Abstract
While all members of the Orchidaceae are fully dependent on mycorrhizal fungi during their achlorophyllous juvenile stages, mature plants may remain fully myco-heterotrophic, become fully autotrophic or develop a nutritional mode where the carbon gain through photosynthesis is complemented by organic carbon from fungal partners. This so-called partial myco-heterotrophy is intriguingly complex. Current knowledge indicates a large range in the proportion of fungus-derived carbon between and within partially myco-heterotrophic plant species. However, the driving factors for this variation are so far mostly unknown. Here we show for two green species of the orchid genus Cephalanthera that light availability is the major determinant of the degree of myco-heterotrophy. Using leaf stable isotope natural abundance analysis together with time-integrated microscale light climate monitoring we could demonstrate that there is a sensitive reaction to varying light availability within forests. Low light levels result in strong myco-heterotrophy while higher irradiances successively drive the orchids towards autotrophy. Our results demonstrate that partial myco-heterotrophy in these species is not a static nutritional mode but a flexible mechanism driven by light availability which allows a balanced usage of carbon resources available in nature.
Keywords: partial myco-heterotrophy, Orchidaceae, stable isotopes, carbon gain, irradiance, mycorrhiza
1. Introduction
Since the recent discovery of a novel nutritional mode in the world's largest plant family, the Orchidaceae, a dogma in plant sciences meaning that green plants (with the exception of some hemiparasites) are autotrophic is no longer valid (G. Gebauer in Whitfield 2007). Although green plants are able to photosynthesize, some specialized terrestrial orchids have recently been shown to additionally use an underground carbon source—their mycorrhizal fungi (Gebauer & Meyer 2003). A switch of their mycorrhizal associates from typical Rhizoctonia species (a polyphyletic group of fungi) to ectomycorrhizal partners that are simultaneously associated with trees enables the looting of organic nutrients (Bidartondo et al. 2004). Analogue mechanisms have in the meantime also been found in some green pyroloids (Ericaceae, Tedersoo et al. 2007; Zimmer et al. 2007; Hynson et al. 2009) and ongoing investigations continually reveal further species that exhibit this complex form of nutrition (Bidartondo et al. 2004; Julou et al. 2005; Abadie et al. 2006; Zimmer et al. 2008), which can be referred to as partial myco-heterotrophy (Gebauer & Meyer 2003). Although it can be hypothesized that many more green plants from diverse taxa may up to now unnoticeably gain organic compounds through myco-heterotrophic means, we know very little on the factors driving this phenomenon.
Stable isotope natural abundances in organism tissues are a convenient tool to study the use of isotopically distinguished nutrient sources. The incorporation of fungus-derived carbon, for example, is reflected by the green plants' leaf isotope signature since fungal tissues are enriched in the heavy carbon stable isotope 13C relative to accompanying fully autotrophic plants (Högberg et al. 1999). Previous studies indicate a large range in the proportion of fungus-derived carbon between and within partially myco-heterotrophic species (Gebauer & Meyer 2003; Bidartondo et al. 2004; Julou et al. 2005; Abadie et al. 2006; Tedersoo et al. 2007; Zimmer et al. 2007, 2008; Cameron et al. 2009; Hynson et al. 2009) but the driving factors for this variation remain mostly unknown.
Zimmer et al. (2007) suggested a negative relationship between the quantity of carbon gain from fungi and light availability in the wintergreen Orthilia secunda based on results of a previous comparison of three independent investigations on the trophic status of green orchids at different forest types by Gebauer (2005), who raised the hypothesis that the exploitation of mycorrhizal fungi might be affected by the prevalent light climate. To test this hypothesis experimentally, we combined leaf stable isotope natural abundance analysis with time-integrated microscale light climate monitoring and investigated two partially myco-heterotrophic orchid species (Cephalanthera damasonium and C. rubra) together with 12 fully autotrophic and one fully myco-heterotrophic reference species.
2. Material and methods
(a). Study sites and investigated species
Plant samples were collected in 2007 and 2008 from three forest sites in northeast Bavaria: an open Pinus sylvestris stand, a forest dominated by Fagus sylvatica and a mixed stand composed of several conifers (e.g. P. sylvestris, Picea abies) and broadleaf species (e.g. F. sylvatica, Acer campestre). All sites are located at 480–520 m a.s.l. and characterized by mean annual precipitation of 700–1000 mm and mean annual temperatures of 6–9°C. In total, 224 understorey plant samples were collected from a fully myco-heterotrophic (Neottia nidus-avis, n = 11), a fully autotrophic (Cypripedium calceolus, n = 9) and two partially myco-heterotrophic orchid species (Cephalanthera damasonium, n = 18; C. rubra, n = 18); and from 11 diverse (monocotyledons/dicotyledons, tree saplings/herbs, evergreen/deciduous, ectomycorrhizal/arbuscular- or non-mycorrhizal) autotrophic non-orchid species (A. campestre, n = 9; A. pseudoplatanus, n = 3; Anthericum ramosum, n = 12; Carex flacca, n = 20; Convallaria majalis, n = 11; Euphorbia cyparissias, n = 20; F. sylvatica, n = 48; Fragaria vesca, n = 3; Galium odoratum, n = 10; G. verum, n = 10; Polygala chamaebuxus, n = 22).
(b). Microscale light climate monitoring
For each of the 56 orchid individuals, a 1 m2 plot including two to four autotrophic non-orchids was selected. As soon as the young orchid shoots could be identified, a calibrated light sensor (silicon photodiode BPW 21, Infineon, Germany) connected to a mini data logger (HOBO H8, ONSET, USA) was installed right next to each shoot at about 15 cm height. Irradiance was logged every 15 min from the day of sensor installation until the development of seed capsules (2007: 9 May–20 June; 2008: 18 May–6 July). Measured values were converted into photosynthetic active radiation (µmol photons m−2 s−1) and averaged as daily means (from sunrise to sunset). Because of the equal global solar radiation during each month from May to July in the two sampling years, the measured relative light availability of understorey plants had not to be adjusted (total in 2007: 488 kWh m−2; total in 2008: 494 kWh m−2; weather station of the Ecological-Botanical Garden Bayreuth, 32–40 km apart from the three sampling sites). Furthermore, potential differences in the orchids' developmental stage are known to have no significant influence on their leaf δ13C values as has been found for four C. damasonium individuals that were analysed every month from June to September 2003 at an F. sylvatica-dominated forest in northeast Bavaria (B. Burghardt 2003, unpublished data).
(c). Carbon stable isotope abundance analysis
Leaf samples (and stem samples of the leafless N. nidus-avis) were taken following the criteria described by Gebauer & Meyer (2003). The plant material was oven-dried at 105°C and ground to a fine powder. Relative C isotope abundances were measured with an elemental analyser coupled to a continuous flow isotope ratio mass spectrometer as described in Bidartondo et al. (2004). Measured abundances are denoted as δ values, which were calculated according to the following equation: δ13C = (Rsample/Rstandard − 1) × 1000 [‰], where Rsample and Rstandard are the ratios of heavy isotope to light isotope of the samples and the respective standard. Standard gases were calibrated with respect to international standards by using the reference substances ANU sucrose and NBS 19, provided by the International Atomic Energy Agency (Vienna, Austria).
(d). Data preparation and statistics
To facilitate precise data comparisons between sites and plots, δ values were normalized according to Preiss & Gebauer (2008): δ13C values of the orchids and the non-orchid autotrophic reference plants were used to calculate 13C enrichment factors (ε) of every plant against the mean of the autotrophic plants for each plot: εS = δS − δREF; with S as single value of a sample from an autotrophic, partially or fully myco-heterotrophic orchid and REF as the mean value of all autotrophic reference plants from the respective plot.
Differences between δ13C values of Cephalanthera individuals and autotrophic reference plants were analysed using Mann–Whitney U-tests at different light levels (below and above 200 µmol m−2 s−1). To test for significant (α = 0.05) correlations between measured light availability and δ13C values or enrichment factors (ε), respectively, regression analyses were performed. All statistical tests were conducted using SigmaPlot v. 11.0 (Systat Software, Inc., USA). Means are given as ±1 s.d.
3. Results and discussion
(a). Responses of δ13C to varying irradiance
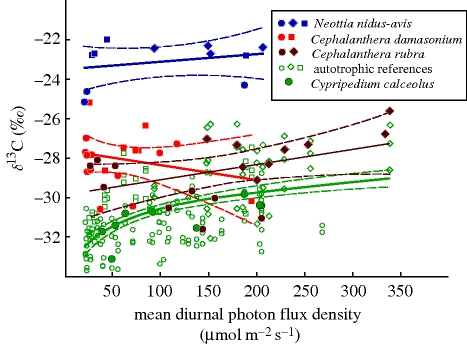
δ13C values in leaves of autotrophic non-orchids ranged from −34.2 to −26.3‰ (figure 1) and showed a significant, positive correlation with light availability (F1,166 = 70.2, R2adj. = 0.293, p < 0.001). These δ13C values and their dependence on light climate are based on the carbon isotope discrimination during C3 photosynthesis (fractionation during carboxylation by Rubisco) and on stomatal regulation, which affects the intercellular partial pressure of CO2 (Farquhar et al. 1989). Leaf isotope signatures of the fully autotrophic orchid C. calceolus responded in the same way as autotrophic non-orchids (F1,7 = 7.5, R2adj. = 0.518, p = 0.029), demonstrating that members of the Orchidaceae per se do not show any peculiarity in carbon nutrition. This is consistent with the findings of Zimmerman & Ehleringer (1990), who analysed the carbon isotope composition of a Panamanian epiphytic C3 orchid (Catasetum viridiflavum) and found higher δ13C values with increasing irradiance owing to increasing stomatal limitation to photosynthesis.
Figure 1.
δ13C values of 12 autotrophic plant species (n = 177) including the orchid C. calceolus, two partially myco-heterotrophic Cephalanthera spp. (n = 18 each) and the fully myco-heterotrophic orchid N. nidus-avis (n = 11), plotted against mean light availability. Samples were collected from an open P. sylvestris stand (diamonds), a forest dominated by Fagus sylvatica (circles) and a mixed stand (squares) in northeast Bavaria. Regression curves (solid lines) are given with 95% confidence intervals (dashed lines).
The achlorophyllous orchid N. nidus-avis showed the highest δ13C values of all investigated species (−23.1 ± 1.10‰ on average). Such a relative 13C enrichment is characteristic of all fully myco-heterotrophic plants that associate with ectomycorrhizal fungi (Preiss & Gebauer 2008) and fits the food-chain model (Trudell et al. 2003). Since these plants' carbon demand is exclusively covered through organic compounds supplied by fungi, δ13C values of N. nidus-avis are not correlated with the microscale light climate (F1,9 = 0.7, R2adj. < 0.001, p = 0.411; figure 1).
A quite interesting pattern turned out for the two Cephalanthera species. Although these green orchids are able to photosynthesize and a positive correlation of their carbon isotope signatures with light availability just as in other green plants could thus be expected, no significant response to varying irradiance was found (figure 1; C. damasonium: F1,16 = 1.0, R2adj. < 0.001, p = 0.341; C. rubra: F1,16 = 4.0, R2adj. = 0.149, p = 0.064). Their mean δ13C values (C. damasonium: −28.1 ± 1.4‰; C. rubra: −28.6 ± 1.6‰) range between those of fully autotrophic and fully myco-heterotrophic plants as typical for partial myco-heterotrophs. While δ13C values of Cephalanthera individuals and fully autotrophic plants are significantly different at irradiances below 200 µmol m−2 s−1 (p < 0.001), these groups are statistically indistinguishable (p = 0.059) at higher light levels indicating a pronounced shift towards autotrophic nutrition at sufficiently light-exposed sites. However, since it has been shown that irradiance-dependent physiological effects can strongly influence leaf δ13C values, isotope data have to be related to a fine spatial scale before assessing the question whether partial myco-heterotrophy is a flexible or a static nutritional mode.
(b). Effects of irradiance on partial myco-heterotrophy
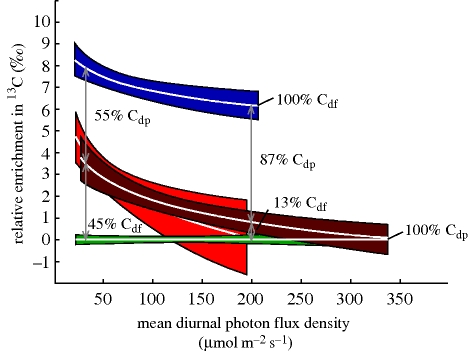
Figure 2 shows the plants' 13C enrichment normalized for environmental changes by relating all isotope data to references of the respective plot as described by Preiss & Gebauer (2008). Owing to referencing δ13C values of N. nidus-avis and the two Cephalanthera species against autotrophic species whose δ13C values increase with increasing irradiance (cp. figure 1), the enrichment factors (ε) of these orchids decrease with increasing light availability. However, there are different physiological processes behind the reactions of Neottia and the Cephalanthera species. While the non-photosynthetic N. nidus-avis completely relies on myco-heterotrophic carbon supplies and constantly gains 100% of its carbon from mycorrhizal fungi, green Cephalanthera species are able to use both the fungal and the atmospheric carbon source. Relating the Cephalantheras' 13C enrichment factors to those of N. nidus-avis shows that the incorporation of organic carbon from fungi is not a constant parameter in these green orchids. As indicated by the arrows (figure 2), Cephalanthera shoots receive about half as much fungus-derived carbon as achlorophyllous plants under low light conditions, while the proportion of heterotrophic nutrition strongly decreases with increasing irradiance. This finding is also supported by previous results of gas-exchange measurements showing that photosynthetic rates in C. damasonium can increase when more light is available (Julou et al. 2005). Thus, the constant δ13C values in Cephalanthera along an irradiance gradient seen in figure 1 are obviously caused by successively lowering the incorporation of 13C-enriched fungal tissue with increasing light availability. During the transfer of organic compounds like amino acids, fully and partially myco-heterotrophic plants are also gaining small amounts of nitrogen affecting their 15N signature. In accordance with the decreasing carbon gain, a trend towards lower 15N enrichment with increasing light availability could be seen in Cephalanthera shoots (not shown). Since weak gains of organic nitrogen were still detectable at high irradiances, it seems as if partially myco-heterotrophic plants do not totally give up their fungal nutrient source. However, we were able to demonstrate that, at high irradiances, above ground parts of adult Cephalanthera plants can cover almost all of their carbon demands through assimilation of atmospheric CO2.
Figure 2.
Correlation between relative enrichments in 13C (ε) calculated per plot (Preiss & Gebauer 2008) and mean light availability based on the data shown in figure 1. Regression lines (±95% confidence intervals) represent the range of isotope signatures of autotrophic (green), partially myco-heterotrophic (light and dark red) and fully myco-heterotrophic plants (blue). Arrows indicate the variable proportion of carbon derived from fungi (Cdf) or photosynthesis (Cdp), respectively.
4. Conclusions
It has been shown that two partially myco-heterotrophic Cephalanthera species strongly supplement their carbon gain through photosynthesis by organic carbon from fungal partners under low light conditions but become almost completely autotrophic when they are exposed to sufficiently high irradiances. This demonstrates that partial myco-heterotrophy is not a strictly static nutritional mode but may be a flexible mechanism allowing a balanced use of carbon resources available in nature. The fact that the degree of myco-heterotrophy may successively change—driven by the prevalent microscale light climate—could explain several discrepancies between previous studies that investigated the trophic status of numerous green Orchidaceae and Ericaceae.
Acknowledgements
This work was supported by the German Research Foundation and contributes to the DFG project GE 565/7-1. The authors would like to thank the technical staff of BayCEER–Isotope Biogeochemistry for skilful assistance in mass spectrometry and Michael Gaag (all University of Bayreuth) for innovative ideas and their implementation during light sensor construction. We gratefully acknowledge permission for orchid sampling by the Regierung von Oberfranken and Mittelfranken.
References
- Abadie J.-C., Püttsepp Ü., Gebauer G., Faccio A., Bonfante P., Selosse A.2006Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and non-photosynthetic individuals. Can. J. Botany 84, 1462–1477 (doi:10.1139/B06-101) [Google Scholar]
- Bidartondo M. I., Burghardt B., Gebauer G., Bruns T. D., Read D. J.2004Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc. R. Soc. Lond. B 271, 1799–1806 (doi:10.1098/rspb.2004.2807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D. D., Preiss K., Gebauer G., Read D. J.2009The chlorophyll-containing orchid Corallorhiza trifida derives little carbon through photosynthesis. New Phytol. 183, 358–364 (doi:10.1111/j.1469-8137.2009.02853.x) [DOI] [PubMed] [Google Scholar]
- Farquhar G. D., Ehleringer J. R., Hubick K. T.1989Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537 (doi:10.1146/annurev.pp.40.060189.002443) [Google Scholar]
- Gebauer G.2005Partnertausch im dunklen Wald–Stabile Isotope geben neue Einblicke in das Ernährungsverhalten von Orchideen. In Rundgespräche der Kommission für Ökologie, vol. 30(ed. Bayerische Akademie der Wissenschaften), pp. 55–67 München, Germany: Verlag Dr. Friedrich Pfeil [Google Scholar]
- Gebauer G., Meyer M.200315N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol. 160, 209–223 (doi:10.1046/j.1469-8137.2003.00872.x) [DOI] [PubMed] [Google Scholar]
- Högberg P., Plamboeck A. H., Taylor A. F. S., Fransson P. M. A.1999Natural 13C abundance reveals trophic status of fungi and host-origin of carbon in mycorrhizal fungi in mixed forests. Proc. Natl Acad. Sci. USA 96, 8534–8539 (doi:10.1073/pnas.96.15.8534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynson N. A., Preiss K., Gebauer G., Bruns T. D.2009Isotopic evidence of full and partial myco-heterotrophy in the plant tribe Pyroleae (Ericaceae). New Phytol. 182, 719–726 (doi:10.1111/j.1469-8137.2009.02781.x) [DOI] [PubMed] [Google Scholar]
- Julou T., Burghardt B., Gebauer G., Berveiller D., Damesin C., Selosse A.2005Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol. 166, 639–653 (doi:10.1111/j.1469-8137.2005.01364.x) [DOI] [PubMed] [Google Scholar]
- Preiss K., Gebauer G.2008A methodological approach to improve estimates of nutrient gains by partially myco-heterotrophic plants. Isotopes Environ. Health Stud. 44, 393–401 (doi:10.1080/10256010802507458) [DOI] [PubMed] [Google Scholar]
- Tedersoo L., Pellet P., Kõljalg U., Selosse A.2007Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia 151, 206–217 (doi:10.1007/s00442-006-0581-2) [DOI] [PubMed] [Google Scholar]
- Trudell S. A., Rygiewicz P. T., Edmonds R. L.2003Nitrogen and carbon stable isotope abundances support the myco-heterotrophic nature and host-specificity of certain achlorophyllous plants. New Phytol. 160, 391–401 (doi:10.1046/j.1469-8137.2003.00876.x) [DOI] [PubMed] [Google Scholar]
- Whitfield J.2007Underground networking. Nature 449, 136–138 (doi:10.1038/449136a) [DOI] [PubMed] [Google Scholar]
- Zimmer K., Hynson N. A., Gebauer G., Allen E. B., Allen M. F., Read D. J.2007Wide geographical and ecological distribution of nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. New Phytol. 175, 166–175 (doi:10.1111/j.1469-8137.2007.02065.x) [DOI] [PubMed] [Google Scholar]
- Zimmer K., Meyer C., Gebauer G.2008The ectomycorrhizal specialist orchid Corallorhiza trifida is a partial myco-heterotroph. New Phytol. 178, 395–400 (doi:10.1111/j.1469-8137.2007.02362.x) [DOI] [PubMed] [Google Scholar]
- Zimmerman J. K., Ehleringer J. R.1990Carbon isotope ratios are correlated with irradiance levels in the Panamanian orchid Catasetum viridiflavum. Oecologia 83, 247–249 (doi:10.1007/BF00317759) [DOI] [PubMed] [Google Scholar]