Abstract
Animals from invertebrates to humans benefit from information conspecifics make available, including information produced inadvertently. While inadvertent social information may frequently be exploited in nature, experiments have rarely been conducted in the wild to examine how such information helps animals in their natural ecology. Here I report a series of field experiments on free-living terrestrial hermit crabs (Coenobita compressus), showing how these asocial invertebrates learn the locations of their most essential resources, food and shelter, using inadvertent cues from conspecific competitors. Crabs have limited abilities to locate resources individually, but as they coalesce on a resource, their aggregation can be noticed by passing foragers, tipping them off about the discovery. Foragers were strongly attracted to experimentally simulated aggregations in which crabs were tethered to the same spot and in which the resources normally found beneath aggregations were excluded. Simulated aggregations of crabs whose shells were removed were likewise attractive, more than even these sought-after-shelters themselves. Experiments that simulated the chemical and visual cues of aggregations independently revealed that foragers oriented to aggregations primarily by sight, cueing in on the jostling competitive activity of the aggregation. Although crabs have not been selected to recruit others to newly discovered resources, their natural ecology has provided a setting where competitors regularly help one another by means of inadvertent social information.
Keywords: collective behaviour, crustaceans, cues, foraging ecology, inadvertent social information, social learning
1. Introduction
The ecology of most animals presents a multitude of challenges, some of which can be solved most effectively by consulting the behaviour of conspecifics (Giraldeau 1997; Dall et al. 2005). Others' behavioural activities provide a valuable form of ‘social information’ (Bonnie & Earley 2007; Seppänen et al. 2007), which can effectively reduce individuals' uncertainty about many of the ecological decisions they face, from finding food and mates to avoiding predators (Dall 2005). In certain cases social information involves true signals, acts specialized by natural selection to convey information (Bradbury & Vehrencamp 1998). However, in many cases pertinent information is instead found in ‘cues’ (Seeley 1989), also known as ‘inadvertent social information’ (Danchin et al. 2004). Behaviours in this latter category have not been designed by evolution for the purpose of communication, but they nevertheless provide a rich assortment of potentially useful information. Inadvertent social information, for instance, might enable animals to learn the locations of resources by observing where others are foraging, or update on the quality of a resource patch by monitoring ‘public information’ indicating the success of others within that patch (Valone 1989; Giraldeau & Caraco 2000). Moreover, because inadvertent social information can be generated unavoidably, as a by-product of a performer's routine activities (Laidre 2009), the advantageous use of this information by others need not benefit the performer itself. Indeed, in some cases, the use of inadvertent social information may be parasitic, with users being helped and performers being harmed.
A wide range of taxa, including both animals and plants (Karban & Maron 2002), make use of inadvertent social information. By far the most concentrated studies have been in fishes, birds, rodents and primates, where decades of research has revealed the impact of social influences on these animals' decisions, particularly during foraging (Heyes & Galef 1996; Galef & Giraldeau 2001). The bias towards vertebrate animals in the study of inadvertent social information appears unwarranted, however. Evidence is accumulating to suggest that invertebrates, despite frequently being regarded as less sophisticated in their information-processing abilities, in fact show many of the same capacities as vertebrates for using and learning from social information. For instance, bumble-bees, after watching the flower choices of other bumble-bees, copy these same choices thereby exploiting new and unfamiliar resources (Leadbeater & Chittka 2005; Worden & Papaj 2005). Crickets acquire life-saving information from conspecifics, adaptively increasing their level of caution after being exposed to other crickets that possess knowledge about a predator (Coolen et al. 2005). Octopuses learn from observing others which prey to attack (Fiorito & Scotto 1992), and female flies choose mates based on the preferences of other females (Mery et al. 2009). This evidence for social information use in invertebrates (reviewed in Webster & Fiorito 2001; Chittka & Leadbeater 2005; Leadbeater & Chittka 2007) suggests that underlying ecology, rather than taxonomic divides, might be the core determinant of whether a given species will make use of social information (Coolen et al. 2003).
To date, most studies of social information use and social learning have been conducted in relatively artificial laboratory and captive settings, which provide controlled conditions but are disconnected from the actual ecology of the species being studied. If certain ecological circumstances in fact promote a greater reliance on social information (Giraldeau 1997), then the study of social information use should ideally incorporate field experiments that rigorously examine how and why wild animals naturally avail themselves of such information. The increasing study of social information use in invertebrates provides a rich opportunity to strengthen such connections with ecology, since many invertebrates are naturally more amenable to controlled field experiments on social transmission than are many vertebrates. Ultimately, ecologically grounded field experiments could provide a powerful foundation for broader comparative analyses, unravelling the impact of diverse environmental variables on the distribution and evolution of social information use.
Here I examine the relevance of inadvertent social information in the natural ecology of crustaceans, invertebrates that have been largely neglected in studies of social information use and social learning. Although most crustaceans are water-dwelling and therefore relatively inaccessible to field observation, a number of land crabs exist (Burggren & McMahon 1988) whose whole existence is spent outside of water, except for brief periods during the release of brooding larvae into the ocean. This paper focuses on a land crab species in Costa Rica, the hermit crab Coenobita compressus. Of more than 800 species of hermit crabs worldwide, only the 12 coenobitid species are terrestrial, all inhabiting tropical regions throughout the world where they roam the dry areas of the beach searching for food and shelter (Childress 1972). Shelter, in particular, is a defining feature of hermit crab natural history: hermits inhabit the empty, portable shells of gastropods and these shelters are essential for crabs' survival and reproduction (Hazlett 1981). Both shells and the variety of foods that compose terrestrial hermit crabs' generalist diet are limited resources that are scattered patchily across the beach and are often moved unpredictably by changing tides, frequently becoming visually concealed by sand. Such a heterogeneous and dynamically changing resource distribution, often obscured from view, presents a situation where social information about resource location from conspecifics could be especially valuable.
Hermit crabs, however, are decidedly uncooperative invertebrates, lacking the types of elaborate recruitment signals that eusocial insects deploy to alert nest mates about new resources. Each crab is essentially a ‘rugged individualist’ that wanders widely, does not belong to any central colony, lacks kinship ties and competes intensely with conspecifics (Bright 1966; Ball 1972). In spite of this individualistic organization, there are indications that crabs might still provide useful information to others, albeit inadvertently, and as a consequence might help competitors locate vitally sustaining resources. This study sought, first, to pinpoint the basis of natural aggregations, which have never been examined in detail but are thought to form around resources and so might provide a cue to the valuable items crabs seek. These natural history data then served as the basis for a series of controlled field experiments that followed-up prior reports (Warner 1977; Kurta 1982), which have suggested that crabs may profitably exploit aggregations of conspecifics as a source of information about resource location. The amenability of terrestrial hermit crabs to field experiments, which simulated various features of aggregations, made it possible to examine how different components of social information operate within the crabs' natural ecology, both at a mechanistic and a functional level.
2. Material and methods
(a). Study site and population
The study was conducted during March and April 2008 in the Osa Peninsula of Costa Rica at the Osa Biodiversity Center, a field station and preserve run by the non-profit conservation organization ‘Friends of the Osa’. Observations and experiments were carried out on a 2–3 mile strip of beach that lies immediately south of where the Rio Piro flows out to the Pacific Ocean and continues up to the Rio Los Sambos. This area contains abundant terrestrial hermit crabs (C. compressus). During mid-day, when the sun is most intense, the hermit crabs remain resting within the forest under the cover of fallen palm fronds and coconuts; but from the early evening (approx. 16.30 h) until mid morning (approx. 11.00 h) they roam the beach, foraging for food and shells.
(b). Natural aggregations
During foraging, terrestrial hermit crabs are typically spaced out individually, with distances of 50 cm or more from any given crab to its nearest neighbour (most crabs in the population are <5 cm in body length; M. E. Laidre 2008, personal observation). While pairs of immediately adjacent crabs are rare, occurring only fleetingly as two individuals pass by one another, congregations of three or more crabs can persist stably. The combined activity of such gatherings (electronic supplementary material, video S1) stands out prominently against the backdrop of otherwise solitary foragers. In the first phase of the study, I systematically quantified the size of such natural aggregations, conducting transects up and down the beach just after sunrise (05.00 h) and just before sunset (17.45 h). An aggregation was operationally defined as any clustering of three or more hermit crabs within an area of 10 × 10 cm. Aggregations could be readily detected from a distance of several metres away, and upon spotting one I immediately approached it and counted the number of crabs present by picking up each one in sequence and tossing it away from the pile. After reaching the bottom of the pile I then identified and recorded the resource around which the crabs were aggregated (typically the resource was not visible to start, since crabs were completely covering it, tumbling over one another to gain access).
(c). Field observations and general experimental protocol
A series of experiments was carried out to investigate whether crabs cue in on aggregations, using them as beacons that indicate the location of otherwise difficult-to-detect resources. These experiments were motivated by preliminary observations of two types. First, hermit crabs were unable to discover naturally available or freshly provided food or shell resources over extended periods, frequently passing right by the resource (within 0.5 m) and still failing to detect it. Isolated resources, without accompanying social cues, thus appeared easily overlooked by crabs. Second, solitary foragers, upon coming within just 1–2 m of an aggregation, were seen changing direction, moving in a beeline directly towards the aggregation and then joining in the tussle to gain access to the resource. Foragers that joined an aggregation in some cases even neglected nearby resources of the very same type, which lay uncontested <0.5 m from the location of the aggregation. A resource hidden beneath a pile of competing conspecifics somehow appeared more salient to foragers. The purpose of the experiments was therefore to: (i) rigorously test whether the aggregations themselves—independent of the resource—were providing information to solitary foragers about the availability of the valuable item which lay beneath them; (ii) isolate the mechanism by which solitary foragers were able to find their way to aggregations; and (iii) evaluate the fitness consequences for each party, both the information providers and the information users.
Four experiments were conducted to narrow down the features of aggregations that drew in other crabs and the mechanisms by which crabs oriented to them. The design of each experiment involved the same core setup (electronic supplementary material, figure S1): four quadrats (0.60 × 0.60 m) were spaced 2 m apart, placed along a transect in the beach sand that was parallel to and 5 m from the edge of the forest (defined by where the base of the palm trees began). The 2 m distance between the quadrats was chosen since natural observations suggested that crabs could detect aggregations only from about 1 m away. The quadrats were made by lightly pressing a wooden dowel into the sand until it made an imprint only a few millimetres deep. This depression was too slight to prevent the crabs from moving over it, but it was enough to be seen by an observer hidden in the forest. For each experiment, two of the quadrats were assigned as controls, one of them being completely empty and the other having some of the materials that were used to simulate aggregations in the other two quadrats. The ordering of quadrats was randomly assigned before each experiment, and there were 20–22 replicates across all four experiments.
Before beginning an experiment, I first cleared the entire area within 2 m of the quadrats of any food, shells or other debris. After setting the quadrats, I then went to the edge of the forest where I could watch the crabs through binoculars from 5 m away without disturbing them. Each experiment lasted 30 min. During the first 10 min I simply waited in the forest, allowing the crabs to return to their normal searching routine after the disruption of my extended movements while clearing the area and setting each quadrat. Next, I collected 10 min of baseline-control data on the number of hermit crabs present in each quadrat at 1 min intervals. During this period no materials had yet been placed inside any of the quadrats, so the data indicate the general background activity of crabs while foraging solitarily. At the end of the baseline-control period (‘time-zero’ in figures 1 and 2), I immediately approached the quadrats and placed, in under 30 s, the prepared materials (detailed below for each experiment). These actions were too brief to cause foraging crabs to leave the area; they retained their positions and only ducked inside their shells momentarily. After placing the materials I then returned to my observing position in the forest and for another 10 min I continued recording the same data on the number of crabs at 1 min intervals in each quadrat. Finally, at the end of the experiment, I went to each quadrat and counted the number of hermit crabs by hand. All experiments were carried out in preferred search locations for the hermit crabs: shaded areas on the beach during low tide, with the ocean generally being 50 m or more distant from the quadrat location. The experiments were conducted during daylight (between 07.00–11.00 and 16.30–17.30 h) when hermit crabs were actively searching these areas.
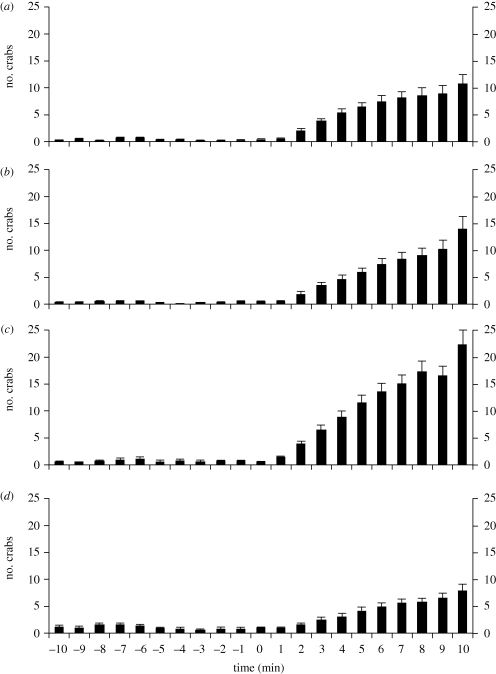
Figure 1.
The number of free-roaming hermit crabs present inside quadrats 10 min before and after the placement of (a) a dowel with three strands of fishing line that had crabs (naturally shelled) tethered to each end; (b) a dowel with six strands of fishing line that had crabs (naturally shelled) tethered to each end; (c) a dowel with three strands of fishing line that had shell-less crabs (their shells having been removed) tethered to each end; and (d) a dowel with three strands of fishing line that had empty shells tethered to each end. Conditions (a,b) are from experiment 1 and conditions (c,d) are from experiment 2 (the controls for each experiment are in electronic supplementary material, figures S5 and S6 respectively). n = 20 replicates for each condition. Bars show mean + s.e. at each minute.
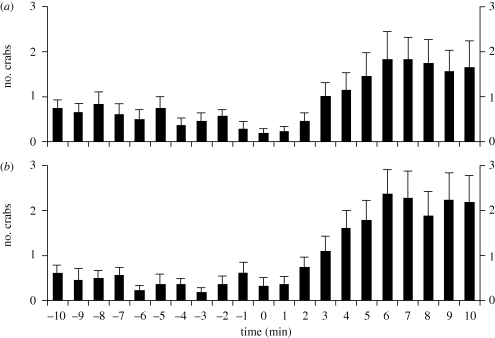
Figure 2.
The number of free-roaming hermit crabs present inside quadrats 10 min before and after the placement of (a) a glass that had three crabs (naturally shelled) beneath it and (b) a glass with six crabs (naturally shelled) beneath it. Both conditions are from experiment 4 (the controls for this experiment are in electronic supplementary material, figure S8). n = 22 replicates for each condition. Bars show mean + s.e. at each minute. Note that the scale of the y-axis has been increased relative to figure 1 to better depict the change between the baseline-control period and the subsequent period.
(d). Experiment 1: tethered crabs
Experiment 1 aimed to simulate aggregations independent of any external resource. This was accomplished (cf. Kurta 1982) by tethering several crabs so that they were effectively stuck next to one another in a single pile. Crabs were tethered by tying fishing line around the distal-most segment of their leg and then tying the other end of the line to a dowel (0.30 m in length), which was inserted half-way into the sand in the centre of the quadrat (electronic supplementary material, figure S2 and video S2). One quadrat ‘control 1’ was completely empty. A second quadrat ‘control 2’ had a dowel with three fishing-line strands attached to it, but nothing was tethered to the ends of these strands. The two other quadrats each had a dowel with attached fishing-line strands and with crabs tethered to the ends of these strands. One quadrat had three tethered crabs and the other had six tethered crabs, each crab tethered individually to its own fishing-line strand. The fishing-line strands (both in the control quadrat and in the two condition quadrats) were 0.20 m in length. The crabs that were tethered were medium-sized, with shell diameters of approximately 2 cm or less. It was predicted that the simulated aggregations of three and six tethered crabs would provide a strong attractive stimulus to solitary foragers, with the number of crabs significantly increasing in both these quadrats from the baseline-control period to the subsequent period. By contrast, it was predicted that the two control quadrats would show no such effect.
(e). Experiment 2: tethered shell-less crabs and empty shells
Although experiment 1 simulated aggregations in which there was no external food or empty shell resource, these aggregations might still be supposed to contain a resource: the shell on each tethered crab's back. Importantly, in experiment 1 no tethered crab ever lost its shell during the course of the experiments, suggesting that any attraction of solitary foragers to the simulated aggregations was not based on these foragers trying to steal the shells of tethered crabs. Nevertheless, experiment 2 was carried out to examine whether simulated aggregations of crabs that possessed no shells (‘naked’ crabs) would still be an attractive stimuli to solitary foragers. The quadrats for control 1 and control 2 remained identical to those in experiment 1, just the two condition quadrats changed: one condition quadrat had three shell-less crabs (each individually tethered to the dowel) and the other condition quadrat had the three empty shells that were taken from those same crabs. Since the empty shells could not be tethered simply by tying the fishing line around them, the end of each fishing line strand was instead glued to each shell with clear waterproof glue (Loctite control gel). It was predicted that the tethered empty shells would be a less attractive stimulus to solitary foragers, since these shells would not—at least initially—exhibit the jostling movements typical of an aggregation. But if multiple foragers happened upon these empty shells and entered them, then these foragers would effectively become tethered crabs, stuck in one spot, and therefore would act as a simulated aggregation. It was therefore expected that tethered empty shells would still be somewhat attractive, given that they could eventually manifest the same movement properties of an aggregation. In comparison, the attractiveness of the tethered shell-less crabs was predicted to be stronger, at least as strong as that of the tethered shelled crabs in experiment 1. For despite not possessing shells these crabs would still be engaged in the continuous commotion that characterizes aggregations.
(f). Experiment 3: chemical cues
Experiment 3 was designed to examine the mechanisms used by solitary foragers to orient to aggregations. In particular, this experiment tested the potential role of chemical cues emitted by the concentrated activity of aggregated crabs. The setup used to isolate possible chemical cues (and to simultaneously exclude any visual signs of an aggregation) was as follows: an opaque (white) plastic cup (base diameter: 6 cm, height: 11 cm, top diameter: 9 cm) was attached to a dowel (length: 0.30 m) by sticking the dowel through the middle of the cup. The dowel was then inserted half-way into the sand in the centre of the quadrat, with the cup itself positioned 5 cm above the ground (electronic supplementary material, figure S3). Thirty holes (approx. 0.5 cm diameter each) were poked into the sides and base of the cup. These holes were too small to visually reveal the internal contents of the cup, but were large enough to enable even a light breeze to carry out the scent of whatever was inside. I confirmed that this setup did in fact enable significant chemical cues to emanate, of the type that the crabs could readily detect. Terrestrial hermit crabs are known to be attracted to the smell of recently killed conspecifics (Small & Thacker 1994). When I placed a single smashed specimen in the setup there was, within minutes, a strong and immediate recruitment of solitary foragers from the surrounding vicinity. Hence, if the crabs are lured to aggregations by the scent of their live, congregated conspecifics, then the setup should have been capable of showing this.
In the quadrats, control 1 was empty as usual, and control 2 had the aforementioned opaque cup setup without anything inside the cup. Of the two condition quadrats, one condition had three live crabs inside the opaque cup setup. It was predicted that if the chemical cues from an aggregation of live crabs are what attract solitary foragers, then the number of crabs within this quadrat would significantly increase from the baseline-control period to the subsequent period. However, if crabs are not attracted to aggregations based on chemical cues alone, then no such effect was predicted to occur. The other quadrat condition had an identical opaque cup setup, but in this case there were no crabs inside the cup. Instead, one of the crabs' staple foods was placed inside: the fruit of a ripe beach almond (Terminalia catappa), which was cut up to maximize odour transmission. I included this food condition since, in natural aggregations, food resources are generally invisible, being completely covered by the aggregated crabs (electronic supplementary material, video S1). As a consequence of such physical concealment, in most natural cases the only available cue to a food resource's existence (other than social cues) would be the smell of the food. Thus, this last quadrat condition tested whether solitary foragers were able to pick up on the scent of one of their most sought after food resources. Natural observations (see above) had suggested they might not.
(g). Experiment 4: visual cues
Experiment 4 was designed to further examine the mechanisms that solitary foragers use to orient to aggregations. This experiment focused in particular on an aggregation's visual cues, which were prominent to a human observer and which potentially could also be prominent to the hermit crabs themselves. The following setup was used to isolate an aggregation's visual cues (and to simultaneously exclude possible chemical cues): a transparent shot glass (base diameter: 7 cm, height: 9 cm, top diameter: 9 cm) was turned upside down, with hermit crabs beneath it, and it was placed on a circular plastic substrate (diameter: 11 cm), which was on level with the ground (electronic supplementary material, figure S4 and video S3). The aggregation's visual image projected clearly through the glass while the plastic substrate prevented the crabs inside from digging up dirt, which would have blocked their image. I independently verified that this setup did in fact keep chemical cues effectively trapped inside, such that the visual image projecting through the glass was the sole indication of the aggregation's presence. As noted above, terrestrial hermit crabs are known to be attracted to the smell of a recently killed conspecific (Small & Thacker 1994). After placing a single smashed specimen beneath the shot glass setup, there was no recruitment of solitary foragers from the surrounding vicinity for more than 15 min. However, after lifting the shot glass, so that the chemical cues would be free to emanate, there was, within minutes, strong and immediate recruitment. Hence, if the crabs are lured to aggregations solely by the sight of their live, congregated conspecifics, then the setup should have been capable of showing this.
In the quadrats, control 1 was empty as usual, and control 2 had the aforementioned shot glass setup and plastic substrate, just with nothing beneath the shot glass. Both the condition quadrats had hermit crabs beneath the shot glass, one condition with three hermit crabs and the other with six. It was predicted that if the sight of an aggregation of live crabs is what attracts solitary foragers, then the number of crabs within these two condition quadrats would significantly increase from the baseline-control period to the subsequent period.
(h). Statistical analyses
Across all four experiments, I tested the effect of each of the control quadrats and each of the condition quadrats by comparing the number of crabs in a given quadrat at the end of the baseline-control period to the number of crabs in this same quadrat at the absolute end of the experiment. Two specific times were chosen for comparison: (i) time 0 (in which crabs were counted immediately before I placed the experimental materials into the quadrats); and (ii) 10 min after time 0 (in which crabs were counted in the final sample point of the experiment). These two times represented the instances in which I was standing directly above the quadrats. At time 0 there was usually (96% of n = 328) just two crabs or less per quadrat, which I could readily count in a single overhead scan before placing in the materials, and at time-(+10), when many crabs had potentially accumulated in response to an experimental condition, I could count the crabs by hand, tossing individuals away from a pile one by one. Other time intervals besides these two required that counts be made from a distance while I observed through binoculars; so if a quadrat had already amassed a large pile of crabs, then slight underestimates were possible if some crabs happened to be covering others. Hence, for the purpose of statistical comparison I focus exclusively on the counts made at time 0 and time-(+10), where an underestimate was not possible. t-tests were used to compare these two time points, since the results were the same when more complex statistical tests were carried out (in which quadrat and replicate number were incorporated as random variables). The Bonferroni method was applied to control the overall α level within each experiment at 0.05; thus only individual quadrats with p < 0.0125 were deemed significant. Figures in §2 graph the counts made across all time intervals (−10 to +10), documenting the temporal dynamics of crab numbers in each quadrat.
3. Results
(a). Natural aggregations
Aggregations (n = 65) were composed of 10.9 ± 1.4 crabs (mean ± s.e.), ranging from the lower-bound of three crabs up to 70. The majority of aggregations (70.8%) were based around a single, clumped piece of food. Foods included an eclectic array of items that terrestrial hermit crabs consume in their omnivorous diet: beach almonds, flowers of beach almond, figs, palm nuts and coconuts, plantains, jicaro (Amphitecna latifolia), sea turtle eggs and dead baby turtles, dead shore crabs, dead insects and horse droppings. The most aggregations accounted for by any single resource (53.9%) were those for beach almond. Aggregations that did not involve food items (29.2%) were based on either newly discovered empty shells or shells that had become empty after their owner was apparently forcibly evicted, and was waiting naked within the aggregation itself.
(b). Experiment 1: tethered crabs
Neither of the control quadrats in experiment 1 showed a change in the number of crabs between the baseline-control period and the subsequent period (t-test for control 1: t = 0, d.f. = 38, p = 1; electronic supplementary material, figure S5a; t-test for control 2: t = 0.33, d.f. = 38, p = 0.75; electronic supplementary material, figure S5b). Across all controls for experiments 1–4, the average number of crabs in a control quadrat at any given time point in the experiment was generally (86.3% of n = 168 time points) less than one crab per quadrat (elecronic supplementary material, figures S5–S8). Moreover, no control quadrat at any time point for any experiment ever reached an average of even two crabs per quadrat, obtaining maximally an average of only 1.95 crabs per quadrat. By contrast, in the two conditions in experiment 1 with tethered crabs there were accumulations of up to 45 crabs per quadrat at the end of the experiment, and both conditions showed significant increases in the number of crabs within their respective quadrats from the baseline-control period to the subsequent period (t-test for three crabs tethered: t = 5.77, d.f. = 38, p < 0.0001; figure 1a; t-test for six crabs tethered: t = 5.52, d.f. = 38, p < 0.0001; figure 1b). Simulated aggregations were therefore extremely attractive to solitary foragers despite the unnatural situation of there being no actual resource beneath them.
(c). Experiment 2: tethered shell-less crabs and empty shells
Neither of the control quadrats showed a change in the number of crabs between the baseline-control period and the subsequent period (t-test for control 1: t = 0.95, d.f. = 38, p = 0.35; electronic supplementary material, figure S6a; t-test for control 2: t = 0.44, d.f. = 38, p = 0.66; electronic supplementary material, figure S6b). By contrast, in the tethered shell-less crab condition there was a significant increase in the number of crabs within the quadrat from the baseline-control period to the subsequent period (t-test for three shell-less crabs tethered: t = 7.69, d.f. = 38, p < 0.0001; figure 1c). There was also a significant increase in the quadrat with the tethered empty shells (t-test for three empty shells tethered: t = 5.02, d.f. = 38, p < 0.0001; figure 1d). However, the quadrat with the shell-less tethered crabs accumulated an average of nearly three times more crabs than the quadrat with the tethered empty shells, a difference that was highly significant (t-test of the final sample points between the shell-less crab condition and the empty shell condition: t = 4.64, d.f. = 38, p < 0.0001; cf. figure 1c,d). A simulated aggregation of shell-less crabs was therefore a more attractive stimulus to solitary foragers than freely available shell resources. The effect of the empty shells seemed to result only because solitary foragers occasionally encountered these shells, fidgeting with and entering them, such that the shells no longer remained stationary, instead exhibiting some of the movement characteristic of aggregations.
(d). Experiment 3: chemical cues
Neither of the control quadrats nor the condition quadrats showed a change in the number of crabs between the baseline-control period and the subsequent period (t-test for control 1: t = 0.99, d.f. = 38, p = 0.33; electronic supplementary material, figure S7a; t-test for control 2: t = 0.79, d.f. = 38, p = 0.44; electronic supplementary material, figure S7b; t-test for three crabs in white cup: t = 0.47, d.f. = 38, p = 0.64; electronic supplementary material, figure S7c; t-test for beach almond in white cup: t = 1.70, d.f. = 38, p = 0.10; electronic supplementary material, figure S7d). Thus, neither the scent of an aggregation of crabs nor the scent of the crabs' staple food was sufficient to attract solitary foragers.
(e). Experiment 4: visual cues
Neither of the control quadrats showed a change in the number of crabs between the baseline-control period and the subsequent period (t-test for control 1: t = 0.27, d.f. = 42, p = 0.79; electronic supplementary material, figure S8a; t-test for control 2: t = 0, d.f. = 42, p = 1; electronic supplementary material, figure S8b). By contrast, in the conditions with aggregations kept beneath a glass there was a nearly significant trend (t-test for three crabs under glass: t = 2.37, d.f. = 42, p = 0.0226; figure 2a) and a significant increase (t-test for six crabs under glass: t = 3.02, d.f. = 42, p = 0.0043; figure 2b) in the number of crabs within each quadrat from the baseline-control period to the subsequent period. The visual image of an aggregation of crabs was therefore sufficient to attract solitary foragers. However, in comparison with the level of accumulation found in the conditions in experiments 1 and 2, in which crabs were tethered, visual cues did not generate substantial accumulations of crabs by the end of the experiment (t-test of the final sample points between the condition with three crabs tethered and the condition with three crabs under glass: t = 5, d.f. = 40, p < 0.0001; cf. figures 1a and 2a; t-test of the final sample points between the condition with six crabs tethered and the condition with six crabs under glass: t = 4.93, d.f. = 40, p < 0.0001; cf. figures 1b and 2b). Although solitary foragers oriented to glasses containing aggregations, soon after reaching the glass they departed the region, typically only circling the glass one to several times while trying ineffectively to claw their way into the aggregation (electronic supplementary material, video S3). Without being able to enter into the cluster itself and physically rummage for a resource, they had no incentive to stay.
(f). Final accumulation
For those conditions in which there was a significant change from the baseline-control period to the subsequent period, there was also a significant difference across these conditions in their final accumulations at the last sample point in the experiment (one-way analysis of variance: F6,137 = 23.57, p < 0.0001; electronic supplementary material, figure S9). When arranged from least-to-most in their final accumulation, the order was: (i) the two conditions with crabs under glass (experiment 4); (ii) the condition with empty shells tethered (experiment 2); (iii) the two conditions with naturally shelled crabs tethered (experiment 1); and finally (iv) the condition with shell-less crabs tethered (experiment 2). That a simulated aggregation of three shell-less crabs generated the greatest accumulation (significantly exceeding a simulated aggregation of three shelled crabs, t-test: t = 3.48, d.f. = 38, p = 0.0013), suggested that solitary foragers do not use the presence of resources to orient to aggregations. Crabs are lured in solely the presence and activity of conspecifics, even those lacking their natural shells.
4. Discussion
Social information transmission is a powerful process that can lead to the spread of valuable ecological knowledge across populations. Among invertebrates, the most elaborate cases of social information transmission occur in the eusocial insects, which have evolved an array of signals—encompassing visual, chemical and tactile components—that are designed for recruiting colony members to newly discovered resources (e.g. trail pheromones in ants and the waggle dance in honeybees; Hölldobler & Wilson 2008). Impressively, tropical hermit crabs can outdo sympatric eusocial insects in the speed with which they form large assemblies at food resources deposited at the nexus of the beach and forest (J. T. Longino, Neotropical myrmecologist 2008, personal communication). Given the radically different social organizations of hermit crabs and eusocial insects, how and why are crabs luring in conspecifics?
At a proximate level, solitary foragers appear to detect perturbations in the density of surrounding foragers, investigating areas of high clustering and activity, where aggregations have recently formed around resources. Supporting this, simulated aggregations with tethered crabs reproduced recruit numbers similar to natural aggregations. While crabs were unable to orient to simulated aggregations based on smell, they did readily navigate towards aggregations that were visible only. The tip-off appears to be the collective jostling and commotion of many crabs in a concentrated locale, since several stationary shells that initially lacked such activity were significantly less effective than shell-less but otherwise moving crabs. The fact that shell-less crabs were the most attractive aggregation might be a consequence of greater activity levels of crabs that are naked compared with crabs that are shelled: the former are particularly desperate for shelter, which might cause them to struggle more, thereby enhancing the cues that draw in surrounding crabs.
For an aggregation to build up and sustain its size, secondary tactile feedback appears critical. Thus, in experiment 4, the glass setup was unable to generate aggregations on the order of the tethering setup, evidently because it precluded tactile contact between the attracted foragers and those clustered beneath the glass. Visual elements therefore may only provide the initial cue that attracts crabs to an inchoate, budding aggregation around a resource; tactile contact may be necessary thereafter to keep crabs at the aggregation, rummaging for the resource.
Although in the experiments themselves crabs did not learn anything from approaching the simulated aggregations (given that resources had been purposely excluded beforehand), the data on natural aggregations (see §3) indicate that crabs which approach conspecific congregations will learn the location of a valuable resource, typically food. Such a process of ‘local enhancement’ (Thorpe 1963) or ‘area copying’ (Giraldeau 1997) is thought to occur widely across taxa, requiring only that ‘after, or during a demonstrator's presence, or interaction with objects, at a particular location, an observer is more likely to visit or interact with objects at that location.’ (Hoppitt & Laland 2008, p. 109). In captivity, local enhancement is probably common, and while local enhancement has also been reported from many observational field studies (Hoppitt & Laland 2008) as well as a few manipulative studies of wild fishes (Reader et al. 2003), this study provides experimental evidence for the dynamics of local enhancement in a free-living invertebrate that is largely asocial, lacking any group structure even approximating the shoals of fishes.
Terrestrial hermit crabs' reliance on socially acquired information, in spite of an asocial organization, appears fundamentally related to their natural ecology, where the locations of essential resources like food and shelter are haphazard, change constantly and are difficult to track down individually. It would be revealing in future studies to monitor individual crabs over time and quantify their foraging success in the absence and presence of social cues. Given the difficult ecological circumstances that crabs face, social cues would be expected to improve foraging success, such that crabs' reliance on social information would be adaptive for information users. But what are the fitness consequences for those that generate the information?
At an ultimate level, the use of inadvertent social information can vary from situations where both bystanders and performers mutually benefit to situations where bystanders effectively parasitize information, inflicting a cost on performers (Danchin et al. 2004). The terrestrial hermit crabs' use of social information may be more consistent with the latter, exploitative category. There was little indication that crabs within an aggregation benefited by attracting conspecifics to the resource site. Crabs compete intensely to access the single resource beneath their aggregation, knocking others out of the way with hard strikes as they tumble in a melee to grasp the resource. Later recruits seem to only add to the antagonism, inflicting a cost on those already present, since they reduce the portion of the food resource each crab stands to acquire. Hypothetical ways that crabs might benefit from attracting recruits should be quantified in future studies, but three can be commented upon here. First, aggregated crabs might benefit from a predation-based ‘dilution effect’ (Hamilton 1971). Predation, however, was never observed during daylight foraging—the time when aggregations are visually attractive. Second, crabs might benefit by having assistance in tearing into tough-skinned foods. But the most common food crabs aggregated around naturally (beach almond) always involved a ripe specimen, which solitary individuals readily rip open. Third, crabs might benefit from exchanging shells while they are aggregated, with more recruits widening the scope for exchange. Fine-grained video analysis of natural aggregations will be necessary to determine whether and how often crabs switch shells inside aggregations. In this study though, tethered crabs were stuck in the centre of aggregations that lacked any other resource besides these crabs' shells and yet these crabs always retained their shells by the end of the experiment. It seems possible, therefore, that the solitary foragers that orient to aggregations are the sole beneficiaries in the relationship. If so, why do crabs within aggregations continue generating information that harms their personal fitness?
The answer appears to be that crabs simply cannot help but help: it would be all but impossible for crabs to ‘erase’ the relevant information-bearing cues that impose a cost on them, since these cues are based on their very presence at a resource. Their natural competitive activity while tussling to access the resource is what provides the inadvertent social information which helps surrounding foragers orient in from the fringe of aggregations. In this regard, it is telling that crabs have not evolved a more prominent form of attraction, transforming cues into signals like the eusocial insects, since this evolutionary transition is thought to necessitate a benefit to the signalling party (Bradbury & Vehrencamp 1998). Notably, hermit crabs do possess rich repertoires of other signals, especially formalized agonistic behaviours, so they appear to have no shortage of ‘raw material’ that could be ritualized into recruitment signals (Hazlett 1972). However, without a gain for recruiting others, attractive signals are not to be expected, and the signals hermit crabs do have function not to attract conspecifics from far away but rather to ward off conspecifics that have already arrived at a resource (Laidre 2007). Thus, although at an evolutionary level, crabs have no intention to help one another; their underlying ecology has provided an arrangement where this occurs regularly through the use of inadvertent social information.
Acknowledgements
I thank the Costa Rican Ministry of Environment and Energy (Ministerio de Ambiente y Energia, MINAE) for permitting me to conduct this study, as well as the Osa Biodiversity Center (OBC) and Friends of the Osa (FOO). I am grateful to Guido Saborío for loaning binoculars and kindly helping with logistics; Adrian Forsyth for loaning a spotting scope; Dennis Vasquez for coordinating permits; Jennifer Cruz and Trond Larsen for pre-travel advice; Aida Bustamante and Ricardo Moreno for information about the locality; Manuel Ramirez for allowing me to work on his land and use his beach house; Rick Stanley and Nick Smith for field assistance; Daniel Stanton, Maria Echeverry and Sergio Cordoba for translating documents into Spanish; and Catherine Markham and Daniel Stanton for the tip about the field site and its crabs. The manuscript benefited from critiques of two anonymous reviewers, discussions with Rachael Winfree and Jessica Yorzinski, and comments from Iain Couzin and the Collective Animal Behaviour Laboratory during an EEB seminar. Supported by an NSF Graduate Research Fellowship and a Princeton University Graduate School Centennial Fellowship in the Sciences and Engineering.
References
- Ball E. E.1972Observations on the biology of the hermit crab, Coenobita compressus H. Milne Edwards (Decapoda: Anomura), on the west coast of the Americas. Rev. Trop. Biol. 20, 265–273 [Google Scholar]
- Bonnie K. E., Earley R. L.2007Expanding the scope for social information use. Anim. Behav. 74, 171–181 (doi:10.1016/j.anbehav.2006.12.009) [Google Scholar]
- Bradbury J. W., Vehrencamp S. L.1998Principles of animal communication Sunderland, MA: Sinauer Associates [Google Scholar]
- Bright D. B.1966The land crabs of Costa Rica. Rev. Trop. Biol. 14, 183–203 [Google Scholar]
- Burggren W. W., McMahon B. R. (eds) 1988Biology of the land crabs New York, NY: Cambridge University Press [Google Scholar]
- Childress J. R.1972Behavioral ecology and fitness theory in a tropical hermit crab. Ecology 53, 960–964 (doi:10.2307/1934316) [Google Scholar]
- Chittka L., Leadbeater E.2005Social learning: public information in insects. Curr. Biol. 15, R869–R871 (doi:10.1016/j.cub.2005.10.018) [DOI] [PubMed] [Google Scholar]
- Coolen I., Van Bergen Y., Day R. L., Laland K. N.2003Species difference in adaptive use of public information in sticklebacks. Proc. R. Soc. Lond. B 270, 2413–2419 (doi:10.1098/rspb.2003.2525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen I., Dangles O., Casas J.2005Social learning in noncolonial insects? Curr. Biol. 15, 1931–1935 (doi:10.1016/j.cub.2005.09.015) [DOI] [PubMed] [Google Scholar]
- Dall S. R. X.2005Defining the concept of public information. Science 308, 353–354 [DOI] [PubMed] [Google Scholar]
- Dall S. R. X., Giraldeau L.-A., Olsson O., McNamara J. M., Stephens D. W.2005Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- Danchin E., Giraldeau L.-A., Valone T. J., Wagner R. H.2004Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- Fiorito G., Scotto P.1992Observational learning in Octopus vulgaris. Science 256, 545–547 (doi:10.1126/science.256.5056.545) [DOI] [PubMed] [Google Scholar]
- Galef B. G., Jr, Giraldeau A.2001Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 (doi:10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
- Giraldeau A.1997The ecology of information use. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 42–68 Oxford, UK: Blackwell Science [Google Scholar]
- Giraldeau L. A., Caraco T.2000Social foraging theory Princeton, NJ: Princeton University Press [Google Scholar]
- Hamilton W. D.1971Geometry for the selfish herd. J. Theor. Biol. 31, 295–311 (doi:10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- Hazlett B. A.1972Ritualization in marine crustacea. In Behavior of marine animals, vol. 1 (eds Winn H. E., Olla B. L.), pp. 97–125 New York, NY: Plenum Press [Google Scholar]
- Hazlett B. A.1981The behavioral ecology of hermit crabs. Ann. Rev. Ecol. Syst. 12, 1–22 (doi:10.1146/annurev.es.12.110181.000245) [Google Scholar]
- Heyes C. M., Galef B. G., Jr (eds) 1996Social learning in animals: the roots of culture San Diego, CA: Academic Press [Google Scholar]
- Hölldobler B., Wilson E. O.2008The superorganism: the beauty, elegance, and strangeness of insect societies New York, NY: W.W. Norton [Google Scholar]
- Hoppitt W., Laland K. N.2008Social processes influencing learning in animals: a review of the evidence. Adv. Stud. Behav. 38, 105–165 (doi:10.1016/S0065-3454(08)00003-X) [Google Scholar]
- Karban R., Maron J.2002The fitness consequences of interspecific eavesdropping between plants. Ecology 83, 1209–1213 [Google Scholar]
- Kurta A.1982Social facilitation of foraging behavior by the hermit crab, Coenobita compressus, in Costa Rica. Biotropica 14, 132–136 (doi:10.2307/2387742) [Google Scholar]
- Laidre M. E.2007Vulnerability and reliable signaling in conflicts between hermit crabs. Behav. Ecol. 18, 736–741 (doi:10.1093/beheco/arm040) [Google Scholar]
- Laidre M. E.2009Informative breath: olfactory cues sought during social foraging among old world monkeys (Mandrillus sphinx, M. leucophaeus, and Papio anubis). J. Comp. Psychol. 123, 34–44 (doi:10.1037/a0013129) [DOI] [PubMed] [Google Scholar]
- Leadbeater E., Chittka L.2005A new mode of information transfer in foraging bumblebees? Curr. Biol. 15, R447–R448 (doi:10.1016/j.cub.2005.06.011) [DOI] [PubMed] [Google Scholar]
- Leadbeater E., Chittka L.2007Social learning in insects: from miniature brains to consensus building. Curr. Biol. 17, R703–R713 (doi:10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- Mery F., Varela S. A. M., Danchin E., Blanchet S., Parejo D., Coolen I., Wagner R. H.2009Public versus personal information for mate copying in an invertebrate. Curr. Biol. 19, 730–734 (doi:10.1016/j.cub.2009.02.064) [DOI] [PubMed] [Google Scholar]
- Reader S. M., Kendal J. R., Laland K. N.2003Social learning of foraging sites and escape routes in wild Trinidadian guppies. Anim. Behav. 66, 729–739 (doi:10.1006/anbe.2003.2252) [Google Scholar]
- Seeley T. D.1989The honey bee colony as a superorganism. Am. Scient. 77, 546–553 [Google Scholar]
- Seppänen J.-T., Forsman J. T., Mönkkönen M., Thomson R. L.2007Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88, 1622–1633 (doi:10.1890/06-1757.1) [DOI] [PubMed] [Google Scholar]
- Small M. P., Thacker R. W.1994Land hermit-crabs use odors of dead conspecifics to locate shells. J. Exp. Mar. Biol. Ecol. 182, 169–182 (doi:10.1016/0022-0981(94)90049-3) [Google Scholar]
- Thorpe W. H.1963Learning and instinct in animals, 2nd edn.London, UK: Methuen [Google Scholar]
- Valone T. J.1989Group foraging, public information, and patch estimation. Oikos 56, 357–363 (doi:10.2307/3565621) [Google Scholar]
- Warner G. F.1977The biology of crabs New York, NY: Van Nostrand-Reinhold [Google Scholar]
- Webster S. J., Fiorito G.2001Socially guided behaviour in non-insect invertebrates. Anim. Cogn. 4, 69–79 (doi:10.1007/s100710100108) [Google Scholar]
- Worden B. D., Papaj D. R.2005Flower choice copying in bumble-bees. Biol. Lett. 1, 504–507 (doi:10.1098/rsbl.2005.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]