, an in vitro study showed that fascin, plastin and α-actinin produced actin bundles with different mechanical properties, such as stiffness (Claessens et al. 2006). Thus, different actin bundling proteins may facilitate the production of actin bundles with specific properties uniquely required at a site. While it is difficult to dissect the significance of different actin bundling proteins at the ES in vivo, the use of primary cultured Sertoli cells which possess ES and mimic the BTB in vivo, in conjunction with techniques such as overexpression and RNAi knock-down of key actin regulatory proteins, may offer insight on their roles in ES dynamics.
Small GTPases are implicated in regulating junction dynamics in the seminiferous epithelium (Lui et al. 2003a). For instance, RhoB is localized to the site of the apical ES, associating with elongating spermatids, and its level is markedly reduced surrounding elongated spermatids in stages VII–VIII tubules (Lui et al. 2003b). It is well established that Rho GTPases are important regulators of stress fibre formation. The activation of downstream effectors, such as Rho kinase and the formin mDia, promotes stress fibre formation via several mechanisms. These include elongation of straight actin filaments, inhibition of actin depolymerization and induction of contractility (Burridge & Wennerberg 2004; Jaffe & Hall 2005). Although actin bundles at the ES are not stress fibres due to their lack of contractility, it is of interest to note that drug-induced apical ES disruption coincides with an increase in the RhoB protein level which promotes stress fibre formation. Following the administration of adjudin, a drug known to specifically perturb anchoring junctions in the testis in particular the apical ES to induce rapid loss of spermatids from the seminiferous epithelium (Cheng et al. 2005), RhoB was shown to be induced in the testis but not in other organs, as well as the protein levels of its effectors ROCK1 and LIMK1 which were also upregulated (Lui et al. 2003b). While this observation is contradictory with the reported function of RhoB in promoting net actin elongation, this nevertheless suggests the participation of RhoB in some aspect of ES dynamics prior to spermatid depletion from the epithelium. Since only the total protein levels of RhoB and its effectors were examined, future studies are needed to define changes in intrinsic GTPase activity.
(ii). Ectoplasmic specialization disassembly and tubulobulbar complex formation
During stage VIII of the seminiferous epithelial cycle in rats, BTB restructuring allows the transit of preleptotene/leptotene spermatocytes, while elongated spermatids are released at the opposite end of the Sertoli cell. To facilitate these events, it is envisioned that the junctional plaque and the underlying actin bundles at the apical and the basal ES undergo extensive remodelling. Since the previous detection of gelsolin at the apical ES is probably due to an artefact (Guttman et al. 2007), other candidates that provide actin severing or depolymerizing activities during ES disassembly are likely to exist but these have yet to be identified.
Several components and regulators of the actin-related protein (Arp) 2/3 complex have been localized to the apical TBC, and they probably contribute to the formation of the highly branched actin network at the site. These include Arp3 (D'Souza et al. 2009), N-WASP (Wiskott–Aldrich syndrome protein; Young et al. 2009), cortactin (Young et al. 2009) and Rac1 (Chapin et al. 2001; see table 1). The Arp2/3 complex is an actin nucleation and branching machinery found abundantly in lamellipodia at the leading edge of motile cells, which contain branched actin filaments (Chhabra & Higgs 2007). This complex consists of seven subunits, including two actin-related proteins Arp2 and Arp3, which form a pseudo actin dimer serving as the starting point for the addition of actin subunits. This leads to the nucleation of a new actin filament, or a branch on a pre-existing filament at an angle of about 70° (Weaver et al. 2003). The crystal structure of the Arp2/3 complex suggests that upon binding of activators such as WASP, the complex undergoes a conformational change that closes the gap between the two Arp subunits, thereby forming the pseudo actin dimer (Rodal et al. 2005). Activators of the Arp2/3 complex include two major protein families, namely WASP and cortactin, which in turn are regulated by small GTPases such as Rac and Cdc42 (Weaver et al. 2003; Burridge & Wennerberg 2004). However, much work is needed to define the activation mechanism and the contribution of the Arp2/3 complex during the formation of the apical TBC.
Recently, the actin regulator Eps8 (Epidermal growth factor receptor pathway substrate 8), detected in both Sertoli (see figure 2) and germ cells, has been reported to play an important role in maintaining germ cell adhesion and BTB integrity in rat testes (Lie et al. 2009). While the precise mechanism by which Eps8 functions in the seminiferous epithelium as an actin regulator remains unclear, it was demonstrated that in vivo knockdown of Eps8 by the intratesticular injection of siRNA was detrimental to the integrity of actin-based cell junctions, leading to germ cell sloughing and BTB damage, consistent with findings in vitro (Lie et al. 2009). Studies in other systems have illustrated Eps8 to function via multiple actin regulating mechanisms. Eps8 displays actin barbed-end capping activity (Disanza et al. 2004), and participates in Eps8-Abi1-Sos1 mediated Rac GTPase activation (Scita et al. 2001), both of which facilitate the formation of a branched actin network. On the other hand, it also activates and synergizes with IRSp53 in actin bundling (Disanza et al. 2006). Thus, Eps8 may exert different mechanisms of action relating to apical ES formation and disassembly, and apical TBC formation. For instance, Eps8 could promote actin bundling in the ES, possibly via IRSp53 (Disanza et al. 2006), to help maintain the paracrystalline array of F-actin which is needed for the homeostasis of the apical ES. Conversely, F-actin branch formation during TBC formation might be facilitated by the barbed-end capping activity of Eps8, and Rac activation induced by the Eps8-Abi1-Sos1 trimeric complex. To determine whether Eps8 has any role in the transition of ES to TBC, additional research is needed to identify the functional partners of Eps8 at these sites and their mechanisms of action.
3. Microtubule dynamics in the seminiferous epithelium
Similar to F-actin, MTs are also polymers with intrinsic polarity, assembled by heterodimers of α- and β-tubulin arranged end-to-end in proto-filaments, which associate laterally to form a hollow cylindrical polymer (Desai & Mitchison 1997). The fast growing plus ends display alternating phases of growth and shrinkage, a phenomenon known as dynamic instability, while the slow growing minus ends are usually anchored to other cellular structures such as the MT organizing centre (MTOC). The arrangement of MTs in Sertoli cells is typical of polarized epithelial cells, that is, they exist in a parallel array and run along the long axis of the cell with minus ends pointing apically (Redenbach & Vogl 1991; Bartolini & Gundersen 2006). This is in contrast to migrating or undifferentiated cells, in which MTs extend radially away from the centrosome with plus ends pointing towards the cell surface (Bartolini & Gundersen 2006). Because of the non-centrosomal MT organization in Sertoli cells, the MTOC has not yet been identified. Interestingly, MTs are known to associate with the apical ES and are proposed to participate in the translocation of developing spermatids across the seminiferous epithelium during spermiogenesis (Amlani & Vogl 1988), as discussed in the next section.
The importance of MTs in Sertoli–germ cell adhesion has been demonstrated largely by toxicology studies. For instance, the MT disrupting agents carbendazim and colchicine, administered by intraperitoneal and intratesticular injection, respectively, both led to germ cell sloughing in the seminiferous epithelium (Correa et al. 2002). On the other hand, oral administration of 2,5-hexanedione caused testicular injury at subneurotoxic doses with several MT related abnormalities, resulting in adverse effects on tubulin assembly dynamics and impaired MT motor transport (Hall et al. 1995). The function of MT-associated proteins in the seminiferous epithelium has not been studied extensively, but several key observations are noteworthy. CLIP-170, an MT plus-end-tracking protein implicated in MT rescue and the regulation of dynactin accumulation at MT tips (Lansbergen et al. 2004) is proposed to participate in spermatid differentiation and sperm head shaping. Male CLIP-170 knockout mice are subfertile, with abnormal manchette features and aberrant MT localization in post-step 8 spermatids (Akhmanova et al. 2005). Another MT plus-end-tracking protein, EB1, has also been demonstrated to play a role in spermatogenesis. To study the function of EB1 in testicular Sertoli cells in vivo, a dominant negative mutant of EB1 was introduced to Sertoli cells, but not germ cells, by lentivirus infection (Wang et al. 2008). Sertoli cells expressing the EB1 mutant displayed abnormal rod-like cell shape with less processes, and the resulting tubules were less circular compared to normal tubules. Thus, EB1 helps maintaining the normal epithelial morphology in the testis, but the mechanism has yet to be elucidated.
4. Crosstalk between different cytoskeletal elements during spermatogenesis
Numerous studies in multiple epithelia over the past decade have supported the concept that crosstalk exists among the actin-, MT- and IF-based cytoskeleton. These three networks function with each other to regulate different cellular processes, such as signalling, cell adhesion, cell motility, maintenance of cell polarity and protein targeting (Chang & Goldman 2004; Etienne-Manneville 2004; Ezratty et al. 2005; Li & Gundersen 2008; Arnold 2009). In some cases, two different cytoskeletal components are influenced by a common regulator, such as the dual role of formins in actin polyermization and MT stabilization (Bartolini & Gundersen in press). In the seminiferous epithelium, crosstalk between cytoskeletal networks is also marked by various observations. By co-immunoprecipitation, several actin-associated proteins, such as zyxin and WASP, were found to physically interact with vimentin and tubulin in seminiferous tubules (Lee et al. 2004). Furthermore, MT disruption by intratesticular injection of colchicine caused a stage-dependent collapse of IFs (Allard et al. 1993). While these are only some examples of cytoskeletal crosstalk in the testis, the following sections discuss two phenomena which probably involve the interaction between MTs and actin in Sertoli cells (see figure 1).
(a). Microtubule-based transport of elongating/elongated spermatids
MTs in different cell types are organized into radial or parallel arrays. Either way, the manner in which MTs extend across a cell makes them an ideal network for intracellular trafficking events. Cargo proteins transported via MTs include vesicles, organelles, and even other cytoskeletal components, such as F-actin and IFs (Waterman-Storer et al. 2000; Chang & Goldman 2004). In a cell-free system using Xenopus egg extract, F-actin bundles exhibited a jerking motion when they associated with motile MTs, but a straight gliding motion when they associated with a stationary MT lattice (Waterman-Storer et al. 2000). Movement of cargo along the MT in both directions (i.e. anterograde and retrograde) is mediated by the corresponding plus-end and minus-end directed motor proteins kinesin and cytoplasmic dynein (Wu et al. 2006). Distinct from movement based on motor proteins, some MT-binding proteins display MT lattice diffusion as another mode of transport (Cooper & Wordeman 2009).
At present, spermatid translocation in the seminiferous epithelium during spermiogenesis is hypothesized to occur via MT-based transport of the apical ES. As described above, the apical ES is a testis-specific actin-based junction type at the Sertoli cell–elongating/elongated spermatid interface. Since actin bundles in the ES are non-contractile in nature (Vogl et al. 2008), the propulsion of spermatids across the seminiferous epithelium during spermiogenesis and at spermiation cannot be attributed to actomyosin-induced contractility. Instead, it was hypothesized that the ‘down and then up’ translocation of elongating/elongated spermatids is achieved by the gliding of the entire apical ES structure together with the attached spermatid along MTs within the Sertoli cell, which run parallel to the longitudinal cell axis and serve as tracks for spermatid movement, possibly driven by motor proteins such as dynein and kinesin (Amlani & Vogl 1988; Guttman et al. 2000; Vaid et al. 2007b). In the apical part of the Sertoli cell, MTs are closely associated with neighboring apical ES junction plaques (Guttman et al. 2000). Early studies demonstrated that the intratesticular injection of taxol (an MT stabilizer) led to the retention of step 19 spermatids deep within Sertoli cells crypts, instead of being transported towards the tubule lumen (Russell et al. 1989). Indeed, when mechanically isolated spermatids which retained their associated ES from the Sertoli cell were immobilized in a flow chamber, movement of fluorescently labelled MTs across the spermatid surface was observed in the presence of exogenous ATP (Beach & Vogl 1999). This illustrated that a force can be generated between ES-bearing spermatids and MTs that causes their movement in opposite directions. Subsequent studies have localized the MT-based motor proteins dynein and kinesin to the cytoplasmic face of the endoplasmic reticulum within the apical ES, which is the proposed site where MTs contact the ES (Guttman et al. 2000; Vaid et al. 2007b). It is probable that these motor proteins drive the translocation of the apical ES, together with the attached spermatid, along the MT in both directions, but this has to be confirmed by functional in vivo experiments. Furthermore, it will be of great interest to identify other adaptors/scaffold proteins and the upstream regulators participating in this mechanism of spermatid movement during spermiogenesis.
(b). Possible role of microtubules in apical ectoplasmic specialization restructuring
Given the remarkable similarity in molecular composition between the apical ES and focal adhesions (Mruk & Cheng 2004a), it is not surprising that they share common mechanisms in the regulation of cell adhesion. One of the untapped areas of research worth exploring is the possible role of MTs in facilitating apical ES disassembly, reminiscent of focal adhesion disassembly in motile cells which also use MTs. Focal adhesions are actin-based cell-matrix anchoring junctions, with the receptor module (e.g. integrin-based protein complex) linked to contractile actin stress fibres via adaptors such as vinculin (Geiger et al. 2009). For migrating cells to move forward, nascent focal adhesions are formed at the leading edge, while focal adhesions at the cell rear must disassemble, which are targeted by the approaching ends of growing MTs (Kaverina et al. 1999; Etienne-Manneville 2004; Broussard et al. 2008). This MT-targeted focal adhesion disassembly is dependent on dynamin and FAK, and is likely to involve FAK-Src and ERK signalling (Webb et al. 2004; Ezratty et al. 2005). One plausible mechanism is that the induction of MT catastrophe facilitates the release of signalling molecules transported to the MT plus end (Wu et al. 2006; Efimov et al. 2008), which in turn destabilize the junction. The requirement of dynamin in this process might be partly due to its role in regulating dynamic instability of MTs (Tanabe & Takei 2009).
In the seminiferous epithelium, the apical ES not only contains structural proteins found at focal adhesions (e.g. the integrin/laminin complex, Yan & Cheng 2006), it is also equipped with molecules implicated in focal adhesion disassembly (e.g. FAK, c-Src). The disassembly of the apical ES takes place at stages VII–VIII of the seminiferous epithelial cycle, during the time that the apical ES disintegrates to be replaced by apical TBCs at this site (Russell 1979). Immunofluorescence staining indeed illustrates close association between MT tips and the heads of elongate spermatids in stage VII tubules (Beardsley et al. 2006). Interestingly, dynamin II and activated FAK (phosphorylated at Tyr397) are localized predominantly to the site of apical ES in stages VII–VIII tubules (Siu et al. 2003; Lie et al. 2006). It is also noteworthy that in rats treated with adjudin, a drug that specifically induces apical ES disassembly without affecting the BTB, the protein level of pFAK-Tyr397 in the testis is induced even before spermatid loss is detected (Siu et al. 2003).
However, as opposed to motile cells, MTs in Sertoli cells display non-centrosomal organization similar to other epithelial cell types, with their minus ends oriented apically (Redenbach & Vogl 1991). Because minus ends do not polymerize in vivo (Dammermann et al. 2003), it is unlikely that Sertoli cell MTs undergo elongation and targeting in a manner comparable to motile cells. In contrast, MT minus ends are less dynamic in nature and require anchorage for stabilization, and in some cases can attach to cell junctions. For instance, a population of MT minus ends in Caco cells is anchored to cadherin-based AJs via the PLEKHA7/Nezha protein complex (Meng et al. 2008). PLEKHA7 was first identified as a p120-catenin interacting protein, and its binding partner designated Nezha was also found to bind the minus end of MTs, forming a link between AJ and MTs. This interaction is apparently indispensible for the integrity of AJs, implying a role for MT minus ends in the maintenance of cell junctions. Although it is not known whether MT minus ends participate in junction restructuring, it is conceivable that the connection of cell junctions via MT minus ends to another part of the cell may facilitate the rapid delivery of restructuring/disassembly signals directly to cell junctions. Thus, it will be of great interest to test the possibility that MTs participate in the maintenance or disassembly of the apical ES.
5. Future perspectives
A major challenge in studying the Sertoli cell cytoskeleton is the complex organization of actin, MT and IF in vivo, which can only be partially mimicked by in vitro cell culture systems. However, in vivo experiments are difficult to perform because of the lack of suitable models, and data obtained from such experiments can be difficult to interpret because of the cyclical nature of the seminiferous epithelium. When a long period is needed for treatments to take effect, the time lapse between the initial treatment and data collection may already encompass several stages in the seminiferous epithelial cycle. Therefore, small molecule inhibitors are attractive to reproductive biologists, which can be directly injected into the testis to produce rapid and local changes. This method has also been used to study actin and MT dynamics, such as the intratesticular administration of cytochalasin D (Russell et al. 1988). With the advent of large scale automatic screening, it is now considerably easier to identify chemical entities with defined effects (see table 2), such as the inhibition of N-WASP by wiskostatin (Peterson et al. 2004). Small molecule inhibitors can also be a valuable tool for functional studies of cytoskeletal dynamics in the testis in vivo, and in some cases may even surpass the use of conditional knockouts, because an instantaneous response may lower the possibility of a redundant gene/protein rescuing the knocked-down gene/protein. Furthermore, given the rapid cell division taking place during spermatogenesis, small molecules influencing MT dynamics (see table 2) can be exploited to develop novel contraceptives, arresting germ cell development by perturbing mitotic/meiotic spindles. While targeting of the cytoskeleton is successful in cancer chemotherapy and is still the centre of attention in anti-tumour drug development (Jordan & Wilson 2004), any detrimental side effects, even moderate, would not be acceptable in contraceptive development. To circumvent systemic effects, it is necessary to optimize novel drug delivery methods to the testis in the future, such as by topical administration and targeting testis-specific receptors.
Table 2.
Small molecules that affect actin and MT dynamics.
| molecule | compound type | effect | mechanism | typical dose in culture | references |
|---|---|---|---|---|---|
| F-actin destabilizing | |||||
| cytochalasin-D | other | inhibition of actin polymerization | F-actin barbed end binding | micromolar | Brenner & Korn (1979) |
| latrunculin-A | macrolide | inhibition of actin polymerization | altering actin monomer subunit interface | micromolar | Morton et al. (2000) |
| misakinolide-A | macrolide | inhibition of actin polymerization | F-actin barbed end binding | nanomolar | Terry et al. (1997) |
| swinholide-A | macrolide | actin depolymerization | F-actin severing | nanomolar | Saito et al. (1998) |
| mycalolide-B | macrolide | actin depolymerization | F-actin severing; G-actin sequestering | nanomolar-micromolar | Saito et al. (1994) |
| F-actin stabilizing | |||||
| phalloidin | bicyclic peptide | inhibition of actin depolymerization | reducing dissociation rate constant at F-actin ends | micromolar | Coluccio & Tilney (1984) |
| jasplakinolide | cyclic peptide | induction of actin polymerization | decreasing critical concentration for monomer addition | nanomolar | Bubb et al. (1994) |
| indirect effects on actin | |||||
| blebbistatin | other | inhibition of non-muscle myosin II | inhibiting phosphate release after ATP hydrolysis | micromolar | Kovacs et al. (2004) |
| wiskostatin | halogenated carbazole derivative | inhibition of N-WASP | stabilizing autoinhibited conformation | micromolar | Peterson et al. (2004) |
| MT destabilizing | |||||
| colchicine | vinca alkaloid | inhibition of MT growth (low conc.)/ MT depolymerization (high conc.) | inhibiting lateral contacts between colchicine bound tubulin in MT | micromolar | Ravelli et al. (2004) |
| carbendazim | benzimidazole | inhibition of MT growth | n.a. | micromolar | Davidse (1986) |
| MT stabilizing | |||||
| paclitaxel (taxol) | taxane | suppression of dynamic MT behaviour | MT stabilization upon binding to β-tubulin | nanomolar | Xiao et al. (2006) |
| 2,5-hexanedione | n-hexane | enhanced MT polymerization | increasing rate of tubulin nucleation | micromolar | Boekelheide (1987) |
| mixed effects on MT | |||||
| nocodazole | benzimidazole | MT depolymerization (micromolar)/ MT stabilization (nanomolar) | n.a. | nanomolar–micromolar | Jordan et al. (1992), Vasquez et al. (1997) |
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (NICHD, U54 HD029 990, Project 5 and R01 HD056 034 to CYC; R03 HD061 401 to DDM) and Hong Kong Research Grants Council (HKU 7693/07M to WML).
Footnotes
One contribution of 17 to a Theme Issue ‘The biology and regulation of spermatogenesis’.
References
- Akhmanova A., et al. 2005The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev. 19, 2501–2515 (doi:10.1101/gad.344505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard E. K., Johnson K. J., Boekelheide K.1993Colchicine disrupts the cytoskeleton of rat testis seminiferous epithelium in a stage-dependent manner. Biol. Reprod. 48, 143–153 (doi:10.1095/biolreprod48.1.143) [DOI] [PubMed] [Google Scholar]
- Amlani S., Vogl A. W.1988Changes in the distribution of microtubules and intermediate filaments in mammalian Sertoli cells during spermatogenesis. Anat. Rec. 220, 143–160 (doi:10.1002/ar.1092200206) [DOI] [PubMed] [Google Scholar]
- Arnold D. B.2009Actin and microtubule-based cytoskeletal cues direct polarized targeting of proteins in neurons. Sci. Signal 2, e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin C. W., Cheng C. Y., Musto N. A., Gunsalus G. L.1988The Sertoli cell. In The physiology of reproduction, vol. 1 (eds Knobil E., Neill J. D., Ewing L. L., Greenwald G. S., Markert C. L., Pfaff D. W.), pp. 933–974 New York, NY: Raven Press [Google Scholar]
- Bartles J. R., Wierda A., Zheng L.1996Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J. Cell Sci. 109, 1229–1239 [DOI] [PubMed] [Google Scholar]
- Bartolini F., Gundersen G. G.2006Generation of noncentrosomal microtubule arrays. J. Cell Sci. 119, 4155–4163 (doi:10.1242/jcs.03227) [DOI] [PubMed] [Google Scholar]
- Bartolini F., Gundersen G. G.In press Formins and microtubules. Biochim. Biophys. Acta (doi:10.1016/j.bbamcr.2009.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach S. F., Vogl A. W.1999Spermatid translocation in the rat seminiferous epithelium: Coupling membrane trafficking machinery to a junction plaque. Biol. Reprod. 60, 1036–1046 (doi:10.1095/biolreprod60.4.1036) [DOI] [PubMed] [Google Scholar]
- Beardsley A., Robertson D. M., O'Donnell L.2006A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J. Endocrinol. 190, 759–770 (doi:10.1677/joe.1.06867) [DOI] [PubMed] [Google Scholar]
- Boekelheide K.19872,5-hexanedione alters microtubule assembly. Toxicol. Appl. Pharmacol. 88, 383–396 (doi:10.1016/0041-008X(87)90213-4) [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D.1979Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. J. Biol. Chem. 254, 9982–9985 [PubMed] [Google Scholar]
- Broussard J. A., Webb D. J., Kaverina I.2008Asymmetric focal adhesion disassembly in motile cells. Curr. Opin. Cell Biol. 20, 85–90 (doi:10.1016/j.ceb.2007.10.009) [DOI] [PubMed] [Google Scholar]
- Bubb M. R., Senderowicz A. M. J., Sausville E. A., Duncan K. L. K., Korn E. D.1994Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269, 14 869–14 871 [PubMed] [Google Scholar]
- Burridge K., Wennerberg K.2004Rho and Rac take center stage. Cell 116, 167–179 (doi:10.1016/S0092-8674(04)00003-0) [DOI] [PubMed] [Google Scholar]
- Cavey M., Rauzi M., Lenne P., Lecuit T.2008A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453, 751–756 (doi:10.1038/nature06953) [DOI] [PubMed] [Google Scholar]
- Chang L., Goldman R. D.2004Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 5, 601–613 (doi:10.1038/nrm1438) [DOI] [PubMed] [Google Scholar]
- Chapin R. E., Wine R. N., Harris M. W., Borchers C. H., Haseman J. K.2001Structure and control of a cell–cell adhesion complex associated with spermiation in rat seminiferous epithelium. J. Androl. 22, 1030–1052 [DOI] [PubMed] [Google Scholar]
- Chen B., Li A., Wang D., Wang M., Zheng L., Bartles J. R.1999Espin contains an additional actin-binding site in its N terminus and is a major actin-bundling protein of the Sertoli cell–spermatid ectoplasmic specialization junctional plaque. Mol. Biol. Cell 10, 4327–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y., Mruk D. D., Silvestrini B., Bonanomi M., Wong C. H., Siu M. K. Y., Lee N. P. Y., Mo M. Y.2005AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception 72, 251–261 (doi:10.1016/j.contraception.2005.03.008) [DOI] [PubMed] [Google Scholar]
- Chhabra E. S., Higgs H. N.2007The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 9, 1110–1121 (doi:10.1038/ncb1007-1110) [DOI] [PubMed] [Google Scholar]
- Claessens M. M. A. E., Bathe M., Frey E., Bausch A. R.2006Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nat. Mater. 5, 748–753 (doi:10.1038/nmat1718) [DOI] [PubMed] [Google Scholar]
- Coluccio L. M., Tilney L. G.1984Phalloidin enhances actin assembly by preventing monomer dissociation. J. Cell Biol. 99, 529–535 (doi:10.1083/jcb.99.2.529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. R., Wordeman L.2009The diffusive interaction of microtubule binding proteins. Curr. Opin. Cell Biol. 21, 68–73 (doi:10.1016/j.ceb.2009.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa L. M., Nakai M., Strandgaard C. S., Hess R. A., Miller M. G.2002Microtubules of the mouse testis exhibit differential sensitivity to the microtubule disruptors carbendazim and colchicine. Toxicol. Sci. 69, 175–182 (doi:10.1093/toxsci/69.1.175) [DOI] [PubMed] [Google Scholar]
- D'Souza R., Pathak S., Upadhyay R., Gaonkar R., D'souza S., Sonawane S., Gill-Sharma M., Balasinor N. H.2009Disruption of tubulobulbar complex by high intratesticular estrogens leading to failed spermiation. Endocrinology 150, 1861–1869 (doi:10.1210/en.2008-1232) [DOI] [PubMed] [Google Scholar]
- Dammermann A., Desal A., Oegema K.2003The minus end in sight. Curr. Biol. 13, R614–R624 (doi:10.1016/S0960-9822(03)00530-X) [DOI] [PubMed] [Google Scholar]
- Davidse L. C.1986Benzimidazole fungicides: mechanism of action and biological impact. Annu. Rev. Phytopathol. 24, 43–65 (doi:10.1146/annurev.py.24.090186.000355) [Google Scholar]
- Desai A., Mitchison T. J.1997Microtubule polymerization dynamics. Annu. Rev. Cell. Dev. Biol. 13, 83–117 (doi:10.1146/annurev.cellbio.13.1.83) [DOI] [PubMed] [Google Scholar]
- Disanza A., et al. 2004Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat. Cell Biol. 6, 1180–1188 (doi:10.1038/ncb1199) [DOI] [PubMed] [Google Scholar]
- Disanza A., et al. 2006Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat. Cell Biol. 8, 1337–1347 (doi:10.1038/ncb1502) [DOI] [PubMed] [Google Scholar]
- Efimov A., Schiefermeier N., Grigoriev I., Ohi R., Brown M. C., Turner C. E., Small J. V., Kaverina I.2008Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. J. Cell Sci. 121, 196–204 (doi:10.1242/jcs.012666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S.2004Actin and microtubules in cell motility: which one is in control? Traffic 5, 470–477 (doi:10.1111/j.1600-0854.2004.00196.x) [DOI] [PubMed] [Google Scholar]
- Ezratty E. J., Partridge M. A., Gundersen G. G.2005Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581–590 (doi:10.1038/ncb1262) [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Suetsugu S., Miki H., Fukami K., Endo T., Takenawa T.2001A novel neural Wiskott–Aldrich syndrome protein (N-WASP) binding protein, WISH, induces Arp2/3 complex activation independent of Cdc42. J. Cell Biol. 152, 471–482 (doi:10.1083/jcb.152.3.471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Spatz J. P., Bershadsky A. D.2009Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 (doi:10.1038/nrm2593) [DOI] [PubMed] [Google Scholar]
- Grove B. D., Vogl A. W.1989Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J. Cell Sci. 93, 309–323 [DOI] [PubMed] [Google Scholar]
- Guttman J. A., Kimel G. H., Vogl A. W.2000Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations). J. Cell Sci. 113, 2167–2176 [DOI] [PubMed] [Google Scholar]
- Guttman J., Takai Y., Vogl A. W.2004aEvidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol. Reprod. 71, 548–559 (doi:10.1095/biolreprod.104.028803) [DOI] [PubMed] [Google Scholar]
- Guttman J. A., Obinata T., Shima J., Griswold M., Vogl A. W.2004bNon-muscle cofilin is a component of tubulobulbar complexes in the testis. Biol. Reprod. 70, 805–812 (doi:10.1095/biolreprod.103.022723) [DOI] [PubMed] [Google Scholar]
- Guttman J. A., Vaid K. S., Vogl A. W.2007A re-evaluation of gelsolin at ectoplasmic specializations in Sertoli cells: the influence of serum in blocking buffers on staining patterns. Anat. Rec. 290, 324–329 (doi:10.1002/ar.20441) [DOI] [PubMed] [Google Scholar]
- Hall E. S., Hall S. J., Boekelheide K.19952,5-Hexanedione exposure alters microtubule motor distribution in adult rat testis. Fundam. Appl. Toxicol. 24, 173–182 (doi:10.1006/faat.1995.1021) [DOI] [PubMed] [Google Scholar]
- Hara Y., Yamagata K., Oguchi K., Baba T.2008Nuclear localization of profilin III-ArpM1 complex in mouse spermiogenesis. FEBS Lett. 582, 2998–3004 (doi:10.1016/j.febslet.2008.07.058) [DOI] [PubMed] [Google Scholar]
- Hasson T., Walsh J., Cable J., Mooseker M. S., Brown S. D. M., Steel K. P.1997Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil. Cytoskeleton 37, 127–138 (doi:10.1002/(SICI)1097-0169(1997)37:2%3C127::AID-CM5%3E3.0.CO;2-5) [DOI] [PubMed] [Google Scholar]
- Heid H. W., Figge U., Winter S., Kuhn C., Zimbelmann R., Franke W. W.2002Novel actin-related proteins Arp-T1 and Arp-T2 as components of the cytoskeletal calyx of the mammalian sperm head. Exp. Cell Res. 279, 177–187 (doi:10.1006/excr.2002.5603) [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A.2005Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (doi:10.1146/annurev.cellbio.21.020604.150721) [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Wilson L.2004Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–265 (doi:10.1038/nrc1317) [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Thrower D., Wilson L.1992Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. J. Cell Sci. 102, 401–416 [DOI] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Small J. V.1999Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146, 1033–1043 (doi:10.1083/jcb.146.5.1033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera I. A., Su L., Bilinska B., Cheng C. Y., Mruk D. D.2009An in vivo study on adjudin and blood-testis barrier dynamics. Endocrinology 150, 4724–4733 (doi:10.1210/en.2008-1779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M., Toth J., Hetenyi C., Malnasi-Csizmadia A., Sellers J. R.2004Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279, 35 557–35 563 (doi:10.1074/jbc.M405319200) [DOI] [PubMed] [Google Scholar]
- Kussel-Andermann P., El-Amraoui A., Safieddine S., Nouaille S., Perfettini I., Lecuit M., Cossart P., Wolfrum U., Petit C.2000Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex. EMBO J. 19, 6020–6029 (doi:10.1093/emboj/19.22.6020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen G., et al. 2004Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J. Cell Biol. 166, 1003–1014 (doi:10.1083/jcb.200402082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. P. Y., Mruk D. D., Conway A. M., Cheng C. Y.2004Zyxin, axin and Wiskott–Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J. Androl. 25, 200–215 [DOI] [PubMed] [Google Scholar]
- Li R., Gundersen G. G.2008Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat. Rev. Mol. Cell Biol. 9, 860–873 (doi:10.1038/nrm2522) [DOI] [PubMed] [Google Scholar]
- Lie P. P. Y., Xia W., Wang C. Q. F., Mruk D. D., Yan H. H. N., Wong C., Lee W. M., Cheng C. Y.2006Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood–testis barrier in adult rat testes. J. Endocrinol. 191, 571–586 (doi:10.1677/joe.1.06996) [DOI] [PubMed] [Google Scholar]
- Lie P. P. Y., Mruk D. D., Lee W. M., Cheng C. Y.2009Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood–testis barrier integrity in the seminiferous epithelium. FASEB J. 23, 2555–2567 (doi:10.1096/fj.06-070573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui W., Lee W. M., Cheng C. Y.2003aRho GTPases and spermatogenesis. Biochim. Biophys. Acta 1593, 121–129 [DOI] [PubMed] [Google Scholar]
- Lui W., Lee W. M., Cheng C. Y.2003bSertoli–germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol. Reprod. 68, 2189–2206 (doi:10.1095/biolreprod.102.011379) [DOI] [PubMed] [Google Scholar]
- Mege R., Gavard J., Lambert M.2006Regulation of cell–cell junctions by the cytoskeleton. Curr. Opin. Cell Biol. 18, 541–548 (doi:10.1016/j.ceb.2006.08.004) [DOI] [PubMed] [Google Scholar]
- Meng W., Mushika Y., Ichii T., Takeichi M.2008Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell–cell contacts. Cell 135, 948–959 (doi:10.1016/j.cell.2008.09.040) [DOI] [PubMed] [Google Scholar]
- Mironova E., Millette C. F.2008Expression of the diaphanous-related formin proteins mDia1 and mDia2 in the rat testis. Dev. Dyn. 237, 2170–2176 (doi:10.1002/dvdy.21622) [DOI] [PubMed] [Google Scholar]
- Morton W. M., Ayscough K. R., Mclaughlin P. J.2000Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2, 376–378 [DOI] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y.2004aCell–cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol. Metab. 15, 439–447 [DOI] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y.2004bSertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 (doi:10.1210/er.2003-0022) [DOI] [PubMed] [Google Scholar]
- Obermann H., Raabe I., Balvers M., Brunswig B., Schulze W., Kirchhoff C.2004Novel testis-expressed profilin IV associated with acrosome biogenesis and spermatid elongation. Mol. Hum. Reprod. 11, 53–64 (doi:10.1093/molehr/gah132) [DOI] [PubMed] [Google Scholar]
- Pellegrin S., Mellor H.2007Actin stress fibres. J. Cell Sci. 120, 3491–3499 (doi:10.1242/jcs.018473) [DOI] [PubMed] [Google Scholar]
- Peterson J. R., Bickford L. C., Morgan D., Kim A. S., Ouerfelli O., Kirschner M. W., Rosen M. K.2004Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat. Struct. Mol. Biol. 11, 747–755 (doi:10.1038/nsmb796) [DOI] [PubMed] [Google Scholar]
- Ravelli R. B. G., Gigant B., Curmi P. A., Jourdain I., Lachkar S., Sobel A., Knossow M.2004Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428, 198–202 (doi:10.1038/nature02393) [DOI] [PubMed] [Google Scholar]
- Rawe V. Y., Ramalho-Santos J., Payne C., Chemes H. E., Schatten G.2004WAVE1, an A-kinase anchoring protein, during mammalian spermatogenesis. Hum. Reprod. 19, 2594–2604 (doi:10.1093/humrep/deh513) [DOI] [PubMed] [Google Scholar]
- Redenbach D. M., Vogl A. W.1991Microtubule polarity in Sertoli cells: a model for microtubule-based spermatid transport. Eur. J. Cell Biol. 54, 277–290 [PubMed] [Google Scholar]
- Revenu C., Athman R., Robine S., Louvard D.2004The co-workers of actin filaments: from cell structures to signals. Nat. Rev. Mol. Cell Biol. 5, 1–12 [DOI] [PubMed] [Google Scholar]
- Rodal A. A., Sokolova O., Robins D. B., Daugherty K. M., Hippenmeyer S., Riezman H., Grigorieff N., Goode B. L.2005Conformational changes in the Arp2/3 complex leading to actin nucleation. Nat. Struct. Mol. Biol. 12, 26–31 (doi:10.1038/nsmb870) [DOI] [PubMed] [Google Scholar]
- Russell L. D.1979Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cell in rat testis. Anat. Rec. 194, 213–232 (doi:10.1002/ar.1091940204) [DOI] [PubMed] [Google Scholar]
- Russell L. D., Goh J. C., Rashed R. M., Vogl A. W.1988The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol. Reprod. 39, 105–118 (doi:10.1095/biolreprod39.1.105) [DOI] [PubMed] [Google Scholar]
- Russell L. D., Saxena N. K., Turner T. T.1989Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell 21, 361–379 (doi:10.1016/0040-8166(89)90051-7) [DOI] [PubMed] [Google Scholar]
- Saito S., Watabe S., Ozaki H., Fusetani N., Karaki H.1994Mycalolide B, a novel actin depolymerizing agent. J. Biol. Chem. 269, 29 710–29 714 [PubMed] [Google Scholar]
- Saito S., Watabe S., Ozaki H., Kobayashi M., Suzuki T., Kobayashi H., Fusetani N., Karaki H.1998Actin-depolymerizing effect of dimeric macrolides, bistheonellide A and swinholide A. J. Biochem. 123, 571–578 [DOI] [PubMed] [Google Scholar]
- Scita G., et al. 2001An effector region in Eps8 is responsible for the activation of the Rac-specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J. Cell Biol. 154, 1031–1044 (doi:10.1083/jcb.200103146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G., Zheng L., Loomis P. A., Mugnaini E., Bartles J. R.2006aEspins and the actin cytoskeleton of hair cell stereocilia and sensory cell microvilli. Cell Mol. Life Sci. 63, 2329–2341 (doi:10.1007/s00018-006-6148-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G., Zheng L., Mugnaini E., Bartles J. R.2006bDifferential expression of espin isoforms during epithelial morphogenesis, stereociliogenesis and postnatal maturation in the developing inner ear. Dev. Biol. 291, 83–95 (doi:10.1016/j.ydbio.2005.12.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu M. K. Y., Mruk D. D., Lee W. M., Cheng C. Y.2003Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex-associated proteins. Endocrinology 144, 2141–2163 (doi:10.1210/en.2002-221035) [DOI] [PubMed] [Google Scholar]
- Sossey-Alaoui K., Su G., Malaj E., Roe B., Cowell J. K.2002WAVE3, an actin-polymerization gene, is truncated and inactivated as a result of a constitutional t(1;13)(q21;q12) chromosome translocation in a patient with ganglioneuroblastoma. Oncogene 21, 5967–5974 (doi:10.1038/sj.onc.1205734) [DOI] [PubMed] [Google Scholar]
- Takahashi H., Koshimizu U., Miyazaki J., Nakamura T.2002Impaired spermatogenic ability of testicular germ cells in mice deficient in the LIM-kinase 2 gene. Dev. Biol. 241, 259–272 (doi:10.1006/dbio.2001.0512) [DOI] [PubMed] [Google Scholar]
- Tanabe K., Takei K.2009Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J. Cell Biol. 185, 939–948 (doi:10.1083/jcb.200803153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Iguchi N., De Carvalho C. E., Tadokoro Y., Yomogida K., Nishimune Y.2003Novel actin-like proteins T-actin 1 and T-actin 2 are differentially expressed in the cytoplasm and nucleus of mouse haploid germ cells. Biol. Reprod. 69, 475–482 (doi:10.1095/biolreprod.103.015867) [DOI] [PubMed] [Google Scholar]
- Terry D. R., Spector I., Higa T., Bubb M. R.1997Misakinolide A is a marine macrolide that caps but does not sever filamentous actin. J. Biol. Chem. 272, 7841–7845 [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Vranich K. A., Shaw M. K., Guild G. M.1998Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J. Cell Biol. 143, 121–133 (doi:10.1083/jcb.143.1.121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J., Toshima J. Y., Takeuchi K., Mori R., Mizuno K.2001Cofilin phosphorylation and actin reorganization activites of testicular protein kinase 2 and its predominant expression in testicular Sertoli cells. J. Biol. Chem. 276, 31 449–31 458 (doi:10.1074/jbc.M102988200) [DOI] [PubMed] [Google Scholar]
- Tubb B., Mulholland D. J., Vogl A. W., Lan Z., Niederberger C., Cooney A., Bryan J.2002Testis fascin (FSCN3): a novel paralog of the actin-bundling protein fascin expressed specifically in the elongate spermatid head. Exp. Cell Res. 275, 92–109 (doi:10.1006/excr.2002.5486) [DOI] [PubMed] [Google Scholar]
- Vaid K. S., Guttman J. A., Babyak N., Deng W., McNiven M. A., Mochizuki N., Finlay B. B., Vogl A. W.2007aThe role of dynamin 3 in the testis. J. Cell. Physiol. 210, 644–654 (doi:10.1002/jcp.20855) [DOI] [PubMed] [Google Scholar]
- Vaid K. S., Guttman J. A., Singaraja R. R., Vogl A. W.2007bA kinesin is present at unique Sertoli/spermatid adherens junctions in rat and mouse testes. Biol. Reprod. 77, 1037–1048 (doi:10.1095/biolreprod.107.063735) [DOI] [PubMed] [Google Scholar]
- Vasquez R. J., Howell B., Yvon A. C., Wadsworth P., Cassimeris L.1997Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 8, 973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velichkova M., Guttman J. A., Warren C., Eng L., Kline K., Vogl A. W., Hasson T.2002A human homologue of Drosophila kelch associates with myosin-VIIa in specialized adhesion junctions. Cell Motil. Cytoskeleton 51, 147–164 (doi:10.1002/cm.10025) [DOI] [PubMed] [Google Scholar]
- Vogl A. W., Soucy L. J.1985Arrangement and possible function of actin filament bundles in ectoplasmic specializations of ground squirrel Sertoli cells. J. Cell Biol. 100, 814–825 (doi:10.1083/jcb.100.3.814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl A. W., Pfeiffer D. C., Mulholland D., Kimel G., Guttman J.2000Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch. Histol. Cytol. 63, 1–15 (doi:10.1679/aohc.63.1) [DOI] [PubMed] [Google Scholar]
- Vogl A. W., Vaid K. S., Guttman J.2008The Sertoli cell cytoskeleton. In Molecular mechanisms in spermatogenesis. (ed. Cheng C. Y.), pp. 186–211 Austin, TX: Landes Bioscience/Springer Science + Business Media LLC [Google Scholar]
- Wang F., Zhang Q., Cao J., Huang Q., Zhu X.2008The microtubule plus end-binding protein EB1 is involved in Sertoli cell plasticity in testicular seminiferous tubules. Exp. Cell Res. 314, 213–226 (doi:10.1016/j.yexcr.2007.09.022) [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C., Duey D. Y., Weber K. L., Keech J., Cheney R. E., Salmon E. D., Bement W. M.2000Microtubules remodel actomyosin networks in Xenopus egg extracts via two mechanisms of F-actin transport. J. Cell Biol. 150, 361–376 (doi:10.1083/jcb.150.2.361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A. M., Young M. E., Lee W., Cooper J. A.2003Integration of signals to the Arp2/3 complex. Curr. Opin. Cell Biol. 15, 23–30 (doi:10.1016/S0955-0674(02)00015-7) [DOI] [PubMed] [Google Scholar]
- Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F.2004FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6, 154–161 (doi:10.1038/ncb1094) [DOI] [PubMed] [Google Scholar]
- Wu X., Xiang X., Hammer J. A., III2006Motor proteins at the microtubule plus-end. Trends Cell Biol. 16, 135–143 (doi:10.1016/j.tcb.2006.01.004) [DOI] [PubMed] [Google Scholar]
- Xia W., Wong E. W. P., Mruk D. D., Cheng C. Y.2009TGF-β3 and TNFα perturb blood–testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev. Biol. 327, 48–61 (doi:10.1016/j.ydbio.2008.11.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Verdier-Pinard P., Fernandez-Fuentes N., Burd B., Angeletti R., Fiser A., Horwitz S. B., Orr G. A.2006Insights into the mechanism of microtubule stabilization by taxol. Proc. Natl. Acad. Sci. USA 103, 10 166–10 173 (doi:10.1073/pnas.0603704103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H. N., Cheng C. Y.2006Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J. Biol. Chem. 281, 17 286–17 303 (doi:10.1074/jbc.M513218200) [DOI] [PubMed] [Google Scholar]
- Yan H. H. N., Mruk D. D., Lee W. M., Cheng C. Y.2006Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays 29, 36–48 (doi:10.1002/bies.20513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H. N., Mruk D. D., Lee W. M., Cheng C. Y.2008Blood–testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 22, 1945–1959 (doi:10.1096/fj.06-070342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazama F., Sawada H., Hirosawa K., Hayashi Y., Nishida T.1991Deep-etch visualization of the Sertoli cell (blood–testis) barrier in the boar. Tissue Cell 23, 235–246 (doi:10.1016/0040-8166(91)90078-8) [DOI] [PubMed] [Google Scholar]
- Young J. S., Guttman J. A., Vaid K. S., Shahinian H., Vogl A. W.2009Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol. Reprod. 80, 153–161 (doi:10.1095/biolreprod.108.070615) [DOI] [PubMed] [Google Scholar]