Abstract
The physiological function of spermatogenesis in Caenorhabditis elegans, Drosophila melanogaster and mammals is to produce spermatozoa (1n, haploid) that contain only half of the genetic material of spermatogonia (2n, diploid). This half number of chromosomes from a spermatozoon will then be reconstituted to become a diploid cell upon fertilization with an egg, which is also haploid. Thus, genetic information from two parental individuals can be passed onto their offspring. Spermatogenesis takes place in the seminiferous epithelium of the seminiferous tubule, the functional unit of the mammalian testis. In mammals, particularly in rodents, the fascinating morphological changes that occur during spermatogenesis involving cellular differentiation and transformation, mitosis, meiosis, germ cell movement, spermiogenesis and spermiation have been well documented from the 1950s through the 1980s. During this time, however, the regulation of, as well as the biochemical and molecular mechanisms underlying these diverse cellular events occurring throughout spermatogenesis, have remained largely unexplored. In the past two decades, important advancements have been made using new biochemical, cell and molecular biology techniques to understand how different genes, proteins and signalling pathways regulate various aspects of spermatogenesis. These include studies on the differentiation of spermatogonia from gonocytes; regulation of spermatogonial stem cells; regulation of spermatogonial mitosis; regulation of meiosis, spermiogenesis and spermiation; role of hormones (e.g. oestrogens, androgens) in spermatogenesis; transcriptional regulation of spermatogenesis; regulation of apoptosis; cell–cell interactions; and the biology of junction dynamics during spermatogenesis. The impact of environmental toxicants on spermatogenesis has also become an urgent issue in the field in light of declining fertility levels in males. Many of these studies have helped investigators to understand important similarities, differences and evolutionary relationships between C. elegans, D. melanogaster and mammals relating to spermatogenesis. In this Special Issue of the Philosophical Transactions of the Royal Society B: Biological Sciences, we have covered many of these areas, and in this Introduction, we highlight the topic of spermatogenesis by examining its past, present and future.
Keywords: spermatogenesis, seminiferous, mitosis, meiosis, differentiation
1. Spermatogenesis: the past
Perhaps, two of the most important discoveries in the past century in the field of spermatogenesis were the identification of the seminiferous epithelial cycle in mammals (LeBlond & Clermont 1952; Clermont 1972; De Kretser & Kerr 1988; Clermont et al. 1993), as well as the hypothalamic–pituitary–testicular axis that regulates spermatogenesis (Sharpe 1994). Without a doubt, these two findings have helped investigators to understand different cellular events that occur during spermatogenesis and to identify critical regulatory biomolecules and/or pathways with the use of staged seminiferous tubules isolated by transillumination stereomicroscopy (Parvinen 1982). Indeed, a recent study using this 6-decade-old technique has helped investigators to identify different sets of genes that can potentially regulate different cellular events during spermatogenesis (Johnston et al. 2008). On the other hand, this technique, powerful as it is, still has its inherent limitations. For instance, at stage VIII of the seminiferous epithelial cycle, spermiation and blood–testis barrier (BTB) restructuring are known to take place simultaneously (LeBlond & Clermont 1952; Hess & de Franca 2008). Thus, stage VIII tubules isolated from the testis would be useful to identify genes and/or proteins that regulate these two cellular events. It is worth noting, however, that other important cellular events also occur in the seminiferous epithelium at stage VIII that are unrelated to spermiation and BTB restructuring. These include germ cell cycle progression associated with the differentiation of preleptotene to leptotene spermatocytes, preparation of genetic material in pachytene spermatocytes for differentiation into diplotene spermatocytes at stage XIII and the eventual transformation of step 8 spermatids into step 9 spermatids. This is in addition to the occurrence of other Sertoli–Sertoli and Sertoli–germ cell interactions, as well as other paracrine and autocrine events (De Kretser & Kerr 1988; De Kretser 1990).
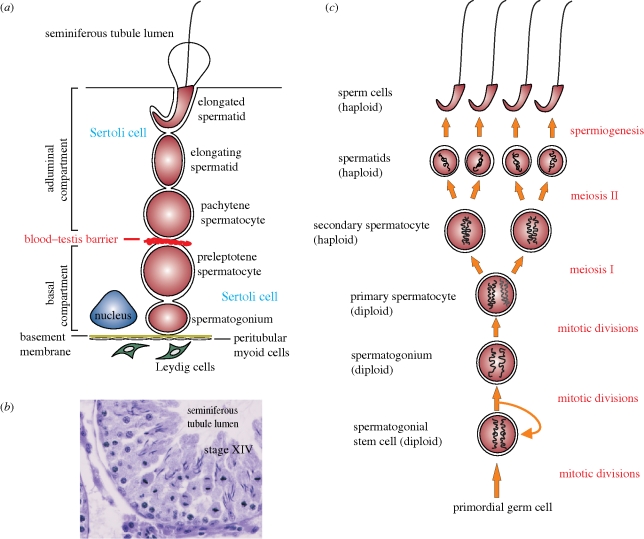
Figure 1 depicts key cellular events occurring during spermatogenesis in the seminiferous epithelium of the rat testis. Figure 1a is a schematic drawing illustrating the relative location of germ cells at different stages of their development where Leydig cells, under the influence of LH, undergo steroidogenesis to produce testosterone and oestradiol-17β that play a critical role in the regulation of spermatogenesis. The BTB situated near the basement membrane segregates the epithelium into a basal and an apical (adluminal) compartment. As such, meiosis which occurs at stage XIV of the seminiferous epithelial cycle in the rat (figure 1b), together with the subsequent steps of post-meiotic germ cell development, are all sequestered from the systemic circulation, that is, they take place in a specialized microenvironment behind the BTB known as the adluminal compartment (figure 1a). Figure 1c depicts some of the key cellular events of spermatogenesis in rats, and these events are also applicable to other species including Caenorhabditis elegans and Drosophila melanogaster.
Figure 1.
The biology of spermatogenesis in the rat. (a) Schematic drawing of the seminiferous epithelium from a seminiferous tubule in the adult rat testis, illustrating the morphological features of different germ cells during development and their intimate relationship with the Sertoli cell. Leydig cells that produce testosterone and oestradiol-17β via steroidogenesis are restricted to the interstitium. The blood–testis barrier (BTB) comprising tight junctions, basal ectoplasmic specializations and desmosome-gap junctions physically divides the seminiferous epithelium into a basal and an apical (adluminal) compartment. (b) Cross-section of a stage XIV tubule from an adult rat testis during which time meiosis occurs. Several newly formed spermatids from secondary spermatocytes at anaphase are clearly visible in this stage. (c) Drawing depicting the process that occurs in male germ cells during spermatogenesis.
2. Spermatogenesis: the present
In this Special Issue, we have compiled several articles with the aim of providing up-to-date reviews in the field, especially in light of recent technological advancements that have helped us to better understand spermatogenesis. These include the use of proteomics and genomics (Calvel et al. 2010) and mouse genetic models (Verhoeven et al. 2010) to examine different aspects of spermatogenesis. There are also other facets of spermatogenesis that have gained momentum in recent years such as the evolution of spermatogenesis in C. elegans, D. melanogaster and mammals (White-Cooper & Bausek 2010); regulation of apoptosis (Shaha et al. 2010); roles of oestrogens (Carreau et al. 2010; Carreau & Hess 2010), aromatase (Carreau et al. 2010) and androgens (Verhoeven et al. 2010; Walker 2010) in spermatogenesis; non-genomic action of testosterone and its impact on spermatogenesis (Walker 2010); regulation of mitosis and meiosis during spermatogenesis (Wolgemuth & Roberts 2010); impact of environmental toxicants and lifestyle effects on spermatogenesis (Sharpe 2010); spermatogonial stem cell biology (Phillips et al. 2010); transcriptional regulation of spermatogenesis (Bettegowda & Wilkinson 2010); and the roles of actin (Lie et al. 2010), gap junctions (Pointis et al. 2010), tight junctions (Morrow et al. 2010; Mruk & Cheng 2010) and anchoring junctions (Kopera et al. 2010; Mruk & Cheng 2010) in spermatogenesis.
As depicted in figure 1c which summarizes different cellular events that occur during spermatogenesis, it is obvious that some of the events listed on the right panel, such as spermiogenesis and its related cellular processes (e.g. spermatid adhesion to Sertoli cells) can become prime targets to disrupt spermatogenesis, leading to male infertility. Indeed, in recent years there has been an interest to develop new approaches for male contraception in order to provide additional means to reduce overpopulation and/or control population growth, particularly in developing countries, so that limited global resources in the globe can sustain the world's population. Herein, we wish to use a recent example to illustrate the importance of studying spermatogenesis, which can lead to exciting developments in a related field, such as male contraception. In the 1970s, there was an interest to develop male contraceptive drugs based on the core structure of indazole-3-carboxylic acid, such as lonidamine [1-(2,4-dichlorobenzyl)-indazole-3-carboxylic acid] (Palazzo et al. 1966; Corsi & Palazzo 1976). In rodents, lonidamine and its analogues were shown to be potent compounds that induced spermatid loss from the seminiferous epithelium without affecting cell adhesion in other organs (Silvestrini 1981; Silvestrini et al. 1984). In the late 1980s, after much experimentation, we noticed that this class of compounds primarily affected a unique ultrastructure in the seminiferous epithelium known as the apical ectoplasmic specialization (apical ES), a testis-specific atypical adherens junction (AJ) type (Russell & Peterson 1985; Cheng & Mruk 2002; Mruk & Cheng 2008; Vogl et al. 2008; Wong et al. 2008; Wong & Cheng 2009). However, lonidamine was subsequently shown to be toxic for use as a male contraceptive. Instead, it was developed into an anti-cancer drug whose mechanism of action is to inhibit hexokinase activity during mitochondrial respiration in cancer cells that were first sensitized with X-irradiation, which caused mitochondria to condense (note: condensed mitochondria are also present abundantly in elongating/elongated spermatids) (Silvestrini 1981; Silvestrini et al. 1984). We subsequently screened several dozen new analogues of lonidamine by investigating whether these compounds could induce the steady-state mRNA and/or protein level of testin, a protein localizing to the apical ES whose expression is tightly coupled to the integrity of this cell junction (Cheng & Bardin 1987; Cheng et al. 1989; Grima et al. 1997, 1998). Based on a series of studies that spanned almost 10 years, we selected adjudin (formerly called AF-2364) [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] as our candidate compound, which was shown to induce testin expression by as much as 10- to 50-fold (Cheng et al. 2001), and this finding of testin induction by adjudin was subsequently confirmed in a gene profiling study (Xia et al. 2007). Our goal was to identify a new compound that would specifically affect Sertoli–germ cell adhesion, thereby defoliating developing spermatids from the epithelium (Cheng et al. 2001). Indeed, in a study using 3[H]-adjudin to assess its tissue distribution, this drug distributed evenly among all organs in the adult rat. While 3[H]-adjudin was not specifically uptaken by the testis, it only perturbed cell adhesion in the testis, particularly at the apical ES, and induced premature release of elongating/elongated spermatids from the seminiferous epithelium (Chen et al. 2003; Cheng et al. 2005). These findings were subsequently confirmed by licensed toxicologists in different toxicity studies (Mruk et al. 2006), as well as in recent studies from other laboratories (Tash et al. 2008a; Sarkar & Mathur 2009). In essence, adjudin is the hydrazide form of lonidamine. The similarity between adjudin and lonidamine is somewhat analogous to aspirin and its natural compound, salicyclic acid. Salicyclic acid, the active ingredient in extracts of willow bark or leaves is highly toxic; but aspirin, the acetylated form of salicyclic acid which was prepared by Felix Hoffman at the Bayer company in Germany in 1897 and became one of the most widely used non-steroid anti-inflammatory drugs, is not toxic (Vane & Botting 2003). In recent years, another analogue of indazole-carboxylic acid, known as gamendazole, has also emerged as a candidate for male contraception (Tash et al. 2008a,b). Gamendazole is significantly more potent than adjudin because it has an additional trifluoro group in the indazole ring (Tash et al. 2008a), thus making it more biologically active versus lonidamine, the free acid derivative of indazole-carboxylic acid and adjudin, the hydrazide derivative of lonidamine (Silvestrini et al. 1984; Cheng et al. 2001). While this trifluoro group increases gamendazole's potency by as much as approximately two- to eightfold (6–25 mg kg−1 b.w. for gamendazole versus 37.5–50 mg kg−1 b.w. for adjudin in order for these compounds to effectively deplete germ cells from the seminiferous epithelium in virtually all seminiferous tubules in the testis) (Cheng et al. 2001, 2005; Tash et al. 2008a), mortality (i.e. three out of five deaths representing a 60% mortality rate) was observed in rats treated with gamendazole at 200 mg kg−1 b.w. but not with adjudin in the same study (Tash et al. 2008a). In the study on adjudin, two acute toxicity studies performed by licensed toxicologists using mice (n = 3) and rats (n = 10) at doses of up to 1000 and 2000 mg kg−1 b.w., respectively, reported that there were no fatalities in both studies (Mruk et al. 2006). Additionally, a subchronic toxicity study performed by licensed toxicologists at 50 mg kg−1 b.w. for 29 days in male (n = 10) and female (n = 10) rats also reported no mortality (Mruk et al. 2006). These findings also illustrate that much research is needed to correlate results based on structure–activity studies in order to identify the best candidate compounds for human use. It is our belief that different formulations of either one of these two compounds will emerge as a drug that can induce reversible fertility in humans. At the very least, these examples illustrate that there are exciting developments in the field regarding the intervention of spermatogenesis that can induce reversible male infertility. However, it must be noted that none of these studies could have been performed without some understanding of the biology of adhesive junctions in the seminiferous epithelium, as this knowledge forms the basis of our understanding of spermatogenesis as depicted in figure 1.
Moreover, other important studies relating to spermatogenesis, such as the biology of spermatogonial stem cells (Kanatsu-Shinohara et al. 2008), have significantly impacted other studies in the field, such as the possible use of spermatogonial stem cells for therapeutic applications (i.e. producing non-reproductive tissues for transplantation applications) (Simon et al. 2009). Some of these areas of research are discussed in this Special Issue.
3. Spermatogenesis: the future
As briefly outlined above, there are many areas that require additional research. For instance, while we have acquired a great deal of knowledge about the transcriptional regulation of spermatogenesis during the past decade, only a few genes (or gene sets) are known to regulate spermatogenesis at the transcriptional level. Moreover, how these genes interact with each other to regulate diverse cellular events during spermatogenesis such as mitosis, meiosis and spermiogenesis remains largely unknown. On the other hand, while the mechanisms that regulate the BTB, which is constituted by coexisting tight junctions, desmosome-gap junctions and basal ectoplasmic specializations between adjacent Sertoli cells, are becoming clearer, there are virtually no functional studies in the literature which attempt to understand the biology of drug transport across this barrier. Without this additional information, it is difficult to develop new contraceptives and/or therapeutic drugs that can target the seminiferous epithelium behind the BTB in order to exert their effects on developing germ cells and/or germ cell tumours, respectively. However, we remain hopeful that significant advances will continue to be made in the next decade.
Footnotes
One contribution of 17 to a Theme Issue ‘The biology and regulation of spermatogenesis’.
References
- Bettegowda A., Wilkinson M. F.2010Transcription and post-transcriptional regulation of spermatogenesis. Phil. Trans. R. Soc. B 365, 1637–1651 (doi:10.1098/rstb.2009.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvel P., Rolland A., Jégou B., Pineau C.2010Testicular postgenomics: targeting the regulation of spermatogenesis. Phil. Trans. R. Soc. B 365, 1481–1500 (doi:10.1098/rstb.2009.0294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S., Hess R.2010Oestrogens and spermatogenesis. Phil. Trans. R. Soc. B 365, 1517–1535 (doi:10.1098/rstb.2009.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S., Wolczynski S., Galeraud-Denis I.2010Aromatase, oestrogens and human male reproduction. Phil. Trans. R. Soc. B 365, 1571–1579 (doi:10.1098/rstb.2009.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M., Lee N. P. Y., Mruk D. D., Lee W. M., Cheng C. Y.2003Fer kinase/Fer T and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol. Reprod. 69, 656–672 (doi:10.1095/biolreprod.103.016881) [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Bardin C. W.1987Identification of two testosterone-responsive testicular proteins in Sertoli cell-enriched culture medium whose secretion is suppressed by cells of the intact seminiferous tubule. J. Biol. Chem. 262, 12 768–12 779 [PubMed] [Google Scholar]
- Cheng C. Y., Mruk D. D.2002Cell junction dynamics in the testis: Sertoli–germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874 (doi:10.1152/physrev.00009.2002) [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Grima J., Stahler M. S., Lockshin R. A., Bardin C. W.1989Testins are two structurally related Sertoli cell proteins whose secretion is tightly coupled to the presence of germ cells. J. Biol. Chem. 264, 21 386–21 393 [PubMed] [Google Scholar]
- Cheng C. Y., et al. 2001Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol. Reprod. 65, 449–461 (doi:10.1095/biolreprod65.2.449) [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Mruk D. D., Silvestrini B., Bonanomi M., Wong C. H., Siu M. K. Y., Lee N. P. Y., Mo M. Y.2005AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception 72, 251–261 (doi:10.1016/j.contraception.2005.03.008) [DOI] [PubMed] [Google Scholar]
- Clermont Y.1972Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 52, 198–235 [DOI] [PubMed] [Google Scholar]
- Clermont Y., Oko R., Hermo L.1993Cell biology of mammalian spermatogenesis. In Cell and molecular biology of the testis (eds Desjardins C., Ewing L.). New York, NY: Oxford University Press [Google Scholar]
- Corsi G., Palazzo G.19761-Halobenzyl-1H-indazole-3-carboxylic acids. A new class of antispermatogenic agents. J. Med. Chem. 19, 778–783 (doi:10.1021/jm00228a008) [DOI] [PubMed] [Google Scholar]
- De Kretser D.1990Germ cell–Sertoli cell interactions. Reprod. Fertil. Dev. 2, 225–235 (doi:10.1071/RD9900225) [DOI] [PubMed] [Google Scholar]
- De Kretser D., Kerr J.1988The cytology of the testis. In The physiology of reproduction (eds Knobil E., Neill J., Ewing L., Greenwald G., Markert C., Pfaff D.). New York, NY: Raven Press [Google Scholar]
- Grima J., Zhu L., Cheng C. Y.1997Testin is tightly associated with testicular cell membrane upon its secretion by Sertoli cells whose steady-state mRNA level in the testis correlates with the turnover and integrity of inter-testicular cell junctions. J. Biol. Chem. 272, 6499–6509 (doi:10.1074/jbc.272.10.6499) [DOI] [PubMed] [Google Scholar]
- Grima J., Wong C. C., Zhu L. J., Zong S. D., Cheng C. Y.1998Testin secreted by Sertoli cells is associated with the cell surface, and its expression correlates with the disruption of Sertoli–germ cell junctions but not the inter-Sertoli tight junction. J. Biol. Chem. 273, 21 040–21 053 (doi:10.1074/jbc.273.33.21040) [DOI] [PubMed] [Google Scholar]
- Hess R., de Franca L.2008Spermatogenesis and cycle of the seminiferous epithelium. In Molecular mechanisms in spermatogenesis (ed. Cheng C. Y.), pp. 1–15 Austin, TX: Landes Bioscience/Springer Science [Google Scholar]
- Johnston D. S., Wright W. W., Dicandeloro P., Wilson E., Kopf G. S., Jelinsky S. A.2008Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc. Natl Acad. Sci. USA 105, 8315–8320 (doi:10.1073/pnas.0709854105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Takehashi M., Shinohara T.2008Brief history, pitfalls, and prospects of mammalian spermatogonial stem cell research. Cold Spring Harb. Symp. Quant. Biol. 73, 17–23 [DOI] [PubMed] [Google Scholar]
- Kopera I. A., Bilinska B., Cheng C. Y., Mruk D.2010Sertoli–germ cell junctions in the testis: a review of recent data. Phil. Trans. R. Soc. B 365, 1593–1605 (doi:10.1098/rstb.2009.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlond C., Clermont Y.1952Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. NY Acad. Sci. 55, 548–573 (doi:10.1111/j.1749-6632.1952.tb26576.x) [DOI] [PubMed] [Google Scholar]
- Lie P. P. Y., Mruk D. D., Lee W. M., Cheng C. Y.2010Cytoskeletal dynamics and spermatogenesis. Phil. Trans. R. Soc. B 365, 1581–1592 (doi:10.1098/rstb.2009.0261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. M. K., Mruk D., Cheng C. Y., Hess R. A.2010Claudin and occludin expression and function in the seminiferous epithelium. Phil. Trans. R. Soc. B 365, 1679–1696 (doi:10.1098/rstb.2010.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y.2008Anchoring junctions as drug targets: role in contraceptive development. Pharmacol. Rev. 60, 146–180 (doi:10.1124/pr.107.07105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y.2010Tight junctions in the testis: new perspectives. Phil. Trans. R. Soc. B 365, 1621–1635 (doi:10.1098/rstb.2010.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D. D., Wong C. H., Silvestrini B., Cheng C. Y.2006A male contraceptive targeting germ cell adhesion. Nat. Med. 12, 1323–1328 (doi:10.1038/nm1420) [DOI] [PubMed] [Google Scholar]
- Palazzo G., Corsi G., Baiocchi L., Silvestrini B.1966Synthesis and pharmacological properties of 1-substituted 3-dimethylaminoalkoxy-1H-indazoles. J. Med. Chem. 9, 38–41 (doi:10.1021/jm00319a009) [DOI] [PubMed] [Google Scholar]
- Parvinen M.1982Regulation of the seminiferous epithelium. Endocr. Rev. 3, 404–417 (doi:10.1210/edrv-3-4-404) [DOI] [PubMed] [Google Scholar]
- Phillips B. T., Gassei K., Orwig K. E.2010Spermatogonial stem cell regulation and spermatogenesis. Phil. Trans. R. Soc. B 365, 1663–1678 (doi:10.1098/rstb.2010.0026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointis G., Gilleron J., Carette D., Segretain D.2010Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Phil. Trans. R. Soc. B 365, 1607–1620 (doi:10.1098/rstb.2009.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L., Peterson R.1985Sertoli cell junctions: morphological and functional correlates. Int. Rev. Cytol. 94, 177–211 (doi:10.1016/S0074-7696(08)60397-6) [DOI] [PubMed] [Google Scholar]
- Sarkar O., Mathur P. P.2009Adjudin-mediated germ cell depletion alters the anti-oxidant status of adult rat testis. Mol. Reprod. Dev. 76, 31–37 (doi:10.1002/mrd.20928) [DOI] [PubMed] [Google Scholar]
- Shaha C., Tripathi R., Mishra D.2010Male germ cell apoptosis: regulation and biology. Phil. Trans. R. Soc. B 365, 1501–1515 (doi:10.1098/rstb.2009.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M.1994Regulation of spermatogenesis. In The physiology of reproduction (eds Knobil E., Neill J. D.), pp. 1363–1434 New York, NY: Raven Press [Google Scholar]
- Sharpe R. M.2010Environmental/lifestyle effects on spermatogenesis. Phil. Trans. R. Soc. B 365, 1697–1712 (doi:10.1098/rstb.2009.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini B.1981Basic and applied research in the study of indazole carboxylic acids. Chemotherapy 27(Suppl. 2), 9–20 [DOI] [PubMed] [Google Scholar]
- Silvestrini B., Palazzo G., De Gregorio M.1984Lonidamine and related compounds. Prog. Med. Chem. 21, 111–135 (doi:10.1016/S0079-6468(08)70408-9) [PubMed] [Google Scholar]
- Simon L., Ekman G. C., Kostereva N., Zhang Z., Hess R. A., Hofmann M. C., Cooke P. S.2009Direct transdifferentiation of stem/progenitor spermatogonia into reproductive and nonreproductive tissues of all germ layers. Stem Cells 27, 1666–1675 (doi:10.1002/stem.93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tash J. S., Attardi B., Hild S. A., Chakrasali R., Jakkaraj S. R., Georg G. I.2008aA novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol. Reprod. 78, 1127–1138 (doi:10.1095/biolreprod.106.057810) [DOI] [PubMed] [Google Scholar]
- Tash J. S., et al. 2008bGamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP90AB1 (HSP90BETA) and EEFA1 (eEF1A), and stimulates Il1a transcription in rat Sertoli cells. Biol. Reprod. 78, 1139–1152 (doi:10.1095/biolreprod.107.062679) [DOI] [PubMed] [Google Scholar]
- Vane J. R., Botting R. M.2003The mechanism of action of aspirin. Thromb. Res. 110, 255–258 (doi:10.1016/S0049-3848(03)00379-7) [DOI] [PubMed] [Google Scholar]
- Verhoeven G., Willems A., Denolet E., Swinnen J. V., De Gendt K.2010Androgens and spermatogenesis: lessons from transgenic mouse models. Phil. Trans. R. Soc. B 365, 1537–1556 (doi:10.1098/rstb.2009.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl A., Vaid K., Guttman J.2008The Sertoli cell cytoskeleton. In Molecular mechanisms in spermatogenesis (ed. Cheng C. Y.), pp. 186–211 Austin, TX: Landes Bioscience/Springer Science [Google Scholar]
- Walker W. H.2010Non-classical actions of testosterone and spermatogenesis. Phil. Trans. R. Soc. B 365, 1557–1569 (doi:10.1098/rstb.2009.0258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Cooper H., Bausek N.2010Evolution and spermatogenesis. Phil. Trans. R. Soc. B 365, 1465–1480 (doi:10.1098/rstb.2009.0323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Roberts S. S.2010Regulating mitosis and meiosis in the male germ line: critical functions for cyclins. Phil. Trans. R. Soc. B 365, 1653–1662 (doi:10.1098/rstb.2009.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. W. P., Cheng C. Y.2009Polarity proteins and cell–cell interactions in the testis. Int. Rev. Cell. Mol. Biol. 278, 309–353 (doi:10.1016/S1937-6448(09)78007-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. W. P., Mruk D. D., Cheng C. Y.2008Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem. Biophys. Acta 1778, 692–708 (doi:10.1016/j.bbamem.2007.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Mruk D. D., Lee W. M., Cheng C. Y.2007Unraveling the molecular targets pertinent to junction restructuring events during spermatogenesis using the Adjudin-induced germ cell depletion model. J. Endocrinol. 192, 563–583 (doi:10.1677/joe-06-0158) [DOI] [PMC free article] [PubMed] [Google Scholar]