Abstract
Integral membrane proteins that contribute to function of the blood–testes barrier (BTB) in mice include claudins 3, 5 and 11 and occludin. Although claudin 11 is expressed throughout all stages of the seminiferous epithelial cycle, claudins 3 and 5 have specific expression at stage VIII. These differences in protein expression suggest that the interactions among, and functions of, these integral membrane proteins may shift over the course of the seminiferous epithelial cycle. Also, differences in expression among rodent species and men may make interpretation of studies across species challenging. This review will discuss the characteristics of claudins and occludin; the expression, regulation and function of these integral membrane proteins in the seminiferous epithelium; and how these properties relate to the unique features of BTB.
Keywords: blood–testis barrier, tight junction, seminiferous epithelial cycle, spermatocyte migration
1. Introduction
Claudins (Furuse et al. 1998a,b) and occludin (Furuse et al. 1993) were the first tight junctional integral membrane proteins identified. Tight junction permeability and epithelial barrier function are primarily mediated by claudins, and the claudin isoforms expressed in a tissue determine the tissue-specific barrier characteristics (for review, see Krause et al. 2008). The importance of occludin in mediating epithelial barrier function appears to be tissue dependent (Saitou et al. 2000; Schulzke et al. 2005). One of those tissues is the seminiferous epithelium. The tight junctions between the Sertoli cells of the seminiferous epithelium, which form the blood–testes barrier (BTB), have several morphological and functional differences when compared with the characteristics of other epithelial barriers. The purpose of this review article is to discuss the expression and function of claudins and occludin in mammalian seminiferous epithelium and how these characteristics relate to the unique properties of the BTB.
2. Characteristics of claudins and occludin
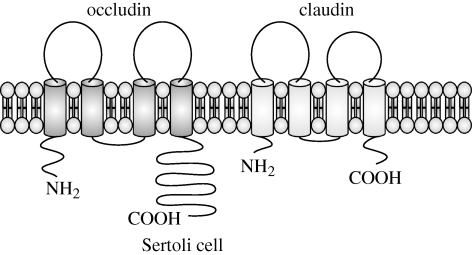
Claudins and occludin have similar topographical features, including four transmembrane domains, two extracellular loops, a short intracytoplasmic loop and cytoplasmic N- and C-termini (figure 1; Tsukita et al. 2001). Both of these proteins interact with scaffolding proteins via their C-termini (figure 2; Tsukita et al. 2001). However, there is no sequence homology between the claudin family and occludin (Furuse et al. 1998a), and the claudins (MW = 20–27 kDa) are also smaller than occludin (MW = 65 kDa; Furuse et al. 1993; Van Itallie & Anderson 2006). There are 24 members of the claudin family, and an identifying characteristic is a conserved residue sequence of W-nx-GLW-nx-C-nx-C in the first extracellular domain (Van Itallie & Anderson 2006).
Figure 1.
Comparison of occludin and claudin topography. Both occludin and claudins have similar topography, with cytoplasmic N- and C-termini, four transmembrane domains, two extracellular loops, and a short cytoplasmic loop. However, there is no sequence homology between these proteins, and the longer cytoplasmic termini in occludin give it a substantially larger molecular mass than the molecular mass of claudin.
Figure 2.
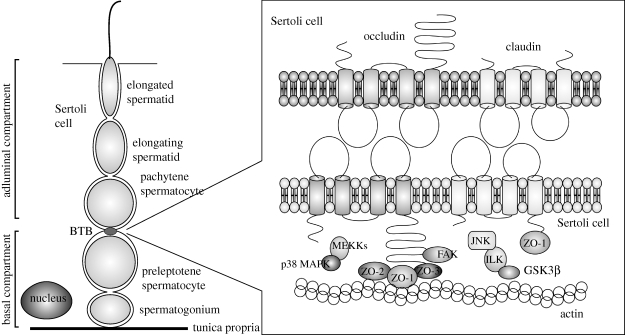
Location of occludin and claudin in the seminiferous epithelium. Occludin- and claudin-based tight junctions are components of the BTB, which is located apical to preleptotene spermatocytes and basal to pachytene spermatocytes in the seminiferous epithelium. Both occludin and claudin interact with scaffolding proteins (ZO-1, -2, and -3) and occludin interacts with the signalling molecule FAK. These interactions with scaffolding proteins connect occludin and claudin to the actin cytoskeleton and allow localization of cell signalling molecules to tight junctions. Through these various interactions, tight junctions can influence cell cycle pathways.
Claudins, occludin and members of the junctional adhesion molecule (JAM) family are the most studied and characterized of the tight junctional integral membrane proteins (Paris et al. 2008). Of these proteins, claudins are of primary importance for optimal establishment of tight junctions. JAM proteins are not sufficient for tight junction formation. Numerous primary and established fibroblast cell lines endogenously express JAM-A or JAM-C, yet these cells do not form tight junctions (Morris et al. 2006). Fibroblasts that are transfected with occludin can form tight junction strands, albeit small and few in number (Furuse et al. 1998b). However, occludin is not necessary for the formation or maintenance of tight junctions, as demonstrated by occludin disrupted murine embryonic stem cells (Saitou et al. 1998) and occludin knockout mice (Saitou et al. 2000; Schulzke et al. 2005). Morphologically normal tight junctions are formed in both of these models, albeit with various degrees of physiological dysfunction among epithelia, including the seminiferous epithelium, in occludin knockout mice (Saitou et al. 2000).
The phenotypes of claudin knockout mice reveal the importance of specific claudins for regulating barrier integrity of distinct epithelial tissues. For example, claudin 1 knockout mice die shortly after birth due to disruption of epidermal barrier function and resultant dehydration (Furuse et al. 2002), whereas in claudin 5 knockout mice, the neonatal lethality is attributable to disruption of endothelial barrier integrity in the central nervous system and resultant loss of blood–brain barrier function (Nitta et al. 2003). Claudin 11 knockout mice are viable, but have locomotion defects and male sterility due to lack of tight junctions in the myelin sheaths of the central nervous system and between Sertoli cells of the testes, respectively (Gow et al. 1999).
The interactions between the claudin proteins within a tissue determine the barrier properties of an epithelial tissue (Krause et al. 2008). The interactions can be described by the plane in which they occur (i.e. cis within the same cell membrane or trans between opposing cell membranes) and by the type of interacting claudins (homophilic between two claudins of the same isoform or heterophilic between two claudins of different isoforms; Krause et al. 2008). Paracellular ion pores are formed by trans-interactions between the first extracellular loops of certain claudins, whereas tightness against solute diffusion is determined by the respective trans-interactions of both extracellular loops (Krause et al. 2008). Both trans- and cis-interactions among claudins are important for formation, morphology and stability of the tight junction strands (Krause et al. 2008; Mrsny et al. 2008).
The importance of occludin–occludin interactions to epithelial barrier function is uncertain. Disruption of these interactions with peptides that correspond to the first (Tavelin et al. 2003; Everett et al. 2006) or second (Wong & Gumbiner 1997; Nusrat et al. 2005) extracellular loop or a monoclonal antibody against the second extracellular loop (Tokunaga et al. 2007) decreases localization of occludin to the cell membrane. Alterations in epithelial barrier function associated with these disruptions in occludin interactions and localization may actually be attributable to altered localization of other tight junctional proteins. Treatment of T84 human intestinal cell cultures with a peptide corresponding to part of the second extracellular loop of occludin alters localization of claudin 1, JAM-A, and the scaffolding protein zona occludens 1 (ZO-1) and increases barrier permeability (Nusrat et al. 2005). However, treatment with a monoclonal antibody against that loop does not alter localization of those proteins nor barrier permeability (Tokunaga et al. 2007).
In addition to claudin–claudin and occludin–occludin interactions, claudin–occludin interactions may also occur. Occludin can interact with a peptide that corresponds to the first extracellular loop of claudin 1 (Mrsny et al. 2008), and claudin 1 and JAM-A can interact with a peptide that corresponds to the second extracellular loop of occludin (Nusrat et al. 2005). The relative importance of the various types of interactions among the claudins and occludin to tight junction formation, stability and function remain to be determined.
Claudins and occludin have additional functions in addition to mediating tight junction permeability (table 1). Indeed, prior to being identified as integral membrane proteins of tight junctions, some claudin proteins had been characterized as a marker of androgen withdrawal in rat ventral prostate (claudin 3; Briehl & Miesfeld 1991), as receptors for Clostridium perfringens enterotoxin (claudins 3 and 4; Katahira et al. 1997a,b), and as one of several proteins whose genes are deleted in velo–cardio–facial syndrome in humans (claudin 5; Sirotkin et al. 1997). In some tissues, the additional functions of occludin may be more important than any contribution occludin may make toward barrier tightness. In gastric epithelia of mice, occludin appears to be necessary for proper differentiation rather than for barrier function (Schulzke et al. 2005). Disruption of occludin–occludin interactions in monolayers of a human intestinal epithelial cell line alters cell polarity without affecting barrier permeability (Tokunaga et al. 2007).
Table 1.
Roles of occludin and claudins in addition to mediating barrier permeability.
| function | protein |
|---|---|
| coactivator for soluble matrix metalloproteinases | claudins 1, 2, 3, 4 and 5 (Miyamori et al. 2001; Takehara et al. 2009) |
| coreceptor for hepatitis C virus | claudins 1, 6 and 9 (Evans et al. 2007; Zheng et al. 2007); occludin (Ploss et al. 2009) |
| mediate cell adhesion (weak) | claudins 1, 2 and 3 (Kubota et al. 1999) |
| mediate cell cycle regulation | claudin 11 (Mazaud-Guittot et al. 2010) |
| mediate cell differentiation | occludin (Schulzke et al. 2005) |
| mediate cell polarity | occludin (Tokunaga et al. 2007) |
| modulate cell migration and invasion | claudins 2, 3, 4 and 11 (Michl et al. 2003; Agarwal et al. 2005, 2009; Mima et al. 2008) |
| receptor for Clostridium perfringens enterotoxin | claudins 3 and 4 (Katahira et al. 1997a,b) |
3. Blood–testis barrier
The BTB is formed by tight junctions between adjacent Sertoli cells and divides the seminiferous epithelium into basal and adluminal compartments (Mruk & Cheng 2004). Spermatogonia and pre-leptotene spermatocytes are below in the basal compartment, and leptotene and later spermatocytes and spermatids are above, in the adluminal compartment. This physical separation establishes the distinct microenvironments needed for the different germ cell types (Onoda et al. 1990; Mruk & Cheng 2004). The BTB also protects the primary spermatocytes and haploid spermatids from potentially harmful chemicals by limiting the movement of intercellular molecules from the interstitial space into the adluminal compartment (Mruk & Cheng 2004; Su et al. 2009). In association with other immunoprotective mechanisms, the BTB protects the haploid spermatids, which express ‘foreign’ proteins, from immunological attack and helps to establish testicular immune privilege (Fijak & Meinhardt 2006).
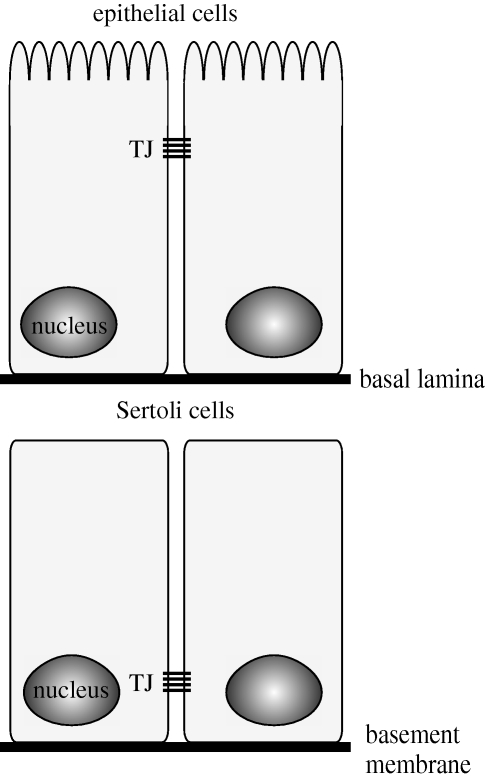
Compared with the tight junctions of other polarized epithelial cells, the tight junctions between Sertoli cells have several unique features. In other polarized epithelia, tight junctions are located in a circumferential band towards the apical membrane; whereas in Sertoli cells, the tight junctions are located in a circumferential band toward the basement membrane (figure 3; Gilula et al. 1976; Wong et al. 2008). In other polarized epithelia, the adherens junction band and then the desmosome band are located just basal to the tight junction band, and gap junctions are located throughout the lateral cell membrane. Although the bands of tight junctions, adherens junctions, and desmosomes support each other functionally through interactions with scaffolding proteins and the cytoskeleton, these different types of cell–cell contact are physically separate from each other in most polarized epithelia (Wong et al. 2008). However, at the BTB in Sertoli cells, the tight junctions and specialized adherens junctions (basal ectoplasmic specialization and basal tubulobulbar complex), as well as the basal gap junctions and desmosome-like junctions are all intermingled with each other (Russell & Peterson 1985; Parreira et al. 2002; Wong et al. 2008). In addition to the tight junctions, the basal ectoplasmic specialization (Yan & Cheng 2005; Lie et al. 2006; Yan et al. 2007) and desmosome-like junctions (Li et al. 2009) appear to be integral, functional components of the BTB. Another feature of the tight junctions and other components of the BTB is the need to allow for the cyclic passage of spermatocyte cohorts while maintaining barrier integrity (Mruk & Cheng 2004).
Figure 3.
Comparison of distribution of tight junctional complexes between the seminiferous epithelium and other epithelia. Unlike other epithelia in which tight junctions are located apically, in the seminiferous epithelium tight junctions are located basally.
4. Claudin and occludin contributions to blood–testis barrier integrity
Claudins 3 (Meng et al. 2005), 5 (Morrow et al. 2009), and 11 (Gow et al. 1999) and occludin (Saitou et al. 2000; Chung et al. 2001) contribute to BTB integrity. The contributions of claudins 3 and 5 were determined in mice via deletion of genes for transcription factors that are upstream regulators of the claudins (androgen receptor (AR) for claudin 3; Meng et al. 2005) and ets variant 5 (ETV5) for claudin 5; Morrow et al. 2009). Mice that are gene-deleted for AR in the Sertoli cells and for ETV5 in all cells have deficient BTBs as demonstrated by leakage of interstitially injected tracers into the seminiferous tubule lumens. Males in both of these lines are infertile. However, the infertility is not primarily attributable to the barrier deficiency. Mice with AR specifically deleted from Sertoli cells are infertile due to the inability of the Sertoli cells to nurture the germ cells past the round spermatid stage (Holdcraft & Braun 2004). The transcription factor ETV5 is needed in Sertoli cells (Chen et al. 2005) and germ cells (Tyagi et al. 2009) for proper regulation of the spermatogonial stem cell niche and renewal of spermatogonial stem cells.
The contribution of claudin 11 and occludin to BTB integrity was determined by gene-deletion in mice (Gow et al. 1999; Mazaud-Guittot et al. 2010). Tight junction strands between adjacent Sertoli cells from testes of claudin 11 knockout mice are not detected on ultrastructural examination (Gow et al. 1999). At the light microscopic level, morphological differences between the testes of wild-type and claudin 11 knockout mice are first detectable at postnatal day 20 (Mazaud-Guittot et al. 2010). In both prepubertal and adult claudin 11 knockout mice, the lumens of the seminiferous tubules are narrowed and often filled with aggregates of Sertoli cells (Gow et al. 1999; Mazaud-Guittot et al. 2010). Round spermatids are the most mature germ cell type detected, and there is evidence of increased germ cell apoptosis (Mazaud-Guittot et al. 2010). As expected with this level of testicular phenotype severity, claudin 11 knockout mice are sterile (Gow et al. 1999). In occludin knockout mice, the testes appear histologically normal at six weeks of age (Saitou et al. 2000). However, at 40 to 60 weeks of age the seminiferous tubules are atrophied and have a Sertoli-cell only phenotype. Despite the apparently normal spermatogenesis in young occludin knockout mice, these male mice are sterile.
An adult model of occludin disruption in the testes of rats also demonstrates the importance of occludin to BTB integrity (Chung et al. 2001). In this model, rat testes are injected with a synthetic peptide that corresponds to a portion of the second extracellular loop of occludin and disrupts occludin–occludin interactions. Loss of elongate spermatids can be detected by post-injection day 8, with loss of spermatocytes and spermatids in all tubules by day 27, reappearance of spermatocytes by day 47, and complete recovery by day 68. The time points with disrupted spermatogenesis correspond with time points in which there are increases in tracer leakage across the BTB in treated testes.
The integral membrane proteins JAM-A and JAM-B are also found at the BTB (Mruk & Cheng 2004). However, the contribution of these JAMs to BTB integrity is uncertain. Mice gene-deleted for JAM-B have normal fertility (Sakaguchi et al. 2006), and mice gene-deleted for JAM-A have normal testes morphology, with the subfertility in the male mice attributable to the role of JAM-A in regulating spermatozoal motility (Shao et al. 2008). Whether this reflects redundancy between JAM-A and JAM-B in regulating the BTB (Shao et al. 2008) or that JAMs have a minimal contribution to BTB integrity remains to be determined.
5. Claudin and occludin expression in the testes
Claudin mRNAs that have been identified in rodent testis via Northern blot analyses include claudins 1, 2, 3, 4, 5, 7, 8 and 11 (Furuse et al. 1998a; Morita et al. 1999a,b). Additionally, claudins 10, 12 and 23 have been detected by microarray analysis (Singh et al. 2009). Of those, the mRNA and protein expression patterns of claudins 1, 3, 5 and 11 have been further characterized.
Claudin 1 mRNA and protein are detected in mouse testes at postnatal day 3, are increased at postnatal day 10, but are decreased on postnatal days 16 and 30, compared with day 3 mRNA and protein expression levels (Gye 2003b). These data were generated from whole testes extracts and were not normalized for the number the Sertoli cells. Thus, the decrease in claudin 1 expression in testes from 16- and 30-day-old mice probably represents a dilutional effect as rapid germ cell proliferation causes a relative increase in the numbers of germ cells and a relative decrease in the numbers of Sertoli cells (Gye 2003b). In rats, expression of claudin 1 protein adjusted for testes weight increases from postnatal day 16–35 and then plateaus in adulthood (Yan et al. 2008a). Although claudin 1 protein expression was determined by Western blot analyses in mice and rats, determination of the cell-specific expression pattern via immunohistochemical analyses was not performed. Whether claudin 1 is a functional component of the BTB or not remains to be determined.
Claudin 3 mRNA is first detected in mouse testes at postnatal day 15, has an apparent peak at day 20, and then decreases to levels comparable to day 15 during puberty and adulthood (Meng et al. 2005). In primary cultures of Sertoli cells from 19- to 21-day-old rats, claudin 3 mRNA expression was not detected (Kaitu'u-Lino et al. 2007). On Western blot analyses, claudin 3 protein in mouse testes is first detected at day 15, has a peak at day 25, and then gradually decreases to levels comparable to day 20 during puberty and adulthood (Meng et al. 2005), whereas in rat testes the peak is detected at postnatal day 35 (Yan et al. 2008a). The data for the Western blots and the mouse qPCR analyses were generated from whole testes extracts and were not normalized for the number of Sertoli cells.
Immunofluorescence analyses of claudin 3 expression in mouse testes revealed interesting patterns. Claudin 3 is first detected by this method at postnatal day 15 and is seen at all levels of the seminiferous tubules (Meng et al. 2005). At day 20, the claudin 3 immunosignal is localized to the area of the BTB (Meng et al. 2005). In adult mice, claudin 3 expression is stage-specific and is observed during the stages when the preleptotene and leptotene spermatocytes migrate across the BTB (Meng et al. 2005). During stage VII, claudin 3 is detected just apical to the preleptotene spermatocytes, whereas during stage VIII and early stage IX, the immunosignal is seen both apically and basally to the preleptotene and leptotene spermatocytes, and during late stage IX and early stage X, claudin 3 is localized basal to the leptotene spermatocytes (Komljenovic et al. 2009). This expression pattern led to the conclusion that claudin 3 could be used as a marker to delineate the intermediate compartment that was hypothesized by Russell (1977). Claudin 3 immunosignal was not detected at the BTB at other seminiferous epithelial stages in mice (Meng et al. 2005), and was not detected in the seminiferous epithelium at all in rats (Kaitu'u-Lino et al. 2007). In Djungarian hamsters (Phodopus sungorus), claudin 3 is transiently expressed at the BTB during simulated transitioning from short-day to long-day photoperiods (Tarulli et al. 2008); Djungarian hamsters are seasonal breeders in which active spermatogenesis is induced by long-day (16 h of light) photoperiods and subsequent FSH secretion. Claudin 3 in testes is also associated with interstitial cells and spermatids in mice (Meng et al. 2005) and Djungarian hamsters (Tarulli et al. 2008) and interstitial cells in rats (Kaitu'u-Lino et al. 2007).
The differences in claudin 3 protein expression in the seminiferous epithelium among mice, rats and hamsters suggest that the role of claudin 3 in the BTB may be species-specific. However, fixation technique is important for detection of claudin 5 in the seminiferous epithelium (Morrow et al. 2009), and if this is a factor for the inability to detect claudin 3 in rat seminiferous epithelium is unknown. The inability to detect claudin 3 mRNA in primary cultures of rat Sertoli cells (Kaitu'u-Lino et al. 2007) suggests that rats do not express claudin 3 in their seminiferous epithelium. However, claudin 3 protein is detected on Western blot analysis of whole testes extracts from rats (Yan et al. 2008a). Whether these differences between these rat studies reflect contributions from the interstitial compartment for claudin 3 expression on Western blot analysis or in vivo versus in vitro differences in claudin 3 expression in rat Sertoli cells remains to be determined.
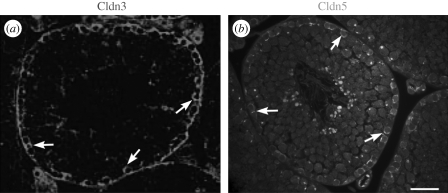
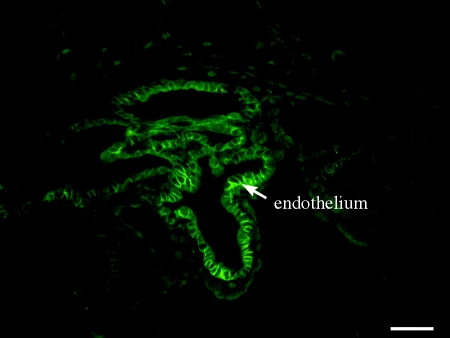
Claudin 5 protein expression in mouse testes has several interesting patterns (Morrow et al. 2009). In testes from 8-day-old pups, it is seen at all levels of the seminiferous epithelium surrounding all of the germ cells. In adult testes, claudin 5 protein localizes to the BTB with stage-specific expression at stages VIII and IX, similar to claudin 3 expression (Meng et al. 2005; figure 4). Claudin 5 protein is also associated with spermatogonial cell membranes and cytoplasm and an unknown perinuclear organelle in spermatocytes, cells that do not have tight junctions. Immunocytochemistry on enriched populations of germ cells and Sertoli cells isolated from 8-day-old pups revealed claudin 5 expression in spermatogonia, spermatocytes and Sertoli cells. The specificity of the immunostain was confirmed by use of preincubation of the claudin 5 antibody with the immunogenic peptide. Claudin 5 protein is also expressed in testicular vascular endothelium and rete testis epithelium.
Figure 4.
Claudin 3 (red) and 5 (green) protein expressions in mouse testes at stage VIII. Expressions of both claudins peak during Stage VIII of the seminiferous epithelial cycle (arrows), with expressions not detectable to minimal at other stages. (a) Claudin 3 image reprinted from Meng et al. (2005) (Copyright © 2005, National Academy of Sciences, USA). (b) Claudin 5 image reprinted from Morrow et al. (2009). Scale bar, 40 µm.
Because claudin 5 protein is expressed in multiple cell types in the testes, interpreting developmental claudin 5 mRNA expression is challenging. In whole testes extracts, claudin 5 mRNA has its peak expression on postnatal day 0 and declines to a plateau of about half the day 0 level at day 12 and in older mice (figure 5). However, when these claudin 5 mRNA data are normalized for the number of seminiferous tubules, claudin 5 mRNA expression remains constant in 0-day-old through 12-day-old mice and then increases at day 16 to reach a plateau of about 3 to 3.5 times the day 0 level in 20-day-old and adult mouse testes (Morrow et al. 2009). Because germ cells also express claudin 5, normalization by a Sertoli cell marker adjusts claudin 5 mRNA to the level of the seminiferous tubule and not the Sertoli cell (Morrow et al. 2009); thus the increase in claudin 5 expression mRNA may also have contributions from germ cells. The normalization also assumes that relative claudin 5 mRNA contributions from the seminiferous epithelial and vascular endothelial compartments remain consistent throughout development.
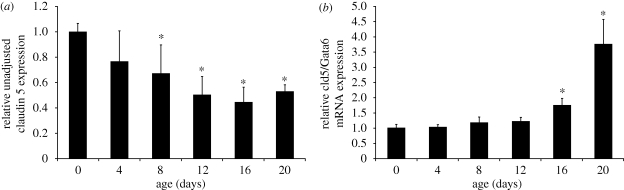
Figure 5.
Developmental claudin 5 mRNA expression in mouse testes (n = 2–4 mice/group). (a) Unadjusted and (b) adjusted claudin 5 expression in whole testes extracts relative to expression values on day 0. To adjust for a germ cell dilution effect that occurs with age, claudin 5 expression was determined relative to Gata 6, a gene expressed in Sertoli cells but not germ cells. The adjusted claudin 5 value in panel b is thus normalized for the number of seminiferous tubules. * p < 0.05 compared with day 0 value. Modified from Morrow et al. (2009).
Claudin 11 mRNA expression is first detected prenatally in mouse testes at postcoitum day 12, at about the same time as the formation of the testes cords (Hellani et al. 2000). Postnatally, unadjusted claudin 11 mRNA expression peaks between days 6 and 15 and then declines through puberty to reach a low plateau in adulthood (Hellani et al. 2000). When developmental claudin 11 mRNA expression is normalized for the number of Sertoli cells, expression increases from postnatal day 0 through adulthood (Johnston et al. 2004; Willems et al. 2009).
Claudin 11 protein is first detectable in mouse testes by use of immunohistochemical staining at postnatal day 13 (Mazaud-Guittot et al. 2010). At this age, the immunosignal is throughout the entire seminiferous epithelium, from the basement membrane to the lumen. By day 20 and in adult mice, claudin 11 staining is primarily localized to the area of the BTB (Morita et al. 1999b; Hellani et al. 2000; Mazaud-Guittot et al. 2010), with fainter staining observed between Sertoli cells at the middle and apical regions in 1 report (Hellani et al. 2000). In Djungarian hamsters exposed to long-day photoperiods and undergoing active spermatogenesis, claudin 11 localization at the BTB is basal to the germ cells at the basement membrane during stages I–III and XI–XII, apical to those germ cells at stages VII–VIII, and intermediate to those germ cells at stages IV–VI and IX–X (Tarulli et al. 2008). Claudin 11 protein expression can be detected at all stages of the seminiferous epithelium cycle in mice (Hellani et al. 2000; Meng et al. 2005; Mazaud-Guittot et al. 2010), men (Fink et al. 2009) and Djungarian hamsters during active spermatogenesis (Tarulli et al. 2008). At the ultrastructural level, claudin 11 immunogold labelling is associated with the tight junction strands between Sertoli cells in mice (Morita et al. 1999b). In all of these reports, claudin 11 protein was only detected in Sertoli cells.
Occludin protein is detected via immunofluorescence in the testes cords of mice at embryonic day 13.5 (observation of vaginal plug = embryonic day 0.5; Cyr et al. 1999). At this time point and also at embryonic day 16.5, occludin immunostain is diffuse in the gonocyte cytoplasm. However, at embryonic day 18.5, the immunostain appears as a filiform-like network in the Sertoli cell cytoplasm with no signal associated with the gonocytes. The filiform staining pattern continues to be observed in neonatal, prepubertal, and adult mice, albeit with decreasing intensity as the mice age (Cyr et al. 1999). By postnatal day 14, occludin immunostain is detected as focal, wavy bands toward the base of tubules that contain more advanced germ cells. By postnatal day 23 and in adult mice, these bands are present in all tubules at all stages of the seminiferous epithelial cycle. Occludin is also expressed in testicular vascular endothelium and rete testes epithelium in mice (Cyr et al. 1999). On Western blot analysis of whole testes extracts from rats, unadjusted occludin expression increases from postnatal days 18–35, and then plateaus in adulthood (Yan et al. 2008a).
Similar to mice, occludin immunostain is detected at all stages of the seminiferous epithelial cycle in dogs (Gye 2004) and Korean wild rabbits (Lepus sinensis coreanus; Yoon et al. 2009). However, in rats, occludin expression is stage-specific, with immunostain not detectable in Stage VIII tubules but strongly expressed at all other stages (Li et al. 2006). Occludin expression is also stage-specific in Djungarian hamsters exposed to long-day photoperiods; the stage distribution was not reported (Tarulli et al. 2008). Interestingly, occludin is not expressed in the seminiferous tubules of guinea pigs (Cavia porcellus) and men (Moroi et al. 1998).
These differences in occludin expression among species imply that the importance of occludin in regulating BTB integrity may be species-specific. In species and stages in which occludin is not detected, the functions of occludin would be superseded by claudins or JAMs. Alternatively, the lack of occludin detection in men and guinea pigs may be attributable to occludin splice variants in the seminiferous epithelium that are not recognized by antibodies against the C-terminus of wild-type occludin (Moroi et al. 1998). Splice variants with altered C-termini have been identified from various human cell lines (Mankertz et al. 2002; Gu et al. 2008); however, the distribution of these variants in the testes has not been evaluated. Differences in the expression patterns among the rodent species may also be attributable to differences in antibody specificity among the antibodies used in the various studies (Ramos-Vara 2005).
6. Regulation of claudin and occludin expression in the testes
There are myriad factors that regulate claudin and occludin transcription, translation and cellular localization in the testes (see Mruk & Cheng 2004; Yan et al. 2008b; Lie et al. 2009 for reviews on regulation and dynamic restructuring of the BTB). This review will focus on the transcription factors, reproductive hormones and cellular associations that are important for claudin and occludin expression in the testes.
(a). Testosterone and androgen receptor
Testosterone is important for maintaining the integrity of the BTB (Janecki et al. 1991). Indeed, testosterone supplementation is able to counteract the disruptive effects of cadmium in primary cultures of rat Sertoli cells (Chung & Cheng 2001). The effects of testosterone and AR signalling on mRNA expression, protein expression, and protein localization of tight junctional proteins, including claudins 1, 3 and 11 and occludin, have been the focus of numerous studies (table 2).
Table 2.
Effects of testosterone and AR signalling on expression of BTB integral membrane mRNA and proteins. AR, androgen receptor; SCARKO, Sertoli cell-specific androgen receptor knockout.
| protein | endpoint | treatment | effecta | reference |
|---|---|---|---|---|
| claudin 1 | mRNA expression | flutamide treatment of adult rats | — | Gye & Ohsako (2003) |
| testosterone supplementation to primary cultures of mouse Sertoli cells | day 2: ↑; day 4: — | Gye (2003b) | ||
| bicompartmental coculture of mouse Sertoli and Leydig cells | day 2: — | Gye (2003a) | ||
| claudin 3 | mRNA expression | mice with hypomorphic AR allele | — | Eacker et al. (2007) |
| SCARKO mice (Braun line) | ↓ | Meng et al. (2005) | ||
| testosterone supplementation of TM4 Sertoli cells | 48 h: ↑ | Meng et al. (2005) | ||
| protein expression | SCARKO mice (Braun line) | ↓ | Meng et al. (2005) | |
| protein localization | SCARKO mice (Braun line) | not visualized | Meng et al. (2005) | |
| claudin 11 | mRNA expression | mice with loss of AR signalling in all cells | — | Johnston et al. (2004), Tan et al. (2005) |
| SCARKO mice (Verhoeven line; Chang line) | ↓ | Tan et al. (2005), Wang et al. (2006) | ||
| flutatmide treatment of adult rats | — | Gye & Ohsako (2003) | ||
| bicompartmental coculture of mouse Sertoli and Leydig cells | day 2: ↑ | Gye (2003b) | ||
| testosterone supplementation to primary cultures of rat Sertoli cells | 4–24 h: —; 48–96 h: ↑ | Florin et al. (2005) | ||
| day 0–3: —; day 4–7: ↑; day 9: ↑; day 13: ↑ | Kaitu'u-Lino et al. (2007) | |||
| primary rat Sertoli cells treated with testosterone on days 1–5, followed by flutatmide treatment on days 6–9, then testosterone on days 10–13 of culture | compared with media control cells: day 9: —; day 13: — | Kaitu'u-Lino et al. (2007) | ||
| compared with continuous testosterone supplementation: day 9: ↓; day 13: ↓ | ||||
| flutamide treatment of primary cultures of rat Sertoli cells on days 6–9 of culture | day 9: —; day 13: — | Kaitu'u-Lino et al. (2007) | ||
| protein localization | testosterone supplementation to primary cultures of rat Sertoli cells | increased localization to the cell membrane at days 5, 9 and 13 | Kaitu'u-Lino et al. (2007) | |
| primary rat Sertoli cells treated with testosterone on days 1–5, followed by flutatmide treatment on days 6–9, then testosterone on days 10–13 of culture | compared with continuous testosterone supplementation: decreased localization to the cell membrane on day 9, similar localization on day 13 | Kaitu'u-Lino et al. (2007) | ||
| flutamide treatment of primary cultures of rat Sertoli cells on days 6–9 of culture | decreased localization to the cell membrane on day 9 | Kaitu'u-Lino et al. (2007) | ||
| occludin | mRNA expression | flutatmide treatment of adult rats | ↓ | Gye & Ohsako (2003) |
| SCARKO mice (Chang line) | ↓ | Wang et al. (2006) | ||
| testosterone supplementation to primary cultures of rat Sertoli cells | 10–24 h: ↑ | Chung & Cheng (2001) | ||
| day 1–7: — | Kaitu'u-Lino et al. (2007) | |||
| protein expression | testosterone supplementation to primary cultures of rat Sertoli cells | day 1: ↑; day 5: — | Yan et al. (2008a) | |
| protein localization | testosterone supplementation to primary cultures of rat Sertoli cells | increased endocytosis at 15, 30 and 60 min; no change in endocytosis at 180 min | Yan et al. (2008a) | |
| increased recycling from endosomes to cell membrane at 5 and 15 min | (Yan et al. 2008a) | |||
| increased localization to the cell membrane at day 7 | (Kaitu'u-Lino et al. 2007) | |||
| primary rat Sertoli cells treated with testosterone on days 1–5, followed by flutatmide treatment on days 6–9, then testosterone on days 10–13 of culture | compared with continuous testosterone supplementation: decreased localization to the cell membrane on day 9, similar localization on day 13 | (Kaitu'u-Lino et al. 2007) |
a↑: increased expression; ↓, decreased expression; —, no change in expression.
In primary cultures of mouse Sertoli cells, testosterone supplementation or coculturing with Leydig cells has negligible effects on the expression of claudin 1 mRNA (Gye 2003a,b). Treatment of adult rats with the AR antagonist flutamide for 6 days did not significantly affect the levels of claudin 1 mRNA in whole testes extracts (Gye & Ohsako 2003). AR signalling does not appear to alter claudin 1 mRNA expression.
Claudin 3 dependence on AR signalling was detected in one line of Sertoli cell-specific AR knockout mice, in which claudin 3 mRNA was reduced by 10-fold and claudin 3 protein was not detected by use of immunofluorescence in testes from 8-week-old mice (Meng et al. 2005). Because of altered splicing of the floxed AR allele, the parental AR-flox mice have reduced pancellular AR signalling; this means that in addition to absent AR signalling in the Sertoli cells, the other cells of this line of Sertoli cell-specific AR knockout mice have reduced AR signalling (Holdcraft & Braun 2004). Interestingly, although claudin 3 mRNA is downregulated in the testes of Sertoli cell-specific AR knockout mice, it is not downregulated in the testes of parental AR-flox mice (Eacker et al. 2007). The parental AR-flox mice have greatly increased levels of serum testosterone, compared with wild-type mice (Holdcraft & Braun 2004). Assuming this reflects increased testosterone concentration in the testes, this may provide adequate stimulation for claudin 3 transcription in the Sertoli cells of the parental AR-flox mice. Testosterone supplementation to TM4 Sertoli cells transiently transfected with an AR expressing plasmid increased claudin 3 mRNA expression after 48 h, compared with values in non-supplemented cells (Meng et al. 2005). Although numerous potential AR response elements in the promoter region of mouse claudin 3 suggest that AR may directly mediate claudin 3 transcription (Eacker et al. 2007), this has yet to be confirmed. Also, results of microarray analysis of the testes of two other lines of Sertoli cell-specific AR knockout mice did not reveal changes in claudin 3 mRNA expression (Tan et al. 2005; Wang et al. 2006).
Claudin 11 responsiveness to AR signalling was detected by numerous in vitro models, in which primary cultures of mouse or rat Sertoli cells, receiving testosterone supplementation, had increased claudin 11 mRNA and protein expression, compared with expression values in cells that did not receive testosterone (Gye 2003a; Florin et al. 2005; Kaitu'u-Lino et al. 2007). There was also increased localization of claudin 11 protein to the cell membrane in cultured rat Sertoli cells that received testosterone (Kaitu'u-Lino et al. 2007). Addition of the AR antagonist flutamide and removal of testosterone to cultured rat Sertoli cells that had been previously supplemented with testosterone caused claudin 11 mRNA expression to decrease to levels comparable to cells grown in non-supplemented media (Kaitu'u-Lino et al. 2007). Interestingly, when the flutamide was removed and testosterone resupplemented to the cells, claudin 11 mRNA expression was not different compared with levels during flutamide treatment and to levels in non-supplemented control cells at the same time point (Kaitu'u-Lino et al. 2007). Flutamide treatment had no effect on basal levels of claudin 11 mRNA expression in cells that did not receive testosterone (Kaitu'u-Lino et al. 2007).
The role of AR in mediating claudin 11 expression in vivo is less clear. In mice in which AR signalling is absent in all cells of the body, through either a natural mutation in AR (Tfm mice; Johnston et al. 2004) or through experimental gene deletion (Tan et al. 2005), there was no difference in the amount of claudin 11 mRNA per Sertoli cell, compared with values in wild-type mice. Also, treatment of adult rats with flutamide for 6 days did not change testicular claudin 11 mRNA expression (Gye & Ohsako 2003). However, in two of three different Sertoli cell-specific AR knockout mouse lines, claudin 11 mRNA expression is decreased in the testes (Tan et al. 2005; Wang et al. 2006); in the third line, claudin 11 mRNA expression was not reported to be changed on microarray analysis (Eacker et al. 2007). The effect of suppressed AR signalling on claudin 11 protein expression and localization in the aforementioned knockout mice and flutamide-treated rat models was not evaluated.
In vitro results reveal that testosterone and AR signalling can increase testicular claudin 11 mRNA expression. However, in vivo results suggest that multiple factors, in addition to AR signalling, control basal expression of claudin 11 mRNA. It is probable that in some mouse lines or treatment models, these factors can maintain basal claudin 11 mRNA expression in the absence of AR signalling. Indeed, results of an analysis of the core region of the claudin 11 promoter revealed 2 GATA/NF-Y binding sites and did not reveal any AR response elements (Lui et al. 2007). Both GATA/NF-Y binding sites are needed for optional transcription activity (Lui et al. 2007). It remains to be determined whether AR can bind and mediate claudin 11 transcription at non-core regions of the promoter or whether AR mediation of claudin 11 transcription is indirect.
Testicular occludin mRNA expression is decreased in adult rats treated with the AR antagonist flutamide for 6 days, compared with values in untreated rats (Gye & Ohsako 2003). Testicular occludin mRNA expression is also decreased at postnatal day 10.5 in a line of Sertoli cell-specific AR knockout mice (Wang et al. 2006). In primary cultures of Sertoli cells from 20-day-old rats, testosterone supplementation stimulated a mild increase in occludin mRNA expression in one experiment (Chung & Cheng 2001) and had no effect in another experiment (Kaitu'u-Lino et al. 2007). Whether AR-mediated signalling of occludin transcription is direct or indirect, and its relative contribution to basal occludin levels, remain to be determined. In primary Sertoli cell cultures from 20-day-old rats, testosterone stimulates a transient increase in occludin protein expression (Yan et al. 2008a) and regulates protein recycling and localization to the cell membrane (Kaitu'u-Lino et al. 2007; Yan et al. 2008a).
Protein localization appears to be the primary mechanism through which testosterone increases BTB integrity (table 2). In primary cultures of rat Sertoli cells, which were supplemented with testosterone on days 1–5, then treated with flutamide on days 6–9, and resupplemented with testosterone on days 10–13, mRNA expression of claudin 11 remained suppressed on day 13, whereas claudin 11 protein had re-localized to the cell membrane (Kaitu'u-Lino et al. 2007). In contrast, occludin protein was transiently increased in primary cultures of rat Sertoli cells after testosterone supplementation, with high levels of occludin endocytosis and recycling to the cell membrane (Yan et al. 2008a). What effect disrupted testosterone and AR signalling have on protein localization of claudin 11 and occludin in vivo remains to be determined.
(b). Other transcription factors
The transcription factor SOX8 regulates claudin 3 expression in an age-dependent manner (Singh et al. 2009). Male mice gene-deleted for SOX8 have defects in spermiation, dysregulation of the seminiferous epithelial cycle, sloughing of spermatocytes and round spermatids and testicular atrophy, all of which worsen as the mice age (O'Bryan et al. 2008). In testes from 10- and 20-day-old mice, claudin 3 mRNA expression is greater in SOX8 knockout mice than in heterozygote control mice. However, after establishment of the BTB, this expression pattern changes such that testicular claudin 3 mRNA expression is greater in heterozygote controls than in SOX8 knockout mice during puberty and adulthood (Singh et al. 2009). The mechanisms that are involved in the switch of SOX8 regulation of claudin 3 transcription after establishment of the BTB remain to be determined, as is a determination of whether SOX8 regulation is direct or indirect.
In mice gene-deleted for the transcription factor ETV5, claudin 5 protein is not expressed in the seminiferous epithelium but is expressed in vascular endothelium and rete testes epithelium (figure 6; Morrow et al. 2009). Thus, claudin 5 expression is ETV5-dependent in the seminiferous epithelium but ETV5-independent in vascular endothelium and rete testes epithelium. Analyses of the claudin 5 promoter in various endothelial cell models reveal a variety of binding sites and transcription factors that can regulate claudin 5 expression (Fontijn et al. 2008; Burek & Forster 2009). What role these other transcription factors have in mediating ETV5-independent expression of claudin 5 in testicular vascular endothelium and rete testis epithelium remains to be determined. In wild-type mice, claudin 5 protein is expressed in both Sertoli cells and germ cells (Morrow et al. 2009). It is not known if ETV5 is required in both of these cell types for each to express claudin 5, or if ETV5 expression in 1 cell type with subsequent intercellular signals to the other is sufficient for claudin 5 expression in the seminiferous epithelium. It also needs to be determined if ETV5 regulation of claudin 5 transcription within a Sertoli or germ cell is direct or indirect.
Figure 6.
Claudin 5 expression in ETV5 knockout mice. Claudin 5 immunostain (green) is detectable in the rete testes epithelium of ETV5 knockout mice but is not detectable in the seminiferous epithelium (lower right). Claudin 5 is also expressed in testicular vascular endothelium (not shown). Reprinted from Morrow et al. (2009). Scale bar, 50 µm.
Claudin 11 expression in Sertoli cells is influenced by numerous transcription factors and coregulators (Lui et al. 2007). Specifically, the transcription factors GATA, NF-YA, and CREB form a complex that binds to the GATA/NF-Y regions of the claudin 11 promoter and induce transcription of claudin 11 mRNA. Negative control of claudin 11 transcription is mediated by Smad proteins, which bind to the GATA/NF-Y site that is furthest upstream from the origin. Smad proteins recruit the histone deacetylase 1 (HDAC1)/mSin3A complex, thus inducing histone deacetylation with the subsequent increase in DNA winding and hindrance for other transcription factors to bind. Analyses of the reported expression patterns of these transcription factors in the testes suggest that the ratio of the positive to the negative regulatory factors is important for maintaining claudin 11 expression throughout the stages of the seminiferous epithelial cycle (Lui et al. 2007). In gastric epithelial cells, hypermethylation of the claudin 11 promoter is associated with decreased expression of claudin 11 mRNA and protein (Agarwal et al. 2009); whether this is also true for Sertoli cells remains to be determined.
Promoter analysis of occludin in Sertoli cells has not been reported. In vascular endothelial cells, promoter analyses revealed that glucocorticoid receptor is important for occludin expression (Felinski et al. 2008; Harke et al. 2008). Activation by the transcription factor Sp3 and repression by YY1 are important for differential expression of occludin in various endothelial beds (Sade et al. 2009).
(c). Follicle stimulating hormone
The effects of FSH on the mRNA and protein expression of claudins 3 and 11 and occludin have been extensively studied in the Djungarian hamster (table 3), a seasonal breeding rodent (Tarulli et al. 2006, 2008). Increased light exposures, during the long-day photoperiods of summer, stimulate FSH secretion and result in active spermatogenesis. Conversely, FSH secretion is inhibited and spermatogenesis is quiescent during the short-day photoperiods of winter. When exogenous FSH is administered to hamsters exposed to short-day (8 h of light) photoperiods, claudins 3 and 11 and occludin proteins all undergo dramatic relocalization to the BTB. Whereas the claudin 3 protein expression at the BTB is transient, claudin 11 and occludin protein remain localized at the BTB in hamsters exposed to long-day (16 h of light) photoperiods. Through the relocalization of these and other proteins to the BTB, exposure to FSH changes the permeability of the BTB from leaky to tight. Interestingly, FSH suppresses the mRNA expression of claudins 3 and 11 and occludin in these hamsters, in that the mRNA expression values are lower in hamsters exposed to long-day photoperiods compared with values in those exposed to short-day photoperiods. Thus in Djungarian hamsters, increased mRNA expressions of claudins 3 and 11 and occludin do not indicate increased BTB functionality. It was noted that in Djungarian hamsters, the responses of testicular claudins 3 and 11 and occludin to FSH stimulation occur during a time period when intratesticular testosterone levels are low (Tarulli et al. 2008). This was interpreted as a difference between Djungarian hamsters and other rodent species regarding the importance of testosterone in establishing and maintaining the BTB.
Table 3.
Effects of FSH on expression of claudins 3 and 11 and occludin in Djungarian hamsters (Tarulli et al. 2006, 2008).
| effecta,b |
||||||
|---|---|---|---|---|---|---|
| claudin 3 |
claudin 11 |
occludin |
||||
| treatment | mRNA expression | protein localization | mRNA expression | protein localization | mRNA expression | protein localization |
| exposure of Djungarian hamsters to 16 h light per day for 12 weeks to stimulate endogenous FSH release | ↓ | associated with elongate spermatids | ↓ | expression at BTB | ↓ | stage-specific BTB expression |
| exposure of Djungarian hamsters to 8 h light per day for 12 weeks to suppress endogenous FSH release | ↑ | expression at apical regions of Sertoli cells | ↑ | cytoplasmic expression | ↑ | cytoplasmic expression |
| exogenous FSH given to Djungarian hamsters exposed to 8 h light per day for 12 weeks | day 2: expression at apical regions of Sertoli cells and at BTB; | days 2 and 4: relocalization from cytoplasm to BTB | days 2 and 4: expressed at apical regions of Sertoli cells and at BTB | |||
| day 4: diminished apical expression, strong BTB expression | day 10: expression at BTB similar to that of long-day photoperiod hamsters | day 10: diminished apical expression, strong BTB expression | ||||
| day 10: variable expression at BTB | ||||||
a↑: increased expression; ↓, decreased expression; —, no change in expression.
bmRNA expression is on a Sertoli cell basis.
Experiments evaluating the effects of FSH on claudin 11 mRNA expression in mice and rats have had variable results (table 4). In cultured mouse Sertoli cells, FSH supplementation decreases claudin 11 mRNA expression during the first 24 h, with values returning to baseline by 48 h (Hellani et al. 2000). In cultured rat Sertoli cells supplemented with FSH for 7 days, claudin 11 mRNA expression is similar to unsupplemented cells for the first 2 days, increases on day 3, and remains elevated through day 7 (Kaitu'u-Lino et al. 2007). Differences between these in vitro studies were attributed to differing culture conditions (Kaitu'u-Lino et al. 2007). In vivo, FSH suppresses testicular claudin 11 mRNA expression in Djungarian hamsters but stimulates claudin 11 expression in mice, as claudin 11 mRNA expression per Sertoli cell is decreased in mice gene-deleted for the β-subunit of FSH or for the FSH receptor, compared with values in adult wild-type mice (Johnston et al. 2004). Differences in FSH signalling among various lines of AR knockout mice, attributable to different levels of FSH secretion or Sertoli cell FSH receptors, were considered a potential reason for different expression levels of claudin 11 mRNA per Sertoli cell among those lines (Tan et al. 2005). The effects of FSH on claudin 11 protein localization in mice and rat Sertoli cells has not been reported. Regarding occludin, in primary cultures of rat Sertoli cells, supplementation with FSH for up to 7 days has no effect on its mRNA expression (Kaitu'u-Lino et al. 2007).
Table 4.
Effects of FSH on mRNA expression of claudin 11 and occludin in mice and rats.
| mRNA | treatment | effecta | reference |
|---|---|---|---|
| claudin 11 | FSH supplementation of primary cultures of mouse Sertoli cells | 6–24 h: ↓; 1 h, 48 h: — | Hellani et al. (2000) |
| FSH supplementation of primary cultures of rat Sertoli cells | day 1 and 2: —; days 3–7: ↑ | Kaitu'u-Lino et al. (2007) | |
| gene deletion of the β-subunit of FSH in mice | ↑ | Johnston et al. (2004) | |
| gene deletion of the FSH receptor | ↑ | Johnston et al. (2004) | |
| occludin | FSH supplementation of primary cultures of rat Sertoli cells | days 1–7: — | Kaitu'u-Lino et al. (2007) |
a↑: increased expression; ↓, decreased expression; —, no change in expression.
(d). Germ cells
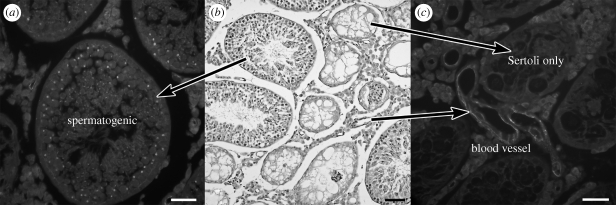
Germ cells are necessary for expression of claudin 5 in murine seminiferous epithelium. In the testes of W/Wv mice that received spermatogonial stem cell transplants, there is a mixture of aspermic tubules and tubules in which spermatogenesis has been successfully established. Claudin 5 protein is not detected in aspermic tubules but it is detected in tubules with spermatogenesis (figure 7; Morrow et al. 2009). Similar to wild-type mice, both Sertoli cells and germ cells appear to express claudin 5 protein. The germ cell stimulatory signal that induces claudin 5 expression in Sertoli cells remains to be determined.
Figure 7.
Effect of germ cells on claudin 5 expression. Representative images from W/Wv mice that received spermatogonial stem cell transplants from wild-type donors showing (a) expression of claudin 5 in tubules in which spermatogenesis was established; scale bar, 50 µm; (b) mixture of spermatogenic and aspermic tubules (PAS/hematoxylin); scale bar, 100 µm; (c) absence of claudin 5 in aspermic tubules; scale bar, 75 µm. Claudin 5 is also expressed in the vascular endothelium. (Note: the images are from different mice; modified from Morrow et al. (2009).)
Postmeiotic germ cells have an inhibitory effect on claudin 11 expression in rat seminiferous epithelium, whereas spermatogonia and premeiotic spermatocytes have no effect on claudin 11 expression (Florin et al. 2005). There is no difference in claudin 11 mRNA and protein expression per Sertoli cell between irradiated testes of rats killed 10 days after local X-ray irradiation and non-irradiated testes of control rats; at this time point spermatogonia are absent but spermatocytes and spermatids are present (Florin et al. 2005). In rats killed 45 days after testes irradiation, claudin 11 mRNA and protein expression per Sertoli cell are increased, compared with values in non-irradiated testes; the irradiated testes have spermatogonia and spermatocytes by not postmeiotic spermatids at this time point (Florin et al. 2005). In cocultures of Sertoli cells and either spermatocytes or spermatids, claudin 11 mRNA expression is not different in spermatocyte cocultures, whereas it is decreased in spermatid cocultures, compared with values in Sertoli cell-only cultures (Florin et al. 2005). Among the proteins produced by round spermatids, growth differentiation factor 9 (GDF9) is a candidate for the claudin 11 inhibitory factor. Addition of GDF9 to primary cultures of rat Sertoli cells decreases claudin 11 protein expression and localization to the cell membrane; localization of occludin and ZO-1 were also affected (Nicholls et al. 2009).
In men, the neoplastic germ cells of testicular intraepithelial neoplasia (TIN) lesions also affect Sertoli cell claudin 11 expression and localization. Compared with the expression in tubules with normal spermatogenesis, claudin 11 mRNA and protein are both increased in tubules with TIN (Fink et al. 2009). However, claudin 11 protein localization in tubules with TIN is substantially decreased at the BTB and increased in the cytoplasm (Fink et al. 2009). This altered claudin 11 localization is associated with similar alterations in ZO-1 and -2 localization and leakage of tracer across the BTB (Fink et al. 2006).
7. Claudin functions in the testes
In addition to contributing to BTB integrity, claudin 3 has been proposed as a marker for the hypothetical intermediate compartment (Komljenovic et al. 2009). This transient compartment was described by Lonnie Russell, who observed ultrastructurally normal tight junctions, both apically and basally to the preleptotene and leptotene spermatocytes, during Stages VIII–IX in rats (Russell 1977). In addition to claudin 3, these flanking tight junctions also contain occludin (Mirza et al. 2007). However, there is no evidence that both of the flanking tight junctions are functional at the same time. In a study in which tracers were injected intratubularly, intravascularly, or both, leptotene spermatocytes in Stages IX–X were surrounded by the intravascular tracer that was stopped just above these cells, whereas those in Stage X were surrounded by the intratubular tracer that was stopped just below these cells; the presence of two competent barriers associated with leptotene spermatocytes was not detected (Cavicchia & Sacerdote 1988).
The increase in expression of claudins 3 and 5 during Stage VIII and the known interactions between these claudin isoforms in other epithelia (Coyne et al. 2003; Daugherty et al. 2007) suggest that interactions between claudins 3 and 5 in Sertoli cells may be important for BTB dynamics during migration of preleptotene and leptotene spermatocytes (Morrow et al. 2009). One possible contribution of claudin 3–claudin 5 interactions may be to provide structural integrity during a period when other integral membrane proteins are being rapidly recycled. However, in other cell types, the barrier characteristics produced by claudin 3–claudin 5 interactions is highly dependent on cell type, expression levels of claudin 3 or claudin 5, and expression levels of other claudins (such as claudin 1; Coyne et al. 2003). It cannot be predicted whether the interactions between the extracellular loops of claudins 3 and 5 would induce a ‘leakier’ or ‘tighter’ barrier phenotype between Sertoli cells at Stage VIII versus the barrier phenotype at stages when these proteins are not expressed. Also, current models of BTB dynamic restructuring during Stage VIII suggest that induced expression of basal ectoplasmic specialization proteins may provide a ‘patch’ to maintain barrier integrity while the tight junctions are opened to allow spermatocyte passage (Yan et al. 2008b). In a model in which barrier integrity is maintained by adherens junctional proteins, structural integrity provided by homophilic and heterophilic interactions between claudins 3 and 5 would be minimal.
It is also possible that claudins 3 and 5 expression during Stage VII may contribute to cell signalling pathways rather than providing only direct structural support. The intracellular framework among tight junctional integral membrane and scaffolding proteins acts as a platform for numerous signalling molecules (Zahraoui et al. 2000; Siu et al. 2009). Indeed, claudin 11-based tight junctions are necessary for cell cycle regulation of Sertoli cells, implying a cell signalling function for claudin 11 (Mazaud-Guittot et al. 2010).
The function of claudin 5 in germ cells is unknown. It has been suggested that interactions between claudin 5 and membrane-type matrix metalloproteinases (MT-MMPs) on the germ cell membrane may enable activation of soluble MMPs in the basal compartment (Morrow et al. 2009). Although this interaction between claudins, MT-MMPs and soluble MMPs has been demonstrated in vitro in other epithelial cells, there is no in vivo or in vitro experimental evidence for this model in germ cells.
Claudin 11 is necessary for the morphology of the tight junction strands in the testes. Tight junction strands between Sertoli cells are mostly parallel to each other with few branch points, whereas in other epithelia the tight junction strands have numerous anastomoses (Gilula et al. 1976). Fibroblasts transfected with claudin 11 develop parallel tight junction strands, similar to the strands in Sertoli cells (Morita et al. 1999b). Interestingly, Sertoli cells in claudin 11 knockout mice that are detached from the basement membrane lose polarity and acquire a shape similar to fibroblasts (Mazaud-Guittot et al. 2010).
Claudin 11 tight junctions between Sertoli cells have a role in cell cycle regulation. Sertoli cells in adult claudin 11 knockout mice are able to proliferate in vivo (Mazaud-Guittot et al. 2010). This proliferation is not due to retention of an immature state or to dedifferentiation, as these Sertoli cells express numerous differentiation markers. It was hypothesized that claudin 11 tight junctions enable contact inhibition between Sertoli cells, thereby maintaining the Sertoli cells in a quiescent state (Mazaud-Guittot et al. 2010). Regulation of the seminiferous tubule microenvironment by claudin 11 tight junctions is also important for attachment of the Sertoli cells to the basement membrane, proper epithelial organization, and nurturing of germ cells (Mazaud-Guittot et al. 2010).
8. Future directions
Species comparisons of the claudins and occludin are important, especially in the field of pharmaceutical development. Much information about the BTB, particularly the structures and proteins involved in dynamic restructuring during preleptotene spermatocyte migration, has been discovered from the use of mice and rats (Mruk & Cheng 2004; Lie et al. 2009; Cheng et al. 2010). However, comparison among rodent species reveals differences in the expression of claudin 3 (Meng et al. 2005; Kaitu'u-Lino et al. 2007; Tarulli et al. 2008), and comparison among rodent species and men reveals differences in occludin expression (Moroi et al. 1998; Cyr et al. 1999; Li et al. 2006). Updated characterization of occludin and claudin 11 protein expression and initial characterization of claudins 3 and 5 protein expression in the seminiferous epithelium of men will aid in determining the applicability of discoveries made in rodent models to humans.
Results of investigations of claudin–claudin interactions in the extracellular space have revealed that the types of claudin–claudin interactions and resultant barrier characteristics are a function of the claudin isoforms expressed in the cells (Morita et al. 1999b; Hoevel et al. 2002; Coyne et al. 2003; Van Itallie et al. 2003; Wen et al. 2004). Specifically, claudins 3, 5, and 11 have homophilic interactions with their respective isoforms, claudins 3 and 5 have heterophilic interactions with each other, and the barrier characteristics (neutral molecule flux, transelectrical resistance) are different among cell cultures that express claudin 3 only, claudin 5 only, or both claudins 3 and 5 (Coyne et al. 2003; Morita et al. 1999b). However, it is unknown how the heterophilic and homophilic interactions of claudins 3 and 5 are altered when claudin 11 is also expressed. Investigation of the barrier characteristics of cells expressing various combinations of claudins 3, 5, and 11 would provide insight into the barrier functions of the seminiferous epithelium during the various cycle stages, particularly during preleptotene spermatocyte migration at Stage VIII when expression of claudins 3 and 5 are highest. Cell signalling molecules known to be involved in BTB dynamic restructuring should also be evaluated in addition to the barrier characteristics. This would be helpful in determining the relative structural versus cell signalling roles of claudins 3 and 5 in Sertoli cells and provide for integration of these claudins into current models BTB dynamic restructuring (Yan et al. 2008b).
Germ cells are needed to stimulate expression of claudin 5 by Sertoli cell in vivo (Morrow et al. 2009). It can be presumed that coculture with germ cells or supplementation with the unidentified germ cell-derived stimulant would be necessary for Sertoli cells to express claudin 5 in vitro. Alternatively, until the germ cell-derived stimulant is identified, transfection of Sertoli cells with vectors containing claudin 5 DNA may be necessary to induce claudin 5 expression in Sertoli cell cultures.
Expression of claudin 5 in germ cells, cells that do not have tight junctions, suggests that additional roles for this protein may be found (Morrow et al. 2009). Ultrastructural examination and immunogold analysis to discover the germ cell organelles with which claudin 5 is associated is an initial step towards discovering those roles. Also, in other cell types, the membrane proteins MMP14 (Miyamori et al. 2001) and connexin 43 (Nagasawa et al. 2006) are associated with claudin 5. Interactions between claudin 5 and MMP14 on germ cell membranes to activate pro-MMP2 has been suggested as a germ cell function of claudin 5 (Morrow et al. 2009), and gap junctions derived from connexin 43 are a source of cell–cell communication from Sertoli cells to spermatogonia and spermatocytes (Decrouy et al. 2004). Therefore MMP14 and connexin 43 are recommended as initial candidates for evaluation of colocalization with claudin 5 on germ cell membranes.
The expression of claudin 5 in germ cells along with the expression of claudins 3 and 5 in Sertoli cells during Stage VIII suggests that claudin–claudin interactions between germ cells and Sertoli cells may be a means of guidance during preleptotene spermatocyte migration. However, if claudin–claudin interactions were a guide for germ cell migration, these trans interactions would be different than the interactions proposed by current models. The trans claudin–claudin interactions described by current models result in formation of tight junctions and obliteration of the intercellular space, which are not observed between germ cells and Sertoli cells.
Modalities used to evaluate integral membrane proteins of the BTB include mRNA quantification by use of Northern blot or quantitative PCR analyses, protein quantification via Western blot analysis, and protein localization by use of immunohistochemical analysis. It is interesting that some conditions associated with increased (or decreased) mRNA expression were also associated with decreased (or increased) protein localization to the cell membrane (Tarulli et al. 2006, 2008; Kaitu'u-Lino et al. 2007; Fink et al. 2009). Future studies should be cautious in making statements regarding integral membrane protein functionality that are based solely on mRNA quantification. Ultimately, protein localization analyses should be performed as a more definitive aid in determining protein functionality in the BTB.
9. Conclusion
The recent discovery of claudins 3 and 5 as components of the BTB have increased the complexity involved in studying the function of this unique epithelial barrier. The similar expression patterns of claudins 3 and 5 in murine seminiferous epithelium suggest that interactions between these proteins may be important for mediating BTB dynamics during preleptotene germ cell migration. Future studies should be directed towards understanding the interactions between integral membrane proteins of the BTB and identifying species differences in protein expression and function among the rodent species and men.
Footnotes
One contribution of 17 to a Theme Issue ‘The biology and regulation of spermatogenesis’.
REFERENCES
- Agarwal R., D'Souza T., Morin P. J.2005Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 65, 7378–7385 (doi:10.1158/0008-5472.CAN-05-1036) [DOI] [PubMed] [Google Scholar]
- Agarwal R., et al. 2009Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS ONE 4, e8002 (doi:10.1371/journal.pone.0008002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briehl M. M., Miesfeld R. L.1991Isolation and characterization of transcripts induced by androgen withdrawal and apoptotic cell death in the rat ventral prostate. Mol. Endocrinol. 5, 1381–1388 (doi:10.1210/mend-5-10-1381) [DOI] [PubMed] [Google Scholar]
- Burek M., Forster C. Y.2009Cloning and characterization of the murine claudin-5 promoter. Mol. Cell Endocrinol. 298, 19–24 (doi:10.1016/j.mce.2008.09.041) [DOI] [PubMed] [Google Scholar]
- Cavicchia J. C., Sacerdote F. L.1988Topography of the rat blood–testis barrier after intratubular administration of intercellular tracers. Tissue Cell 20, 577–586 (doi:10.1016/0040-8166(88)90059-6) [DOI] [PubMed] [Google Scholar]
- Chen C., et al. 2005ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 436, 1030–1034 (doi:10.1038/nature03894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y., Wong E. W., Yan H. H., Mruk D. D.2010Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol. Cell Endocrinol. 315, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung N. P., Cheng C. Y.2001Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology 142, 1878–1888 (doi:10.1210/en.142.5.1878) [DOI] [PubMed] [Google Scholar]
- Chung N. P., Mruk D., Mo M. Y., Lee W. M., Cheng C. Y.2001A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood–testis barrier and disrupts spermatogenesis reversibly in vivo. Biol. Reprod. 65, 1340–1351 (doi:10.1095/biolreprod65.5.1340) [DOI] [PubMed] [Google Scholar]
- Coyne C. B., Gambling T. M., Boucher R. C., Carson J. L., Johnson L. G.2003Role of claudin interactions in airway tight junctional permeability. Am. J. Physiol. Lung Cell Mol. Physiol. 285, L1166–L1178 [DOI] [PubMed] [Google Scholar]
- Cyr D. G., Hermo L., Egenberger N., Mertineit C., Trasler J. M., Laird D. W.1999Cellular immunolocalization of occludin during embryonic and postnatal development of the mouse testis and epididymis. Endocrinology 140, 3815–3825 (doi:10.1210/en.140.8.3815) [DOI] [PubMed] [Google Scholar]
- Daugherty B. L., Ward C., Smith T., Ritzenthaler J. D., Koval M.2007Regulation of heterotypic claudin compatibility. J. Biol. Chem. 282, 30 005–30 013 (doi:10.1074/jbc.M703547200) [DOI] [PubMed] [Google Scholar]
- Decrouy X., Gasc J. M., Pointis G., Segretain D.2004Functional characterization of Cx43 based gap junctions during spermatogenesis. J. Cell Physiol. 200, 146–154 (doi:10.1002/jcp.10473) [DOI] [PubMed] [Google Scholar]
- Eacker S. M., Shima J. E., Connolly C. M., Sharma M., Holdcraft R. W., Griswold M. D., Braun R. E.2007Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol. Endocrinol. 21, 895–907 (doi:10.1210/me.2006-0113) [DOI] [PubMed] [Google Scholar]
- Evans M. J., et al. 2007Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446, 801–805 (doi:10.1038/nature05654) [DOI] [PubMed] [Google Scholar]
- Everett R. S., Vanhook M. K., Barozzi N., Toth I., Johnson L. G.2006Specific modulation of airway epithelial tight junctions by apical application of an occludin peptide. Mol. Pharmacol. 69, 492–500 (doi:10.1124/mol.105.017251) [DOI] [PubMed] [Google Scholar]
- Felinski E. A., Cox A. E., Phillips B. E., Antonetti D. A.2008Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp. Eye Res. 86, 867–878 (doi:10.1016/j.exer.2008.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijak M., Meinhardt A.2006The testis in immune privilege. Immunol. Rev. 213, 66–81 (doi:10.1111/j.1600-065X.2006.00438.x) [DOI] [PubMed] [Google Scholar]
- Fink C., Weigel R., Hembes T., Lauke-Wettwer H., Kliesch S., Bergmann M., Brehm R. H.2006Altered expression of ZO-1 and ZO-2 in Sertoli cells and loss of blood–testis barrier integrity in testicular carcinoma in situ. Neoplasia 8, 1019–1027 (doi:10.1593/neo.06559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink C., Weigel R., Fink L., Wilhelm J., Kliesch S., Zeiler M., Bergmann M., Brehm R.2009Claudin-11 is over-expressed and dislocated from the blood–testis barrier in Sertoli cells associated with testicular intraepithelial neoplasia in men. Histochem. Cell Biol. 131, 755–764 (doi:10.1007/s00418-009-0576-2) [DOI] [PubMed] [Google Scholar]
- Florin A., Maire M., Bozec A., Hellani A., Chater S., Bars R., Chuzel F., Benahmed M.2005Androgens and postmeiotic germ cells regulate claudin-11 expression in rat Sertoli cells. Endocrinology 146, 1532–1540 (doi:10.1210/en.2004-0834) [DOI] [PubMed] [Google Scholar]
- Fontijn R. D., Volger O. L., Fledderus J. O., Reijerkerk A., De Vries H. E., Horrevoets A. J.2008SOX-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am. J. Physiol. Heart Circ. Physiol. 294, H891–H900 (doi:10.1152/ajpheart.01248.2007) [DOI] [PubMed] [Google Scholar]
- Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S.1993Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123, 1777–1788 (doi:10.1083/jcb.123.6.1777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita S.1998aClaudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141, 1539–1550 (doi:10.1083/jcb.141.7.1539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Sasaki H., Fujimoto K., Tsukita S.1998bA single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 143, 391–401 (doi:10.1083/jcb.143.2.391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., Noda T., Kubo A., Tsukita S.2002Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 156, 1099–1111 (doi:10.1083/jcb.200110122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula N. B., Fawcett D. W., Aoki A.1976The Sertoli cell occluding junctions and gap junctions in mature and developing mammalian testis. Dev. Biol. 50, 142–168 (doi:10.1016/0012-1606(76)90074-9) [DOI] [PubMed] [Google Scholar]
- Gow A., et al. 1999CNS myelin and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 99, 649–659 (doi:10.1016/S0092-8674(00)81553-6) [DOI] [PubMed] [Google Scholar]
- Gu J. M., Lim S. O., Park Y. M., Jung G.2008A novel splice variant of occludin deleted in exon 9 and its role in cell apoptosis and invasion. FEBS J. 275, 3145–3156 (doi:10.1111/j.1742-4658.2008.06467.x) [DOI] [PubMed] [Google Scholar]
- Gye M. C.2003aChanges in the expression of claudins and transepithelial electrical resistance of mouse Sertoli cells by Leydig cell coculture. Int. J. Androl. 26, 271–278 (doi:10.1046/j.1365-2605.2003.00423.x) [DOI] [PubMed] [Google Scholar]
- Gye M. C.2003bExpression of claudin-1 in mouse testis. Arch. Androl. 49, 271–279 (doi:10.1080/01485010390204913) [DOI] [PubMed] [Google Scholar]
- Gye M. C.2004Expression of occludin in canine testis and epididymis. Reprod. Domest. Anim. 39, 43–47 (doi:10.1046/j.1439-0531.2003.00474.x) [DOI] [PubMed] [Google Scholar]
- Gye M. C., Ohsako S.2003Effects of flutamide in the rat testis on the expression of occludin, an integral member of the tight junctions. Toxicol. Lett. 143, 217–222 (doi:10.1016/S0378-4274(03)00178-4) [DOI] [PubMed] [Google Scholar]
- Harke N., Leers J., Kietz S., Drenckhahn D., Forster C.2008Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Mol. Cell. Endocrinol. 295, 39–47 (doi:10.1016/j.mce.2008.08.011) [DOI] [PubMed] [Google Scholar]
- Hellani A., Ji J., Mauduit C., Deschildre C., Tabone E., Benahmed M.2000Developmental and hormonal regulation of the expression of oligodendrocyte-specific protein/claudin 11 in mouse testis. Endocrinology 141, 3012–3019 (doi:10.1210/en.141.8.3012) [DOI] [PubMed] [Google Scholar]
- Hoevel T., Macek R., Mundigl O., Swisshelm K., Kubbies M.2002Expression and targeting of the tight junction protein CLDN1 in CLDN1-negative human breast tumor cells. J. Cell Physiol. 191, 60–68 (doi:10.1002/jcp.10076) [DOI] [PubMed] [Google Scholar]
- Holdcraft R. W., Braun R. E.2004Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131, 459–467 (doi:10.1242/dev.00957) [DOI] [PubMed] [Google Scholar]
- Janecki A., Jakubowiak A., Steinberger A.1991Regulation of transepithelial electrical resistance in two-compartment Sertoli cell cultures: in vitro model of the blood–testis barrier. Endocrinology 129, 1489–1496 (doi:10.1210/endo-129-3-1489) [DOI] [PubMed] [Google Scholar]
- Johnston H., Baker P. J., Abel M., Charlton H. M., Jackson G., Fleming L., Kumar T. R., O'Shaughnessy P. J.2004Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 145, 318–329 (doi:10.1210/en.2003-1055) [DOI] [PubMed] [Google Scholar]
- Kaitu'u-Lino T. J., Sluka P., Foo C. F., Stanton P. G.2007Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction 133, 1169–1179 (doi:10.1530/REP-06-0385) [DOI] [PubMed] [Google Scholar]
- Katahira J., Inoue N., Horiguchi Y., Matsuda M., Sugimoto N.1997aMolecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J. Cell Biol. 136, 1239–1247 (doi:10.1083/jcb.136.6.1239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira J., Sugiyama H., Inoue N., Horiguchi Y., Matsuda M., Sugimoto N.1997bClostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J. Biol. Chem. 272, 26 652–26 658 (doi:10.1074/jbc.272.42.26652) [DOI] [PubMed] [Google Scholar]
- Komljenovic D., Sandhoff R., Teigler A., Heid H., Just W. W., Gorgas K.2009Disruption of blood–testis barrier dynamics in ether-lipid-deficient mice. Cell Tissue Res. 337, 281–299 (doi:10.1007/s00441-009-0809-7) [DOI] [PubMed] [Google Scholar]
- Krause G., Winkler L., Mueller S. L., Haseloff R. F., Piontek J., Blasig I. E.2008Structure and function of claudins. Biochim. Biophys. Acta 1778, 631–645 [DOI] [PubMed] [Google Scholar]
- Kubota K., Furuse M., Sasaki H., Sonoda N., Fujita K., Nagafuchi A., Tsukita S.1999Ca(2+)-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr. Biol. 9, 1035–1038 (doi:10.1016/S0960-9822(99)80452-7) [DOI] [PubMed] [Google Scholar]
- Li M. W., Xia W., Mruk D. D., Wang C. Q., Yan H. H., Siu M. K., Lui W. Y., Lee W. M., Cheng C. Y.2006Tumor necrosis factor α reversibly disrupts the blood–testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J. Endocrinol. 190, 313–329 (doi:10.1677/joe.1.06781) [DOI] [PubMed] [Google Scholar]
- Li M. W., Mruk D. D., Lee W. M., Cheng C. Y.2009Connexin 43 and plakophilin-2 as a protein complex that regulates blood–testis barrier dynamics. Proc. Natl Acad. Sci. USA 106, 10 213–10 218 (doi:10.1073/pnas.0901700106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie P. P., Xia W., Wang C. Q., Mruk D. D., Yan H. H., Wong C. H., Lee W. M., Cheng C. Y.2006Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood–testis barrier in adult rat testes. J. Endocrinol. 191, 571–586 (doi:10.1677/joe.1.06996) [DOI] [PubMed] [Google Scholar]
- Lie P. P., Cheng C. Y., Mruk D. D.2009Coordinating cellular events during spermatogenesis: a biochemical model. Trends Biochem. Sci. 34, 366–373 (doi:10.1016/j.tibs.2009.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui W. Y., Wong E. W., Guan Y., Lee W. M.2007Dual transcriptional control of claudin-11 via an overlapping GATA/NF-Y motif: positive regulation through the interaction of GATA, NF-YA, and CREB and negative regulation through the interaction of Smad, HDAC1, and mSin3A. J. Cell Physiol. 211, 638–648 (doi:10.1002/jcp.20970) [DOI] [PubMed] [Google Scholar]
- Mankertz J., Waller J. S., Hillenbrand B., Tavalali S., Florian P., Schoneberg T., Fromm M., Schulzke J. D.2002Gene expression of the tight junction protein occludin includes differential splicing and alternative promoter usage. Biochem. Biophys. Res. Commun. 298, 657–666 (doi:10.1016/S0006-291X(02)02487-7) [DOI] [PubMed] [Google Scholar]
- Mazaud-Guittot S., Meugnier E., Pesenti S., Wu X., Vidal H., Gow A., Le Magueresse-Battistoni B.2010Claudin 11 Deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol. Reprod. 82, 202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Holdcraft R. W., Shima J. E., Griswold M. D., Braun R. E.2005Androgens regulate the permeability of the blood–testis barrier. Proc. Natl Acad. Sci. USA 102, 16 696–16 700 (doi:10.1073/pnas.0506084102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl P., et al. 2003Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer. Res. 63, 6265–6271 [PubMed] [Google Scholar]
- Mima S., Takehara M., Takada H., Nishimura T., Hoshino T., Mizushima T.2008NSAIDs suppress the expression of claudin-2 to promote invasion activity of cancer cells. Carcinogenesis 29, 1994–2000 (doi:10.1093/carcin/bgn134) [DOI] [PubMed] [Google Scholar]
- Mirza M., Petersen C., Nordqvist K., Sollerbrant K.2007Coxsackievirus and adenovirus receptor is up-regulated in migratory germ cells during passage of the blood–testis barrier. Endocrinology 148, 5459–5469 (doi:10.1210/en.2007-0359) [DOI] [PubMed] [Google Scholar]
- Miyamori H., Takino T., Kobayashi Y., Tokai H., Itoh Y., Seiki M., Sato H.2001Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J. Biol. Chem. 276, 28 204–28 211 (doi:10.1074/jbc.M103083200) [DOI] [PubMed] [Google Scholar]
- Morita K., Furuse M., Fujimoto K., Tsukita S.1999aClaudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 96, 511–516 (doi:10.1073/pnas.96.2.511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Sasaki H., Fujimoto K., Furuse M., Tsukita S.1999bClaudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J. Cell Biol. 145, 579–588 (doi:10.1083/jcb.145.3.579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi S., Saitou M., Fujimoto K., Sakakibara A., Furuse M., Yoshida O., Tsukita S.1998Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am. J. Physiol. 274, C1708–C1717 [DOI] [PubMed] [Google Scholar]
- Morris A. P., Tawil A., Berkova Z., Wible L., Smith C. W., Cunningham S. A.2006Junctional adhesion molecules (JAMs) are differentially expressed in fibroblasts and co-localize with ZO-1 to adherens-like junctions. Cell Commun. Adhes. 13, 233–247 (doi:10.1080/15419060600877978) [DOI] [PubMed] [Google Scholar]
- Morrow C. M., Tyagi G., Simon L., Carnes K., Murphy K. M., Cooke P. S., Hofmann M. C., Hess R. A.2009Claudin 5 expression in mouse seminiferous epithelium is dependent upon the transcription factor Ets-variant 5 and contributes to blood–testis barrier function. Biol. Reprod. 81, 871–879 (doi:10.1095/biolreprod.109.077040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsny R. J., Brown G. T., Gerner-Smidt K., Buret A. G., Meddings J. B., Quan C., Koval M., Nusrat A.2008A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am. J. Pathol. 172, 905–915 (doi:10.2353/ajpath.2008.070698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y.2004Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 (doi:10.1210/er.2003-0022) [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Chiba H., Fujita H., Kojima T., Saito T., Endo T., Sawada N.2006Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell Physiol. 208, 123–132 (doi:10.1002/jcp.20647) [DOI] [PubMed] [Google Scholar]
- Nicholls P. K., Harrison C. A., Gilchrist R. B., Farnworth P. G., Stanton P. G.2009Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function. Endocrinology 150, 2481–2490 (doi:10.1210/en.2008-1048) [DOI] [PubMed] [Google Scholar]
- Nitta T., Hata M., Gotoh S., Seo Y., Sasaki H., Hashimoto N., Furuse M., Tsukita S.2003Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J. Cell Biol. 161, 653–660 (doi:10.1083/jcb.200302070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A., Brown G. T., Tom J., Drake A., Bui T. T., Quan C., Mrsny R. J.2005Multiple protein interactions involving proposed extracellular loop domains of the tight junction protein occludin. Mol. Biol. Cell. 16, 1725–1734 (doi:10.1091/mbc.E04-06-0465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan M. K., et al. 2008Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev. Biol. 316, 359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda M., Suarez-Quian C. A., Djakiew D., Dym M.1990Characterization of Sertoli cells cultured in the bicameral chamber system: relationship between formation of permeability barriers and polarized secretion of transferrin. Biol. Reprod. 43, 672–683 (doi:10.1095/biolreprod43.4.672) [DOI] [PubMed] [Google Scholar]
- Paris L., Tonutti L., Vannini C., Bazzoni G.2008Structural organization of the tight junctions. Biochim. Biophys. Acta 1778, 646–659 [DOI] [PubMed] [Google Scholar]
- Parreira G. G., Melo R. C., Russell L. D.2002Relationship of Sertoli–Sertoli tight junctions to ectoplasmic specialization in conventional and en face views. Biol. Reprod. 67, 1232–1241 (doi:10.1095/biolreprod67.4.1232) [DOI] [PubMed] [Google Scholar]
- Ploss A., Evans M. J., Gaysinskaya V. A., Panis M., You H., De Jong Y. P., Rice C. M.2009Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457, 882–886 (doi:10.1038/nature07684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Vara J. A.2005Technical aspects of immunohistochemistry. Vet. Pathol. 42, 405–426 (doi:10.1354/vp.42-4-405) [DOI] [PubMed] [Google Scholar]
- Russell L.1977Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am. J. Anat. 148, 313–328 (doi:10.1002/aja.1001480303) [DOI] [PubMed] [Google Scholar]
- Russell L. D., Peterson R. N.1985Sertoli cell junctions: morphological and functional correlates. Int. Rev. Cytol. 94, 177–211 (doi:10.1016/S0074-7696(08)60397-6) [DOI] [PubMed] [Google Scholar]
- Sade H., Holloway K., Romero I. A., Male D.2009Transcriptional control of occludin expression in vascular endothelia: regulation by Sp3 and YY1. Biochim. Biophys. Acta 1789, 175–184 [DOI] [PubMed] [Google Scholar]
- Saitou M., Fujimoto K., Doi Y., Itoh M., Fujimoto T., Furuse M., Takano H., Noda T., Tsukita S.1998Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 141, 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M., Furuse M., Sasaki H., Schulzke J. D., Fromm M., Takano H., Noda T., Tsukita S.2000Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, 4131–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T., et al. 2006Putative ‘stemness’ gene JAM-B is not required for maintenance of stem cell state in embryonic, neural, or hematopoietic stem cells. Mol. Cell Biol. 26, 6557–6570 (doi:10.1128/MCB.00729-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzke J. D., Gitter A. H., Mankertz J., Spiegel S., Seidler U., Amasheh S., Saitou M., Tsukita S., Fromm M.2005Epithelial transport and barrier function in occludin-deficient mice. Biochim. Biophys. Acta 1669, 34–42 [DOI] [PubMed] [Google Scholar]
- Shao M., Ghosh A., Cooke V. G., Naik U. P., Martin-Deleon P. A.2008JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Dev. Biol. 313, 246–255 (doi:10.1016/j.ydbio.2007.10.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P., Harada S., Mishina Y.2009Downstream genes of Sox8 that would affect adult male fertility. Sex Dev. 3, 16–25 (doi:10.1159/000200078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin H., Morrow B., Saint-Jore B., Puech A., Das Gupta R., Patanjali S. R., Skoultchi A., Weissman S. M., Kucherlapati R.1997Identification, characterization, and precise mapping of a human gene encoding a novel membrane-spanning protein from the 22q11 region deleted in velo–cardio–facial syndrome. Genomics 42, 245–251 (doi:10.1006/geno.1997.4734) [DOI] [PubMed] [Google Scholar]
- Siu E. R., Wong E. W., Mruk D. D., Sze K. L., Porto C. S., Cheng C. Y.2009An occludin-focal adhesion kinase protein complex at the blood–testis barrier: a study using the cadmium model. Endocrinology 150, 3336–3344 (doi:10.1210/en.2008-1741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Cheng C. Y., Mruk D. D.2009Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood–testis barrier. Int. J. Biochem. Cell Biol. 41, 2578–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara M., Nishimura T., Mima S., Hoshino T., Mizushima T.2009Effect of claudin expression on paracellular permeability, migration and invasion of colonic cancer cells. Biol. Pharm. Bull. 32, 825–831 (doi:10.1248/bpb.32.825) [DOI] [PubMed] [Google Scholar]
- Tan K. A., De Gendt K., Atanassova N., Walker M., Sharpe R. M., Saunders P. T., Denolet E., Verhoeven G.2005The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 146, 2674–2683 (doi:10.1210/en.2004-1630) [DOI] [PubMed] [Google Scholar]
- Tarulli G. A., Stanton P. G., Lerchl A., Meachem S. J.2006Adult Sertoli cells are not terminally differentiated in the Djungarian hamster: effect of FSH on proliferation and junction protein organization. Biol. Reprod. 74, 798–806 (doi:10.1095/biolreprod.105.050450) [DOI] [PubMed] [Google Scholar]
- Tarulli G. A., Meachem S. J., Schlatt S., Stanton P. G.2008Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reproduction 135, 867–877 (doi:10.1530/REP-07-0572) [DOI] [PubMed] [Google Scholar]
- Tavelin S., Hashimoto K., Malkinson J., Lazorova L., Toth I., Artursson P.2003A new principle for tight junction modulation based on occludin peptides. Mol. Pharmacol. 64, 1530–1540 (doi:10.1124/mol.64.6.1530) [DOI] [PubMed] [Google Scholar]
- Tokunaga Y., Kojima T., Osanai M., Murata M., Chiba H., Tobioka H., Sawada N.2007A novel monoclonal antibody against the second extracellular loop of occludin disrupts epithelial cell polarity. J. Histochem. Cytochem. 55, 735–744 (doi:10.1369/jhc.6A7165.2007) [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M.2001Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293 (doi:10.1038/35067088) [DOI] [PubMed] [Google Scholar]
- Tyagi G., et al. 2009Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis. Biol. Reprod. 81, 258–266 (doi:10.1095/biolreprod.108.075200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C. M., Anderson J. M.2006Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68, 403–429 (doi:10.1146/annurev.physiol.68.040104.131404) [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Fanning A. S., Anderson J. M.2003Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am. J. Physiol. Renal Physiol. 285, F1078–F1084 [DOI] [PubMed] [Google Scholar]
- Wang R. S., et al. 2006Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology 147, 5624–5633 (doi:10.1210/en.2006-0138) [DOI] [PubMed] [Google Scholar]
- Wen H., Watry D. D., Marcondes M. C., Fox H. S.2004Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol. Cell Biol. 24, 8408–8417 (doi:10.1128/MCB.24.19.8408-8417.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems A., De Gendt K., Allemeersch J., Smith L. B., Welsh M., Swinnen J. V., Verhoeven G.2009Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression. Int. J. Androl. [DOI] [PubMed] [Google Scholar]
- Wong V., Gumbiner B. M.1997A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol. 136, 399–409 (doi:10.1083/jcb.136.2.399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. W., Mruk D. D., Cheng C. Y.2008Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim. Biophys. Acta 1778, 692–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H., Cheng C. Y.2005Blood–testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc. Natl Acad. Sci. USA 102, 11 722–11 727 (doi:10.1073/pnas.0503855102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H., Mruk D. D., Lee W. M., Cheng C. Y.2007Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays 29, 36–48 (doi:10.1002/bies.20513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H., Mruk D. D., Lee W. M., Cheng C. Y.2008aBlood–testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 22, 1945–1959 (doi:10.1096/fj.06-070342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H. N., Mruk D. D., Lee W. M., Cheng C. Y.2008bCross-talk between tight and anchoring junctions—lesson from the testis. In Molecular mechanisms in spermatogenesis (ed. Cheng C. Y.), pp. 234–254 Austin, TX: Landes Bioscience and Springer Science + Business Media; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. I., Park C. J., Nah W. H., Gye M. C.2009Expression of occludin in testis and epididymis of wild rabbits, Lepus sinensis coreanus. Reprod. Domest. Anim. 44, 745–750 (doi:10.1111/j.1439-0531.2008.01064.x) [DOI] [PubMed] [Google Scholar]
- Zahraoui A., Louvard D., Galli T.2000Tight junction, a platform for trafficking and signaling protein complexes. J. Cell Biol. 151, F31–F36 (doi:10.1083/jcb.151.5.F31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng A., Yuan F., Li Y., Zhu F., Hou P., Li J., Song X., Ding M., Deng H.2007Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J. Virol. 81, 12 465–12 471 (doi:10.1128/JVI.01457-07) [DOI] [PMC free article] [PubMed] [Google Scholar]