Abstract
Globally, overfishing large-bodied groundfish populations has resulted in substantial increases in their prey populations. Where it has been examined, the effects of overfishing have cascaded down the food chain. In an intensively fished area on the western Scotian Shelf, Northwest Atlantic, the biomass of prey species increased exponentially (doubling time of 11 years) even though the aggregate biomass of their predators remained stable over 38 years. Concomitant reductions in herbivorous zooplankton and increases in phytoplankton were also evident. This anomalous trophic pattern led us to examine how declines in predator body size (approx. 60% in body mass since the early 1970s) and climatic regime influenced lower trophic levels. The increase in prey biomass was associated primarily with declines in predator body size and secondarily to an increase in stratification. Sea surface temperature and predator biomass had no influence. A regression model explained 65 per cent of prey biomass variability. Trait-mediated effects, namely a reduction in predator size, resulted in a weakening of top predation pressure. Increased stratification may have enhanced growing conditions for prey fish. Size-selective harvesting under changing climatic conditions initiated a trophic restructuring of the food chain, the effects of which may have influenced three trophic levels.
Keywords: climate change, trophic interactions, body size, marine fisheries
1. Introduction
Over large spatial and temporal scales, ecosystems are governed by bottom-up forcing, that is, the biomass of top predators is determined by productivity at the lower trophic levels. Under these conditions, positive correlations between time series of predator and prey abundance reflect a stable, resource-controlled trophic structure (Strong 1992). In both terrestrial and marine ecosystems, however, over-exploitation of top predators can create an imbalance between predator and prey abundances, thereby leading to degraded or negative correlations between adjacent trophic levels (Strong 1992). Typically, in such situations, as top predators are removed, their prey and/or competitors increase owing to decreased predation and competitive release (Strong 1992; Schmitz et al. 2000; Berger et al. 2001; Worm & Myers 2003; Bascompte et al. 2005). In some cases, removal of top predators can affect the dynamics of lower trophic levels and transform ecosystems (Frank et al. 2005; Daskalov et al. 2007; Casini et al. 2008). Large-scale oceanographic variability can exacerbate fishing-induced trophic modifications or directly result in a change in composition and abundance of species (e.g. Stenseth et al. 2006; Cloern et al. 2007; Daskalov et al. 2007; Casini et al. 2008, 2009).
Cascading trophic interactions in large marine ecosystems were documented in a four-level food chain on the eastern Scotian Shelf, Northwest Atlantic (Frank et al. 2005). There, a decline in the aggregate biomass of top predators (groundfish) resulted in an explosion in both planktivore and macroinvertebrate abundances, which then precipitated declines in the abundance of large herbivorous copepods, increases in phytoplankton standing stock and a decline in essential nutrients. Other heavily exploited, cold water ecosystems to the north of this area responded similarly (Frank et al. 2006). Farther to the southwest in the Gulf of Maine, a more complex blend of trophic responses has been suggested. Increased stratification (with surface salinity as a proxy) was hypothesized to be the mechanism that enabled a coupled increase in phytoplankton and small copepod biomass (Greene & Pershing 2007).
Large-scale studies of the trophic dynamics of the eastern Scotian Shelf, Gulf of Maine and other large ecosystems have principally focused on density-mediated (i.e. abundance-driven) interactions and/or environmental forcing as the primary causative factors of trophic change (Strong 1992; Schmitz et al. 2000; Berger et al. 2001; Worm & Myers 2003; Bascompte et al. 2005; Frank et al. 2005, 2006; Daskalov et al. 2007; Greene & Pershing 2007; Casini et al. 2008, 2009). On the other hand, trait-mediated interactions (e.g. changes in behaviour or body size) also influence the structure of food webs (Werner & Peacor 2003; Schmitz et al. 2004; Woodward et al. 2005; Urban 2007). Specifically, body size is recognized as a determinant of trophic structure and energy flow in size-structured ecosystems (Economo et al. 2005; Woodward et al. 2005; Brose et al. 2006). In many heavily exploited ecosystems, size-selective fishing has led to rapid temporal reductions in the body size of top predators (Bianchi et al. 2000; Olsen et al. 2004; Shin et al. 2005; Greenstreet & Rogers 2006; Swain et al. 2007; Darimont et al. 2009). Moreover, the rates of phenotypic change in body size and related traits caused by commercial harvesting can be greater than 300 per cent higher than natural rates (Darimont et al. 2009). Size reduction of target species is often the direct result of harvest management plans that are typically characterized by reliance on minimum size limits for the target species (Halliday et al. 1992). Such policies focus fishing effort on larger individuals, yet the consequences of changes in predator size composition on large-scale trophic structure and function remain an outstanding question in ecology (Woodward et al. 2005; Brose et al. 2006).
In the Northwest Atlantic, the western Scotian Shelf (WSS) ecosystem (figure S1 in the electronic supplementary material) is interposed between the eastern Scotian Shelf, where a top-down response to fishing pressure has been documented, and the Gulf of Maine where a mixed top-down/bottom-up response has been suggested. Over the past three and a half decades, during which time fishing pressure on top predators has been similar to that in adjacent ecosystems, a relatively constant top predator biomass has prevailed on the WSS. Over the same interval, a steady and dramatic reduction in the average body size of top predators has occurred and stratification has increased. Despite the biomass stability at the top trophic level, their prey (forage fish, macroinvertebrate and small benthivore fish) biomass increased, herbivorous copepod abundance declined and phytoplankton increased. Except for the top trophic level, the trophic pattern is reminiscent, yet less severe, of what occurred on the eastern Scotian Shelf (Frank et al. 2005).
We used 38-year time series to examine the relationship between the changes in prey biomass on the WSS and four potential forcing mechanisms: predator biomass, predator body size, temperature and stratification. We provide evidence consistent with the following hypothesis: trait-mediated interactions, through the decline in body size of predators, were the dominant factors controlling the temporal increase in prey biomass. Increasing water column stratification had a significant but weaker association with increasing prey biomass. Sea surface temperature (SST) and top predator biomass had a negligible influence. We conclude that the energetic efficiency of this ecosystem has been profoundly reduced by the declines in top predator body size. Our study contributes to the understanding of trophic dynamics by demonstrating that trait-mediated effects, generated by preferentially removing the larger fish, can alter ecosystem balances and may trigger trophic cascades of varying intensity.
2. Material and methods
(a). Oceanographic data
Oceanographic indices were derived from databases available at http://www.mar.dfo-mpo.gc.ca/science/ocean/database/data_query.html. SST was selected as it affects the growth and availability of food of both prey fish and predatory fish. Further, SST and stratification influence the productivity of lower trophic levels (phytoplankton, zooplankton) as well as the extent of benthic pelagic coupling (the extent of mixing from the surface layers to bottom layers), which in turn affects the productivity of planktivores, small benthivores and macroinvertebrates. As SST increases in the spring, density differences between shallow and deep water create a stratified water column, which is broken down by wind and surface cooling in the autumn. Stratification is measured as the difference in density between 50 and 0 m (kg m−3); large positive values reflect stronger stratification. There is less vertical mixing in the water column in highly stratified water. It has been hypothesized that increased stratification leads to a decrease in turbulence and tends to concentrate plankton in the upper water column, leading to higher productivity (Greene & Pershing 2007). In contrast, an equally strong argument could be made that increased stratification leads to a lower supply of nutrient-rich deep water to the surface for use by phytoplankton. While the mechanism of how stratification affects organisms is debatable, the increasing trend observed across the shelf as a result of freshening water originating from the Arctic has been identified as an important influence on the physical properties of North Atlantic ecosystems (Greene et al. 2008).
(b). Lower trophic levels
Abundance estimates of herbivorous zooplankton (Calanus finmarchicus, Calanus hyperboreus, Calanus glacialis and copepodites stages I–V), and greenness (an index of phytoplankton levels) were obtained from the Continuous Plankton Recorder (CPR) survey. The sampling details of the CPR survey are contained in the electronic supplementary material.
(c). Higher trophic levels
Since 1970, the Canadian Department of Fisheries and Oceans has conducted annual, fishery-independent, scientific research vessel (RV) surveys in July on the Scotian Shelf to assess the distribution and abundance of both commercial and non-commercial species. Details of the sampling methodology are provided in the electronic supplementary material.
We derived biomass (stratified weight per tow) and abundance (stratified number per tow) time series (1970–2008) for 53 finfish species from the RV survey database (table S1 in the electronic supplementary material). Abundance information was available for one invertebrate, Homarus americanus (lobster), which has been consistently enumerated and is referred to as ‘macroinvertebrate’. Finfish species were divided into functional groups based on their maximum body size (www.fishbase.org) and diet (Shackell & Frank 2007) because the size range of top predators is large and variability of body size may be obscured by estimating the average body size of all fish. Each group contains commercial and non-commercial fish species. Since Squalus acanthias (dogfish) is partially migratory (Campana et al. 2007), we weighted its biomass by 0.5 to reflect its annual residency (Rago et al. 1998). Following Shackell & Frank (2007), six finfish functional groups were denoted: small (less than 46 cm), medium (46 cm or more and less than 80 cm) and large (80 cm or more) benthivores (primarily consumers of bottom dwellers), piscivores (primarily consumers of fish, i.e. of planktivores), zoopiscivores (consumers of amphipods, euphausiids and small fish, and planktivores (consumers of zooplankton) (table S1 in the electronic supplementary material). For analyses, we pooled the piscivores, zoopiscivores, medium and large benthivores and refer to them as aggregate top predators (see Frank et al. 2006 for rationale). Note that the functional groups divide top predators based on maximum size and diet but the definition of their diets should be considered general; most of these top predators eat both invertebrates and fish in varying proportions (Scharf et al. 2000).
Top predators affect planktivores through predation, and affect small benthivores through predation and competition, but consumption of lobsters by top predators has long been questioned. Convincing evidence was provided from the Gulf of Maine area where predation pressure on larger lobster (50–78 mm carapace lengths) was drastically reduced in the absence of large predatory groundfish such that in areas where there were few predatory groundfish, lobsters were only vulnerable during their earliest life stages (Steneck & Carlton 2001).
(d). Statistical analysis
Our goal was to test whether the aggregate prey biomass responded to intrinsic factors associated with top predator body size, top predator biomass and/or extrinsic oceanographic factors, namely SST and stratification. The model's dependent variable, the aggregate prey biomass, was the sum of the biomasses of planktivores, macroinvertebrates and small benthivores. We did not consider lower trophic levels further because of the 17-year data gap in the phytoplankton and copepod time series.
Each of the independent variables was selected a priori based on its potential influence on prey biomass variability. Top predator body size was the average of the following standardized anomalies: mean length, mean mass and growth of top predator functional groups. The details of estimating size from length frequency distributions are contained in the electronic supplementary material. All variables were log transformed and expressed as standardized anomalies prior to analyses. Standardized anomalies of response and independent variables through time are contained in figure S2 in the electronic supplementary material. The model was aggregate prey biomasst = f(top predator body sizet + stratificationt + top predator biomasst + SSTt), where t = year.
In our initial explorations, we determined that all predictors were adequately explained using a parametric approach. The 1978 observation was omitted from analysis as it was detected as an outlier during initial analyses by disproportionately influencing regression results. As some of the predictors might have been correlated, we used the variance inflation factor (VIF) to estimate the model stability. Values of VIF ≥10 represent highly correlated variables. We also report the correlation among model residuals at lag 1, associated Durbin–Watson statistic and boot-strapped p-value in parentheses.
It is reasonable to expect that a response in prey biomass will lag behind its drivers owing to the biological processes of survival, recruitment and reproduction (e.g. Casini et al. 2008). We explored the effect of lagging predictors on aggregate prey biomass through the use of lagged linear models. The effect of lagging did not change our interpretation of the results appreciably, nor account for more variability; the correlations between the response variable and key predictor variables were of the same magnitude and sign over 1–4-year lags (table S2 in the electronic supplementary material). As Casini et al. (2008) argued, correlations spanning over several lags may reflect cumulative processes that span over several years between the response and independent variables. The simpler model without lags is presented herein.
All analyses were performed using the R statistical software (R Development Core Team 2008) and relevant additional libraries (e.g. Companion to applied regression. R package v. 1.2-12. http://www.r-project.org, http://socserv.socsci.mcmaster.ca/jfox/).
3. Results and discussion
(a). Trends in biomass, abundance and climate
Annual removals of commercial groundfish from the WSS averaged close to 85 000 MT during the 1980s and have steadily declined to an average of 40 000 MT during the last decade. Recent survey estimates of the biomass of cod, one of the dominant top predators, are the lowest ever observed. The system appears to be evolving towards a state characteristic of highly perturbed, colder ecosystems to the north (Petrie et al. 2009). Despite single species declines, the aggregate biomass of top predators has changed only slightly during the past 38 years (coefficient of variation = 8%; figure 1a) owing primarily to rapid, compensatory increases within functional groups (Shackell & Frank 2007).
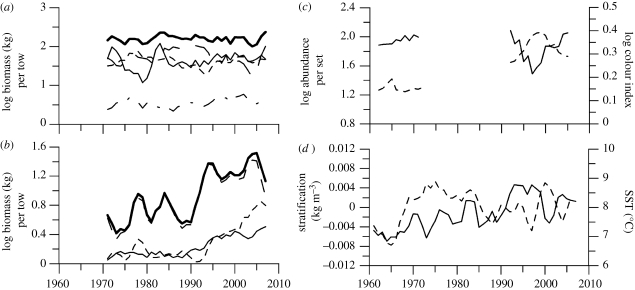
Figure 1.
Trophic and climate dynamics of the WSS, Northwest Atlantic from 1970 to 2008. (a) Aggregate biomass of top predators (sum of piscivores, large benthivores, medium benthivores and zoopiscivores) (thick solid); piscivores (long dash); large benthivores (short dash); medium benthivores (dash dot) and zoopiscivores (thin solid). (b) Aggregate prey biomass (sum of planktivores, macroinvertebrate and small benthivores) (thick solid); planktivores (long dash); macroinvertebrate (short dash); small benthivores (thin solid). (c) Herbivorous copepod abundance (solid) and colour index representative of phytoplankton abundance (dashed). (d) Stratification (solid) and SST (dashed). All lines are 3-year running averages.
While the aggregate top predator biomass has remained constant, the aggregate prey biomass has increased by more than 300 per cent since the early 1990s (figure 1b). In the adjacent lower trophic levels, large herbivorous copepods exhibited a declining trend from the early 1970s to the mid-1990s, followed by a recent increase (figure 1c). At the base trophic level, phytoplankton abundance increased from the early 1970s to the mid-1990s and more recently has declined (figure 1c). The climatic regime as represented by SST and stratification was cooler and less stratified in the 1960s. The SST cooled again from the mid-1980s through to the mid-1990s, while stratification showed a generally increasing trend over the time series (figure 1d). Correlations between adjacent trophic levels were weak but consistently negative (r = −0.12 aggregate top predators versus aggregate prey; r = −0.19 planktivores versus herbivorous copepods; and r = −0.40 herbivorous copepods versus phytoplankton). Both plankton time series had 17-year gaps, severely weakening their utility in examining trophic interactions. However, the opposing variability between adjacent trophic levels is consistent with cascading trophic interactions (Frank et al. 2005).
(b). Trends in size
The mean length and body mass of all fish functional groups declined through time. The declines in the mean length of large benthivores were most rapid (0.42 cm yr−1), (figure 2a), resulting in a 21 per cent decline from the early 1970s to the later 2000s, while zoopiscivores (0.18) and planktivores (0.15) declined more slowly, resulting in 8 and 16 per cent declines from the early 1970s to the 2000s.
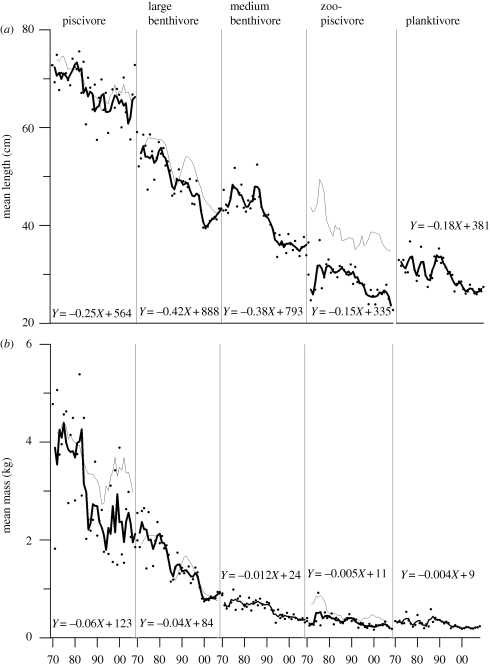
Figure 2.
(a) Mean length (cm) and (b) mean mass (kg) for fish functional groups from 1970 to 2008. Linear regression equations of body size through time are shown. Dots are annual values and lines show 3-year running averages. Grey lines denote the direct measure of growth ((a) length and (b) mass at age 6 as weighted by species biomass within a functional group).
Reductions in body mass ranged from 0.06 kg yr−1 in piscivores to 0.004 kg yr−1 in planktivores (figure 2b). On a percentage basis, reductions in the volumetric body mass for each functional group were more extreme than for linear length. Losses of mass, relative to levels observed during the 1970s, amounted to 59 per cent for the large benthivores, 48 per cent for medium benthivores, 45 per cent for piscivores, while planktivores and zoopiscivores decline 34 and 18 per cent, respectively. The direct measure of growth (mass and length at age 6 as weighted by species biomass within a functional group) underlines the loss of size over time (figure 2a,b). For example, a 6-year-old haddock (Melanogrammus aeglefinus) in the 1970s weighed, on average, 2 kg, but declined to 0.8 kg in the 2000s. The current average top predator body size has converged to the size of fish in the next lowest functional group during the 1970s, illustrating the collapse of size structure within top predator functional groups (figure 2).
Since larger predators can consume more prey per unit time than smaller predators (Sørnes & Aksnes 2004), it is reasonable to assume that larger predators can regulate their prey more efficiently. It is then possible that the declines in predator size (up to 60%) observed in this system resulted in weaker predation pressure, released the prey species from predation and contributed to their 300 per cent increase in biomass. However, planktivore size has declined as well, albeit to a lesser extent. Herring (Clupea harengus) is the dominant component of the planktivore biomass (table S1 in the electronic supplementary material) and are also targeted by size in the fishery. Unlike the top predator groups, planktivore biomass increased substantially so that their decline in size may be a density-dependent response, a response to size-selective fishing, or reflect an increased proportion of younger fish. Nonetheless, smaller predators would now have smaller prey, implying that predator efficiency would be conserved. While the ability of predators to capture prey varies with size (discussed in depth in §3e), it is important to note that predatory ability also depends on physiological condition, which has declined significantly through time in many of the top predators (Shackell & Frank 2007). Their weakened physiological condition, combined with the highly correlated declines in size, is consistent with the notion that the top predators may now have less impact on their prey.
(c). Prey biomass response to predators and climate
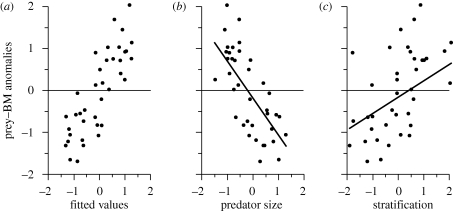
The interannual dynamics of the aggregate prey biomass were strongly and significantly accounted for by the top predator body size, less so by the stratification, and not at all by SST or top predator biomass. The majority of variance was captured by top predator size (table 1 and figure 3; figure S3 in the electronic supplementary material). As the top predator body size declined, and stratification increased, the aggregate prey biomass steadily increased (figure 3). The association between top predator size and the response is steeper than that of stratification. If density-mediated interactions between prey and top predators were important in this system, top predator biomass would have been a significant contributor, but it was not. Our empirical results support the notion that reductions in top predator body size through the 1990s (figure 2) were the dominant factor underlying the explosion of prey biomass and that predator size may be indirectly associated with changes at lower trophic levels (figure 1). Furthermore, the results (figure 3) are consistent with the hypothesis that the response of prey to top predator size resulted from the cumulative impact of lower predator per capita consumption of increasingly available prey, combined with the increasing reproductive potential of the prey populations (Casini et al. 2008).
Table 1.
Results of final model of aggregate prey biomass response to predator size, stratification, predator biomass and SST. Predictors, slopes, slope standard errors (s.e.), slope significance (prob), marginal sums of squares (SS), adjusted R2 value and variance inflation factor (VIF) are indicated. ‘corr among errors’ refers to the temporal correlation of residuals from the model at lag 1, and the Durbin–Watson statistic (D–W) represents a test of whether the autocorrelation is significantly different from 0. The boot-strapped p-value is in parentheses.
| predictor | slope | s.e. | marginal SS | prob | adj. R2 | VIF | corr among errors lag 1 | D–W (Pr) |
|---|---|---|---|---|---|---|---|---|
| intercept | −0.17 | 0.10 | 9.7E − 02 | 0.65 | ||||
| top pred. size | −0.89 | 0.14 | 13.35 | 5.1E − 07 | 1.17 | |||
| stratification | 0.38 | 0.10 | 4.65 | 8.6E − 04 | 1.15 | |||
| top pred. BM | −0.13 | 0.10 | 0.59 | 2.0E − 01 | 1.08 | |||
| SST | 0.04 | 0.10 | 0.06 | 6.8E − 01 | 1.12 | |||
| residual s.e./SS | 0.59 | 11.41 | 0.18 | 1.59 (0.14) | ||||
| on DF | 33 |
Figure 3.
Model summary. Solid line represents prediction of response from independent variables. All variables are expressed as anomalies. (a) Aggregate prey biomass observed versus fitted values. (b) Aggregate prey biomass versus top predator body size, and (c) versus stratification index.
(d). How stratification might lead to increase in prey
Greene & Pershing (2007) hypothesized that increased stratification led to a concentration of phytoplankton in the upper water column owing to a longer residency time. Therefore, stratification may enhance pelagic fish larval survival. Further, if plankton, such as copepods and larval fish, were also concentrated in the upper water column, this would provide adult planktivores with a concentrated food source. While we cannot definitively confirm a mechanism, our results suggest that stratification may contribute to survival, and that the issue should be investigated further.
(e). How top predator size reduction may lead to decline in predatory impact
Larger predators can have stronger per capita effects on prey biomass partly because they can consume more prey per unit time than smaller predators (Sørnes & Aksnes 2004). Larger predators, both within and among species, have been shown to be more successful at capturing prey because they can swim faster, possess higher visual acuity and eat a wider range of prey sizes that may provide a body size refuge beyond which prey are less vulnerable to predation (Cohen et al. 1993; Scharf et al. 2000). Recently, Biro & Post (2009) provided evidence that a genetic component of growth rate, activity and boldness are positively correlated in trout (Oncorhyncus mykiss). They argued that fast growers, who are also more active, consume with greater risk, are less scared and are thus more vulnerable to size-selective fishing. If growth rate of marine fish is related to activity and boldness, the observed decline in growth rates in this study directly reflects a weakened predatory effect.
Using information compiled on FishBase (see the electronic supplementary material), we estimated the relationship between burst swimming speed (swimming that could be maintained for a few seconds only but important for capturing prey) and body length. We found that a reduction from 45 to 35 cm resulted in a 53 per cent decline in burst speed, while a reduction from 40 to 35 cm represented a 25 per cent decline (see the electronic supplementary material). Note that the per cent decline in burst speed assumes a constant length/weight relationship, which has changed (Shackell & Frank 2007). For example, cod (Gadus morhua), haddock and pollock (Theragra chalcogramma) currently weigh 5, 9 and 20 per cent less at 45 cm than at the same length at the beginning of the RV survey time series. That is, the estimated per cent decline in burst speed owing to a decline in length is a minimum estimate given the changes in energy reserves at any given length. Fish with higher energy reserves (better physiological condition) could maintain higher burst speeds longer. In effect, diminished energy reserves, as observed in this system (Shackell & Frank 2007), negatively affect predation rates in addition to reductions in predator size.
(f). Ecosystem implications
The relationship between size reductions among the top predators and the dynamics of lower trophic levels implies that the WSS ecosystem may now be less energetically efficient than its historical state. New production is typically allocated to assimilation (growth of new tissue) or respiration, and both are related to body size. In principle, smaller fish respire a larger fraction of production than larger fish owing to higher metabolic rates per unit mass (Brown et al. 2004). As a result, on the WSS, a higher fraction of new production of top predators is now being lost as respiration rather than assimilated as biomass as was once the case. Reductions in the average body size of the predator complex have also occurred elsewhere in the Northern Hemisphere (Bianchi et al. 2000), often in areas where the biomass of the predator complex has also declined (Frank et al. 2006). Such ecosystems, characterized by the eastern Scotian Shelf, which have experienced a reduction in both predator biomass and average body size (Shackell & Frank 2007), are likely to exhibit the strongest trophic cascades.
4. Summary
A decline in average body size of aggregate top predators was the dominant factor associated with the increase in prey species biomass on the WSS. Stratification may also have contributed to that increase and deserves further investigation. Declines in the abundance of larger zooplankton and increases in phytoplankton concentrations were weakly associated with the increase in prey species, suggesting that a weak trophic cascade has occurred. These conclusions are based on the empirical modelling of a 38-year time series of fish community and oceanographic metrics. A common observation, among systems where interactions among species have been intensively studied, is that there are a few strong and many weak interactions among predator and prey species (Wootton & Emmerson 2005). In this study, we have not explored the notion that some predators may have a larger impact than others, which may be better accomplished with more highly refined data. However, our results represent a first approximation of the effects of predator size on large-scale ecosystem dynamics and as such, contribute to our understanding of how fishing can alter ecosystems.
The fishery has been highly selective for larger individuals in the population, as can be seen by comparing the RV survey data (what is available for capture) versus what is actually captured (i.e. the commercial catch) (figure S4 in the electronic supplementary material). Preferential exploitation of larger fish has produced a suite of smaller top predators whose resultant lowered physiological condition (Shackell & Frank 2007) and reduced prey capture ability imply that they can no longer effectively exploit the increased food base available to them. This is true notwithstanding the increased overall abundance of prey. At their current average body size, predators are only exploiting a fraction of the prey complex potentially available, and this, combined with their reduced physiological condition and efficiency, appears to have reduced their prey capture/conversion capacity.
The loss of energetic efficiency owing to a loss in size translates directly into a reduction in the economic value of groundfish through lost production, reduced marketability (small fish fetch lower unit prices) and export restrictions (minimum groundfish length of 43 cm required by markets in the USA). The end result, in addition to the loss of economic value, is an incipient trophic cascade. If body size declines are permanent, the WSS may have entered a new, perhaps irreversible, productivity regime, as has been argued strongly for the Baltic Sea (Casini et al. 2009). However, unlike the situation in more northerly northwest Atlantic ecosystems that have experienced trophic destabilization, and where there is no evidence of the recovery of top predators, the WSS may be more resilient (Frank et al. 2006). Fishery-induced size changes can be reversible (Conover et al. 2009) and the top predators of the WSS may yet regain their former predatory role.
Acknowledgements
This research was supported by the Department of Fisheries and Oceans, Canada, and Natural Sciences and Engineering Research Council of Canada Discovery grants to K.T.F. and W.C.L. We thank the Editor and two anonymous reviewers, whose comments helped us clarify our ideas.
References
- Bascompte J., Melián C. J., Sala E.2005Interaction strength combinations and the overfishing of a marine food web. Proc. Natl Acad. Sci. USA 102, 5443–5447 (doi:10.1073/pnas.0501562102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J., Stacey P. B., Bellis L., Johnson M. P.2001A mammalian predator–prey imbalance: grizzly bears and wolf extinction affect avian neotropical migrants. Ecol. Appl. 11, 947–960 (doi:10.1890/1051-0761(2001)011[0947:AMPPIG]2.0.CO;2) [Google Scholar]
- Bianchi G., et al. 2000Impact of fishing on size composition and diversity of demersal fish communities. ICES J. Mar. Sci. 57, 558–571 (doi:10.1006/jmsc.2000.0727) [Google Scholar]
- Biro P. A., Post J. R.2009Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl Acad. Sci. USA 105, 2919–2922 (doi:10.1073/pnas.0708159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose U., Williams R. J., Martinez N. D.2006Allometric scaling enhances stability in complex food webs. Ecol. Lett. 9, 1228–1236 (doi:10.1111/j.1461-0248.2006.00978.x) [DOI] [PubMed] [Google Scholar]
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B.2004Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (doi:10.1890/03-9000) [Google Scholar]
- Campana S. E., Gibson A. J. F., Marks L., Joyce W., Rulifson R. A., Dadswell M. J.2007Stock structure, life history, fishery and abundance indices for spiny dogfish (Squalus acanthias) in Atlantic Canada. Canadian Science Advisory Secretariat Research Document 2007/089 [Google Scholar]
- Casini M., Lövgren J., Hjelm J., Cardinale M., Molinero J.-C., Kornilovs G.2008Multi-level trophic cascades in a heavily exploited open marine ecosystem. Proc. R. Soc. B 275, 1793–1801 (doi:10.1098/rspb.2007.1752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini M., Hjelm J., Molinero J.-C., Lövgren J., Cardinale M., Bartolino V., Belgrano A., Kornilovs G.2009Trophic cascades promote threshold-like shifts in pelagic marine ecosystems. Proc. Natl Acad. Sci. USA 106, 197–202 (doi:10.1073/pnas.0806649105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloern J. E., Jassby A. D., Thompson J. K., Hieb K. A.2007A cold phase of the East Pacific triggers new phytoplankton blooms in San Francisco Bay. Proc. Natl Acad. Sci. USA 104, 18 561–18 565 (doi:10.1073/pnas.0706151104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. E., Pimm S. L., Yodzis P., Saldana J.1993Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 62, 67–78 [Google Scholar]
- Conover D. O., Munch S. B., Arnott S. A.2009Reversal of evolutionary downsizing caused by selective harvest of large fish. Proc. R. Soc. B 276, 2015–2020 (doi:10.1098/rspb.2009.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darimont C. T., Carlson S. M., Kinnison M. T., Paquet P. C., Reimchen T. E., Wilmers C. C.2009Human predators outpace other agents of trait change in the wild. Proc. Natl Acad. Sci. USA 106, 952–954 (doi:10.1073/pnas.0809235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalov G. M., Grishin A. N., Rodionov S., Mihneva V.2007Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl Acad. Sci. USA 104, 10 518–10 523 (doi:10.1073/pnas.0701100104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economo E. P., Kerkhoff A. J., Enquist B. J.2005Allometric growth, life-history invariants and population energetics. Ecol. Lett. 8, 353–360 (doi:10.1111/j.1461-0248.2005.00737.x) [Google Scholar]
- Frank K. T., Petrie B., Choi J. S., Leggett W. C.2005Trophic cascades in a formerly cod-dominated ecosystem. Science 308, 1621–1623 (doi:10.1126/science.1113075) [DOI] [PubMed] [Google Scholar]
- Frank K. T., Petrie B., Shackell N. L., Choi J. S.2006Reconciling differences in trophic control in mid-latitude marine ecosystems. Ecol. Lett. 9, 1096–1105 (doi:10.1111/j.1461-0248.2006.00961.x) [DOI] [PubMed] [Google Scholar]
- Greene C. H., Pershing A. J.2007Climate drives sea change. Science 315, 1084–1085 (doi:10.1126/science.1136495) [DOI] [PubMed] [Google Scholar]
- Greene C. H., Pershing A. J., Cronin T. M., Ceci N.2008Arctic climate change and its impacts on the ecology of the North Atlantic. Ecology 89, S24–S38 (doi:10.1890/07-0550.1) [DOI] [PubMed] [Google Scholar]
- Greenstreet S. P. R., Rogers S. I.2006Indicators of the health of the North Sea fish community: identifying reference levels for an ecosystem approach to management. ICES J. Mar. Sci. 63, 573–593 (doi:10.1016/j.icesjms.2005.12.009) [Google Scholar]
- Halliday R. G., Peacock F. G., Burke D. L.1992Development of management measures for the groundfish fishery in Atlantic Canada: a case study of the Nova Scotia inshore fleet. Mar. Policy 16, 411–426 (doi:10.1016/0308-597X(92)90070-6) [Google Scholar]
- Olsen E. M., Heino M., Lilly G. R., Morgan M. J., Brattey J., Ernande B., Dieckmann U.2004Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428, 932–935 (doi:10.1038/nature02430) [DOI] [PubMed] [Google Scholar]
- Petrie B., Frank K. T., Shackell N. L., Leggett W. C.2009Structure and stability in exploited marine fish communities: quantifying critical transitions. Fish. Oceanogr. 18, 83–101 (doi:10.1111/j.1365-2419.2009.00500.x) [Google Scholar]
- Rago P. J., Sosebee K. A., Brodziak J. K. T., Murawski S. A., Anderson E. D.1998Implications of recent increases in catches on the dynamics of Northwest Atlantic spiny dogfish (Squalus acanthias). Fish. Res. 39, 165–181 (doi:10.1016/S0165-7836(98)00181-7) [Google Scholar]
- R Development Core Team 2008R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- Scharf F. S., Juanes F., Rountree R. A.2000Predator size–prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Prog. Ser. 208, 229–248 (doi:10.3354/meps208229) [Google Scholar]
- Schmitz O. J., Hambäck P. A., Beckerman A. P.2000Trophic cascades in terrestrial systems: a review of the effects of carnivore removal on plants. Am. Nat. 155, 141–153 (doi:10.1086/303311) [DOI] [PubMed] [Google Scholar]
- Schmitz O. J., Krivan V., Ovadia O.2004Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163 (doi:10.1111/j.1461-0248.2003.00560.x) [Google Scholar]
- Shackell N. L., Frank K. T.2007Compensation in exploited marine fish communities on the Scotian Shelf, Canada. Mar. Ecol. Prog. Ser. 336, 235–247 (doi:10.3354/meps336235) [Google Scholar]
- Shin Y.-J., Rochet M.-J., Jennings S., Field J. G., Gislason H.2005Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 62, 384–396 (doi:10.1016/j.icesjms.2005.01.004) [Google Scholar]
- Sørnes T. A., Aksnes D. L.2004Predation efficiency in visual and tactile zooplanktivores. Limnol. Oceanogr. 49, 69–75 [Google Scholar]
- Steneck R. S., Carlton J. T.2001Human alterations of marine communities: students beware!. In Marine community ecology (eds Bertness M. D., Gaines S. D., Hay M. E.), pp. 445–468 Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- Stenseth N. C., Llope M., Anadón R., Ciannelli L., Chan K.-S., Hjermann D. Ø., Bagøien E., Ottersen G.2006Seasonal plankton dynamics along a cross-shelf gradient. Proc. R. Soc. B 273, 2831–2838 (doi:10.1098/rspb.2006.3658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D. R.1992Are trophic cascades all wet? Differentiation and donor-control in speciose ecosystems. Ecology 73, 747–754 (doi:10.2307/1940154) [Google Scholar]
- Swain D. P., Sinclair A. F., Hanson J. M.2007Evolutionary response to size-selective mortality in an exploited fish population. Proc. R. Soc. B 274, 1015–1022 (doi:10.1098/rspb.2006.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E. E., Peacor S. D.2003A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100 (doi:10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2) [Google Scholar]
- Woodward G., Ebenman B., Emmerson M., Montoya J. M., Olesen J. M., Valido A., Warren P. H.2005Body size in ecological networks. Trends Ecol. Evol. 20, 402–409 (doi:10.1016/j.tree.2005.04.005) [DOI] [PubMed] [Google Scholar]
- Wootton J. T., Emmerson M.2005Measurement of interaction strength in nature. Annu. Rev. Ecol. Evol. Syst. 36, 419–444 (doi:10.1146/annurev.ecolsys.36.091704.175535) [Google Scholar]
- Worm B., Myers R. A.2003Meta-analysis of cod–shrimp interactions reveals top-down control in oceanic food webs. Ecology 84, 162–173 (doi:10.1890/0012-9658(2003)084[0162:MAOCSI]2.0.CO;2) [Google Scholar]
- Urban M. C.2007Predator size and phenology shape prey survival in temporary ponds. Oecologia 154, 571–580 (doi:10.1007/s00442-007-0856-2) [DOI] [PubMed] [Google Scholar]